Redox-Stable and Multicolor Electrochromic Polyamides with Four Triarylamine Cores in the Repeating Unit
Abstract
1. Introduction
2. Materials and Methods
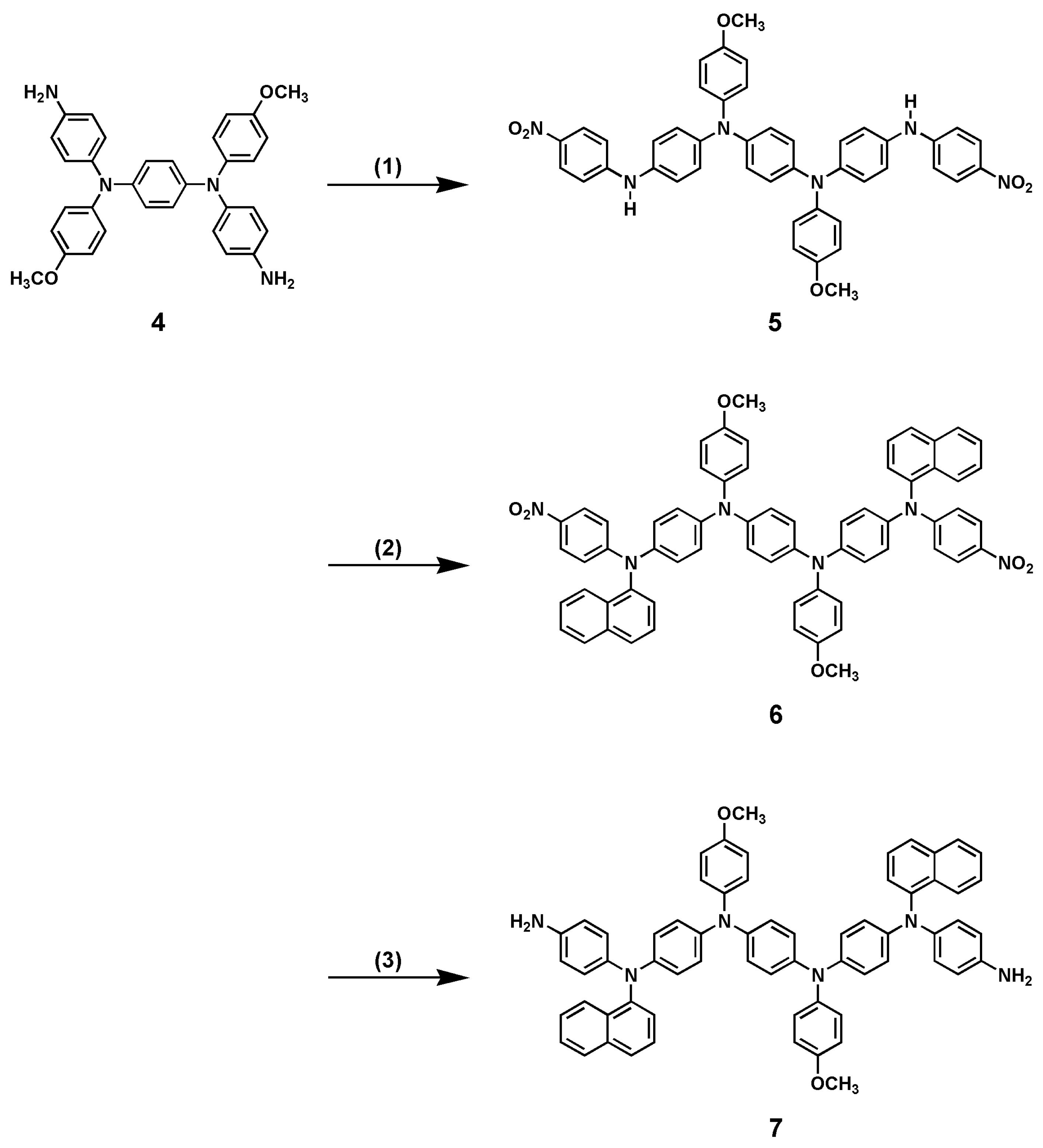
2.1. Synthesis N,N′-Bis(4-methoxyphenyl)-N,N′-bis[4-(4-methoxyphenyl-4′-nitrophenylamino)phenyl]-p-phenylenediamine (2)
2.2. Synthesis of N,N′-Bis(4-methoxyphenyl)-N,N′-bis(4-(4-aminophenyl-4′-methoxyphenylamino)phenyl)-p-phenylenediamine (3)
2.3. Synthesis of N,N′-Bis(4-methoxyphenyl)-N,N′-bis[4-(4-nitrophenylaminopheny]-p-phenylenediamine (5)
2.4. Synthesis of N,N′-Bis(4-methoxyphenyl)-N,N′-bis[4-((1-naphthyl-4-nitrophenyl)amino)phenyl]-p-phenylenediamine (6)
2.5. Synthesis of N,N′-Bis(4-methoxyphenyl)-N,N′-bis(4-((4-aminophenyl-1-naphthyl)amino)phenyl)-p-phenylenediamine (7)
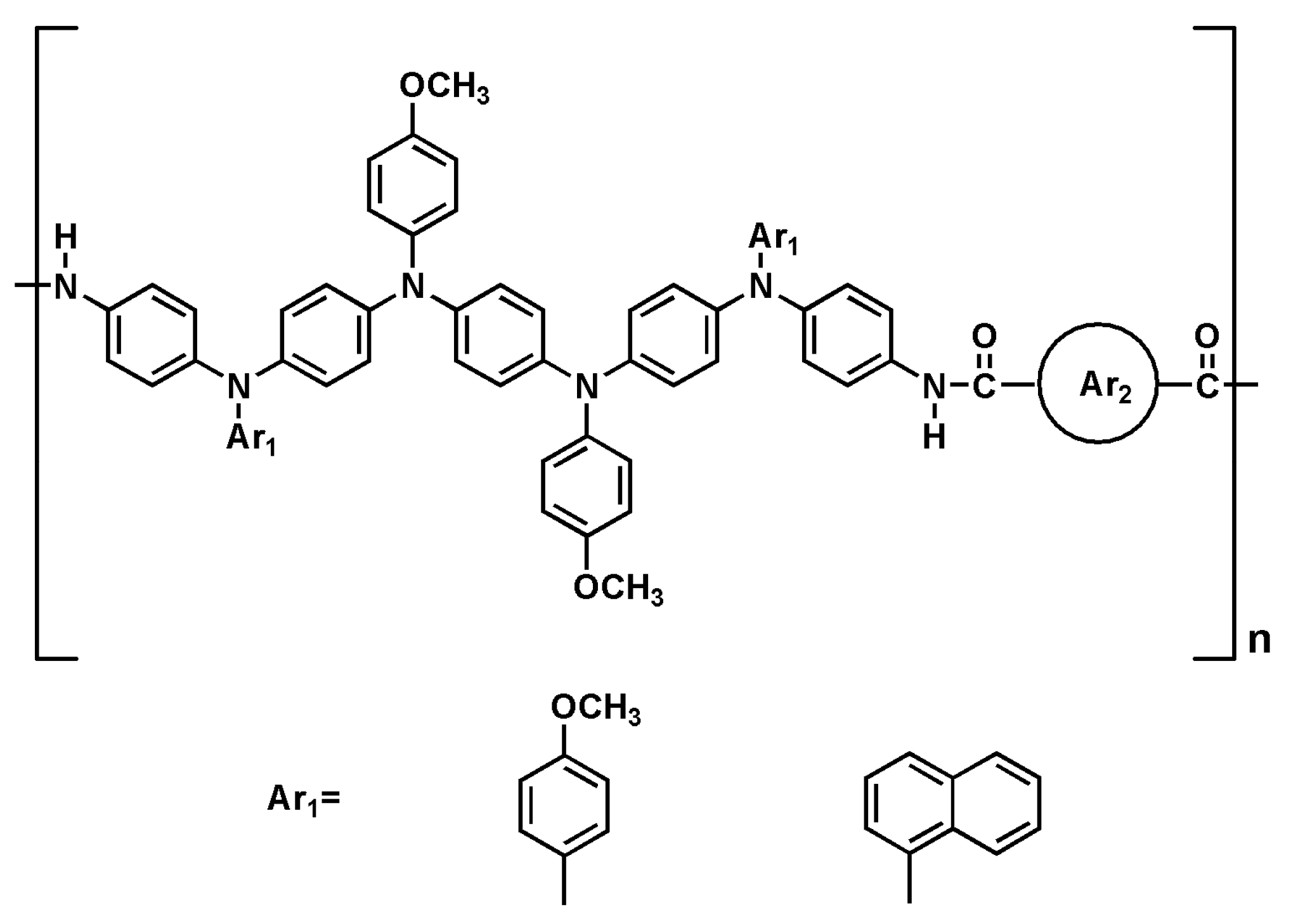
2.6. Polymer Synthesis
2.7. Measurements
3. Results and Discussion
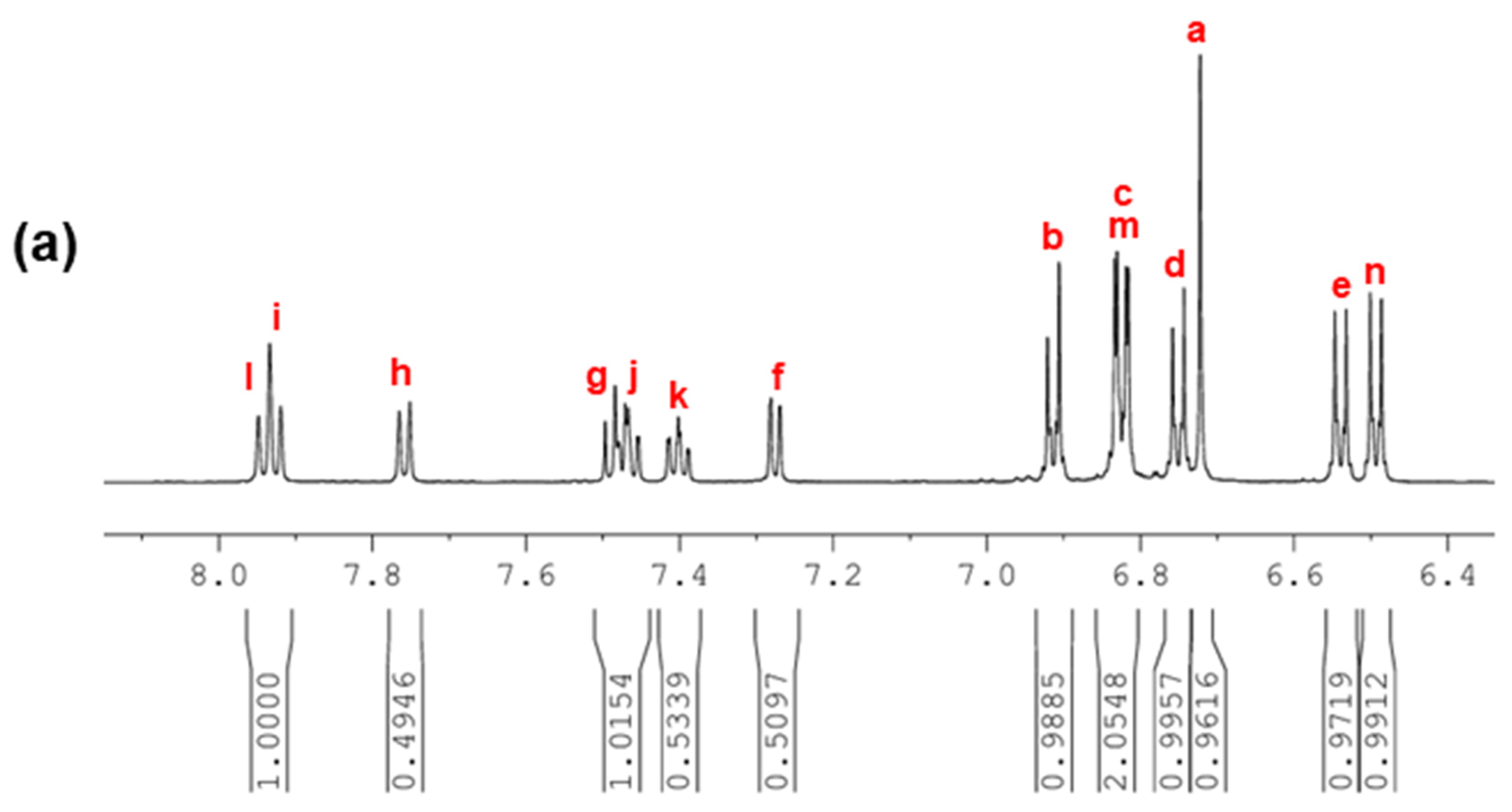
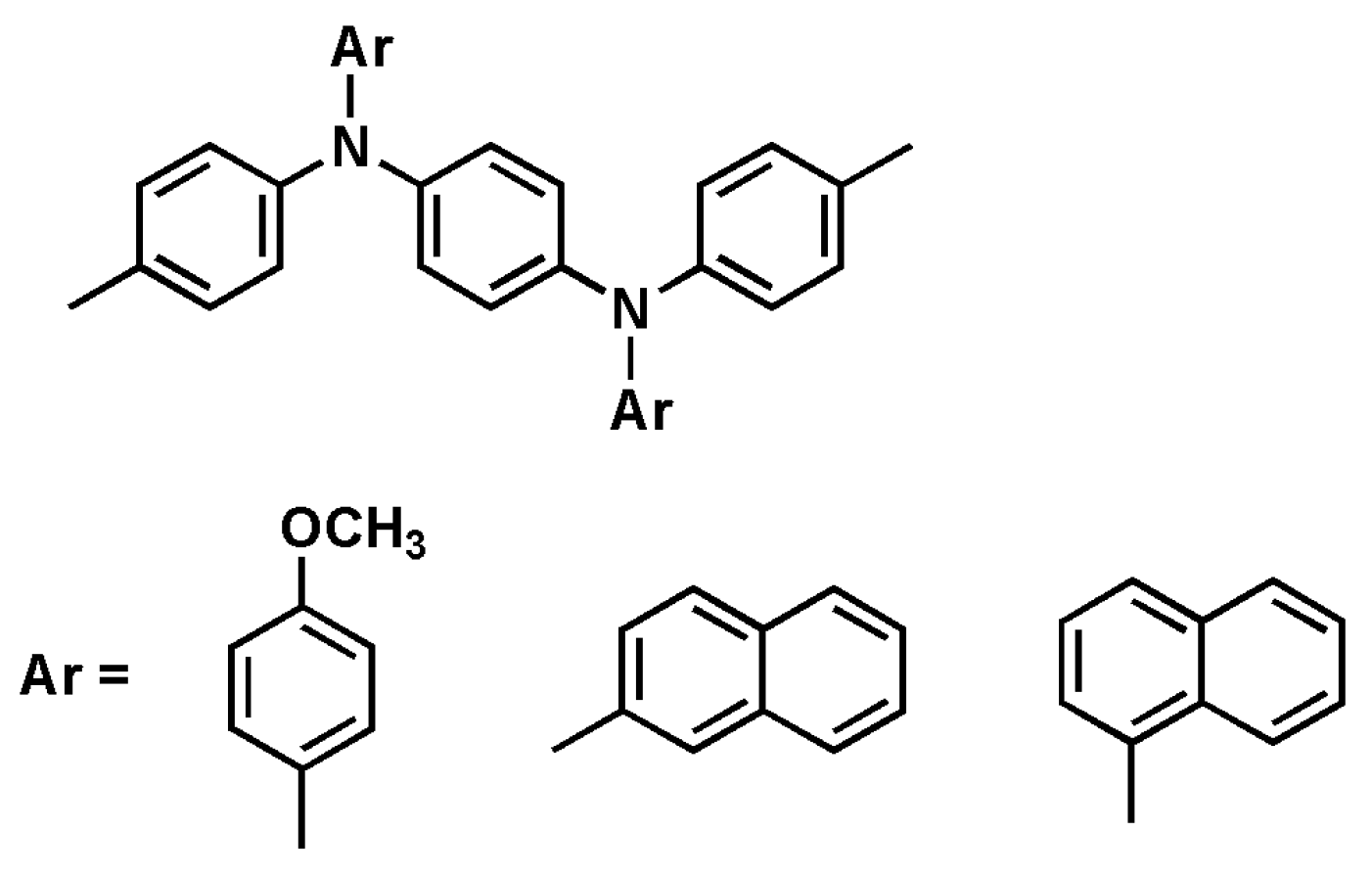
3.1. Monomer Synthesis
3.2. Polymer Synthesis
3.3. Solubility and Thermal Properties
3.4. Electrochemical Properties
3.5. Spectroelectrochemical Properties
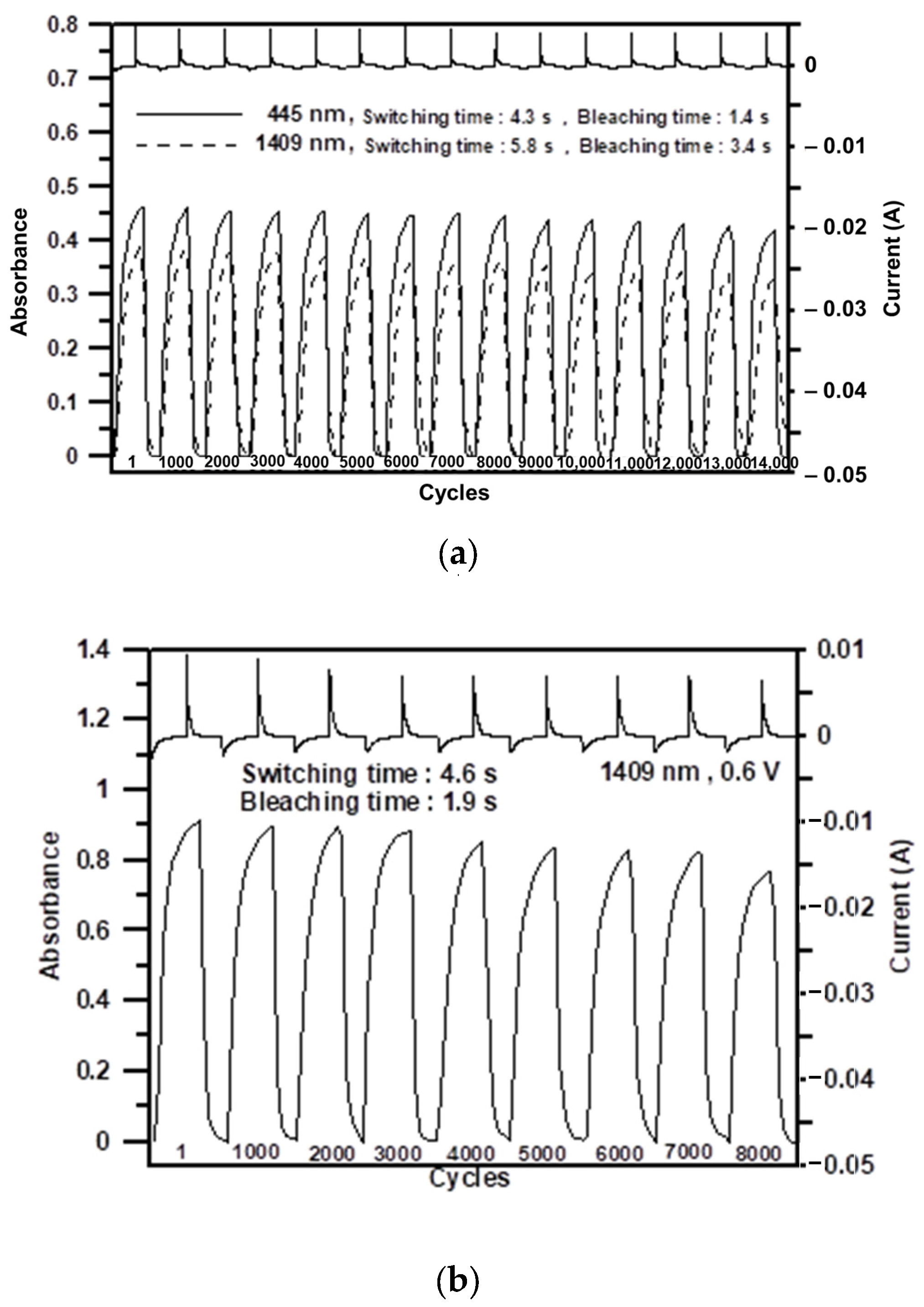
3.6. Electrochromic Switching Properties
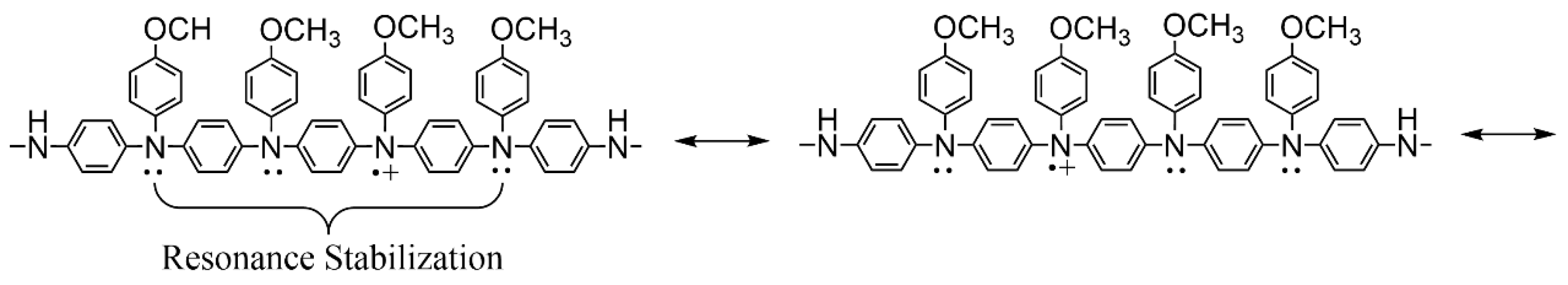
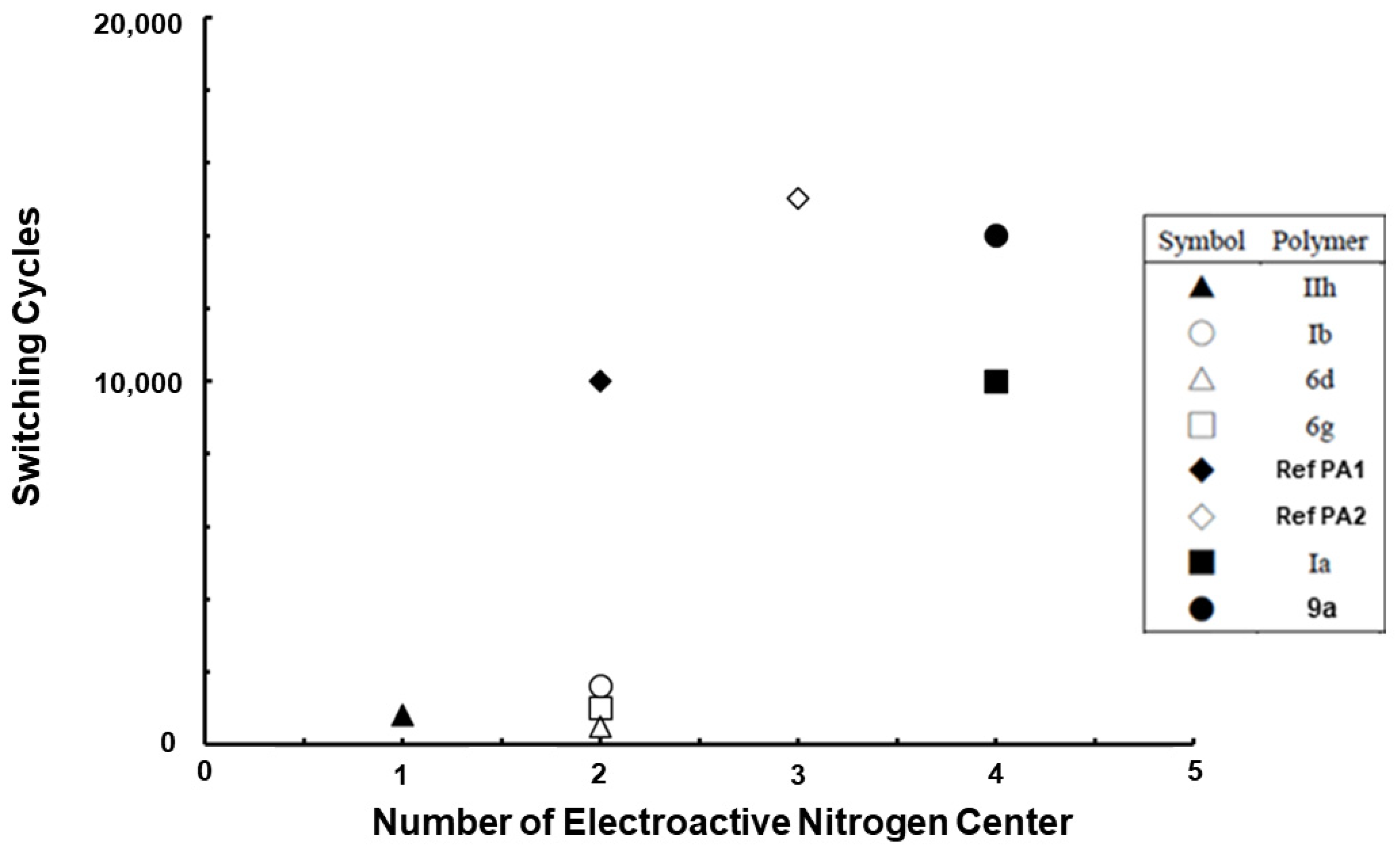
3.7. Relationship between Chemical Structure and Switching Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic Systems and the Prospects for Devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Beaujuge, P.M.; Reynolds, J.R. Color Control in π-Conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Gunbas, G.; Toppare, L. Electrochromic Conjugated Polyheterocycles and Derivatives—Highlights from the Last Decade towards Realization of Long Lived Aspirations. Chem. Commun. 2012, 48, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Neo, W.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated Polymer-Based Electrochromics: Materials, Device Fabrication and Application Prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric Electrochromic Materials with Donor-Acceptor Structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Huang, Z.J.; Li, F.; Xie, J.P.; Mou, H.R.; Gong, C.B.; Tang, Q. Electrochromic Materials Based on Tetra-Substituted Viologen Analogues with Broad Absorption and Good Cycling Stability. Sol. Energy Mater. Sol. Cells 2021, 223, 110968. [Google Scholar] [CrossRef]
