The MUC2 Gene Product: Polymerisation and Post-Secretory Organisation—Current Models
Abstract
1. Introduction
2. Mucin Structure

3. Secreted Mucins
3.1. Secreted Gel-Forming Mucins
3.2. Secreted Non-Gel-Forming Mucins
4. MUC2: The Major Gel-Forming Mucin of the Small Intestine and Colon
Structure and Polymerisation of MUC2 Mucins
5. Small Intestinal Mucus Layer Post-Secretory Organisation
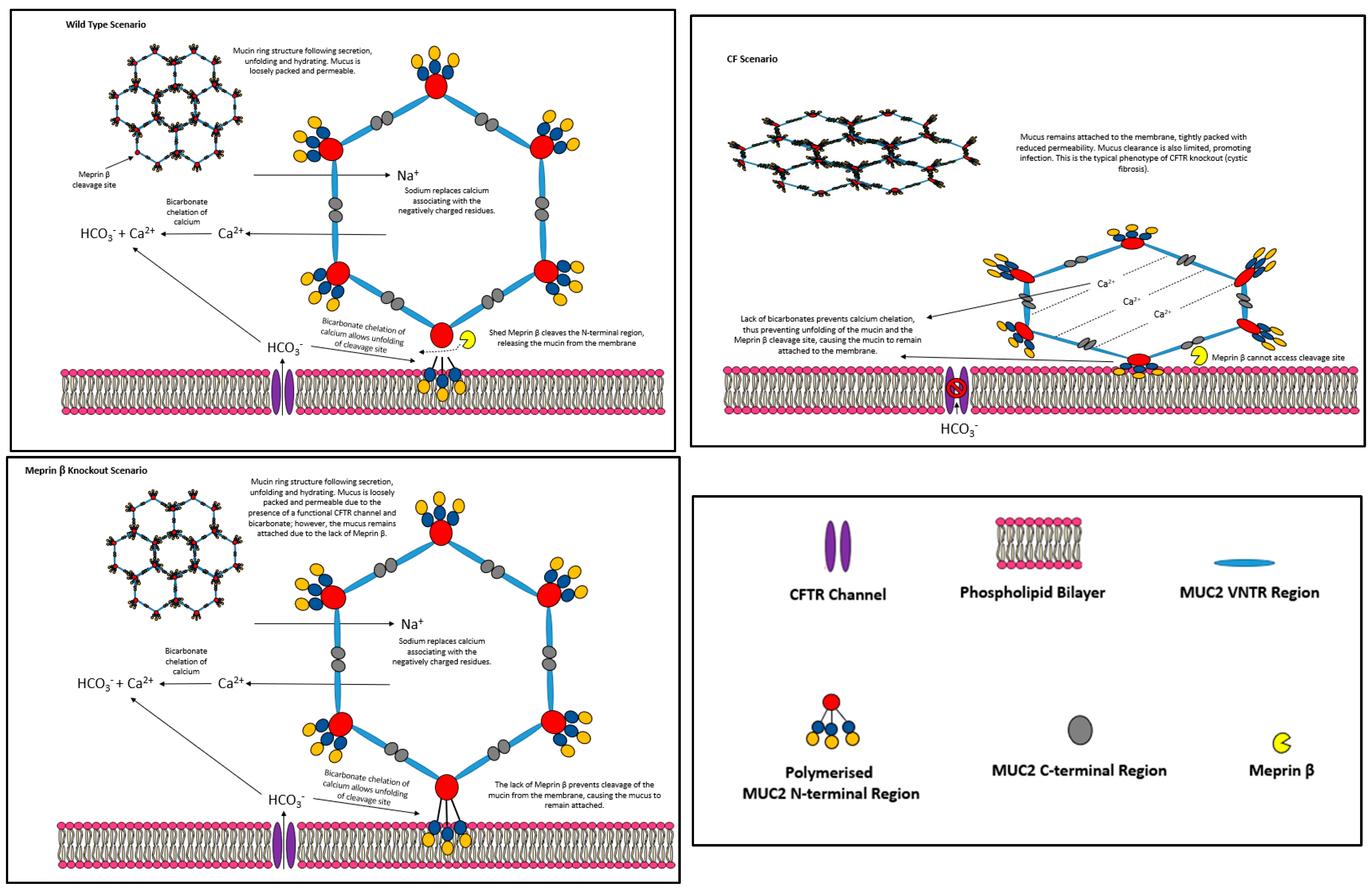
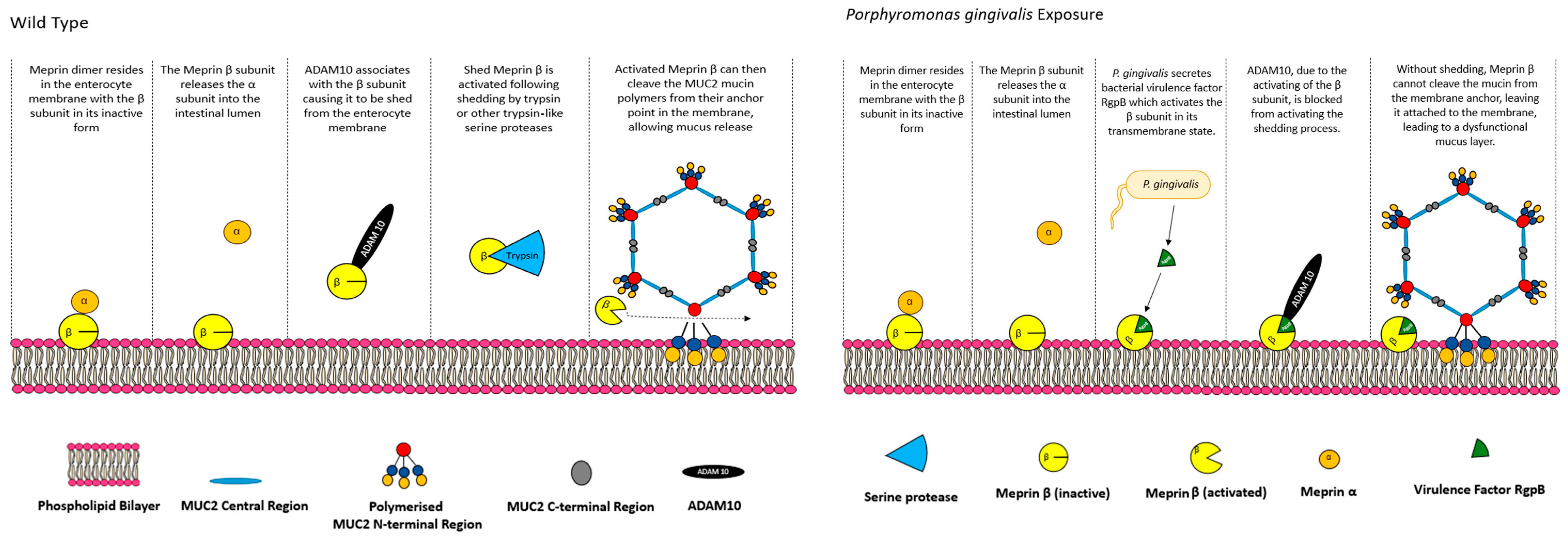
5.1. CFTR, Bicarbonate and Meprin β Mediate Extracellular Release of Membrane-Bound MUC2 Polymers
- Meprin β−\−:
- CFTR−\−:
5.2. Small Intestinal Mucus Layer Organisation
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract–revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Raplee, I.; Walker, L.; Xu, L.; Surathu, A.; Chockalingam, A.; Stewart, S.; Han, X.; Rouse, R.; Li, Z. Emergence of nosocomial associated opportunistic pathogens in the gut microbiome after antibiotic treatment. Antimicrob. Resist. Infect. Control 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rud, T.; Karch, A.; Pieper, D.H.; Vital, M. Pathogenic functions of host microbiota. Microbiome 2018, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; Van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Strugala, V.; Griffin, S.M.; Welfare, M.R.; Dettmar, P.W.; Allen, A.; Pearson, J.P. Esophageal mucin: An adherent mucus gel barrier is absent in the normal esophagus but present in columnar-lined Barrett’s esophagus. Am. J. Gastroenterol. 2001, 96, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Javitt, G.; Khmelnitsky, L.; Albert, L.; Bigman, L.S.; Elad, N.; Morgenstern, D.; Ilani, T.; Levy, Y.; Diskin, R.; Fass, D. Assembly mechanism of mucin and von Willebrand factor polymers. Cell 2020, 183, 717–729.e16. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Chater, P.I.; Wilcox, M.D. The properties of the mucus barrier, a unique gel—How can nanoparticles cross it? Ther. Deliv. 2016, 7, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.-Y.; Wirtz, D.; Hanes, J. Micro-and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef]
- Prasanna, L.C. Analysis of the distribution of mucins in adult human gastric mucosa and its functional significance. J. Clin. Diagn. Res. 2016, 10, AC01–AC04. [Google Scholar] [CrossRef]
- Behera, S.K.; Praharaj, A.B.; Dehury, B.; Negi, S. Exploring the role and diversity of mucins in health and disease with special insight into non-communicable diseases. Glycoconj. J. 2015, 32, 575–613. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Ambort, D.; Johansson, M.E.; Gustafsson, J.K.; Ermund, A.; Hansson, G.C. Perspectives on mucus properties and formation—Lessons from the biochemical world. Cold Spring Harb. Perspect. Med. 2012, 2, a014159. [Google Scholar] [CrossRef]
