Development and Investigation of an Innovative 3D Biohybrid Based on Collagen and Silk Sericin Enriched with Flavonoids for Potential Wound Healing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Microcapsules Preparation
2.2. CollSS-Based Scaffolds Preparation
2.3. The FT-IR Analysis for CollSS-Based Scaffolds
2.4. The Water Uptake Analysis for CollSS-Based Scaffolds
2.5. In Vitro Degradation of CollSS-Based Scaffolds
2.6. Scanning Electron Microscopy for CollSS-Based Scaffolds
2.7. In Vitro Study of Material Biocompatibility
2.8. Quantitative and Qualitative Assessment of Cell Viability, Proliferation, and Cytotoxicity
2.9. Cytoskeleton Development of Keratinocytes in Contact with the Biomaterials
2.10. Gene Expression Evaluation of Apoptotic Markers by qPCR
2.11. Protein Expression Evaluation of Apoptotic Markers by Confocal Microscopy
2.12. Fluorescence Quantification of Cell Imaging
2.13. Evaluation of Anti-Inflammatory Potential of CollSS-CQ Biomaterial
2.14. Evaluation of Antioxidant Potential of CollSS-CQ Biomaterial
2.15. Statistical Analysis
3. Results
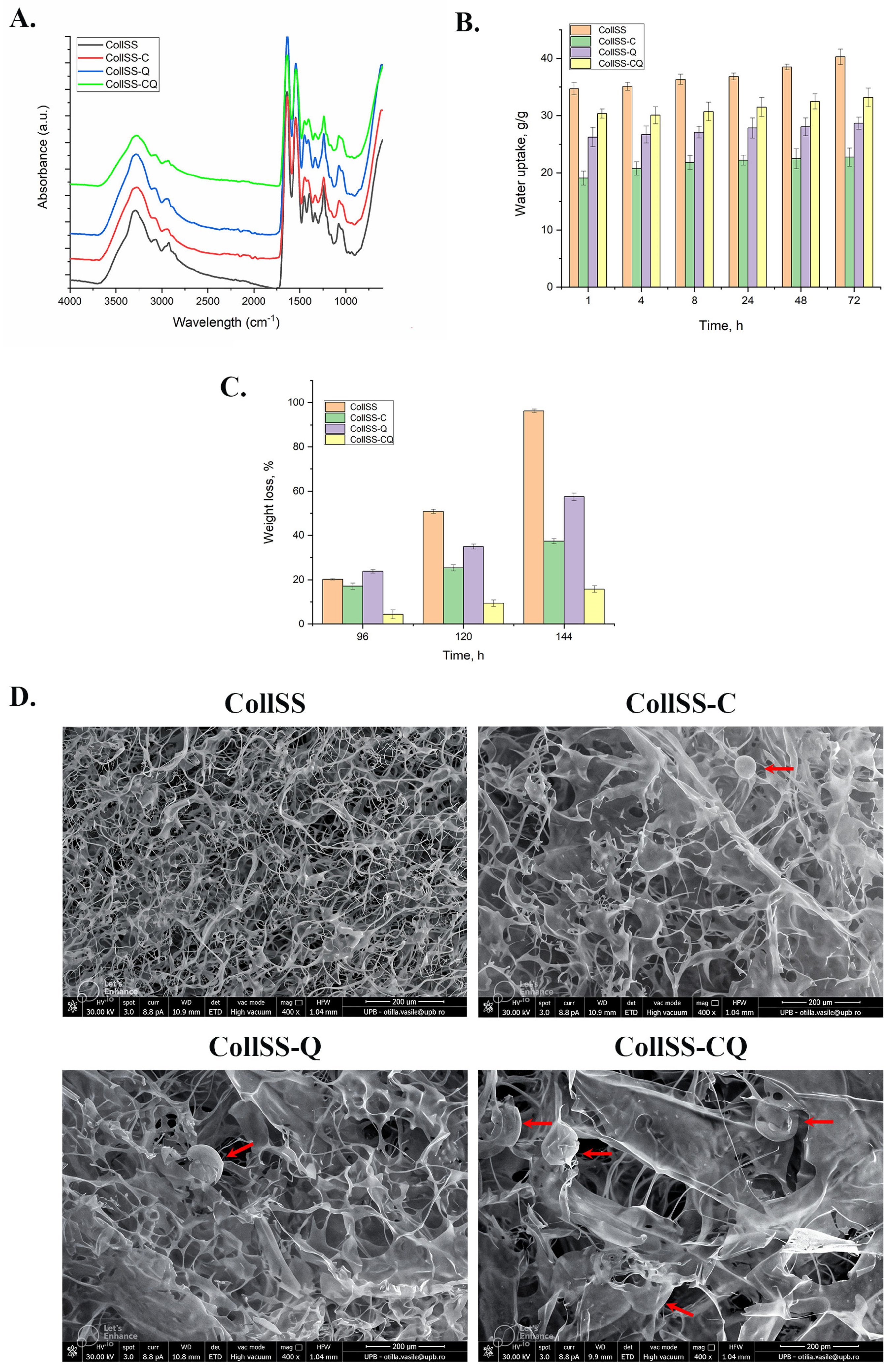
3.1. The FT-IR Analysis for CollSS-Based Scaffolds
3.2. The Water Uptake Analysis for CollSS-Based Scaffolds
3.3. In Vitro Degradation of CollSS-Based Scaffolds
3.4. Scanning Electron Microscopy for CollSS-Based Scaffolds
3.5. In Vitro Assessment of CollSS-Based Materials Biocompatibility and Cytoskeleton Development
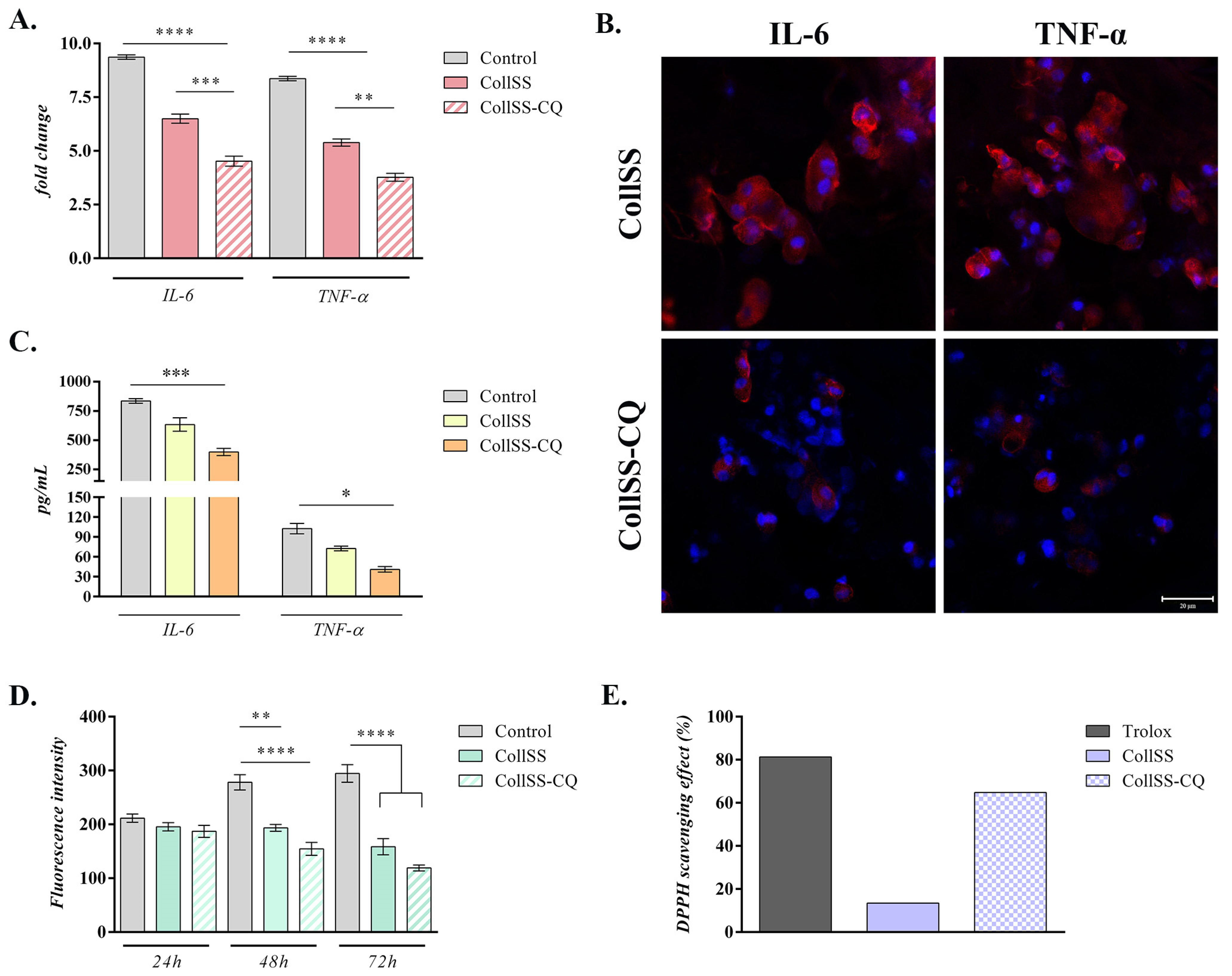
3.6. Evaluation of Apoptotic Markers Gene Expression by qPCR
3.7. Analysis of Anti-Inflammatory Potential for CollSS-CQ
3.8. Analysis of Antioxidant Potential of CollSS-CQ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Ijaola, A.O.; Akamo, D.O.; Damiri, F.; Akisin, C.J.; Bamidele, E.A.; Ajiboye, E.G.; Berrada, M.; Onyenokwe, V.O.; Yang, S.-Y.; Asmatulu, E. Polymeric biomaterials for wound healing applications: A comprehensive review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1998–2050. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2020, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-based formulations for wound healing: A literature review. Life Sci. 2022, 290, 120096. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Wright, M.E.E.; Jeschke, M.G.; Amini-Nik, S. Biomaterials for Skin Substitutes. Adv. Healthc. Mater. 2018, 7, 1700897. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Y.; Zhang, G.; Zhang, Y. Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine. Polymers 2023, 15, 2941. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef]