- Feng, F.; Guo, S.; Ma, D.; Wang, J. An Overview of Electrochromic Devices with Electrolytes Containing Viologens. Sol. Energy Mater. Sol. Cells 2023, 254, 112270. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, Z.; Jia, S.; Shi, X.; Chen, Z.; Jiang, Z. Research Progress in Special Engineering Plastic-Based Electrochromic Polymers. Materials 2024, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Liou, G.S.; Hsiao, S.H. Highly Stable Anodic Green Electrochromic Aromatic Polyamides: Synthesis and Electrochromic Properties. J. Mater. Chem. 2007, 17, 1007–1015. [Google Scholar] [CrossRef]
- Liou, G.S.; Chang, C.W. Highly Stable Anodic Electrochromic Aromatic Polyamides Containing N,N,N′,N′-Tetraphenyl-p-Phenylenediamine Moieties: Synthesis, Electrochemical, and Electrochromic Properties. Macromolecules 2008, 41, 1667–1674. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Solution-Processable Novel Near-Infrared Electrochromic Aromatic Polyamides Based on Electroactive Tetraphenyl-p-Phenylenediamine Moieties. Chem. Mater. 2009, 21, 4062–4070. [Google Scholar] [CrossRef]
- Liou, G.S.; Lin, H.Y. Synthesis and Electrochemical Properties of Novel Aromatic Poly(amine-amide)s with Anodically Highly Stable Yellow and Blue Electrochromic Behaviors. Macromolecules 2009, 42, 125–134. [Google Scholar] [CrossRef]
- Yen, H.J.; Lin, H.Y.; Liou, G.S. Novel Starburst Triarylamine-Containing Electroactive Aramids with Highly Stable Electrochromism in Near-Infrared and Visible Light Regions. Chem. Mater. 2011, 23, 1874–1882. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wu, C.N. Electrochemical and Electrochromic Studies of Redox-Active Aromatic Polyamides with 3,5-Dimethyltriphenylamine Units. J. Electroanal. Chem. 2016, 776, 139–147. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Huang, Y.P. Redox-Active and Fluorescent Pyrene-Based Triarylamine Dyes and Their Derived Electrochromic Polymers. Dyes Pigm. 2018, 158, 368–381. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Chung, H.H. Applications of Tris(4-(thiophen-2-yl)phenyl)amine Dithienylpyrrole-based Conjugated Copolymers in High-Contrast Electrochromic Devices. Polymers 2016, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Kong, L.; Ju, X.; Liu, X.; Zhao, J.; Niu, Q. Multichromic Polymers Containing Alternating Bis(3-Methoxythiophene) and Triphenylamine Based Units with Para-Protective Substituents. Materials 2016, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wang, S.; Wei, D.; Niu, H.; Wang, W.; Bai, X. Multifunctional Polyamides Containing Pyrrole Unit with Different Triarylamine Units Owning Electrochromic, Electrofluorochromic and Photoelectron Conversion Properties. J. Electroanal. Chem. 2018, 812, 132–142. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Design and Preparation of Triphenylamine-Based Polymeric Materials Towards Emergent Optoelectronic Applications. Prog. Polym. Sci. 2019, 89, 250–287. [Google Scholar] [CrossRef]
- Gong, H.; Li, W.; Fu, G.; Zhang, Q.; Jin, Y.; Wang, H. Recent Progress and Advances in Electrochromic Devices Exhibiting Infrared Modulation. J. Mater. Chem. A 2022, 10, 6269–6290. [Google Scholar] [CrossRef]
- Brooke, R.; Mitraka, E.; Sardar, S.; Sandberg, M.; Sawatdee, A.; Berggren, M.; Crispin, X.; Jonsson, M.P. Infrared Electrochromic Conducting Polymer Devices. J. Mater. Chem. C 2017, 5, 5824–5830. [Google Scholar] [CrossRef]
- Hao, Q.; Li, Z.J.; Lu, C.; Sun, B.; Zhong, Y.W.; Wan, L.J.; Wang, D. Oriented Two-Dimensional Covalent Organic Framework Films for Near-Infrared Electrochromic Application. J. Am. Chem. Soc. 2019, 141, 19831–19838. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, Y.; Rajan, K.; Xiao, Z.; Sagar, R.U.R.; Liu, P. Transparent-to-black Electrochromic Smart Windows Based on N, N, N’, N’-Tetraphenylbenzidine Derivatives and Tungsten Trioxide with High Adjustment Ability for Visible and Near-Infrared Light. Sol. Energy Mater. Sol. Cells 2021, 226, 111070. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Zou, X.; Tan, Y.; Jia, C.; Weng, X.; Deng, L. Infrared Electrochromic Materials, Devices and Applications. Appl. Mater. Today 2021, 24, 101073. [Google Scholar] [CrossRef]