- Ambort, D.; van der Post, S.; Johansson, M.E.; MacKenzie, J.; Thomsson, E.; Krengel, U.; Hansson, G.C. Function of the CysD domain of the gel-forming MUC2 mucin. Biochem. J. 2011, 436, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, M.; Ambort, D.; Thomsson, E.; Johansson, M.E.; Hansson, G.C. Increased understanding of the biochemistry and biosynthesis of MUC2 and other gel-forming mucins through the recombinant expression of their protein domains. Mol. Biotechnol. 2013, 54, 250–256. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Ermund, A.; Ambort, D.; Johansson, M.E.; Nilsson, H.E.; Thorell, K.; Hebert, H.; Sjövall, H.; Hansson, G.C. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 2012, 209, 1263–1272. [Google Scholar] [CrossRef]
- Nilsson, H.E.; Ambort, D.; Bäckström, M.; Thomsson, E.; Koeck, P.J.; Hansson, G.C.; Hebert, H. Intestinal MUC2 mucin supramolecular topology by packing and release resting on D3 domain assembly. J. Mol. Biol. 2014, 426, 2567–2579. [Google Scholar] [CrossRef]
- Javitt, G.; Calvo, M.L.G.; Albert, L.; Reznik, N.; Ilani, T.; Diskin, R.; Fass, D. Intestinal gel-forming mucins polymerize by disulfide-mediated dimerization of d3 domains. J. Mol. Biol. 2019, 431, 3740–3752. [Google Scholar] [CrossRef]
- Gallego, P.; Garcia-Bonete, M.J.; Trillo-Muyo, S.; Recktenwald, C.V.; Johansson, M.E.V.; Hansson, G.C. The intestinal MUC2 mucin C-terminus is stabilized by an extra disulfide bond in comparison to von Willebrand factor and other gel-forming mucins. Nat. Commun. 2023, 14, 1969. [Google Scholar] [CrossRef]
- Stanforth, K.; Chater, P.; Brownlee, I.; Wilcox, M.; Ward, C.; Pearson, J. In vitro modelling of the mucosa of the oesophagus and upper digestive tract. Ann. Esophagus 2021, 5, 5958. [Google Scholar] [CrossRef]
- McAuley, J.L.; Linden, S.K.; Png, C.W.; King, R.M.; Pennington, H.L.; Gendler, S.J.; Florin, T.H.; Hill, G.R.; Korolik, V.; McGuckin, M.A. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Investig. 2007, 117, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Thamadilok, S.; Roche-Håkansson, H.; Håkansson, A.P.; Ruhl, S. Absence of capsule reveals glycan-mediated binding and recognition of salivary mucin MUC7 by Streptococcus pneumoniae. Mol. Oral Microbiol. 2016, 31, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Bobek, L.A. Regulation of human MUC7 mucin gene expression by cigarette smoke extract or cigarette smoke and Pseudomonas aeruginosa lipopolysaccharide in human airway epithelial cells and in MUC7 transgenic mice. Open Respir. Med. J. 2010, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-J.; Song, K.S. Effect of MUC8 on airway inflammation: A friend or a foe? J. Clin. Med. 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Algarra, B.; Han, L.; Soriano-Ubeda, C.; Aviles, M.; Coy, P.; Jovine, L.; Jimenez-Movilla, M. The C-terminal region of OVGP1 remodels the zona pellucida and modifies fertility parameters. Sci. Rep. 2016, 6, 32556. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Svensson, F.; Lang, T.; Johansson, M.E.; Hansson, G.C. The central exons of the human MUC2 and MUC6 mucins are highly repetitive and variable in sequence between individuals. Sci. Rep. 2018, 8, 17503. [Google Scholar] [CrossRef] [PubMed]
- Desseyn, J.-L.; Guyonnet-Dupérat, V.; Porchet, N.; Aubert, J.-P.; Laine, A. Human mucin gene MUC5B, the 10.7-kb large central exon encodes various alternate subdomains resulting in a super-repeat structural evidence for a 11p15. 5 gene family. J. Biol. Chem. 1997, 272, 3168–3178. [Google Scholar] [CrossRef]
