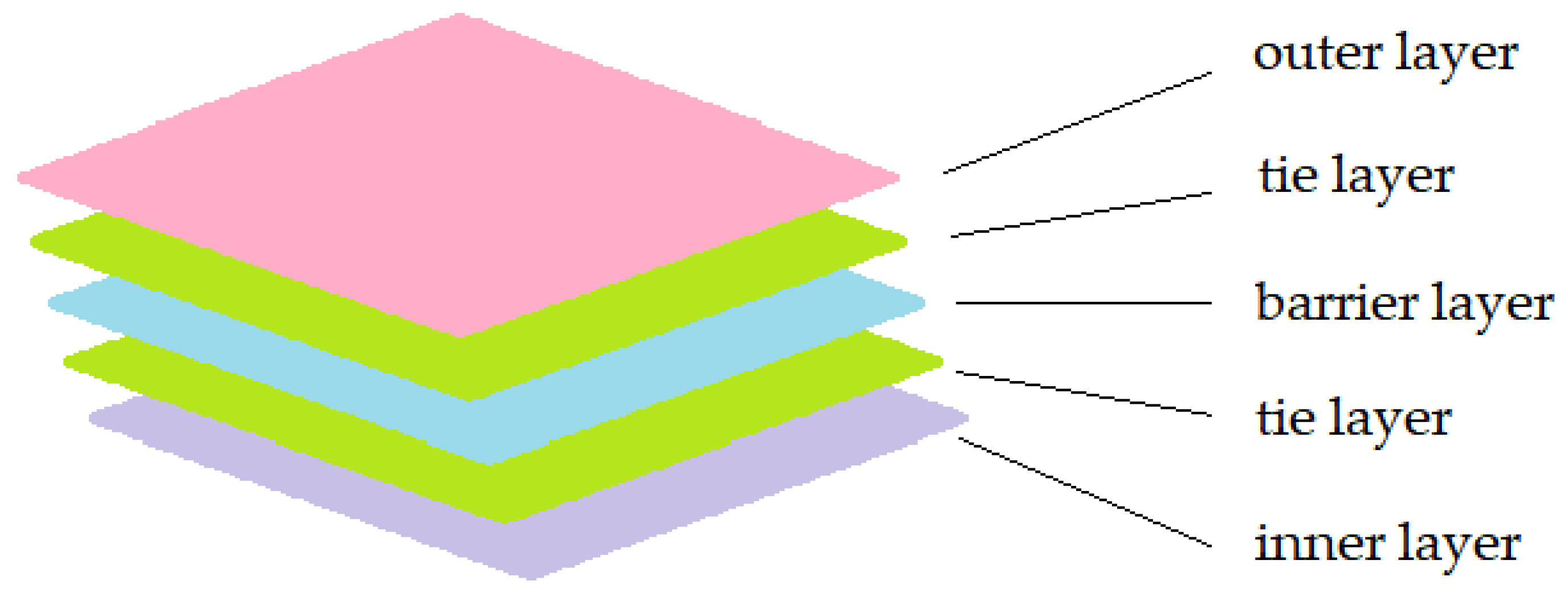
3.1. Delamination
Multilayer packaging often requires the use of adhesives to bond different materials which have different properties. These adhesive layers are called “tie-layers” in a multilayer structure. Commonly used adhesives include acrylics and polyurethanes (PU). Acrylic is more specifically suitable for bonding PE and aluminium, while polyurethane is more versatile and more widely used [
20]. A schematic diagram of multilayer packaging is shown in
Figure 3.
The delamination processes aim to decompose or dissolve the tie-layers from the multilayer structure separating the target materials and keeping them in their original shape and molecular structure. It can be achieved by removing interlayers or by reactions at the interface [
8]. Delamination by dissolving tie-layers is popular because only a small proportion of the structure, (i.e., the adhesive used for bonding dissimilar materials) needs to be removed. The delamination rate mainly depends on ability of the solvent to diffuse in the material and dissolve the tie-layer material [
21]. The access surface area is another important parameter affecting the delamination rate. In all experiments reported in the literature, the multilayer packaging samples were cut into strips or pieces (to increase reaction surface area) and thoroughly immersed in selected solvents [
20,
22,
23,
24,
25,
26,
27,
28,
29,
30].
Acids (including inorganic acids and organic acids) are one of the most commonly used delamination solvents. In 2023, Šleiniūtė et al. [
20] studied the delamination of aluminium-containing multilayer packaging using nitric acid. The role of nitric acid was to dissolve the aluminium layer and break down the structure of PU adhesive. The authors found that the application of ultrasound during the process significantly improved the delamination efficiency of nitric acid, reducing the delamination response duration from 240 min (6 h) to 35 min. They also highlighted that controlling nitrous dioxide gas emissions in the delamination process still requires further research.
