Abstract
Polymers with a low dielectric constant (Dk) are promising materials for high-speed communication networks, which demand exceptional thermal stability, ultralow Dk and dissipation factor, and minimum moisture absorption. In this paper, we prepared a series of novel low-Dk polyimide films containing an MCM-41-type amino-functionalized mesoporous silica (AMS) via in situ polymerization and subsequent thermal imidization and investigated their morphologies, thermal properties, frequency-dependent dielectric behaviors, and water permeabilities. Incorporating 6 wt.% AMS reduced the Dk at 1 MHz from 2.91 of the pristine fluorinated polyimide (FPI) to 2.67 of the AMS-grafted FPI (FPI-g-AMS), attributed to the free volume and low polarizability of fluorine moieties in the backbone and the incorporation of air voids within the mesoporous AMS particles. The FPI-g-AMS films presented a stable dissipation factor across a wide frequency range. Introducing a silane coupling agent increased the hydrophobicity of AMS surfaces, which inhibited the approaching of the water molecules, avoiding the hydrolysis of Si–O–Si bonds of the AMS pore walls. The increased tortuosity caused by the AMS particles also reduced water permeability. All the FPI-g-AMS films displayed excellent thermooxidative/thermomechanical stability, including a high 5% weight loss temperature (>531 °C), char residue at 800 °C (>51%), and glass transition temperature (>300 °C).
1. Introduction
Recently, the remarkable progress of electronic devices capable of high-speed data communication has further increased the demand for electronic substrates with a low dielectric constant (Dk) and high electrical insulation properties [1,2,3]. Reducing the permittivity of materials with electrical insulation properties can improve signal transmission speed and efficiency, which is an effective way to promote the development of high-frequency and high-speed flexible circuit boards [4]. Aromatic polyimides (PIs) have been broadly used as insulating layers and electronic packages in the microelectronic industry and have been proposed as potential candidates for printed circuit boards, flexible display screens, and next-generation interlayer dielectric materials because of their excellent thermal stability, mechanical strength, dielectric properties, and chemical resistance [5,6]. However, conventional PIs have relatively high dielectric constant (Dk) values, ranging from 3.0 to 3.5, owing to the polarization due to their polar imide groups [7,8]. Moreover, hydrophilic moieties, such as carbonyl and amine groups, facilitate the penetration of water molecules into PIs in the ambient atmosphere, leading to an increase in the dielectric constant and functional deterioration [9,10]. Generally, the water absorption rates of the aromatic PI are relatively high, at 2.51% [11], and other examples are 1.91% for 2,2-bis(4-cyanatophenyl)propane [12], 3.4% for CYCOM 977-2 epoxy resin [13], and 10% for polybenzimidazole (PBI) [14]. In contrast, the water absorption rates of poly(ethylene terephthalate) (PET) and poly(phenylene sulfide) (PPS) are relatively low, at 1.1% [15] and 0.026% [16], respectively. Despite its low percentage, water absorption has an obvious influence on the dielectric properties because water molecules have a much higher dielectric constant (Dk = 80) than PIs [5,17,18].
Reducing the density or polarizability are two effective strategies for minimizing the Dk of PIs. It was demonstrated that introducing bulky fluorinated substituents into the backbone can significantly reduce the Dk of PIs due to the strong electronegativity and low atomic polarizability of fluorine [19,20]. In addition, the introduction of fluorine endows PIs with good organo-solubility and lowers water absorption [21]. Nonetheless, fluorinated polyimides (FPIs) generally have poor thermomechanical stability and high chemical sensitivity, which are particularly troublesome for low-Dk materials. Under such circumstances, organic/inorganic hybrids have evolved as an effective alternative owing to their tunable physical properties provided the prudent selection of fillers.
Mesoporous silica, such as polyhedral oligomeric silsesquioxanes, Santa Barbara Amorphous-15, or Mobil Composition of Matter No. 41 (MCM-41), has emerged as a research hotspot for reducing Dk and improving other properties [22,23,24]. Among them, MCM-41 exhibits an intrinsically low Dk of 1.4–2.1 due to its regular arrangement of cylindrical mesopores (3–4 nm) that form a one-dimensional pore system. Furthermore, MCM-41 presents a unique opportunity for preparing organic/inorganic hybrids with an inorganic core containing Si–O–Si bonds and organic functional groups on the periphery, which can increase compatibility with the polymer matrix [25]. Against this background, the introduction of cross-linked MCM-41 could be an effective solution to reduce the Dk and enhance other indispensable properties, including water resistance and thermomechanical stability. The cross-linked structure of MCM-41 can effectively restrict the segmental mobility of FPI chains and prevent the molecular segments from being oriented along the external electric field direction, thus reducing the Dk of FPIs.
In this study, we prepared organic/inorganic hybrids of an FPI and amino-functionalized mesoporous silica MCM-41 (AMS) by in situ polymerization and examined their dielectric properties, including the permittivity and dissipation factor (Df). The Dk at 1 MHz decreased to an ultralow value of 2.67 with a systematic addition of the AMS, with a promisingly low Df. Furthermore, we investigated the influence of AMS particles on the water absorption and thermomechanical stability of the FPI.