- Robin, M.B.; Day, P. Mixed-Valence Chemistry: A Survey and Classification. Adv. Inorg. Chem. Radiochem. 1968, 10, 247–422. [Google Scholar]
- Seo, E.T.; Nelson, R.F.; Fritsch, J.M.; Marcoux, L.S.; Leedy, D.W.; Adams, R.N. Anodic Oxidation Pathways of Aromatic Amines. Electrochemical and Electron Paramagnetic Resonance Studies. J. Am. Chem. Soc. 1966, 88, 3498–3503. [Google Scholar] [CrossRef]
- Nelson, R.F.; Adams, R.N. Anodic Oxidation Pathways of Substituted Triphenylamines II Quantitative Studies of Benzidine Formation. J. Am. Chem. Soc. 1968, 90, 3925–3930. [Google Scholar] [CrossRef]
- Ito, A.; Ino, H.; Tanaka, K.; Kanemoto, K.; Kato, T. Facile Synthesis, Crystal Structures, and High-Spin Cationic States of All-para-Brominated Oligo(N-phenyl-m-aniline)s. J. Org. Chem. 2002, 67, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Hagopian, L.; Kohler, G.; Walter, R.I. Substituent Effects on the Properties of Stable Aromatic Free Radicals. Oxidation-Reduction Potentials of Triarylamine-Triarylaminium Ion Systems. J. Phys. Chem. 1967, 71, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, G.; Meng, H.Q.; Wudl, F. Organic Polymeric Electrochromic Devices: Polychromism with Very High Coloration Efficiency. Chem. Mater. 2004, 16, 574–580. [Google Scholar] [CrossRef]
- Chern, Y.T.; Zhang, S.J.; Ho, S.J.; Shao, Y.J.; Wang, Y.J.; Liou, G.S. Substituents and Resonance Effects on the Electrochemical Stability of Polyelectrochromic Triarylamine-Based Polymers. ACS Appl. Polym. Mater. 2024, 6, 5256–5267. [Google Scholar] [CrossRef]