- Krishn, S.R.; Ganguly, K.; Kaur, S.; Batra, S.K. Ramifications of secreted mucin MUC5AC in malignant journey: A holistic view. Carcinogenesis 2018, 39, 633–651. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Dawson, P.A.; Lourie, R.; Hutson, P.; Tong, H.; Grencis, R.K.; McGuckin, M.A.; Thornton, D.J. Immune-driven alterations in mucin sulphation is an important mediator of Trichuris muris helminth expulsion. PLoS Pathog. 2017, 13, e1006218. [Google Scholar] [CrossRef] [PubMed]
- Honigfort, D.J.; Altman, M.O.; Gagneux, P.; Godula, K. Glycocalyx crowding with mucin mimetics strengthens binding of soluble and virus-associated lectins to host cell glycan receptors. Proc. Natl. Acad. Sci. USA 2021, 118, e2107896118. [Google Scholar] [CrossRef] [PubMed]
- Znamenskaya, Y.; Sotres, J.; Gavryushov, S.; Engblom, J.; Arnebrant, T.; Kocherbitov, V. Water sorption and glass transition of pig gastric mucin studied by QCM-D. J. Phys. Chem. B 2013, 117, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Arike, L.; Holmén-Larsson, J.; Hansson, G.C. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology 2017, 27, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef]
- Steen, P.V.d.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. [Google Scholar] [CrossRef]
- Christlet, T.H.T.; Veluraja, K. Database analysis of O-glycosylation sites in proteins. Biophys. J. 2001, 80, 952–960. [Google Scholar] [CrossRef]
- Brockhausen, I. 6.11—Biosynthesis of Complex Mucin-Type O-Glycans. In Comprehensive Natural Products II; Mander, L., Liu, H.-W., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 6, pp. 315–350. [Google Scholar]
- Van Klinken, B.J.; Van der Wal, J.G.; Einerhand, A.; Büller, H.; Dekker, J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut 1999, 44, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.L.; Jamieson, A.M.; Blackwell, J.; Jentoft, N. The thermal depolymerization of porcine submaxillary mucin. J. Biol. Chem. 1984, 259, 14657–14662. [Google Scholar] [CrossRef]
- Nordman, H.; Davies, J.R.; Lindell, G.; De Bolos, C.; Real, F.; Carlstedt, I. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem. J. 2002, 364, 191–200. [Google Scholar] [CrossRef]
- Zhu, L.; Lee, P.; Yu, D.; Tao, S.; Chen, Y. Cloning and characterization of human MUC19 gene. Am. J. Respir. Cell Mol. Biol. 2011, 45, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Ambort, D.; Johansson, M.E.; Gustafsson, J.K.; Nilsson, H.E.; Ermund, A.; Johansson, B.R.; Koeck, P.J.; Hebert, H.; Hansson, G.C. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. USA 2012, 109, 5645–5650. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Larsson, J.M.H.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Audie, J.; Janin, A.; Porchet, N.; Copin, M.; Gosselin, B.; Aubert, J. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J. Histochem. Cytochem. 1993, 41, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Porchet, N.; Pigny, P.; Buisine, M.-P.; Debailleul, V.; Degand, P.; Laine, A.; Aubert, J.-P. Human mucin genes: Genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem. Soc. Trans. 1995, 4, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.J.; Gould, S.; Harris, A. Developmental expression of mucin genes in the human respiratory tract. Am. J. Respir. Cell Mol. Biol. 1997, 17, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Buisine, M.-P.; Devisme, L.; Copin, M.-C.; Durand-Réville, M.; Gosselin, B.; Aubert, J.-P.; Porchet, N. Developmental mucin gene expression in the human respiratory tract. Am. J. Respir. Cell Mol. Biol. 1999, 20, 209–218. [Google Scholar] [CrossRef]