2. Materials and Methods
2.1. Materials
Sodium silicate solution (~10.6% Na2O and ~26.5% SiO2), cetyltrimethylammonium bromide (CTAB, >98%), and (3-aminopropyl)triethoxysilane (APTES, >99%) were purchased from Sigma-Aldrich (Saint Louis, MO, USA); 4,4′-(hexafluoroisopropylidene) diphthalic anhydride (6FDA, >98.0%) and 4,4′-oxydianiline (ODA, >98%) were purchased from Tokyo Chemical Industry, Co., Ltd. (Tokyo, Japan); and 1-methyl-2-pyrrolidinone (NMP, >99%), toluene (≥99.5%), ethyl alcohol (>99%), acetic acid (>99%), and hydrochloric acid (HCl, 37%) were purchased from Duksan Chemical Co., Ltd. (Incheon, Republic of Korea). Deionized water was obtained from a Milli-Q Ultrapure water purification system (MilliporeSigma, Burlington, Massachusetts, USA). All the chemicals were used without further purification.
2.2. Synthesis of NH2-MCM-41
To synthesize NH2-MCM-41, 3.25 g of CTAB was dissolved in 38 mL of deionized water at 40 °C. Then, 13.9 g of sodium silicate solution was slowly added to the system to induce silicate condensation. Subsequently, the mixture was heated at 100 °C for 24 h under static conditions and then cooled for easy handling, after which its pH was adjusted to 10 with a 50% acetic acid solution. The reaction and pH adjustment were repeated two more times, followed by washing and filtering with deionized water. The product was placed in an oven at 100 °C for 24 h. The sample was subsequently washed and filtered three times using a solution containing 2.5 g of HCl in 100 mL of ethyl alcohol and then dried and calcined at 550 °C in a furnace to remove the CTAB template. The mixture containing 2.5 g of synthesized silica and 2.5 g of APTES was refluxed in 100 mL of toluene for 24 h to obtain the amino-functionalized mesoporous silica MCM-41. The product was washed and filtered three times with ethanol and dried in the oven.
2.3. Synthesis of the NH2-MCM-41-Grafted Fluorinated Polyimide Films
A measured quantity of NH2-MCM-41 (0, 0.5, 1, 3, and 6 wt.%) was dispersed in NMP by sonication for 30 min. Stoichiometric equivalent amounts of ODA (0.6 g, 3 mmol) and 6FDA (1.33 g, 3 mmol) were added into the NH2-MCM-41/NMP suspension. The resulting mixture (11–16 wt.% solid in anhydrous NMP) was mechanically stirred for 24 h in an N2 atmosphere to produce a viscous NH2-MCM-41-grafted fluorinated poly(amic acid) (FPAA) intermediate solution. Subsequently, the homogeneous solution was cast onto a clean glass substrate and thermally cured under a sequential temperature programming (90 °C/2 h, 150 °C/1 h, 200 °C/1 h, 250 °C/30 min, 300 °C/30 min, and 400 °C/30 min). Finally, the resultant NH2-MCM-41-grafted FPI films were self-stripped from the glass substrate by soaking in deionized water and further dried at 80 °C for 24 h in a convection oven.
2.4. Measurements
Transmission electron microscopy (TEM) was performed using a Titan G2 80–200 instrument (FEI, Hillsboro, OR, USA) with ChemiSTEM technology. Scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDS, Ultim Extreme, Oxford Instruments, Abingdon, Oxfordshire, UK) was performed using an SU8220 microscope (Hitachi, Tokyo, Japan) at an acceleration voltage of 10 kV. Fourier-transform infrared (FTIR) spectra were obtained with a Nicolet iS5 (Thermo Fisher Scientific Inc., Waltham, MA, USA). X-ray diffraction (XRD) was conducted on an Empyrean X-ray diffractometer (Malvern Panalytical Ltd, Malvern, UK) with Cu Kα radiation (λ = 1.54 Å). Thermogravimetric analysis (TGA) was conducted with an SDT 650 (TA Instruments, New Castle, DE, USA) under N2 flow at a heating rate of 20 °C/min. Dynamic mechanical analysis (DMA) was conducted with a DMA 850 (TA Instruments) at a heating rate of 3 °C/min with a load frequency of 1 Hz in air. Dielectric properties were measured by a 4294A impedance analyzer (Agilent, Santa Clara, CA, USA) with a 16451B dielectric test fixture (Agilent, Santa Clara, CA, USA) in the frequency range of 40 Hz to 30 MHz at 25 °C. The dielectric tests were performed following the ASTM D150 standard [26]. The dielectric constant (ε′) was calculated as per Equation (1).
where ε0 is the vacuum permittivity (8.85·10−12 F m−1), Cm is the measured capacitance, A is the electrode area, d is the electrode diameter, and t is the film thickness.