- Rózalska, I.; Kulyk, P.; Bajer, I.K. Linear 1,4-Coupled Oligoanilines of Defined Length; Preparation and Spectroscopic Properties. New J. Chem. 2004, 28, 1235–1243. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Yang, C.P.; Lin, W.L. Synthesis and Characterization of New Diphenylfluorene-Based Aromatic Polyamides Derived from 9,9-Bis[4-(4-carboxyhenoxy)phenyl]fluorene. Macromol. Chem. Phys. 1999, 200, 1428–1433. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Chen, W.T. Syntheses and Properties of Novel Fluorinated Polyamides Based on a Bis(ether-carboxylic acid) or a Bis(ether amine) Extended from Bis(4-hydroxyphenyl)phenyl-2,2,2-trifluoroethane. J. Polym. Sci. Polym. Chem. 2003, 41, 420–431. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, Y.C.; Ju, J.J.; Chiang, C.J.; Chern, Y.T. Synthesis, Characterization and Hydrolysis of Aromatic Polyazomethines Containing Non-coplanar Biphenyl Structures. Polymer 2011, 52, 954–964. [Google Scholar] [CrossRef]
- Yamazaki, N.; Higashi, F.; Kawabata, J. Studies on Reactions of the N-Phosphonium Salts of Pyridines. XI. Preparation of Polypeptides and Polyamides by Means of Triaryl Phosphites in Pyridine. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 2149–2154. [Google Scholar]
- Yamazaki, N.; Matsumoto, M.; Higashi, F. Studies on Reactions of the N-Phosphonium Salts of Pyridines. XIV. Wholly Aromatic Polyamides by The Direct Polycondensation Reaction by Using Phosphites in the Presence of Metal Salts. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 1373–1380. [Google Scholar] [CrossRef]
- Lambert, C.; Noll, G. The Class II/III Transition in Triarylamine Redox Systems. J. Am. Chem. Soc. 1999, 121, 8434–8442. [Google Scholar] [CrossRef]
- Yen, H.J.; Guo, S.M.; Liou, G.S. The Mixed-Valence Class I Transition and Electrochemistry of Bis(triphenylamine)-based Aramids Containing Isolated Ether-linkage. J. Polym. Sci. Polym. Chem. 2011, 49, 3805–3816. [Google Scholar] [CrossRef]
- Li, M.; Patra, A.; Sheynin, Y.; Bendikov, M. Hexyl-derivatized Poly(3,4-ethylenedioxyselenophene): Novel Highly Stable Organic Electrochromic Material with High Contrast Ratio, High Coloration Efficiency, and Low-Switching Voltage. Adv. Mater. 2009, 21, 1707–1711. [Google Scholar] [CrossRef]
