Effect of Aminating Lignin Loading with Arbuscular Mycorrhizal Fungi on Soil Aggregate Structure Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Analysis of Soil Aggregate Structure
2.3.1. Determination of the pH Value of Soil
2.3.2. Calculation of Soil Bulk Density
2.3.3. Calculation of Soil Porosity
2.3.4. Soil Water Stability Aggregate Distribution Test
2.4. Aminating Modification of Lignin
2.4.1. Purification of Hydrolysis Lignin
2.4.2. Epoxidation of Lignin
2.4.3. Amination of Epoxy Lignin
2.4.4. FTIR Analysis
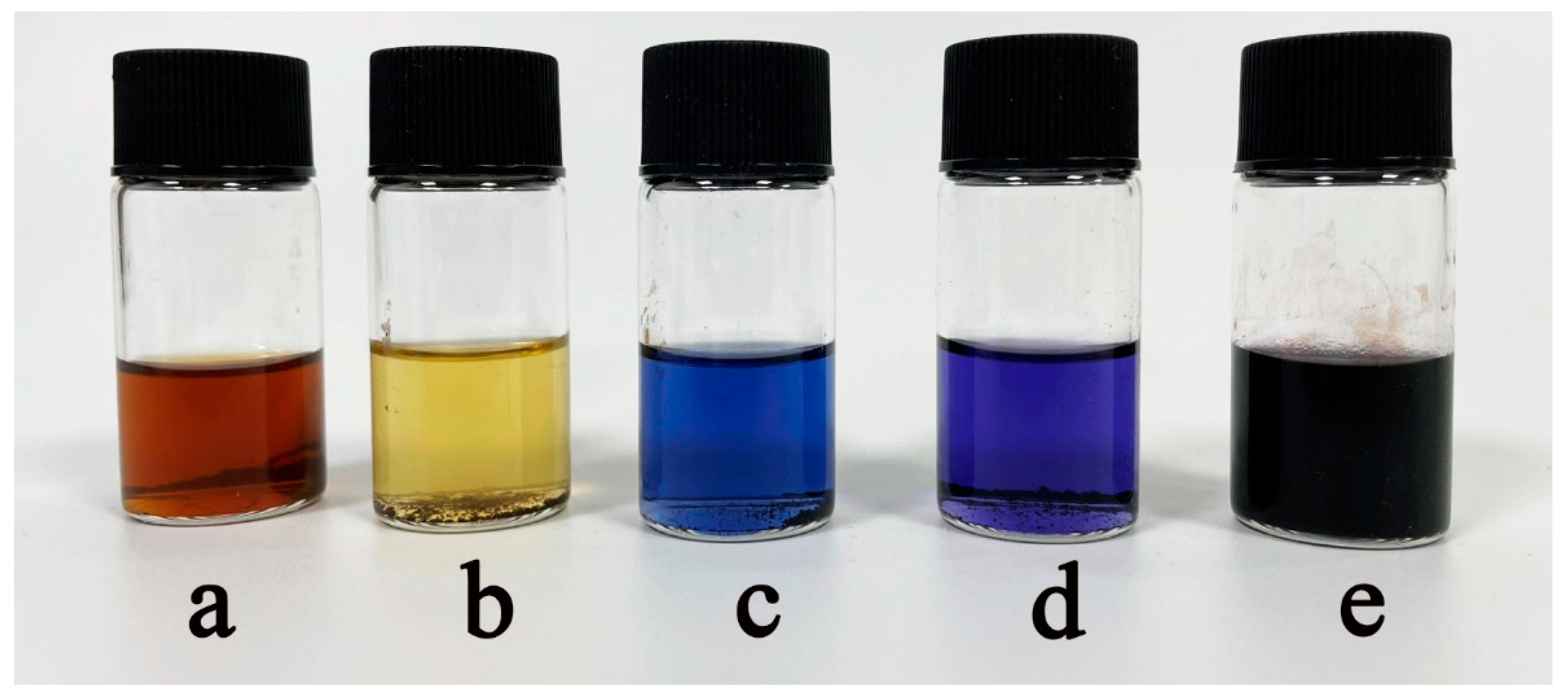
2.4.5. Ninhydrin Test
2.4.6. Zeta Potential
2.5. Loading of Arbuscular Mycorrhizal Fungi
FTIR Analysis
2.6. Investigate the Influence of Different Samples on Soil Aggregate Structure
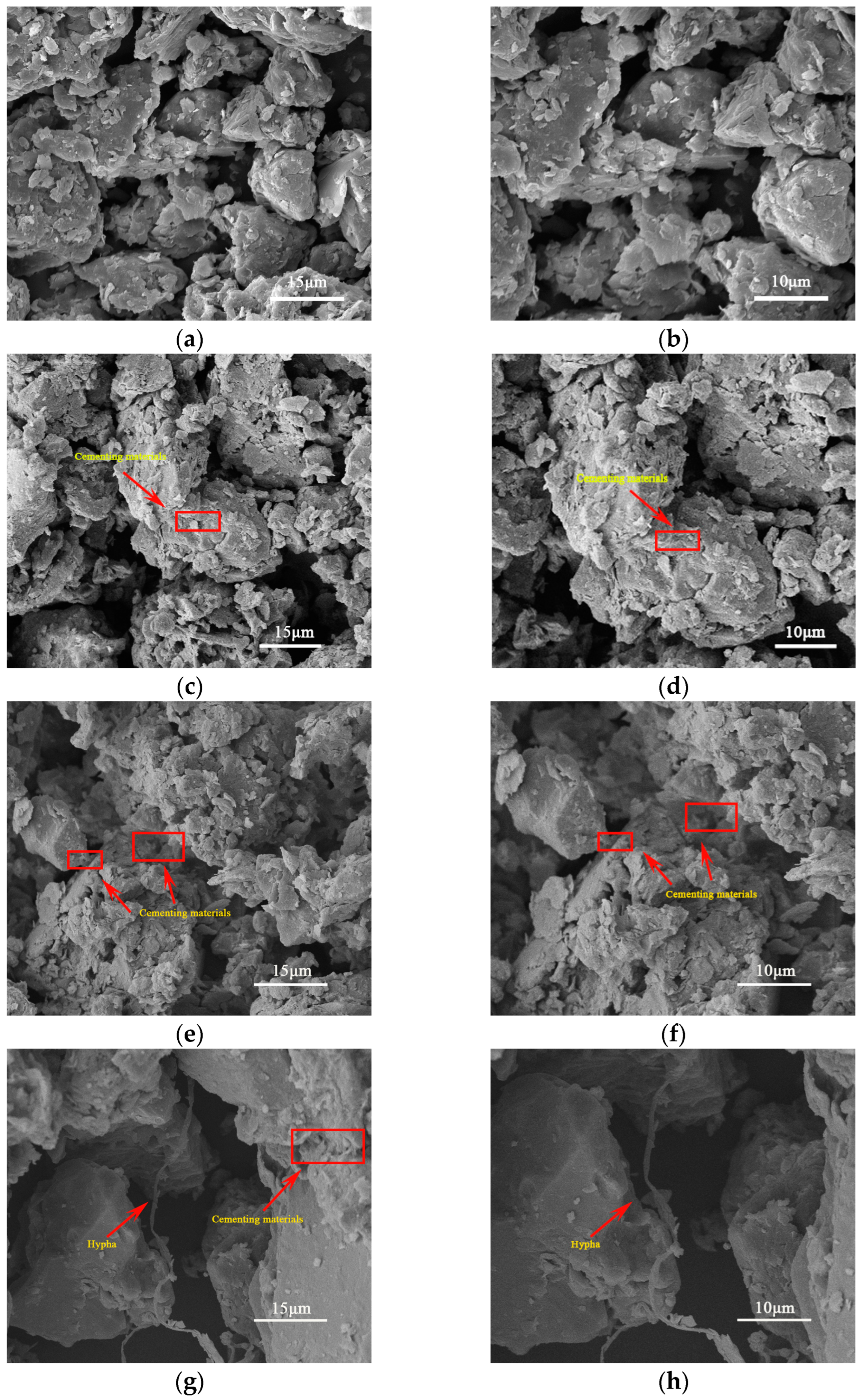
2.6.1. Characterization of Soil Microscopic Morphology
2.6.2. Pretreatment of Potting Soil
2.6.3. Corn Seed Budding
2.6.4. Pot Experiment
2.6.5. Calculation of Soil Bulk Density and Soil Porosity
2.6.6. Determination of Apparent Biomass of Maize Plants
2.6.7. Statistical Analysis
3. Results and Discussion
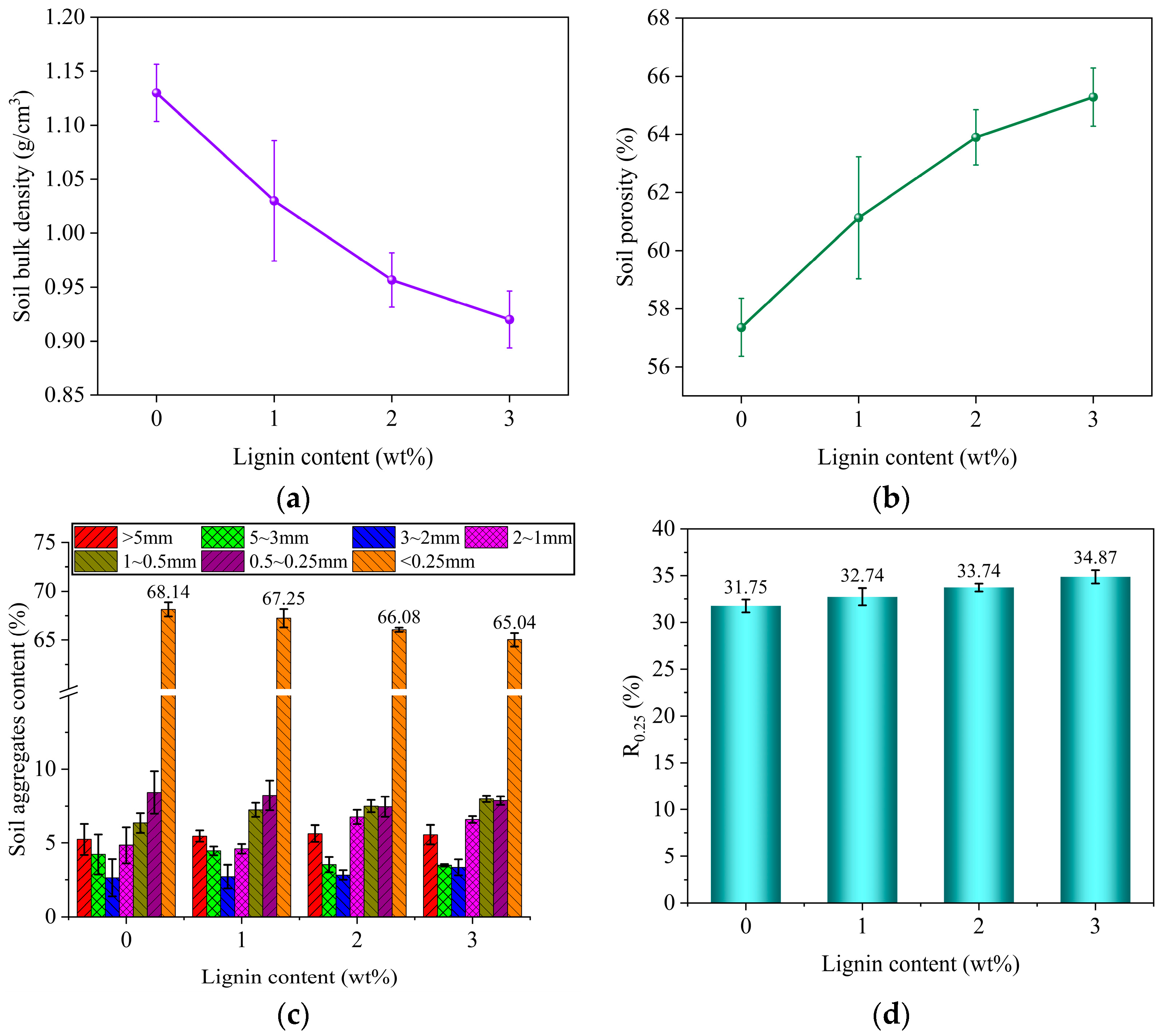
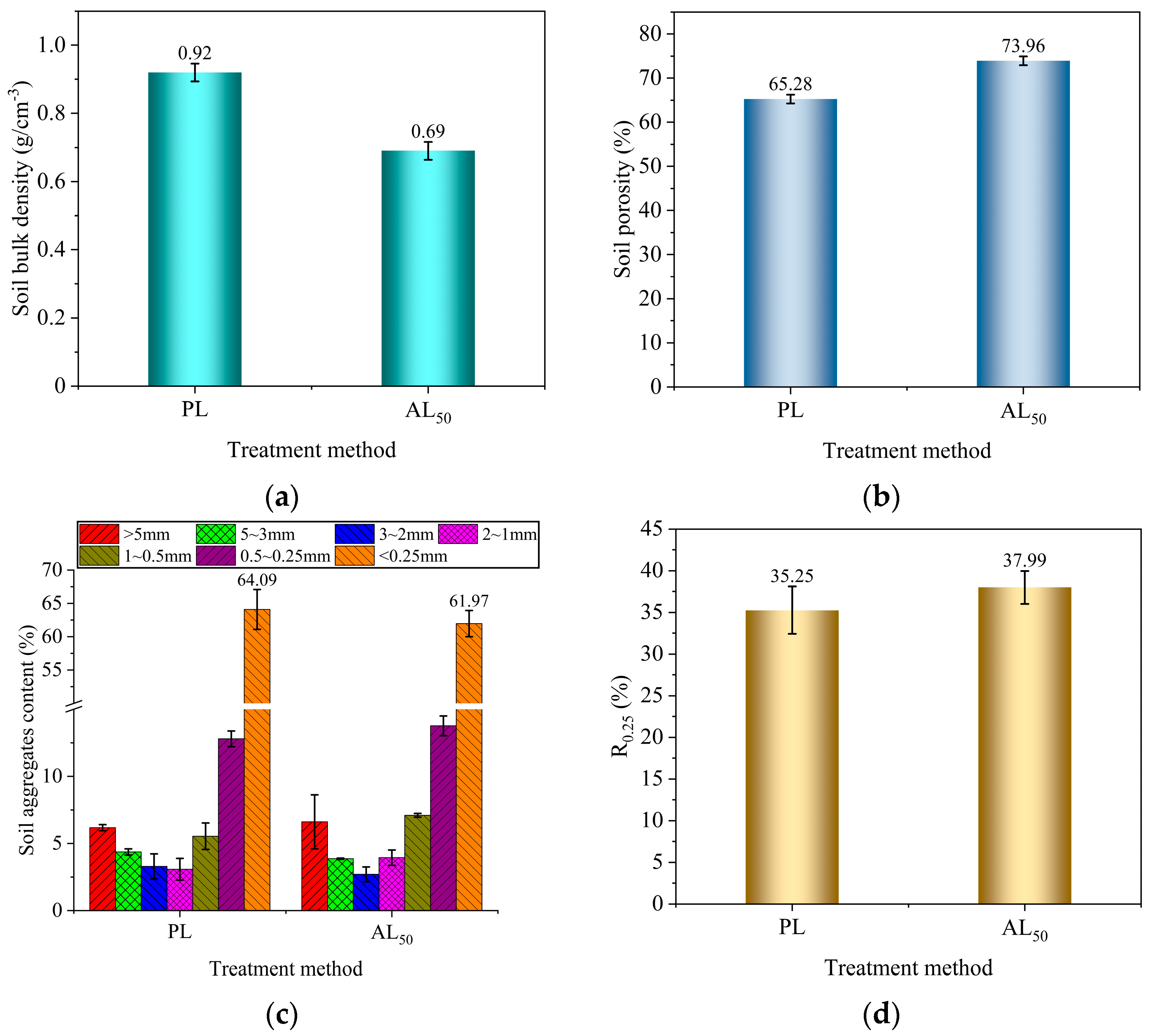
3.1. Analysis of Soil Aggregate Structure
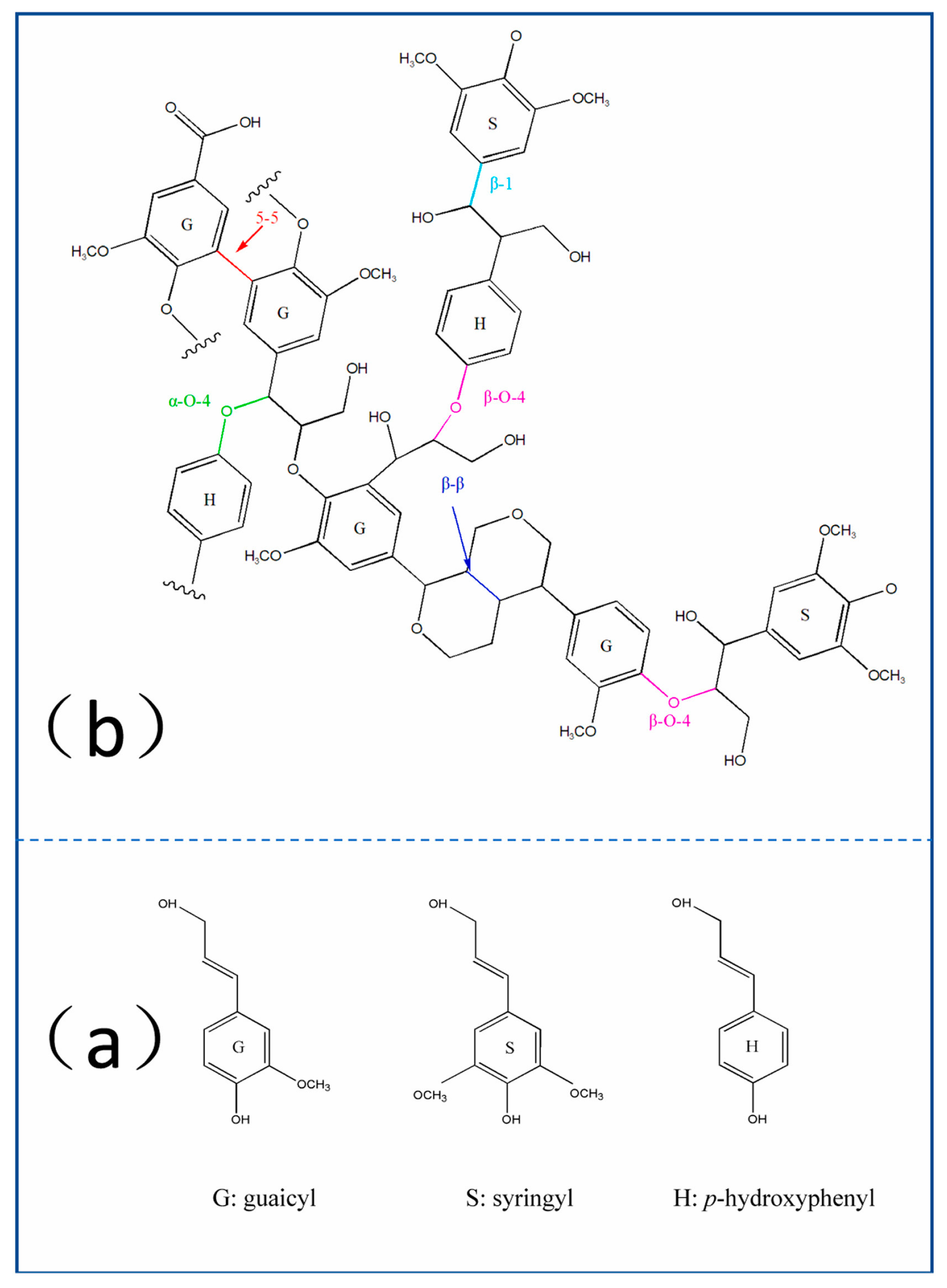
3.2. Aminating Modification of Lignin
3.3. Immobilization of Arbuscular Mycorrhizal Fungi
3.4. Investigation of the Influence of Different Samples on Soil Aggregate Structure
3.5. Investigation of the Influence of Different Samples on Plant Biomass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, M.N.; Nechifor, M.; Tanasa, F.; Zanoaga, M.; McLoughlin, A.; Strózyk, M.A.; Culebras, M.; Teaca, C.A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Phan, D.P.; Sarwar, A.; Tran, M.H.; Lee, O.K.; Lee, E.Y. Valorization of industrial lignin to value-added chemicals by chemical depolymerization and biological conversion. Ind. Crops Prod. 2021, 161, 113219. [Google Scholar] [CrossRef]