Water vapor transmission rates (WVTRs) were measured using a PERMATRAN-W 3/61 (Ametek Mocon, Brooklyn Park, Minnesota, USA) instrument at 38 °C and relative humidity of 100%, following the ASTM F-1249 standard [27]. The WVTR data were normalized to the film thickness to obtain the water vapor permeability (WVP) using Equation (2).
where l is the average film thickness, P is the water vapor pressure at 38 °C, and R1 and R2 represent the moisture gradients.
Surface wettability was analyzed by measuring the Young–Laplace’s contact angle using the sessile drop method at ambient temperature (~23 °C) in the air by Phoenix300 (SEO, Suwon, Republic of Korea). The contact angle was recorded with a droplet volume of 3 μL after 3 s from droplet deposition, with at least five replicate samples. For each reported value, all the measurements taken from different surface locations were averaged, and the standard deviation was calculated.
3. Results and Discussion
The AMS was synthesized via the hydrolysis and condensation of silicate precursors in the presence of cationic surfactants under basic conditions, followed by the post-grafting of organic grapes, as shown in Figure 1a. The TEM micrograph of the AMS particles demonstrates a parallel channel-like porous structure with a regular hexagonal arrangement, which is representative of MCM-41 (Figure 1b). The surface modification of the AMS seems to hardly affect the hexagonal porous structure of MCM-41. The XRD patterns of the non-functionalized mesoporous silica (MS) and AMS are displayed in Figure 1c. Three well-resolved diffraction peaks of (100), (110), and (200) suggest good crystallinity with the ordered hexagonal mesoporous architecture of MS [28,29]. The small diffraction peaks of (110) and (200) were not found in the XRD pattern of AMS, indicating that –NH2 groups on the AMS surface increased the structural disorder. However, the original honeycomb-patterned mesoporous scaffold was not destroyed during the post-grafting amination. Thermogravimetric weight loss curves for MS and AMS are shown in Figure 1d. The TGA curve of the AMS exhibits three weight loss intervals that are distinct from MS: (i) desorption of the water linked to the silica surface in the range of 50–150 °C, (ii) fragmentation of the organic functionalities attached to the AMS surface in the range of 200–600 °C, and (iii) mesoporous structure disruption above 600 °C [24,30].
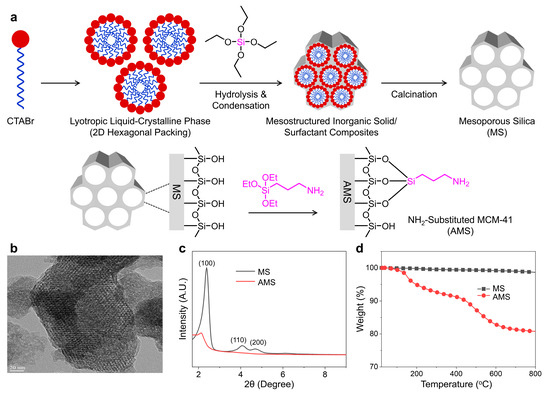
Figure 1.
(a) Schematic illustration of amino-functionalized mesoporous silica (AMS) via the sol–gel reaction catalyzed in a basic medium and post-synthesis grafting method. (b) Transmission electron microscopy of AMS. (c) X-ray diffraction (XRD) patterns and (d) thermogravimetric analysis (TGA) curves of non-functionalized mesoporous silica (MS) and AMS.
Figure 2 shows an overview of the synthesis scheme for the FPI-g-AMS films via in situ polymerization. To achieve a high-molecular-weight FPI, the FPAA intermediate precursor was synthesized using tetracarboxylic dianhydride 6FDA and ODA in a stoichiometric equivalent ratio. The FPAA chain grafting of AMS resulted from the reaction of the amino end group of the AMS with the anhydride-chain end group of the FPAA backbone. The FPAA-g-AMS was dissolved in NMP to form a viscous homogeneous solution. After bar-coating this solution onto a clean glass substrate, the imidization was performed by a step-cure process (90 °C/2 h, 150 °C/1 h, 200 °C/1 h, 250 °C/30 min, 300 °C/30 min, and 400 °C/30 min). The thickness of the cured FPI-g-AMS films was ca. 60 µm.
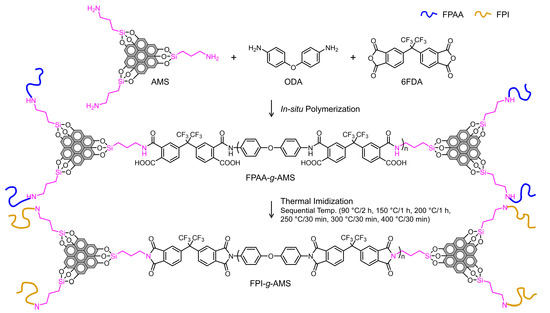
Figure 2.
Synthesis of AMS-grafted fluorinated polyimide films.