- Chang, C.W.; Chung, C.H.; Liou, G.S. Novel Anodic Polyelectrochromic Aromatic Polyamides Containing Pendent Dimethyltriphenylamine Moieties. Macromolecules 2008, 41, 8441–8451. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Liou, G.S.; Wang, H.M. Highly Stable Electrochromic Polyamides Based on N,N-Bis(4-aminophenyl)-N′,N′-bis(4-tert-butylphenyl)-1,4-phenylenediamine. J. Polym. Sci. Polym. Chem. 2009, 47, 2330–2343. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Yeh, S.J. Synthesis and Optoelectronic Properties of Novel Polyamides with 2-Naphthyldiphenylamine Units. Macromol. Chem. Phys. 2014, 215, 705–715. [Google Scholar] [CrossRef]
- Wang, J.M. Highly Stable Electrochromism of New Polyamide Based on Triphenylamine. Master’s Thesis, National Taiwan University of Science and Technology, Taipei, Taiwan, 2013. [Google Scholar]
- Yen, H.J.; Liou, G.S. Novel Thermally Stable Triarylamine-Containing Aromatic Polyamides Bearing Anthrylamine Chromophores for Highly Efficient Green-Light-Emitting Materials. J. Polym. Sci. Polym. Chem. 2008, 46, 7354–7368. [Google Scholar] [CrossRef]
- Kung, Y.C.; Hsiao, S.H. Fluorescent and Electrochromic Polyamides with Pyrenylamine Chromophore. J. Mater. Chem. 2010, 20, 5481–5492. [Google Scholar] [CrossRef]






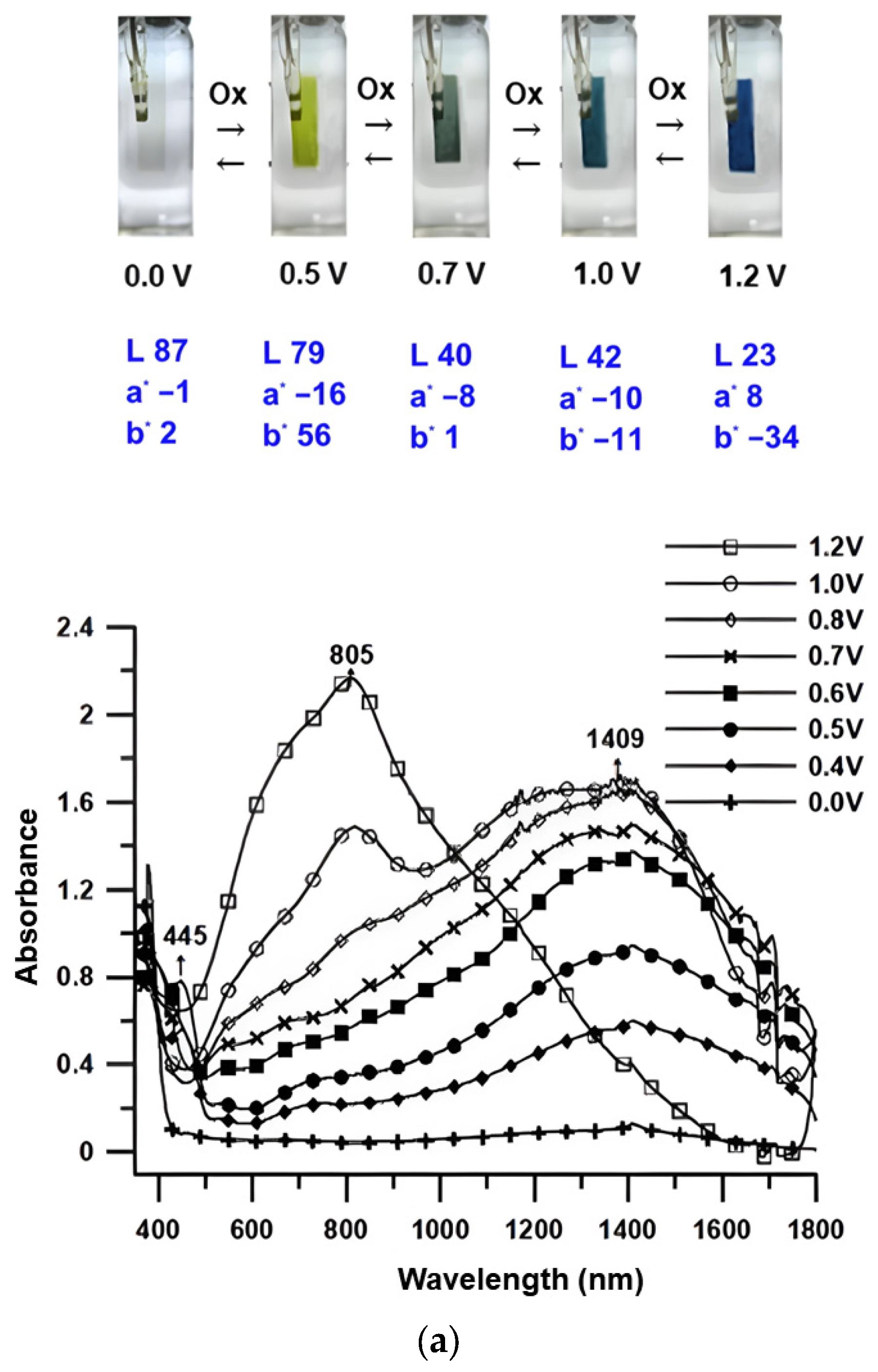
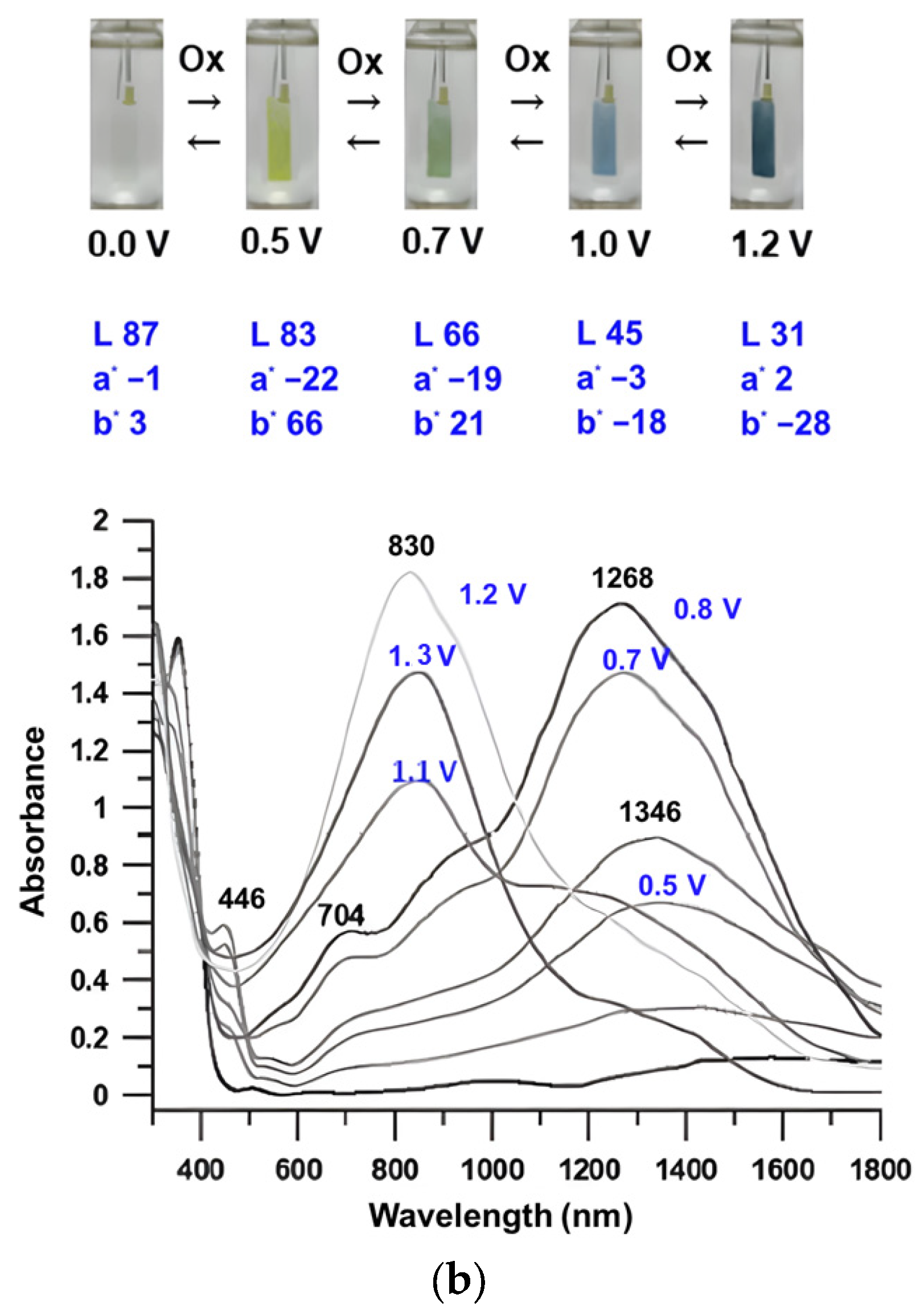


| Solution/Film (nm) | Oxidation Potential (V) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E1/2 c | ||||||||||
| Polymer | λmax a | λonset a | Eg b (eV) | Eonset | 1st | 2nd | 3rd | 4th | HOMO d (eV) | LUMO e (eV) |
| 9a | 277 (319) | 341 (420) | 2.95 | 0.27 | 0.42 | 0.61 | 0.93 | 1.06 | 4.78 | 1.83 |
| 9b | 275 (317) | 365 (418) | 2.97 | 0.26 | 0.42 | 0.62 | 0.93 | 1.05 | 4.78 | 1.81 |
| 9c | 323 (314) | 407 (428) | 2.90 | 0.26 | 0.42 | 0.61 | 0.94 | 1.04 | 4.78 | 1.88 |
| 10d | 343 (355) | 410 (416) | 2.98 | 0.28 | 0.43 | 0.67 | 1.11 | - | 4.79 | 1.81 |
| 10e | 333 (333) | 427 (452) | 2.74 | 0.23 | 0.39 | 0.62 | 1.08 | - | 4.75 | 2.01 |
| 10f | 331 (331) | 429 (440) | 2.82 | 0.21 | 0.38 | 0.62 | 1.08 | - | 4.74 | 1.92 |
| Cycles a | ΔOD1409 b | ΔT(%)1409 c | Q1409 d (mC/cm2) | η e (cm2/C) | Decay1409 f (%) |
|---|---|---|---|---|---|
| 1 | 0.387 (0.463) g | 59.0 (65.6) | 1.27 (1.70) | 305 (272) | 0.00 (0.00) |