- Wang, G.Q.; Kong, X.H.; Zhang, Y.H.; Zhao, Q.M.; Feng, X. Stability and Micro-mechanisms of Lignin-Improved Soil in a Drying-Wetting Environment. Ksce J. Civ. Eng. 2022, 26, 3314–3324. [Google Scholar] [CrossRef]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Abetz, C.; Zech, W. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma 2005, 128, 116–129. [Google Scholar] [CrossRef]
- Premalatha, R.P.; Poorna Bindu, J.; Nivetha, E.; Malarvizhi, P.; Manorama, K.; Parameswari, E.; Davamani, V. A review on biochar’s effect on soil properties and crop growth. Front. Energy Res. 2023, 11, 1092637. [Google Scholar] [CrossRef]
- Martínez, Á.T.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Ibarra, D.; Jiménez-Barbero, J.; del Río, J.C. Monolignol acylation and lignin structure in some nonwoody plants: A 2D NMR study. Phytochemistry 2008, 69, 2831–2843. [Google Scholar] [CrossRef]
- Thevenot, M.; Dignac, M.-F.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of lignin in preparation of slow-release fertilizer: Current status and future perspectives. Ind. Crops Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Park, G.W.; Gong, G.; Joo, J.C.; Song, J.J.; Lee, J.; Lee, J.P.; Kim, H.T.; Ryu, M.H.; Sirohi, R.; Zhuang, X.S.; et al. Recent progress and challenges in biological degradation and biotechnological valorization of lignin as an emerging source of bioenergy: A state-of-the-art review. Renew. Sust. Energ. Rev. 2022, 157, 112025. [Google Scholar] [CrossRef]