- Bhowmick, S.; Scharnweber, D.; Koul, V. Co-cultivation of keratinocyte-human mesenchymal stem cell (hMSC) on sericin loaded electrospun nanofibrous composite scaffold (cationic gelatin/hyaluronan/chondroitin sulfate) stimulates epithelial differentiation in hMSCs: In vitro study. Biomaterials 2016, 88, 83–96. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Hwang, C.; Talbot, S.G.; Hibler, B.P.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications; Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Dinescu, S.; Ignat, S.R.; Lazar, A.D.; Marin, Ș.; Danilă, E.; Marin, M.M.; Udeanu, D.I.; Ghica, M.V.; Albu-Kaya, M.G.; Costache, M. Efficiency of Multiparticulate Delivery Systems Loaded with Flufenamic Acid Designed for Burn Wound Healing Applications. J. Immunol. Res. 2019, 2019, 4513108. [Google Scholar] [CrossRef] [PubMed]
- Albu, M.G.; Ferdes, M.; Kaya, D.A.; Ghica, M.V.; Titorencu, I.; Popa, L.; Albu, L. Collagen Wound Dressings with Anti-Inflammatory Activity. Mol. Cryst. Liq. Cryst. 2012, 555, 271–279. [Google Scholar] [CrossRef]
- Lungu, A.; Albu, M.G.; Stancu, I.C.; Florea, N.M.; Vasile, E.; Iovu, H. Superporous Collagen-sericin Scaffolds. J. Appl. Polym. Sci. 2013, 127, 2269–2279. [Google Scholar] [CrossRef]
- Bryan, M.A.; Brauner, J.W.; Anderle, G.; Flach, C.R.; Brodsky, B.; Mendelsohn, R. FTIR Studies of Collagen Model Peptides: Complementary Experimental and Simulation Approaches to Conformation and Unfolding. J. Am. Chem. Soc. 2007, 129, 7877–7884. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L.S. Collagen Type I Amide I Band Infrared Spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef] [PubMed]
- De Meutter, J.; Goormaghtigh, E. Evaluation of Protein Secondary Structure from FTIR Spectra Improved after Partial Deuteration. Eur. Biophys. J. 2021, 50, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.; Gălăţeanu, B.; Albu, M.; Lungu, A.; Radu, E.; Hermenean, A.; Costache, M. Biocompatibility Assessment of Novel Collagen-Sericin Scaffolds Improved with Hyaluronic Acid and Chondroitin Sulfate for Cartilage Regeneration. Biomed. Res. Int. 2013, 2013, 598056. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; H. Mondal, M.I.; Karim Sheikh, M.R.; Habib, M.A. Extraction, Structural and Functional Properties of Silk Sericin Biopolymer from Bombyx Mori Silk Cocoon Waste. J. Text. Sci. Eng. 2019, 9, 390. [Google Scholar] [CrossRef]