- Gum, J.; Hicks, J.W.; Toribara, N.W.; Siddiki, B.; Kim, Y.S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994, 269, 2440–2446. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Eng, E.T.; Zhu, J.; Lu, C.; Walz, T.; Springer, T.A. Sequence and structure relationships within von Willebrand factor. Blood 2012, 120, 449–458. [Google Scholar] [CrossRef]
- Ridley, C.; Thornton, D.J. Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol./Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 2019, 285, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vilar, J.; Hill, R.L. The structure and assembly of secreted mucins. J. Biol. Chem. 1999, 274, 31751–31754. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, K.; Sargsyan, K.; Grauffel, C.d.; Dudev, T.; Lim, C. Preferred hydrogen-bonding partners of cysteine: Implications for regulating cys functions. J. Phys. Chem. B 2016, 120, 10288–10296. [Google Scholar] [CrossRef] [PubMed]
- Desseyn, J.-L. Mucin CYS domains are ancient and highly conserved modules that evolved in concert. Mol. Phylogenetics Evol. 2009, 52, 284–292. [Google Scholar] [CrossRef]
- Chaudhury, N.M.; Proctor, G.B.; Karlsson, N.G.; Carpenter, G.H.; Flowers, S.A. Reduced mucin-7 (Muc7) sialylation and altered saliva rheology in Sjögren’s syndrome associated oral dryness. Mol. Cell. Proteom. 2016, 15, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Gururaja, T.L.; Ramasubbu, N.; Venugopalan, P.; Reddy, M.S.; Ramalingam, K.; Levine, M.J. Structural features of the human salivary mucin, MUC7. Glycoconj. J. 1998, 15, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Bobek, L.A.; Situ, H. MUC7 20-Mer: Investigation of antimicrobial activity, secondary structure, and possible mechanism of antifungal action. Antimicrob. Agents Chemother. 2003, 47, 643–652. [Google Scholar] [CrossRef]
- Narasimhamurthy, S.; Naganagowda, G.A.; Janagani, S.; Gururaja, T.L.; Levine, M.J. Solution structure of O-glycosylated C-terminal leucine zipper domain of human salivary mucin (MUC7). J. Biomol. Struct. Dyn. 2000, 18, 145–154. [Google Scholar] [CrossRef]
- Hakoshima, T. Leucine zippers. In eLS (Encyclopedia of Life Sciences); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; p. a0005049. [Google Scholar]