- Daassi, R.; Kasangana, P.B.; Khasa, D.P.; Stevanovic, T. Chemical characterization of tropical ramial and trunk woods and their lignins in view of applications in soil amendments. Ind. Crops Prod. 2020, 156, 112880. [Google Scholar] [CrossRef]
- Liu, J.B.; Hwang, Y.S.; Lenhart, J.J. Heteroaggregation of bare silver nanoparticles with clay minerals. Env. Sci-Nano 2015, 2, 528–540. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Vinod, J.S.; Indraratna, B.; Mahamud, M.A.A. Stabilisation of an erodible soil using a chemical admixture. Proc. Inst. Civ. Eng.-Ground Improv. 2010, 163, 43–51. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.L.; Liu, S.Y. Application of biomass by-product lignin stabilized soils as sustainable Geomaterials: A review. Sci. Total Environ. 2020, 728, 138830. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Sui, H.; He, L. One-Step Fabrication of Dual Responsive Lignin Coated Fe3O4 Nanoparticles for Efficient Removal of Cationic and Anionic Dyes. Nanomaterials 2018, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dai, T.; Wang, L.; Li, T.; Qiu, P.; Wang, J.; Wang, Z. Study on characteristics of soil and nutrient losses in Sunjiagou small watershed in cold black soil area. PLoS ONE 2023, 18, e0289479. [Google Scholar]
- Ferrol, N.; Azcón-Aguilar, C.; Pérez-Tienda, J. Review: Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef]
- Wang, H.J.; Frits, P.D.; Jin, Y.C. A win-win technique of stabilizing sand dune and purifying paper mill black-liquor. J. Environ. Sci. 2009, 21, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Law, S.M.; Maherali, H. Variation in glomalin-related soil protein and plant growth response to arbuscular mycorrhizal fungi along a nutrient gradient in temperate grasslands. Plant Soil 2023, 487, 623–637. [Google Scholar] [CrossRef]