- Li, S.-T. Biologic Biomaterials: Tissue-Derived Biomaterials (Collagen). In Biomaterials, 1st ed.; Wong, J.Y., Bronzino, J.D., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Huang, C.; Yuan, W.; Chen, J.; Wu, L.-P.; You, T. Construction of Smart Biomaterials for Promoting Diabetic Wound Healing. Molecules 2023, 28, 1110. [Google Scholar] [CrossRef] [PubMed]
- Dahri, M.; Beheshtizadeh, N.; Seyedpour, N.; Nakhostin-Ansari, A.; Aghajani, F.; Seyedpour, S.; Masjedi, M.; Farjadian, F.; Maleki, R.; Adibkia, K. Biomaterial-based delivery platforms for transdermal immunotherapy. Biomed. Pharmacother. 2023, 165, 115048. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Structural Characterization and Physicochemical Properties of Quercetin–Pb Complex. J. Coord. Chem. 2014, 67, 1449–1462. [Google Scholar] [CrossRef]
- Indira Priyadarsini, K. Chemical and Structural Features Influencing the Biological Activity of Curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.Q.; Niu, J.; Liu, W.; Peng, S.F.; Liu, C.M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, H. Inhibition of Mammalian Collagenase, Matrix Metalloproteinase-1, by Naturally-Occurring Flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, I.; Kurniawan, K.O.; Rullyansyah, S.; Prijo, T.A.; Widyowati, R.; Ekowati, J.; Hestianah, E.P.; Ma’at, S.; Matsunami, K. Anti-aging properties of Curcuma heyneana Valeton & Zipj: A scientific approach to its use in Javanese tradition. J. Ethnopharmacol. 2018, 225, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Brett, D. A Review of Collagen and Collagen-based Wound Dressings. Wounds 2008, 20, 347–356. [Google Scholar] [PubMed]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.; Li, S. Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Cui, Y.; Chen, Y.; Yao, K.; Goh, J.C.H. Effects of the Controlled-Released Basic Fibroblast Growth Factor from Chitosan−Gelatin Microspheres on Human Fibroblasts Cultured on a Chitosan−Gelatin Scaffold. Biomacromolecules 2007, 8, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Nokoorani, Y.D.; Shamloo, A.; Bahadoran, M.; Moravvej, H. Fabrication and characterization of scaffolds containing different amounts of allantoin for skin tissue engineering. Sci. Rep. 2021, 11, 16164. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Hamam, F.; Nasr, A. Curcumin-loaded mesoporous silica particles as wound-healing agent: An In vivo study. Saudi J. Med. Med. Sci. 2020, 8, 17–24. [Google Scholar] [CrossRef]