Ügduler et al. [
22] studied formic acid delamination processes and mechanisms of multilayer packaging containing five constituents. The polymer materials included PET, PE, solvent-based polyurethane (SB-PU) adhesive, and solvent-free polyurethane (SF-PU) adhesive. The authors found that formic acid could diffuse in both polar polymers (e.g., PET) and non-polar polymers (e.g., PE), hypothesising that the good diffusion ability of formic acid may be due to its short alkyl chain. The dissolution of both the SB-PU and the SF-PU in formic acid was proportional to the reaction temperature (50–75 °C) and the formic acid concentration (50–100%). In their further studies, it was found that the solubility of SF-PU in formic acid was smaller than that of SB-PU, also suggesting that it was due to the dense structure of SF-PU, which limits the diffusion of formic acid and its swelling. In addition, the diffusion rate of formic acid in PET-based packages was found to be faster than that in PE-based packages. Thus, the authors concluded that formic acid would be promising for use in large-scale delamination and recycling of PET-based multilayer packaging laminated with SB-PU adhesive.
Apart from organic acids, other organic solvents have also been studied for the delamination of multilayer films. Fávaro et al. [
23] reported the use of acetone to delaminate PE-based aluminium-containing multilayer packaging. Delamination was accomplished by stirring in acetone at 50 °C for 4 h. The adhesive used between layers was SB-PU and the delamination products were PE and aluminium-embedded PET. The report demonstrated that PE can be directly recycled by extrusion and PET can be recycled by chemical depolymerisation, which will be reviewed in the next section.
In 2022, O’Rourke et al. [
24] studied the delamination mechanism of polyamide (PA)/polyolefin (PO) multilayer packaging by ethylene glycol (EG) oligomers. At a temperature (100–150 °C), diethylene glycol (DEG) could result in glycolysis of the solvent-free polyurethane adhesive in both polypropylene (PP) and polyethylene (PE) based multilayer films, thereby separating the PA and the PO layers. The polymer films recovered by this process contain almost no impurities, making it easy to be reused efficiently.
Solvent mixtures were also employed for the delamination of multilayer packaging in the last decade. In 2014, Zhang et al. [
25] studied the interfacial delamination mechanism of an Al-PE multilayer film. The composite film was obtained from a paper-containing multilayer packaging. Before delamination, the paper in the packaging was removed by hydraulic disintegration. The extracted Al-PE multilayer film contained PE, PE adhesive and aluminium foil. A solvent system involving benzene–ethanol–water with a volume ratio of 30:20:50 was used as the separation solution, delaminating PE and aluminium foil. The authors reported swelling of the PE layer and destruction of the PE adhesive layer. Zhang et al. [
25] suggested that the delamination mechanism is the interfacial adhesive failure between the PE and the aluminium foil.
Deep eutectic solvents (DES) which are normally non-flammable, non-toxic, and have low vapour pressure [
26], have been studied in the delamination processes. In 2020, Nieminen et al. [
26] studied the recycling of polyvinyl chloride (PVC) from waste pharmaceutical blister packaging by a DES prepared with choline chloride and lactic acid, where choline chloride acted as a hydrogen bond acceptor and lactic acid acted as a hydrogen bond donor. These two components are considered less hazardous chemicals. The authors found that after recycling, the transparent PVC panels turned slightly white. The explanation for this phenomenon was attributed by the authors to the fact that during recycling the PVC was heated close to its glass transition temperature, which resulted in the increase in the crystallinity of the recycled PVC, thus whitening its appearance. They suggested that re-extrusion can reverse the opacity of the PVC.
Some switchable solvents were also studied as novel solvents to delaminate multilayer packaging in an attempt to ensure the reuse of the solvent without distillation. In 2018, Mumladze and Yousef et al. [
27] proposed a method to separate multilayer packaging using a switchable hydrophilicity solvent (SHS), N,N-dimethylcyclohexylamine (DMCHA). After SHS treatment in ultrasound, floating polymer films (Ethylene-vinyl acetate (EVA) and PET) and precipitated aluminium flakes/particles were separated from the solution. The authors suggested that the application of ultrasound treatment accelerated the delamination of the aluminium barrier layer and polymer layers. The ink, paint and sealing layer (PE) dissolved in the SHS solution were extracted by adding water and bubbling CO
2 for several hours. The properties of DMCHA recovered after removal of CO
2 were determined to be unchanged from the initial DMCHA. Therefore, the authors believed that the recycling of multilayer packaging by using SHS has potential from both economic and environmental perspectives.