The FTIR spectra of the FPI-g-AMS films are presented in Figure 3a. The characteristic absorption peaks at 1784 cm−1 and 1718 cm−1 were observed for all samples, corresponding to asymmetric (imide I) and symmetric (imide II) stretching vibrations of C=O, respectively. The absorption peak near 1370 cm−1, associated with C–N stretching vibration (imide III), appeared after the thermal imidization of the FPAA-g-AMS precursor [31]. Meanwhile, the absorption peak at 1650 cm−1, assigned to amide carbonyl stretching vibration (amide I), disappeared, indicating the complete conversion of the amic acid into imide bonds [32]. The absorption bands arising from the –CF3 group of the 6FDA moiety are located in the region of 1320–1170 cm−1, which overlaps with the peak of the Si–O–Si asymmetric stretching vibration in the FPI-g-AMS films [33]. Additionally, the absorption peak resulting from the stretching vibration of Si–O–Si bonds in AMS around 1070 cm−1 is overlapped by the C–H stretching vibration band in the PI backbone [34,35]. Nonetheless, the peak intensity of Si–O–Si stretching was slightly enhanced with the increasing AMS loading, confirming that the AMS is present in the resulting FPI-g-AMS films.
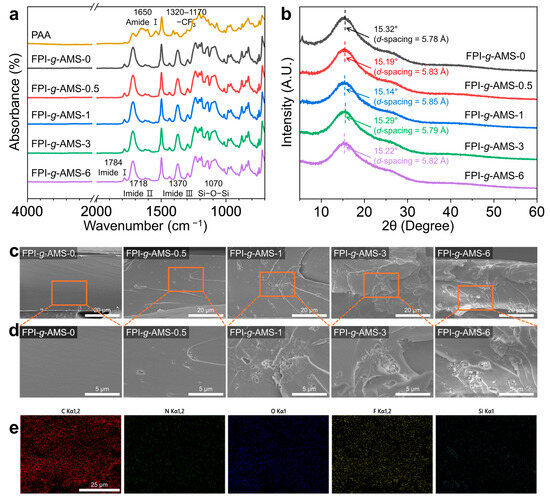
Figure 3.
(a) Fourier-transform infrared spectra and (b) XRD patterns of the FPI-g-AMS films. (c) Low-magnification and (d) high-magnification SEM fracture surface morphologies of the FPI-g-AMS films. (e) Elemental mapping images of the FPI-g-AMS-3 film.
The FPI-g-AMS films exhibited broad halos in the range of 10–30° (2θ), which is attributed to their amorphous nature (Figure 3b). The average interchain distance (d-spacing) of all the samples was calculated from the position of the respective maxima using Bragg’s equation. The interchain d-spacing slightly increased from 5.78 Å for pristine FPI-g-AMS-0 to 5.85 Å for FPI-g-AMS-1, which can be attributed to the increased free volume and loose distribution of PI chains.
Figure 3c,d shows the SEM images of the cross-sectional morphologies of the FPI-g-AMS films. Pristine FPI-g-AMS-0 reveals a relatively smooth and flat fracture surface. However, the fracture surface roughness of the FPI-g-AMS films increased with AMS loading, suggesting that the AMS distorted the path of the crack tip, impeding crack propagation [36]. In addition, the SEM results show that AMS particles are distributed uniformly in the FPI matrix up to 3 wt.% AMS loading. For a highly loaded system (FPI-g-AMS-6), only a little agglomeration was observed in the FPI matrix. The EDX elemental mapping images further demonstrate that AMS particles are homogeneously dispersed in the FPI matrix (Figure 3e), confirming that amino-functionalization enhances the interfacial interaction between the fillers and the polymer chains.
The thermal degradation behaviors of the FPI-g-AMS films are shown in Figure 4a and Table S1. It can be seen that the thermal degradation of all the samples occurs through a single stage in the temperature range of 100–800 °C, which indicates a favorable phase interconnection between the AMS and the FPI matrix. The pristine FPI-g-AMS-0 film started to decompose by 5% (Td5%) at 539 °C and retained 57% char residue at 800 °C. The Td5% values of the FPI-g-AMS films were in the range of 531–536 °C, similar to the pristine FPI-g-AMS-0 film. However, the thermal degradation of the FPI-g-AMS films was accelerated above 600 °C, and char residue at 800 °C slightly decreased with increasing AMS loading. This could be ascribed to the relatively higher thermal conductivity of the mesoporous silica compared to that of the FPI matrix.
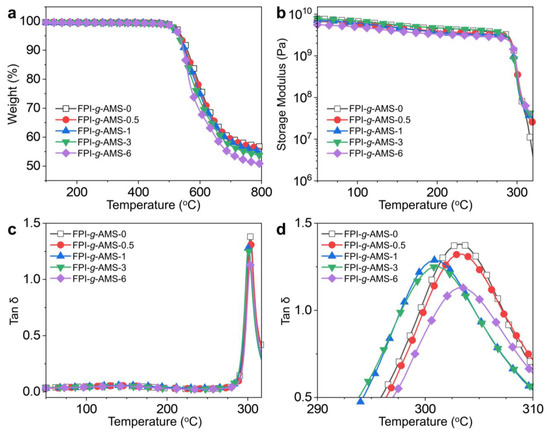
Figure 4.