- Farhat, F.; Sohail, S.S.; Siddiqui, F.; Irshad, R.R.; Madsen, D.Ø. Curcumin in Wound Healing—A Bibliometric Analysis. Life 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, T.; Zhang, G.; Fang, A.; Li, Y.; Wang, S.; Yan, H.; Liang, P.; Lian, J.; Zhang, Y. A native sericin wound dressing spun directly from silkworms enhances wound healing. Colloids Surf. B Biointerfaces 2023, 225, 113228. [Google Scholar] [CrossRef] [PubMed]
- Sapru, S.; Das, S.; Mandal, M.; Ghosh, A.K.; Kundu, S.C. Nonmulberry silk protein sericin blend hydrogels for skin tissue regeneration—In vitro and in vivo. Int. J. Biol. Macromol. 2019, 137, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Huang, S.; Yu, F.; Yu, B.; Zhang, Y.; Tang, J.; Huang, Y.; Xiang, Q. Hybrid Freeze-Dried Dressings Composed of Epidermal Growth Factor and Recombinant Human-Like Collagen Enhance Cutaneous Wound Healing in Rats. Front. Bioeng. Biotechnol. 2020, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Kitamura, M. Anti-apoptotic effect of quercetin: Intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int. 2000, 58, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, X.; Hu, X.; Li, S.; Wang, J. The anti-apoptotic, antioxidant and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 2020, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Kong, B.; Gu, J.-W.; Kuang, Y.-Q.; Cheng, L.; Yang, W.-T.; Xia, X.; Shu, H.-F. Anti-apoptotic and Anti-oxidative Roles of Quercetin After Traumatic Brain Injury. Cell. Mol. Neurobiol. 2014, 34, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-H.; Xue, R.; He, B. Quercetin protects oral mucosal keratinocytes against lipopolysaccharide-induced inflammatory toxicity by suppressing the AKT/AMPK/mTOR pathway. Immunopharmacol. Immunotoxicol. 2021, 43, 519–526. [Google Scholar] [CrossRef]
- Becatti, M.; Prignano, F.; Fiorillo, C.; Pescitelli, L.; Nassi, P.; Lotti, T.; Taddei, N. The Involvement of Smac/DIABLO, p53, NF-kB, and MAPK Pathways in Apoptosis of Keratinocytes from Perilesional Vitiligo Skin: Protective Effects of Curcumin and Capsaicin. Antioxid. Redox Signal. 2010, 13, 1309–1321. [Google Scholar] [CrossRef]
- Ali, A.; Kim, M.J.; Kim, M.Y.; Lee, H.J.; Roh, G.S.; Kim, H.J.; Cho, G.J.; Choi, W.S. Quercetin induces cell death in cervical cancer by reducing O-GlcNAcylation of adenosine monophosphate-activated protein kinase. Anat. Cell Biol. 2018, 51, 274–283. [Google Scholar] [CrossRef]
- Song, W.; Ren, Y.-J.; Liu, L.-L.; Zhao, Y.-Y.; Li, Q.-F.; Yang, H.-B. Curcumin induced the cell death of immortalized human keratinocytes (HaCaT) through caspase-independent and caspase-dependent pathways. Food Funct. 2021, 12, 8669–8680. [Google Scholar] [CrossRef]