The same research group also studied the DMCHA delamination recycling of PVC and PP from waste pharmaceutical blisters [
28]. The recycled polymers were investigated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), and were shown to have good thermal stability and similar glass transition temperatures to the virgin polymers.
Less hazardous amine-free CO
2-switchable hydrophilicity solvents (ASHS) composed of carboxylic acid and sodium hydroxide (NaOH) were introduced by Cunha et al. [
29]. The authors believed that it is possible to use ASHS as a new method for polymer recycling.
In 2023, Vagnoni et al. [
30] proposed a method for delaminating PO-Al multilayer film waste using switchable anionic surfactants, which were prepared by mixing carboxylic acid and base (amine or hydroxide) with an equivalent ratio of 1:1.5. The authors concluded that the optimum combination was C12-TEA (lauric acid-triethanolamine), which could efficiently recover PE from de-pulped food and beverage cartons. In addition, they proposed that C12-TEA is better than most of other switchable systems in terms of human health and environmental friendliness.
In order to facilitate packaging delamination, other efforts have also been made in the design of multilayer structures, including the microperforation technique [
31], reversible cross-linking adhesives [
32,
33], water-based adhesives [
34], etc.
A summary of the solvents used in the studies reviewed above is shown in
Table 2.
The mechanism of delamination is to dissolve or decompose the adhesive layer, which is mainly composed of acrylics or polyurethanes, to separate the main components (e.g., LDPE, PP, aluminium foil, etc.) of the multilayer structural. Since the delamination process is likely to be economically and environmentally friendly, it has become a hot topic of research. A variety of solvents have been studied and tested for the delamination of multilayer packaging:
Single-component solvents, including inorganic acids (e.g., nitric acid), organic acids (e.g., formic acid), and other organic solvents (e.g., acetone, DEG), etc.
Mixed solvents, such as benzene–ethanol–water solution system, and choline chloride–lactic acid deep eutectic solvent.
Recently invented CO2-switchable solvent systems; for example, switchable hydrophilicity solvents, and switchable anionic surfactants.
It is worth mentioning that some studies concluded that the use of ultrasound can significantly accelerate the delamination process.
3.2. Selective Dissolution–Precipitation
The mechanism of selective dissolution–precipitation (SDP) is to dissolve and separate a series of incompatible polymers one after another by using one solvent at different reaction temperatures, or by using different solvents. The recovery of target polymers is normally achieved by precipitation using anti-solvents, or by solvent evaporation [
35]. In real applications of SDP to recycle mixed polymers from a waste stream, all the target polymers should be dissolved and precipitated, aiming to remove impurities and other insoluble plastics [
35].
Tetra Pak
® is a common multilayer packaging for liquid food [
36]. This widely used beverage carton normally consists of 75 wt% of stiff paper, 20 wt% of low-density polyethylene (LDPE) and 5 wt% of aluminium foil [
36]. The paper can be recycled through hydropulping, while the LDPE and aluminium foil remain laminated [
37]. In order to further separate the LDPE and the aluminium, Georgiopoulou et al. [
36] used xylene to dissolve the LDPE from the Al-PE laminates, followed by filtration to recover the aluminium foil, and then precipitated the LDPE using isopropanol as an antisolvent. In their work, the outer PE layer was also dissolved and precipitated. Unlike the fine powder LDPE recovered from Al-PE laminates, the recycled product of the outer PE layer was in the form of lumps. Therefore, the authors believed that the selective dissolution–precipitation process using xylene and isopropanol to recover LDPE is not suitable to remove impurities such as printing inks. They suggested that for high-quality recycling of LDPE, pre-separation of Al-PE laminates and outer PE is necessary.
Samorì et al. [
37] tried different sustainable solvents to recover LDPE and aluminium from de-pulped multilayer packaging. The solvents were used to dissolve the LDPE layer and then separate the multilayer structure into single components. The solvents used were biodiesel, 2-methyl tetrahydrofuran (2-MeTHF), and cyclopentyl methyl ether (CPME). The LDPE was recovered by adding ethanol as an anti-solvent in the biodiesel-based process or by distilling the solvent under vacuum for the other two processes. The authors concluded that among the three solvents, CPME performed best for LDPE solubility and recovery, followed by biodiesel and 2-MeTHF. They believed that the results of their work could widen the choice of solvents for recycling plastic waste.