(a) TGA curves, (b) storage modulus, (c) tan δ, determined by dynamic mechanical analysis, and (d) magnification region of tan δ in the range of 290–310 °C for the FPI-g-AMS-3 film.
Figure 4b–d and Table S1 demonstrate the temperature dependence of the viscoelastic behaviors of the FPI-g-AMS films. The DMA curves for all the samples exhibit glass-to-rubber transitions. As shown in Figure 4b, all the samples had constant dynamic storage moduli (E’) before the glass transition temperature (Tg), reflecting the solid-like properties of the rigid imide backbone. However, above the Tg, the increase in E’ with the addition of AMS is significant, which can be attributed to the good interfacial interaction between the AMS and the FPI matrix. Figure 4c,d show the loss factor (tan δ) of the FPI-g-AMS films. The pristine FPI-g-AMS-0 and FPI-g-AMS films exhibit similar Tg values regardless of the AMS loading. Nevertheless, as the AMS loading in the FPI-g-AMS films increases, the peak intensity of tan δ declines, as clearly shown in Figure 4d. These results suggest weak relaxation of FPI chains near the surface of the AMS around Tg, due to strong filler–polymer interactions.
The frequency dependences of the Dk and Df of the FPI-g-AMS films are shown in Figure 5 and Table S2. The dielectric constants of all the samples declined slightly with increasing frequency. Typically, the Dk of polymeric materials gradually decreased as the frequency increased at constant temperature. This is because the orientation of the dipole moment was not fast enough to keep up with the oscillations of the applied alternating electric field at high frequencies. The dielectric constants of the FPI-g-AMS films at 1 MHz showed a conspicuous decline with increasing AMS loading, i.e., from 2.91 of the pristine FPI-g-AMS-0 film to 2.67 of FPI-g-AMS-6. The dielectric constant was closely related to molecular-orientation-dependent polarization. The well-defined mesoporous architecture of the FPI-g-AMS films considerably reduced the number of polarizing molecules per unit volume. On the other hand, the increase in interfacial polarization due to the high concentration of AMS (>3 wt.%) suppressed the reduction effect of the Dk in the FPI-g-AMS films. This polarization enabled the charged particles to overcome the energy loss caused by the thermal motion induced by the electric field. The dissipation factor quantifies the amount of energy absorbed or dissipated as heat within a dielectric material when subjected to an electric field. The Df also exhibited frequency-dependent characteristics. At high frequencies, the rapid reversal of the polarization direction led to greater energy dissipation as heat, increasing the Df of the polymer. Figure 5b shows that the FPI-g-AMS films had stable and low Df values (~0.0026 at 1 MHz) over a wide frequency range, from 103 Hz to 10 MHz. This observation is attributed to the rigid segmental dynamics of FPI chains grafted to AMS particles.
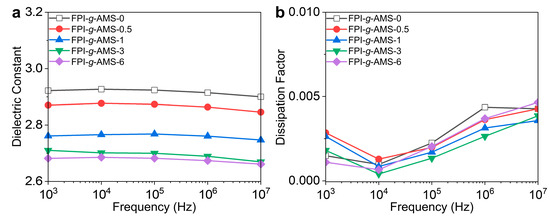
Figure 5.
Frequency-dependent (a) dielectric constant (Dk) and (b) dissipation factor (Df) of the FPI-g-AMS films at room temperature.
Water absorption is a major factor that degrades the electrical performance and reliability of low-Dk polymeric materials in electronic devices, increasing current leakage [37]. The water barrier properties of the FPI-g-AMS films were investigated using surface static water contact angle and WVP analysis. The experimental WVP data calculated from the WVTR was fit to the solution of Fick’s second law of diffusion in Figure 6b, assuming the water vapor concentration to be close to zero at the exit side of the films, as follows [38].
where P° = Jd/p, P = Jsd/p = SD/p, J and Js denote the water vapor flux at time t at steady state, d is the sample thickness, p is the differential water vapor pressure, P is permeability, D is diffusivity, and S is solubility.
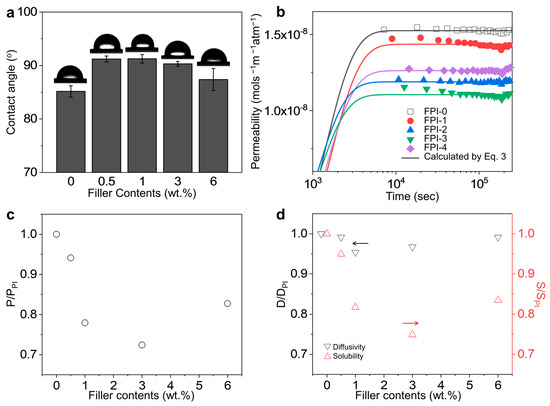
Figure 6.
(a) Surface static water contact angles of the FPI-g-AMS films. (b) Water vapor permeability of the FPI-g-AMS films as a function of time compared to the model calculated by Equation (3). Filler effects on the FPI-g-AMS films: (c) permeability and (d) diffusivity and solubility. Accordingly, the y-axis for diffusivity is on the left in black, while the y-axis for solubility is on the right in red. Each arrow indicates the direction of its respective y-axis.