| 1000 | 0.387 (0.461) | 59.0 (65.4) | 1.27 (1.70) | 305 (272) | 0.00 (0.00) |
| 2000 | 0.381 (0.455) | 58.4 (64.9) | 1.26 (1.68) | 303 (271) | 0.66 (0.37) |
| 3000 | 0.378 (0.455) | 58.1 (64.9) | 1.25 (1.68) | 302 (271) | 0.98 (0.37) |
| 4000 | 0.373 (0.454) | 57.6 (64.8) | 1.24 (1.67) | 301 (271) | 1.31 (0.37) |
| 5000 | 0.372 (0.451) | 57.5 (64.6) | 1.24 (1.67) | 300 (271) | 1.64 (0.37) |
| 6000 | 0.370 (0.447) | 57.3 (64.3) | 1.23 (1.65) | 300 (270) | 1.64 (0.73) |
| 7000 | 0.366 (0.447) | 56.9 (64.3) | 1.22 (1.65) | 300 (270) | 1.64 (0.73) |
| 8000 | 0.365 (0.445) | 56.8 (64.1) | 1.22 (1.65) | 299 (270) | 1.97 (0.73) |
| 9000 | 0.356 (0.439) | 55.9 (63.6) | 1.20 (1.63) | 298 (269) | 2.29 (1.10) |
| 10,000 | 0.348 (0.438) | 55.1 (63.5) | 1.18 (1.63) | 295 (269) | 3.28 (1.10) |
| 11,000 | 0.343 (0.437) | 54.6 (63.4) | 1.17 (1.63) | 294 (269) | 3.61 (1.10) |
| 12,000 | 0.342 (0.433) | 54.5 (63.1) | 1.17 (1.62) | 293 (267) | 3.93 (1.84) |
| 13,000 | 0.340 (0.426) | 54.3 (62.5) | 1.16 (1.61) | 293 (264) | 3.93 (2.94) |
| 14,000 | 0.337 (0.419) | 54.0 (61.9) | 1.15 (1.60) | 292 (262) | 4.26 (3.68) |
| Cycles a | ΔOD1409 b | ΔT (%) c | Q (mC/cm2) d | η (cm2/C) e | Decay (%) f |
|---|---|---|---|---|---|
| 1 | 0.912 | 87.8 | 3.43 | 266 | 0.00 |
| 1000 | 0.898 | 87.4 | 3.40 | 264 | 0.75 |
| 2000 | 0.898 | 87.4 | 3.40 | 264 | 0.75 |
| 3000 | 0.884 | 86.9 | 3.36 | 263 | 1.13 |
| 4000 | 0.854 | 86.0 | 3.32 | 257 | 3.38 |
| 5000 | 0.837 | 85.4 | 3.29 | 255 | 4.13 |
| 6000 | 0.828 | 85.1 | 3.26 | 254 | 4.51 |
| 7000 | 0.823 | 85.0 | 3.24 | 254 | 4.51 |
| 8000 | 0.769 | 83.0 | 3.17 | 243 | 8.65 |
| Cycles a | ΔOD b | ΔT (%) c | Q d (mC/cm2) | η e (cm2/C) | Decay f (%) |
|---|---|---|---|---|---|
| 1 | 0.653 (0.439) g | 77.8 (63.6) | 1.71 (1.86) | 381 (236) | 0.00 (0.00) |
| 1400 | 0.647 (0.432) | 77.5 (63.0) | 1.71 (1.85) | 379 (234) | 0.52 (0.85) |
| 2800 | 0.638 (0.430) | 77.0 (62.8) | 1.69 (1.84) | 377 (234) | 1.05 (0.85) |
| 4200 | 0.629 (0.427) | 76.5 (62.6) | 1.68 (1.83) | 375 (233) | 1.57 (1.27) |
| 4600 | 0.617 (0.421) | 75.8 (62.1) | 1.66 (1.82) | 372 (231) | 2.36 (2.12) |