A number of publications also reported the recycling of single or mixed polymers using the method of selective dissolution–precipitation [
35,
38]. Although multilayer packaging was not included in these papers, the solvents and the experimental parameters are valuable for reference. Pappa et al. [
35] used xylene and isopropanol as solvent and anti-solvent to dissolve and precipitate mixed LDPE, HDPE and PP. The separation procedure is shown in
Figure 4 [
35]. LDPE, HDPE and PP were dissolved in turn by xylene at 85 °C, 100 °C and 135 °C. The authors reported that the polymers recovered by the xylene/isopropanol system retained their value, therefore, they believed that this technique could be applied in the recycling of mixed plastic waste.
Achilias et al. [
38] studied the dissolution–reprecipitation recycling of a series of commonly used polymers from plastic packaging materials. The polymers included LDPE, HDPE, PP, polystyrene (PS), PET and PVC. A summary of the optimum solvent and dissolution–reprecipitation parameters obtained from their research is shown in
Table 3 [
38]. D-limonene as an environmental-friendly solvent was used for the recovery of PS. The authors claimed that the advantages of using this solvent could be the low dissolution temperature, high solubility, low dissolution time and high selectivity. In addition, the D-limonene can be removed by vacuum distillation thus preventing the usage of an anti-solvent.
Aiming at the deconstruction of multilayer films, Walker et al. [
39] addressed a unique strategy named solvent-targeted recovery and precipitation (STRAP). Walker et al. demonstrated the STRAP process by separating a three-component multilayer film consisting of PE, ethylene vinyl alcohol copolymer (EVOH) and PET. The PE fraction was dissolved by toluene at 110 °C and precipitated by acetone. The EVOH was dissolved by dimethyl sulfoxide (DMSO) from the remaining solid and was precipitated by water. The remaining PET was filtered, washed and separated directly without dissolution and precipitation. The authors reported that the polymers recovered by the STRAP process were in a chemically pure form while being cost-competitive with the corresponding virgin materials [
39].
Further research was conducted employing the STRAP process. Sánchez-Rivera et al. [
40] studied the separation of multilayer films with more complex components in 2021 proposing temperature-controlled STRAP to reduce the use of anti-solvents in the recycling of multilayer plastic films. They compared two STRAP processes separating a PE, EVOH, PET and EVA (minor) multilayer film. In STRAP-A, PE and EVOH were selectively dissolved by toluene and DMSO and precipitated by anti-solvents of acetone and water, respectively. This process did not separate PE and EVA adhesive layers as they were dissolved and precipitated together which influenced the purity of the recovered PE. In STRAP-B, PE was precipitated by cooling the solution from 110 °C to 35 °C, while the EVA remained in toluene and was later precipitated in acetone. EVOH was dissolved by the mixture of 60% DMSO–40% water (
v/
v) and precipitated by cooling the solution from 95 °C to 35 °C. The authors found that the yields of the recovered polymers were similar from the two STRAP processes, while STRAP-B, using less anti-solvent, was even more efficient in the separation of PE and EVA. Therefore, they came to the conclusion that the temperature-controlled polymer dissolution and precipitation is a promising optimisation to make STRAP cost competitive and environmentally friendly [
40,
41].
In the same paper, Sánchez-Rivera et al. also reported a STRAP-C process for separating a polyethylene terephthalate glycol (PETG), PE, EVOH, PET and EVA multilayer film [
40]. A PETG selective separation procedure was conducted before STRAP-B. The PETG was dissolved by a solvent mixture of 60% dimethylformamide (DMF)–40% tetrahydrofuran (THF) (
v/
v) and precipitated by n-propanol. In 2023 [
41], they published a study in the separation of a multilayer printed film composed of PE, EVOH, PET and PU-based inks. After the removal of PE and EVOH, the remaining PET and PU inks were separated in γ-Valerolactone (GVL) which successfully removed the PU inks.
The solvents which have been studied for the selective dissolution–precipitation are summarised in
Table 4.
As for the selective dissolution–precipitation method, the commonly used solvents for recycling polyolefin components (including LDPE, HDPE, PP, etc.) from multilayer materials are toluene and xylene, and the anti-solvent can be isopropanol or n-hexane. Some sustainable solvents, such as biodiesel, 2-MeTHF and CPME, have also been explored for the dissolution and separation of LDPE. Except for biodiesel, where the dissolved LDPE requires the addition of ethanol for precipitation, the other two solvents do not require the use of anti-solvents. In addition, the STRAP recycling processes developed for multilayer packaging films laminated with PE, EVOH, PET and EVA have also received certain attention and research. Due to the complexity of the composition and structure of post-consumer multilayer packaging waste, the recycling processes of involving selective dissolution–precipitation are still in the development and experimental stages.