The permeability, diffusivity, and solubility of the FPI-g-AMS films determined from the best fitting of the model calculations are summarized in Table S3. According to the solution–diffusion model, condensable vapors could cause increased polymer chain mobility and plasticization, thus dissolving the rigidified polymer chains at the filler–polymer interface and diffusing into the nm-scale pores of the AMS particles. In addition, once the water vapor enters large-diameter pores, the diffusion rates can be increased dramatically. The WVP of the pristine FPI-g-AMS-0 film is 1.52 × 10–8 mol s−1 m−1 atm−1, as shown in Figure 6b and Table S3. The addition of AMS significantly influenced the WVP values of the FPI-g-AMS films. The largest reduction in WVP (1.10 × 10–8 mol s−1 m−1 atm−1) was observed at 3 wt.% AMS. This could be attributed to the increase in the hydrophobicity of the AMS surfaces by APTES moieties, thus inhibiting the approaching of the water molecules and avoiding the hydrolysis of the Si–O–Si bonds of the AMS pore walls [39]. The increased tortuosity caused by the AMS particles also reduced water permeability. Even though they could diffuse into the AMS pores, the increase in the diffusion path lengths when they traversed the film led to reduced permeabilities. For FPI-g-AMS-6, because of the agglomerated large size of the AMS particles, the AMS surface could not be wrapped perfectly by the FPI chains, leading to the formation of interfacial voids and defects. The water vapor had a tendency to diffuse through these non-selective voids, resulting in higher permeability in FPI-g-AMS-6. These explanations clearly follow from the permeability, diffusivity, and solubility behaviors according to the AMS concentration, as shown in Figure 6b–d. The solubility behavior is similar to that of permeability, whereas the diffusivity of all the samples is nearly identical. This result indicates that when incorporating AMS particles into the FPI matrix, the difference in permeability is ascribed to the solubility rather than the pathway tortuosity effect. The surface static water contact angle also strongly depends on the AMS loading (Figure 6a). We observed that the contact angles of the FPI-g-AMS films were larger than those of the pristine FPI-g-AMS-0 film. However, at 3 wt.% AMS, the contact angle decreased due to the formation of polymer–filler interfacial voids and defects caused by AMS agglomeration. These results are consistent with the diffusion behavior of the water molecules, as shown in Figure 6b–d.
4. Conclusions
We successfully prepared novel low-Dk FPI films containing an MCM-41-type AMS, which exhibited an ultralow dielectric constant of ~2.69 and a very low dissipation factor of ~0.0026 at 1 MHz. The surface of the FPI-g-AMS films exhibited obvious hydrophobic characteristics that endowed the films with outstanding water resistance, with FPI-g-AMS-3 showing a remarkably low water permeability of 1.10·10−8 mol s−1 m−1 atm−1. This could be attributed to an increase in the hydrophobicity of AMS surfaces by the APTES moieties, thus inhibiting the approaching of the water molecules and avoiding the hydrolysis of the Si–O–Si bonds of the AMS pore walls. The increased tortuosity caused by the AMS particles also reduced water permeability. In addition, the FPI-g-AMS films showed high thermooxidative/thermomechanical stability, including a high 5% weight loss temperature (>531 °C), char residue at 800 °C (>51%), and glass transition temperature (>300 °C). Therefore, the introduction of a hydrophobic cross-linked organic/inorganic hybrid network is beneficial to effectively reduce the Dk, improve water resistance, and simultaneously maintain the overall physical properties of PIs. The proposed FPI-g-AMS films showed great potential in the applications of next-generation dielectric materials in the microelectronic industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym16121716/s1, Table S1. Thermal and thermooxidative behaviors of the FPI-g-AMS films; Table S2. Dielectric constant (Dk) and dissipation factor (Df) of the FPI-g-AMS films at a frequency of 1 MHz; Table S3. Water barrier properties of the FPI-g-AMS films.