| 7000 | 0.594 (0.417) | 74.5 (61.7) | 1.62 (1.82) | 368 (230) | 3.41 (2.54) |
| 8400 | 0.558 (0.415) | 72.3 (61.5) | 1.55 (1.81) | 359 (229) | 5.77 (2.97) |
| 9800 | 0.547 (0.407) | 71.6 (60.8) | 1.54 (1.80) | 356 (227) | 6.56 (3.81) |
| 11,200 | 0.527 (0.401) | 70.3 (60.2) | 1.51 (1.78) | 349 (225) | 8.40 (4.66) |
| 12,600 | 0.522 (0.393) | 69.9 (59.5) | 1.51 (1.76) | 347 (224) | 8.92 (5.08) |
| 14,000 | 0.501 (0.383) | 68.4 (58.6) | 1.46 (1.73) | 343 (221) | 9.97 (6.35) |
| Polymer Code | Potential a | λmax b | Absorption Range b |
|---|---|---|---|
| (V) | (nm) | (nm) | |
| 9a | 0.40 | 1409 | 800–1800 |
| Ref PA1 | 0.75 | 1062 | 800–1500 |
| Ref PA2 | 0.40 | 1252 | 800–1700 |
| Polymer | Potential (V) | CE (cm2/C) a | Cycles b | ΔT (%) c | Decay (%) d | Ref. |
|---|---|---|---|---|---|---|
| 9a | 0.0 ↔ 0.40 | 272 | 14,000 | 65.6 | 3.7 | This study |
| Ref PA1 | 0.0 ↔ 0.70 | 388 | 10,000 | 54.0 | 4.9 | [12] |
| Ref PA2 | 0.0 ↔ 0.40 | 195 | 15,000 | 54.0 | 4.1 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chern, Y.-T.; Yen, C.-C.; Wang, J.-M.; Lu, I.-S.; Huang, B.-W.; Hsiao, S.-H. Redox-Stable and Multicolor Electrochromic Polyamides with Four Triarylamine Cores in the Repeating Unit. Polymers 2024, 16, 1644. https://doi.org/10.3390/polym16121644
Chern Y-T, Yen C-C, Wang J-M, Lu I-S, Huang B-W, Hsiao S-H. Redox-Stable and Multicolor Electrochromic Polyamides with Four Triarylamine Cores in the Repeating Unit. Polymers. 2024; 16(12):1644. https://doi.org/10.3390/polym16121644
Chicago/Turabian StyleChern, Yaw-Terng, Chien-Cheng Yen, Jia-Mao Wang, I-Shan Lu, Bo-Wei Huang, and Sheng-Huei Hsiao. 2024. "Redox-Stable and Multicolor Electrochromic Polyamides with Four Triarylamine Cores in the Repeating Unit" Polymers 16, no. 12: 1644. https://doi.org/10.3390/polym16121644
APA StyleChern, Y.-T., Yen, C.-C., Wang, J.-M., Lu, I.-S., Huang, B.-W., & Hsiao, S.-H. (2024). Redox-Stable and Multicolor Electrochromic Polyamides with Four Triarylamine Cores in the Repeating Unit. Polymers, 16(12), 1644. https://doi.org/10.3390/polym16121644