- Zhang, J.M.; Chi, F.Q.; Wei, D.; Zhou, B.K.; Cai, S.S.; Li, Y.; Kuang, E.J.; Sun, L.; Li, L.J. Impacts of Long-term Fertilization on the Molecular Structure of Humic Acid and Organic Carbon Content in Soil Aggregates in Black Soil. Sci. Rep. 2019, 9, 11908. [Google Scholar] [CrossRef]
- Jeewani, P.H.; Luo, Y.; Yu, G.H.; Fu, Y.Y.; He, X.H.; Van Zwieten, L.; Liang, C.; Kumar, A.; He, Y.; Kuzyakov, Y.; et al. Arbuscular mycorrhizal fungi and goethite promote carbon sequestration via hyphal-aggregate mineral interactions. Soil Biol. Biochem. 2021, 162, 108417. [Google Scholar] [CrossRef]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219, 162–167. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Meena, V.S.; Gupta, V.K.; Rana, K.; Choudhary, M.; Tiwari, G.; Mishra, P.K.; Pattanayak, A.; Bisht, J.K.; et al. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Arch. Microbiol. 2020, 202, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 2006, 33, 141–163. [Google Scholar] [CrossRef]
- Assouline, S. Bulk Density of Soils and Impact on Their Hydraulic Properties. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 95–100. [Google Scholar]
- Yusuff, I.M.; Adagunodo, T.A.; Omoloye, M.A.; Olanrewaju, A.M. Interdependency of Soil-gas Radon-222 Concentration on Soil Porosity at different Soil-depths. J. Phys. Conf. Ser. 2019, 1299, 012099. [Google Scholar] [CrossRef]
- Björkman, A. Isolation of Lignin from Finely Divided Wood with Neutral Solvents. Nature 1954, 174, 1057–1058. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and characterization of aminated lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef]