- Bentz, A.B. A Review of Quercetin: Chemistry, Antioxidant Properties, and Bioavailability. J. Young Investig. 2009. Available online: https://www.jyi.org/2009-april/2017/10/15/a-review-of-quercetin-chemistry-antioxidant-properties-and-bioavailability (accessed on 8 April 2024).
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Sharma, M.; Kumar, V.; Joshi, V.G. Topical application of quercetin improves wound repair and regeneration in diabetic rats. Immunopharmacol. Immunotoxicol. 2021, 43, 536–553. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, T.; Li, J.; Xu, Y.; Wang, R.; Ke, Q.; Shen, S.G.F.; Xu, H.; Lin, K. Hierarchical micro/nanofibrous scaffolds incorporated with curcumin and zinc ion eutectic metal organic frameworks for enhanced diabetic wound healing via anti-oxidant and anti-inflammatory activities. Chem. Eng. J. 2020, 402, 126273. [Google Scholar] [CrossRef]
- Chittasupho, C.; Manthaisong, A.; Okonogi, S.; Tadtong, S.; Samee, W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, In Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int. J. Mol. Sci. 2021, 23, 142. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sleiman, L.; Lazăr, A.-D.; Albu-Kaya, M.; Marin, M.M.; Kaya, D.A.; Vasile, O.-R.; Dinescu, S. Development and Investigation of an Innovative 3D Biohybrid Based on Collagen and Silk Sericin Enriched with Flavonoids for Potential Wound Healing Applications. Polymers 2024, 16, 1627. https://doi.org/10.3390/polym16121627
Sleiman L, Lazăr A-D, Albu-Kaya M, Marin MM, Kaya DA, Vasile O-R, Dinescu S. Development and Investigation of an Innovative 3D Biohybrid Based on Collagen and Silk Sericin Enriched with Flavonoids for Potential Wound Healing Applications. Polymers. 2024; 16(12):1627. https://doi.org/10.3390/polym16121627
Chicago/Turabian StyleSleiman, Lea, Andreea-Daniela Lazăr (Popa), Mădălina Albu-Kaya, Minodora Maria Marin, Durmuș Alpaslan Kaya, Otilia-Ruxandra Vasile, and Sorina Dinescu. 2024. "Development and Investigation of an Innovative 3D Biohybrid Based on Collagen and Silk Sericin Enriched with Flavonoids for Potential Wound Healing Applications" Polymers 16, no. 12: 1627. https://doi.org/10.3390/polym16121627
APA StyleSleiman, L., Lazăr, A.-D., Albu-Kaya, M., Marin, M. M., Kaya, D. A., Vasile, O.-R., & Dinescu, S. (2024). Development and Investigation of an Innovative 3D Biohybrid Based on Collagen and Silk Sericin Enriched with Flavonoids for Potential Wound Healing Applications. Polymers, 16(12), 1627. https://doi.org/10.3390/polym16121627