- Lee, H.-M.; Kim, D.H.; Lee, S.H.; Kim, J.M.; Hwang, S.J. MUC8 mucin gene up-regulation in chronic rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2004, 113, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, W.E.; Zlock, L.T.; Morikawa, M.; Lao, A.Y.; Dasari, V.; Widdicombe, J.H. Cystic fibrosis and the relationship between mucin and chloride secretion by cultures of human airway gland mucous cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L402–L414. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, C.-H.; Ryu, J.-H.; Joo, J.H.; Lee, S.-N.; Kim, M.-J.; Lee, J.-G.; Bae, Y.S.; Yoon, J.-H. Crosstalk between platelet-derived growth factor-induced Nox4 activation and MUC8 gene overexpression in human airway epithelial cells. Free. Radic. Biol. Med. 2011, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- O’Day-Bowman, M.B.; Mavrogianis, P.A.; Reuter, L.M.; Johnson, D.E.; Fazleabas, A.T.; Verhage, H.G. Association of oviduct-specific glycoproteins with human and baboon (Papio anubis) ovarian oocytes and enhancement of human sperm binding to human hemizonae following in vitro incubation. Biol. Reprod. 1996, 54, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Bell, A.; Mantle, M.; Pearson, J.P. The structure and physiology of gastrointestinal mucus. In Mucus in Health and Disease—II; Springer: Boston, MA, USA, 1982; pp. 115–133. [Google Scholar]
- Wilcox, M.; Van Rooij, L.; Chater, P.; De Sousa, I.P.; Pearson, J. The effect of nanoparticle permeation on the bulk rheological properties of mucus from the small intestine. Eur. J. Pharm. Biopharm. 2015, 96, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.; Loukoianov, A.; Wachi, S.; Wu, R. Regulation of airway mucin gene expression. Annu. Rev. Physiol. 2008, 70, 405–429. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, K.; Swallow, D.M. Mucin methods: Genes encoding mucins and their genetic variation with a focus on gel-forming mucins. In Mucins; Humana Press: Totowa, NJ, USA, 2012; pp. 1–26. [Google Scholar]
- Nguyen, T.V.; Janssen, M.J.; Gritters, P.; te Morsche, R.H.; Drenth, J.P.; van Asten, H.; Laheij, R.J.; Jansen, J.B. Short mucin 6 alleles are associated with H pylori infection. World J. Gastroenterol. 2006, 12, 6021. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Davies, J.R.; Lindell, G.; Mårtensson, S.; Packer, N.H.; Swallow, D.M.; Carlstedt, I. Studies on the “insoluble” glycoprotein complex from human colon. Identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J. Biol. Chem. 1999, 274, 15828–15836. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.; Fu, J.; Johansson, M.E.; Liu, X.; Gao, N.; Wu, Q.; Song, J.; McDaniel, J.M.; McGee, S.; Chen, W. Core 1–and 3–derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol. 2017, 10, 91–103. [Google Scholar] [CrossRef]
- Ringot-Destrez, B.; Kalach, N.; Mihalache, A.; Gosset, P.; Michalski, J.-C.; Léonard, R.; Robbe-Masselot, C. How do they stick together? Bacterial adhesins implicated in the binding of bacteria to the human gastrointestinal mucins. Biochem. Soc. Trans. 2017, 45, 389–399. [Google Scholar] [CrossRef]
- Banerjee, S.; Bond, J.S. Prointerleukin-18 is activated by meprin β in vitro and in vivo in intestinal inflammation. J. Biol. Chem. 2008, 283, 31371–31377. [Google Scholar] [CrossRef] [PubMed]
- Godl, K.; Johansson, M.E.; Lidell, M.E.; Mörgelin, M.; Karlsson, H.; Olson, F.J.; Gum, J.R.; Kim, Y.S.; Hansson, G.C. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J. Biol. Chem. 2002, 277, 47248–47256. [Google Scholar] [CrossRef] [PubMed]
