Photoluminescent Nanocellulosic Film for Selective Hg2+ Ion Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Luminescent BSA-Protected Gold Nanocluster (BSA-AuNC)
2.3. AuNC/CNF Nanocomposites
2.4. Imaging
2.5. Spectroscopic Analyses
2.6. Mechanical Strength and Printing
3. Results and Discussion
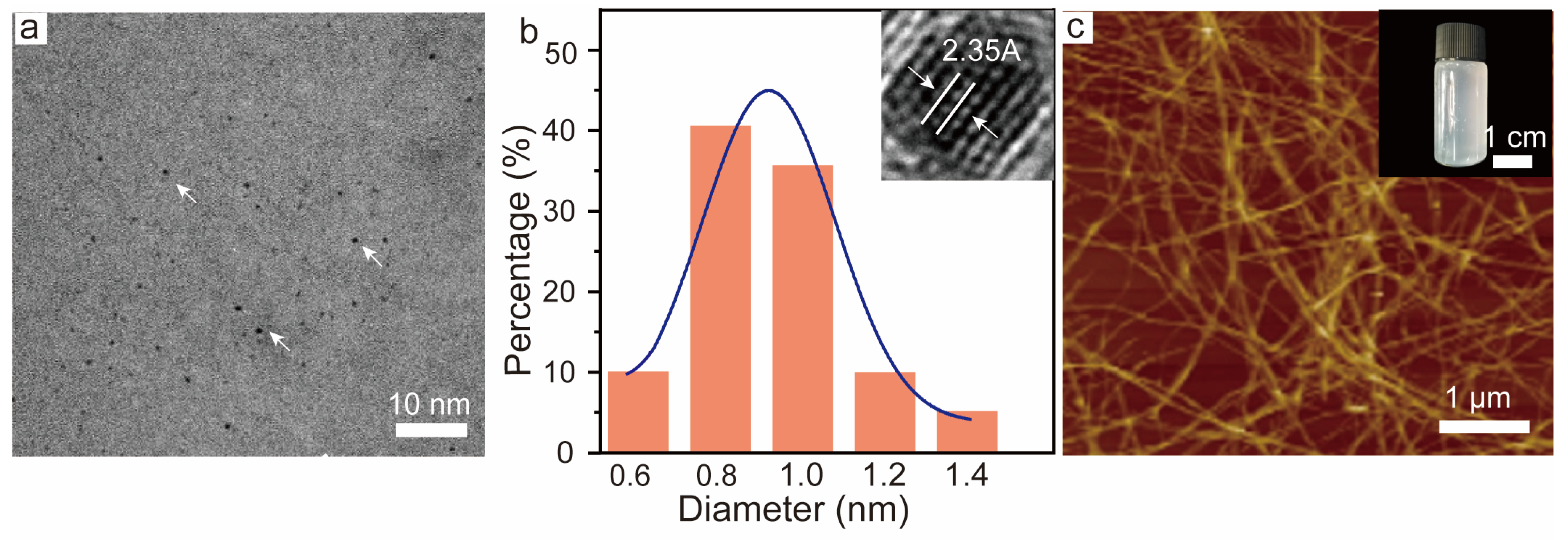
3.1. BSA-Protected Gold Nanoclusters (BSA-AuNCs) and Cellulose Nanofibrils (CNF)
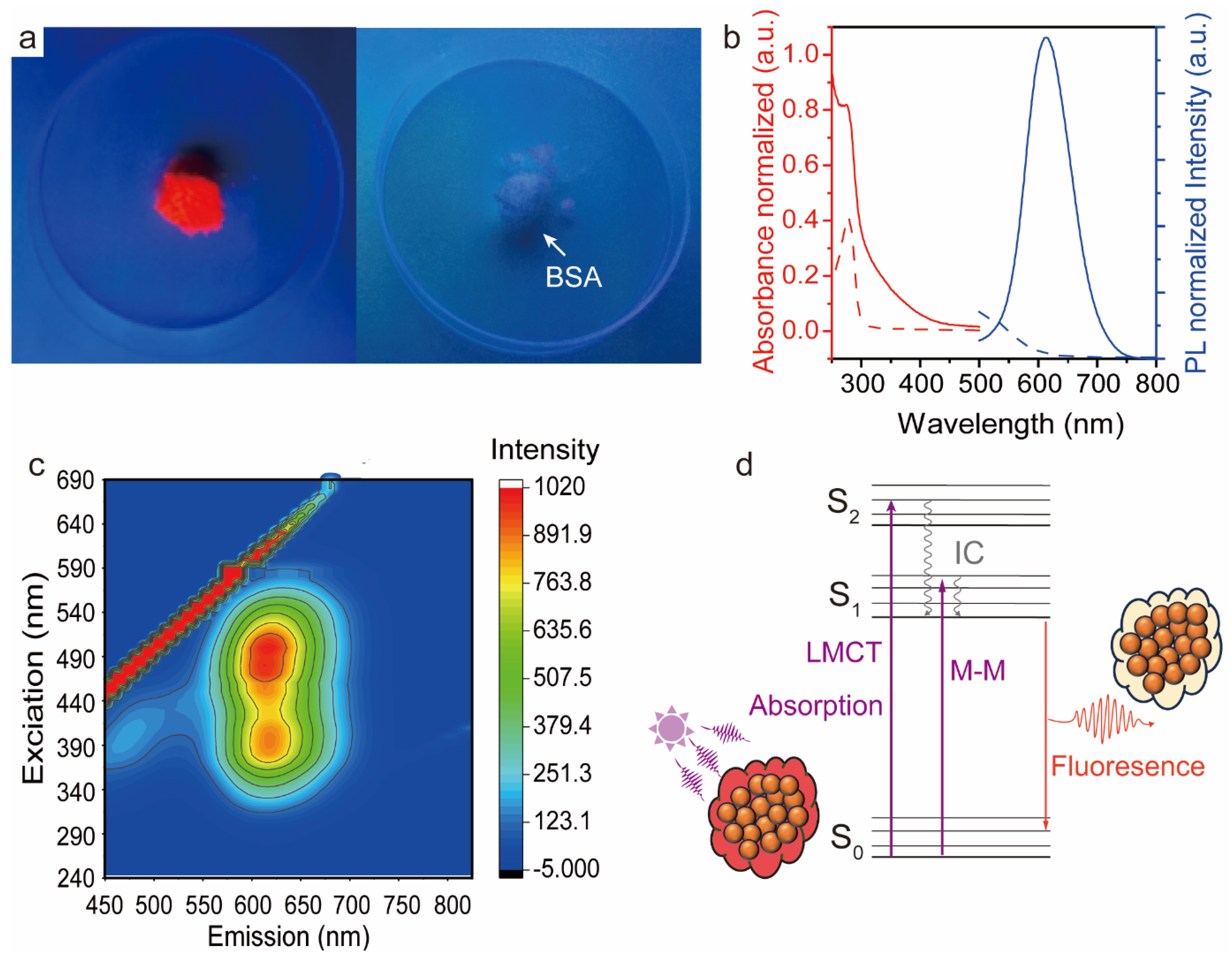