Author Contributions
Conceptualization, J.S.; Investigation, J.S., H.P. and J.H.P.; Writing—original draft, J.S.; Writing—review & editing, K.-H.N.; Visualization, M.K.; Supervision, K.-H.N. and J.-S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Kyungpook National University Research Fund, 2021.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hecht, J. The Bandwidth Bottleneck. Nature 2016, 536, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.G.; Buzzi, S.; Choi, W.; Hanly, S.V.; Lozano, A.; Soong, A.C.K.; Zhang, J.C. What Will 5G Be? IEEE J. Sel. Areas Commun. 2014, 32, 1065–1082. [Google Scholar] [CrossRef]
- Maex, K.; Baklanov, M.R.; Shamiryan, D.; Iacopi, F.; Brongersma, S.H.; Yanovitskaya, Z.S. Low Dielectric Constant Materials for Microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.Y.; Sun, Y.G.; Li, W.Z.; Zhang, X.S.; Luan, J. Synthesis and Applications of Low Dielectric Polyimide. Resour. Chem. Mater. 2023, 2, 49–62. [Google Scholar] [CrossRef]
- Bei, R.; Qian, C.; Zhang, Y.; Chi, Z.; Liu, S.; Chen, X.; Xu, J.; Aldred, M.P. Intrinsic Low Dielectric Constant Polyimides: Relationship between Molecular Structure and Dielectric Properties. J. Mater. Chem. C 2017, 5, 12807–12815. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Yu, W.; Yuan, Z.; Kang, E.T.; Neoh, K.G.; Krauter, B.; Greiner, A. Nanoporous Low-κ Polyimide Films via Poly(Amic Acid)s with Grafted Poly(Ethylene Glycol) Side Chains from a Reversible Addition-Fragmentation Chain-Transfer-Mediated Process. Adv. Funct. Mater. 2004, 14, 471–478. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Han, E.; Niu, H.; Wu, D. Structure-Property Relationship of Low Dielectric Constant Polyimide Fibers Containing Fluorine Groups. Polymer 2020, 206, 122884. [Google Scholar] [CrossRef]
- Kim, S.; Son, J.; Park, H.; Jeong, E.; Nam, K.H.; Bae, J.S. Polymer Concentration and Liquid—Liquid Demixing Time Correlation with Porous Structure of Low Dielectric Polyimide in Diffusion-Driven Phase Separation. Polymers 2022, 14, 1425. [Google Scholar] [CrossRef]
- Song, N.; Shi, K.; Yu, H.; Yao, H.; Ma, T.; Zhu, S.; Zhang, Y.; Guan, S. Decreasing the Dielectric Constant and Water Uptake of Co-Polyimide Films by Introducing Hydrophobic Cross-Linked Networks. Eur. Polym. J. 2018, 101, 105–112. [Google Scholar] [CrossRef]
- Wu, T.; Dong, J.; Gan, F.; Fang, Y.; Zhao, X.; Zhang, Q. Low Dielectric Constant and Moisture-Resistant Polyimide Aerogels Containing Trifluoromethyl Pendent Groups. Appl. Surf. Sci. 2018, 440, 595–605. [Google Scholar] [CrossRef]
- Han, Y.; Ma, Y.; Zhang, J.; Yao, S.; Xu, K. Overall Improvement in Dielectric, Water Resistance and Mechanical Properties of Polyimide Film via Synergy between GO and Sandwich-Type Porous Structure. Chem. Asian J. 2022, 18, e202201130. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Han, Y.; Guo, Y.; Liu, J.; Ding, J.; Li, Y.; Zhou, H.; Zhao, T. Thermal and Water Absorption Properties of Cyanate Ester Resins Modified by Fluoride-Containing and Silicone-Containing Components. Polym. Adv. Technol. 2020, 31, 1245–1255. [Google Scholar] [CrossRef]
- Korkees, F.; Swart, R.; Barsoum, I. Diffusion Mechanism and Properties of Chemical Liquids and Their Mixtures in 977-2 Epoxy Resin. Polym. Eng. Sci. 2022, 62, 1582–1592. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; An, L.; Zhao, G.; Yan, X.H. Physicochemical Properties of Alkaline Doped Polybenzimidazole Membranes for Anion Exchange Membrane Fuel Cells. J. Memb. Sci. 2015, 493, 340–348. [Google Scholar] [CrossRef]
- Wu, T.; Ke, Y. The Absorption and Thermal Behaviors of PET-SiO2 Nanocomposite Films. Polym. Degrad. Stab. 2006, 91, 2205–2212. [Google Scholar] [CrossRef]
- Ana, M.D.; Angel, L.D. High-Performance Aminated Poly (Phenylene Sul Fi de)/ZnO Nanocomposites for Medical Applications. ACS Appl. Mater. Interfaces 2014, 6, 10132–10145. [Google Scholar]
- Joseph, A.M.; Nagendra, B.; Surendran, K.P.; Bhoje Gowd, E. Syndiotactic Polystyrene/Hybrid Silica Spheres of POSS Siloxane Composites Exhibiting Ultralow Dielectric Constant. ACS Appl. Mater. Interfaces 2015, 7, 19474–19483. [Google Scholar] [CrossRef]
- Song, N.; Yao, H.; Ma, T.; Wang, T.; Shi, K.; Tian, Y.; Zhang, B.; Zhu, S.; Zhang, Y.; Guan, S. Decreasing the Dielectric Constant and Water Uptake by Introducing Hydrophobic Cross-Linked Networks into Co-Polyimide Films. Appl. Surf. Sci. 2019, 480, 990–997. [Google Scholar] [CrossRef]