- Qi, X.; Gou, J.L.; Chen, X.M.; Xiao, S.Q.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.W.; Han, M.W.; Luo, X.G. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Dai, Z.M.; Guo, X.; Lin, J.H.; Wang, X.; He, D.; Zeng, R.J.; Meng, J.; Luo, J.P.; Delgado-Baquerizo, M.; Moreno-Jiménez, E.; et al. Metallic micronutrients are associated with the structure and function of the soil microbiome. Nat. Commun. 2023, 14, 8456. [Google Scholar] [CrossRef]
- Cavalli, J.P.; Reichert, J.M.; Rodrigues, M.F.; de Araújo, E.F. Composition and functional soil properties of arenosols and acrisols: Effects on eucalyptus growth and productivity. Soil Till. Res. 2020, 196, 104439. [Google Scholar] [CrossRef]
- Martín, M.Á.; Reyes, M.; Taguas, F.J. Estimating soil bulk density with information metrics of soil texture. Geoderma 2017, 287, 66–70. [Google Scholar] [CrossRef]
- Beare, M.H.; Hendrix, P.F.; Coleman, D.C. Water-Stable Aggregates and Organic Matter Fractions in Conventional- and No-Tillage Soils. Soil Sci. Soc. Am. J. 1994, 58, 777–786. [Google Scholar] [CrossRef]
- Plante, A.F.; McGill, W.B. Soil aggregate dynamics and the retention of organic matter in laboratory-incubated soil with differing simulated tillage frequencies. Soil Till. Res. 2002, 66, 79–92. [Google Scholar] [CrossRef]
- Olayemi, O.P.; Kallenbach, C.M.; Wallenstein, M.D. Distribution of soil organic matter fractions are altered with soil priming. Soil Biol. Biochem. 2022, 164, 108494. [Google Scholar] [CrossRef]
- Heo, J.W.; An, L.L.; Chen, J.S.; Bae, J.H.; Kim, Y.S. Preparation of amine-functionalized lignins for the selective adsorption of Methylene blue and Congo red. Chemosphere 2022, 295, 133815. [Google Scholar] [CrossRef]