- Noone, P.G.; Knowles, M.R. ‘CFTR-opathies’: Disease phenotypes associated with cystic fibrosis transmembrane regulator gene mutations. Respir. Res. 2001, 2, 328. [Google Scholar] [CrossRef] [PubMed]
- Furnari, M.; De Alessandri, A.; Cresta, F.; Haupt, M.; Bassi, M.; Calvi, A.; Haupt, R.; Bodini, G.; Ahmed, I.; Bagnasco, F. The role of small intestinal bacterial overgrowth in cystic fibrosis: A randomized case-controlled clinical trial with rifaximin. J. Gastroenterol. 2019, 54, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Assis, D.N.; Debray, D. Gallbladder and bile duct disease in cystic fibrosis. J. Cyst. Fibros. 2017, 16, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Fatehi, M.; Linsdell, P. Mechanism of direct bicarbonate transport by the CFTR anion channel. J. Cyst. Fibros. 2009, 8, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A. Bicarbonate secretion: It takes two to tango. Nat. Cell Biol. 2004, 6, 292–294. [Google Scholar] [CrossRef]
- Singh, A.K.; Riederer, B.; Chen, M.; Xiao, F.; Krabbenhöft, A.; Engelhardt, R.; Nylander, O.; Soleimani, M.; Seidler, U. The switch of intestinal Slc26 exchangers from anion absorptive to HCO3− secretory mode is dependent on CFTR anion channel function. Am. J. Physiol. Cell Physiol. 2010, 298, C1057–C1065. [Google Scholar] [CrossRef]
- Quinton, P.M. Cystic fibrosis: Impaired bicarbonate secretion and mucoviscidosis. Lancet 2008, 372, 415–417. [Google Scholar] [CrossRef]
- George, T.; Brady, M.F. Ethylenediaminetetraacetic Acid (EDTA). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Schütte, A.; Ermund, A.; Becker-Pauly, C.; Johansson, M.E.; Rodriguez-Pineiro, A.M.; Bäckhed, F.; Müller, S.; Lottaz, D.; Bond, J.S.; Hansson, G.C. Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc. Natl. Acad. Sci. USA 2014, 111, 12396–12401. [Google Scholar] [CrossRef]
- Sterchi, E.E.; Stöcker, W.; Bond, J.S. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol. Asp. Med. 2008, 29, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Lottaz, D.; Hahn, D.; Müller, S.; Müller, C.; Sterchi, E.E. Secretion of human meprin from intestinal epithelial cells depends on differential expression of the α and β subunits. Eur. J. Biochem. 1999, 259, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.D.; Bond, J.S. Activation mechanism of meprins, members of the astacin metalloendopeptidase family. J. Biol. Chem. 1997, 272, 28126–28132. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Jin, G.; Bradley, S.G.; Matters, G.L.; Gailey, R.D.; Crisman, J.M.; Bond, J.S. Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G273–G282. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.-N.; Becker, C.; Lottaz, D.; Köhler, D.; Yiallouros, I.; Krell, H.-W.; Sterchi, E.E.; Stöcker, W. Human meprin alpha and beta homo-oligomers: Cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem. J. 2004, 378, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Becker-Pauly, C.; Barré, O.; Schilling, O.; auf dem Keller, U.; Ohler, A.; Broder, C.; Schütte, A.; Kappelhoff, R.; Stöcker, W.; Overall, C.M. Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol. Cell. Proteom. 2011, 10, M111.009233. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.; Haun, R.S.; Ludwig, A.; Shah, S.V.; Kaushal, G.P. ADAM10 is the major sheddase responsible for the release of membrane-associated meprin A. J. Biol. Chem. 2014, 289, 13308–13322. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar, H.; Basbaum, C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 2002, 8, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.J. Role of ADAM10 in intestinal crypt homeostasis and tumorigenesis. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2228–2239. [Google Scholar] [CrossRef]
- Jefferson, T.; Auf dem Keller, U.; Bellac, C.; Metz, V.V.; Broder, C.; Hedrich, J.; Ohler, A.; Maier, W.; Magdolen, V.; Sterchi, E. The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin β and ADAM10. Cell. Mol. Life Sci. 2013, 70, 309–333. [Google Scholar] [CrossRef]
- Wichert, R.; Ermund, A.; Schmidt, S.; Schweinlin, M.; Ksiazek, M.; Arnold, P.; Knittler, K.; Wilkens, F.; Potempa, B.; Rabe, B. Mucus detachment by host metalloprotease meprin β requires shedding of its inactive pro-form, which is abrogated by the pathogenic protease RgpB. Cell Rep. 2017, 21, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- Jäckle, F.; Schmidt, F.; Wichert, R.; Arnold, P.; Prox, J.; Mangold, M.; Ohler, A.; Pietrzik, C.U.; Koudelka, T.; Tholey, A. Metalloprotease meprin β is activated by transmembrane serine protease matriptase-2 at the cell surface thereby enhancing APP shedding. Biochem. J. 2015, 470, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef] [PubMed]
- Strong, T.; Boehm, K.; Collins, F. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J. Clin. Investig. 1994, 93, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.W.; Ridley, C.; Collins, R.; Roseman, A.; Ford, R.; Thornton, D.J. The MUC5B mucin polymer is dominated by repeating structural motifs and its topology is regulated by calcium and pH. Sci. Rep. 2019, 9, 17350. [Google Scholar] [CrossRef] [PubMed]
- Asker, N.; Axelsson, M.A.; Olofsson, S.O.; Hansson, G.C. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J. Biol. Chem. 1998, 273, 18857–18863. [Google Scholar] [CrossRef] [PubMed]
- Croset, A.; Delafosse, L.; Gaudry, J.P.; Arod, C.; Glez, L.; Losberger, C.; Begue, D.; Krstanovic, A.; Robert, F.; Vilbois, F.; et al. Differences in the glycosylation of recombinant proteins expressed in HEK and CHO cells. J. Biotechnol. 2012, 161, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Blundell, P.A.; Lu, D.; Dell, A.; Haslam, S.; Pleass, R.J. Choice of Host Cell Line Is Essential for the Functional Glycosylation of the Fc Region of Human IgG1 Inhibitors of Influenza B Viruses. J. Immunol. 2020, 204, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Böhm, E.; Seyfried, B.K.; Dockal, M.; Graninger, M.; Hasslacher, M.; Neurath, M.; Konetschny, C.; Matthiessen, P.; Mitterer, A.; Scheiflinger, F. Differences in N-glycosylation of recombinant human coagulation factor VII derived from BHK, CHO, and HEK293 cells. BMC Biotechnol. 2015, 15, 87. [Google Scholar] [CrossRef]
- Ermund, A.; Gustafsson, J.K.; Hansson, G.C.; Keita, Å.V. Mucus properties and goblet cell quantification in mouse, rat and human ileal Peyer’s patches. PLoS ONE 2013, 8, e83688. [Google Scholar] [CrossRef]