- Li, H.; Bao, F.; Lan, X.; Li, S.; Zhu, H.; Li, Y.; Wang, M.; Zhu, C.; Xu, J. Fluorinated Polyimide with Triphenyl Pyridine Structure for 5G Communications: Low Dielectric, Highly Hydrophobic, and Highly Transparent. Eur. Polym. J. 2023, 197, 112327. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Lv, X.; Wang, K.; Yin, H.; Qiu, S.; Li, J.; Zhang, G.; Sun, R. Polyimides with Low Dielectric Constants and Dissipation Factors at High Frequency Derived from Novel Aromatic Diamines with Bistrifluoromethyl Pendant Groups. Polym. Chem. 2023, 14, 3862–3871. [Google Scholar] [CrossRef]
- Shioda, T.; Takamatsu, N.; Suzuki, K.; Shichijyo, S. Influence of Water Sorption on Refractive Index of Fluorinated Polyimide. Polymer 2002, 44, 137–142. [Google Scholar] [CrossRef]
- Chan Hwang, Y.; Khim, S.; Min Sohn, J.; Nam, K.H. Controllable Growth of Porous Morphology in Low Dielectric Polyimides via Thermal-Driven Spontaneous Phase Separation. Eur. Polym. J. 2023, 195, 112195. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X. Novel Low-κ Polyimide/Mesoporous Silica Composite Films: Preparation, Microstructure, and Properties. Polymer 2007, 48, 318–329. [Google Scholar] [CrossRef]
- Khim, S.; Hwang, Y.C.; Choi, J.; Park, H.; Nam, K.H. Temperature-Invariant Large Broadband Polyimide Dielectrics with Multimodal Porous Networks. ACS Appl. Polym. Mater. 2023, 5, 4159–4169. [Google Scholar] [CrossRef]
- Seo, K.; Nam, K.H.; Han, H. Proton Transport in Aluminum-Substituted Mesoporous Silica Channel-Embedded High-Temperature Anhydrous Proton-Exchange Membrane Fuel Cells. Sci. Rep. 2020, 10, 10352. [Google Scholar] [CrossRef] [PubMed]
- ASTM D150; Standard Test Methods for AC Loss Characteristics and Permittivity (Dielectric Constant) of Solid Electrical Insulation. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM F-1249; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2020.
- Li, X.; Han, C.; Zhu, W.; Ma, W.; Luo, Y.; Zhou, Y.; Yu, J.; Wei, K. Cr(VI) Removal from Aqueous by Adsorption on Amine-Functionalized Mesoporous Silica Prepared from Silica Fume. J. Chem. 2014, 2014, 765856. [Google Scholar] [CrossRef]
- Chen, X.; Ching, W.K.; Lam, K.F.; Wei, W.; Yeung, K.L. An Investigation of the Selective Adsorptions of Metals on Mesoporous NH2-MCM-41. J. Phys. Chem. C 2016, 120, 18365–18376. [Google Scholar] [CrossRef]
- Nhavene, E.P.F.; Andrade, G.F.; Arantes Faria, J.A.Q.; Gomes, D.A.; de Sousa, E.M.B. Biodegradable Polymers Grafted onto Multifunctional Mesoporous Silica Nanoparticles for Gene Delivery. ChemEngineering 2018, 2, 24. [Google Scholar] [CrossRef]
- Nam, K.H.; Lee, W.; Seo, K.; Han, H. Residual Stress Behavior and Physical Properties of Colorless and Transparent Polyimide Films. Polym. Korea 2014, 38, 510–517. [Google Scholar] [CrossRef]
- Pryde, C.A. IR Studies of Polyimides. I. Effects of Chemical and Physical Changes during Cure. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 711–724. [Google Scholar] [CrossRef]
- Moon, K.H.; Chae, B.; Kim, K.S.; Lee, S.W.; Jung, Y.M. Preparation and Characterization of Transparent Polyimide-Silica Composite Films Using Polyimide with Carboxylic Acid Groups. Polymers 2019, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.T.; Chen, W.C.; Liaw, D.J.; Lu, H.Y. Synthesis and Properties of New Polyimide-Silica Hybrid Films through Both Intrachain and Interchain Bonding. Polymer 2003, 44, 7079–7087. [Google Scholar] [CrossRef]
- Zou, L.; Roddecha, S.; Anthamatten, M. Morphology, Hydration, and Proton Transport in Novel Sulfonated Polyimide-Silica Nanocomposites. Polymer 2009, 50, 3136–3144. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Li, L.; Zhou, J. In Situ Synthesis and Mechanical, Thermal Properties of Polyimide Nanocomposite Film by Addition of Functionalized Graphene Oxide. Polym. Compos. 2016, 37, 907–914. [Google Scholar] [CrossRef]
- Jiang, L.-Y.; Leu, C.-M.; Wei, K.-H. Layered Silicates / Fluorinated Polyimide. Adv. Mater. 2002, 14, 426–429. [Google Scholar] [CrossRef]
- Jin, J.U.; Lee, D.H.; Nam, K.H.; Yu, J.; Kim, Y.K.; Goh, M.; Kim, S.G.; Lee, H.S.; Ku, B.C.; You, N.H. Methylpiperidine-Functionalized Graphene Oxide for Efficient Curing Acceleration and Gas Barrier of Polymer Nanocomposites. Appl. Surf. Sci. 2019, 464, 509–515. [Google Scholar] [CrossRef]
- Martin, P.; Rafti, M.; Marchetti, S.; Fellenz, N. MCM-41-Based Composite with Enhanced Stability for Cr(VI) Removal from Aqueous Media. Solid State Sci. 2020, 106, 106300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).