- Pang, C.; You, H.; Lei, S.; Su, F.; Liang, L.; Li, Z.; Lin, X.; Zhang, Y.; Zhang, H.; Pan, X.; et al. Chemically tailored molecular surface modification of bamboo pulp fibers for manipulating the electret performance of electret filter media. Carbohydr. Polym. 2024, 330, 121830. [Google Scholar] [CrossRef] [PubMed]
- Garbowski, T.; Bar-Michalczyk, D.; Charazińska, S.; Grabowska-Polanowska, B.; Kowalczyk, A.; Lochyński, P. An overview of natural soil amendments in agriculture. Soil Till. Res. 2023, 225, 105462. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.T.H.; Toan, N.C.; Kajitvichyanukul, P. Enhanced paraquat removal from contaminated water using cell-immobilized biochar. Clean Technol. Environ. Policy 2021, 24, 1073–1085. [Google Scholar] [CrossRef]
- Fang, L.; Cao, Y.; Huang, Q.; Walker, S.L.; Cai, P. Reactions between bacterial exopolymers and goethite: A combined macroscopic and spectroscopic investigation. Water Res. 2012, 46, 5613–5620. [Google Scholar] [CrossRef]
- Chen, J.; Min, F.F.; Liu, L.Y.; Liu, C.F.; Lu, F.Q. Experimental investigation and DFT calculation of different amine/ammonium salts adsorption on kaolinite. Appl. Surf. Sci. 2017, 419, 241–251. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Luo, J.J.; Peng, F.T.; Xiao, Y.S.; Du, A.Q. Application of Bag-Controlled Release Fertilizer Facilitated New Root Formation, Delayed Leaf, and Root Senescence in Peach Trees and Improved Nitrogen Utilization Efficiency. Front. Plant Sci. 2021, 12, 627313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cordero, I.; Bardgett, R.D. Defoliation and fertilisation differentially moderate root trait effects on soil abiotic and biotic properties. J. Ecol. 2023, 111, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.; Emami, H.; Fotovat, A.; Khorassani, R. Effect of different K:Na ratios in soil on dispersive charge, cation exchange and zeta potential. Eur. J. Soil Sci. 2018, 70, 311–320. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, R.; Li, X.; Zhang, J. Potential of arbuscular mycorrhizal fungi for soil health. Pedosphere 2024, 34, 279–288. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Wang, H.R.; Liang, J.M.; Huo, P.J.; Zhang, L.D.; Fan, X.L.; Sun, S.L. Understanding the cadmium passivation and nitrogen mineralization of aminated lignin in soil. Sci. Total Environ. 2023, 873, 162334. [Google Scholar] [CrossRef]








| pH Value | Soil Bulk Density (g/cm3) | Soil Porosity (%) |
|---|---|---|
| 5.99 | 1.3 | 51 |
| Peak/cm−1 | Corresponding Group |
|---|---|
| 1463, 1510 | C=C |
| 1698 | C=O |
| 3432 | O–H, N–H |
| 1125, 1029 | C–O–C |
| 1245 | C–O |
| 748 | C–Cl |
| 1633 | N–H |
| Peak/cm−1 | Corresponding Group |
|---|---|
| 1635 | C=O |
| 1420 | C-N |
| 1238 | -COOH |
| Lignin Content (wt%) | Mean Weight Diameter (mm) | Geometric Mean Diameter (mm) |
|---|---|---|
| 0 | 0.76 ± 0.029 a | 0.68 ± 0.020 a |
| 1 | 0.82 ± 0.019 b | 0.69 ± 0.005 ab |
| 2 | 0.84 ± 0.011 b | 0.70 ± 0.003 b |
| 3 | 0.85 ± 0.022 b | 0.71 ± 0.012 b |
| Treatment Method | Mean Weight Diameter (mm) | Geometric Mean Diameter (mm) |
|---|---|---|
| PL | 0.85 ± 0.030 | 0.69 ± 0.021 |
| AL50 | 0.90 ± 0.013 | 0.71 ± 0.011 |
| m (AMF)–m (AL) (wt%) | Mean Weight Diameter (mm) | Geometric Mean Diameter (mm) |
|---|---|---|
| 0:1 | 0.87 ± 0.030 a | 0.70 ± 0.005 a |
| 1:1 | 0.91 ± 0.063 a | 0.72 ± 0.026 a |
| 2:1 | 0.94 ± 0.037 a | 0.73 ± 0.016 a |
| 3:1 | 1.29 ± 0.260 b | 0.79 ± 0.043 b |
| Treatment Method | Stem Diameter (mm) | Plant Height (cm) | Root Dry Weight (g) | Shoot Dry Weight (g) | Root–Shoot Ratio |