- Porter, E.; Bevins, C.L.; Ghosh, D.; Ganz, T. The multifaceted Paneth cell. Cell. Mol. Life Sci. 2002, 59, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, N.; Furman, M.; Karanika, E.; Phillips, A.; Bates, A.W. Paneth cell metaplasia in newly diagnosed inflammatory bowel disease in children. BMC Gastroenterol. 2014, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Cyrany, J.; Kohoutova, D.; Förstl, M.; Rejchrt, S.; Kvetina, J.; Vorisek, V.; Kopacova, M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010, 16, 2978. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Isomoto, H.; Shikuwa, S.; Hayashi, T.; Inoue, N.; Yamaguchi, N.; Ohnita, K.; Nanashima, A.; Ito, M.; Nakao, K. Peyer’s patches in the terminal ileum in ulcerative colitis: Magnifying endoscopic findings. J. Clin. Biochem. Nutr. 2010, 46, 111–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jordan, N.; Newton, J.; Pearson, J.; Allen, A. A novel method for the visualization of the in situ mucus layer in rat and man. Clin. Sci. 1998, 95, 97–106. [Google Scholar] [CrossRef]
- Hall, B.; Limaye, A.; Kulkarni, A.B. Overview: Generation of gene knockout mice. Curr. Protoc. Cell Biol. 2009, 44, 19.12.1–19.12.17. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, K.M.; Marburger, K.; Intody, Z.; Wilson, J.H. Manipulating the mammalian genome by homologous recombination. Proc. Natl. Acad. Sci. USA 2001, 98, 8403–8410. [Google Scholar] [CrossRef]
- Krych, L.; Hansen, C.H.F.; Hansen, A.K.; van den Berg, F.W.J.; Nielsen, D.S. Quantitatively Different, yet Qualitatively Alike: A Meta-Analysis of the Mouse Core Gut Microbiome with a View towards the Human Gut Microbiome. PLoS ONE 2013, 8, e62578. [Google Scholar] [CrossRef]
| Membrane-Tethered Cell Surface | Secreted Gel-Forming | Secreted Non-Gel-Forming |
|---|---|---|
| MUC1 | MUC2 | MUC7 |
| MUC3A | MUC5AC | MUC8 |
| MUC3B | MUC5B | MUC9 |
| MUC4 | MUC6 | |
| MUC12 | MUC19 | |
| MUC13 | ||
| MUC14 | ||
| MUC15 | ||
| MUC16 | ||
| MUC17 | ||
| MUC18 | ||
| MUC19 | ||
| MUC20 | ||
| MUC21 | ||
| MUC22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanforth, K.J.; Zakhour, M.I.; Chater, P.I.; Wilcox, M.D.; Adamson, B.; Robson, N.A.; Pearson, J.P. The MUC2 Gene Product: Polymerisation and Post-Secretory Organisation—Current Models. Polymers 2024, 16, 1663. https://doi.org/10.3390/polym16121663
Stanforth KJ, Zakhour MI, Chater PI, Wilcox MD, Adamson B, Robson NA, Pearson JP. The MUC2 Gene Product: Polymerisation and Post-Secretory Organisation—Current Models. Polymers. 2024; 16(12):1663. https://doi.org/10.3390/polym16121663
Chicago/Turabian StyleStanforth, Kyle J., Maria I. Zakhour, Peter I. Chater, Matthew D. Wilcox, Beth Adamson, Niamh A. Robson, and Jeffrey P. Pearson. 2024. "The MUC2 Gene Product: Polymerisation and Post-Secretory Organisation—Current Models" Polymers 16, no. 12: 1663. https://doi.org/10.3390/polym16121663
APA StyleStanforth, K. J., Zakhour, M. I., Chater, P. I., Wilcox, M. D., Adamson, B., Robson, N. A., & Pearson, J. P. (2024). The MUC2 Gene Product: Polymerisation and Post-Secretory Organisation—Current Models. Polymers, 16(12), 1663. https://doi.org/10.3390/polym16121663