|---|---|---|---|---|---|
| Untreated | 1.63 ± 0.122 a | 25.50 ± 0.764 a | 0.04 ± 0.005 a | 0.23 ± 0.014 a | 0.16 ± 0.012 a |
| PL | 2.03 ± 0.065 a | 30.10 ± 1.258 b | 0.07 ± 0.003 b | 0.29 ± 0.002 b | 0.23 ± 0.011 b |
| AL50 | 2.64 ± 0.025 b | 36.30 ± 1.041 c | 0.10 ± 0.004 c | 0.33 ± 0.015 c | 0.30 ± 0.009 c |
| AMF@AL(0:1) | 2.68 ± 0.112 b | 36.90 ± 1.000 c | 0.11 ± 0.007 c | 0.35 ± 0.033 cd | 0.31 ± 0.050 c |
| AMF@AL(1:1) | 2.85 ± 0.350 bc | 38.10 ± 2.466 c | 0.12 ± 0.014 d | 0.37 ± 0.025 de | 0.33 ± 0.023 c |
| AMF@AL(2:1) | 3.09 ± 0.377 cd | 40.60 ± 2.255 c | 0.14 ± 0.002 e | 0.42 ± 0.024 e | 0.35 ± 0.021 cd |
| AMF@AL(3:1) | 3.47 ± 0.239 d | 43.90 ± 3.122 d | 0.25 ± 0.009 f | 0.61 ± 0.011 f | 0.41 ± 0.009 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Xu, T.; Wang, S.; Bian, H.; Dai, H. Effect of Aminating Lignin Loading with Arbuscular Mycorrhizal Fungi on Soil Aggregate Structure Improvement. Polymers 2024, 16, 1701. https://doi.org/10.3390/polym16121701
Hu C, Xu T, Wang S, Bian H, Dai H. Effect of Aminating Lignin Loading with Arbuscular Mycorrhizal Fungi on Soil Aggregate Structure Improvement. Polymers. 2024; 16(12):1701. https://doi.org/10.3390/polym16121701
Chicago/Turabian StyleHu, Chenghui, Tingting Xu, Shumei Wang, Huiyang Bian, and Hongqi Dai. 2024. "Effect of Aminating Lignin Loading with Arbuscular Mycorrhizal Fungi on Soil Aggregate Structure Improvement" Polymers 16, no. 12: 1701. https://doi.org/10.3390/polym16121701
APA StyleHu, C., Xu, T., Wang, S., Bian, H., & Dai, H. (2024). Effect of Aminating Lignin Loading with Arbuscular Mycorrhizal Fungi on Soil Aggregate Structure Improvement. Polymers, 16(12), 1701. https://doi.org/10.3390/polym16121701