3.2. BSA-AuNC Photoluminescence
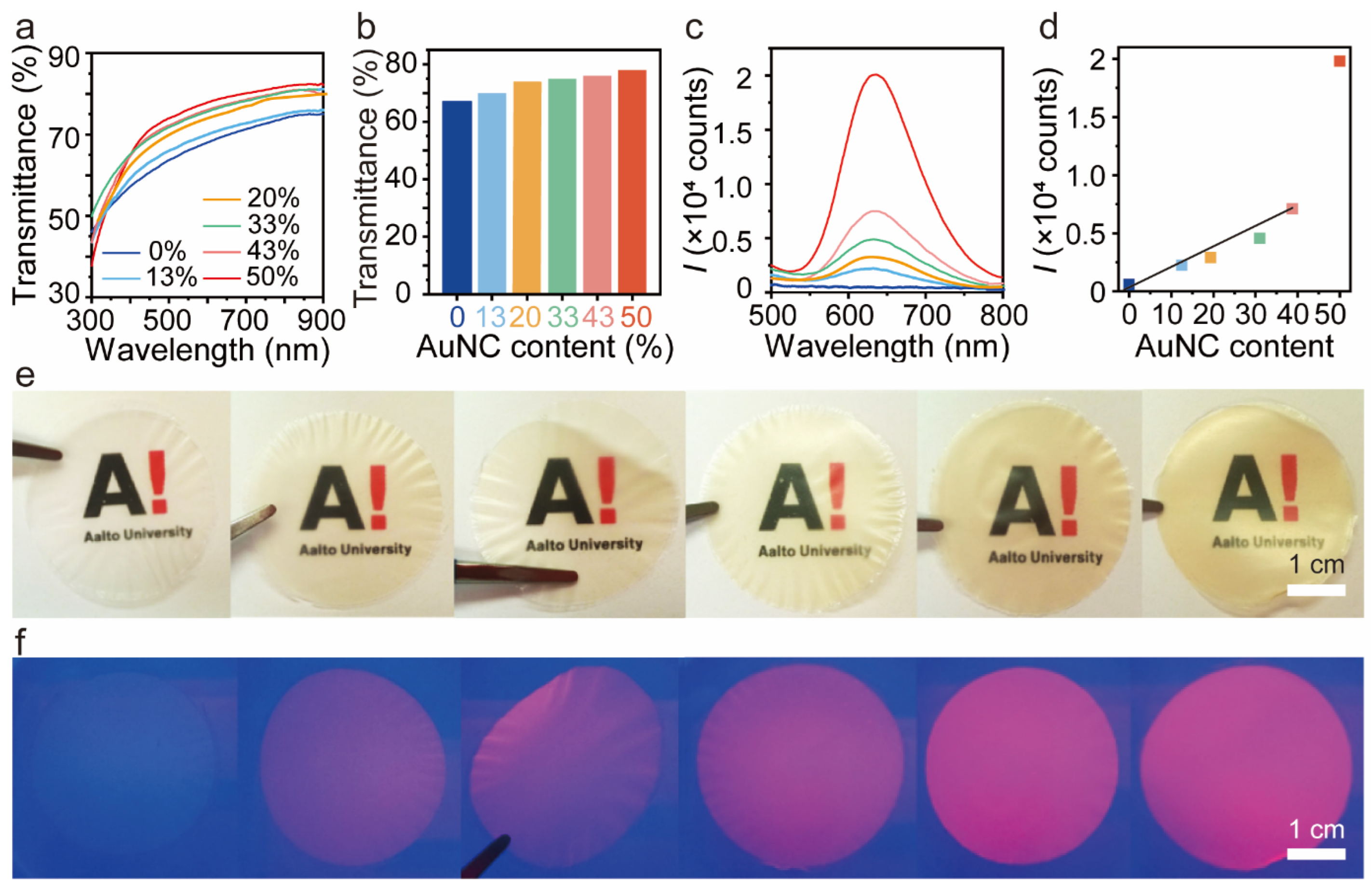
3.3. Photoluminescent BSA-AuNC/CNF Hybrid Film
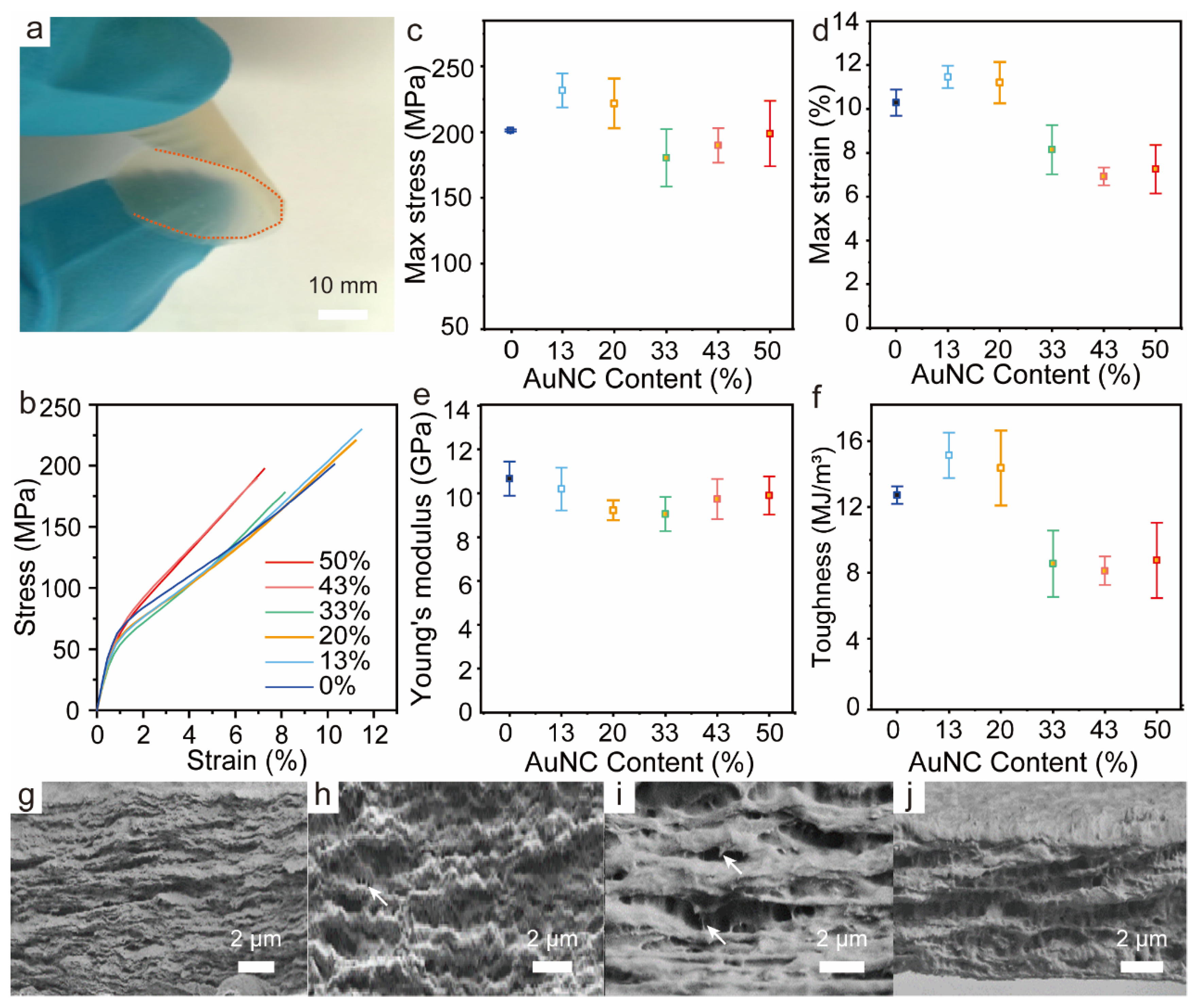
3.4. Mechanical Strength of BSA-AuNC/CNF Films
3.5. Hg2+ Detection with Printed BSA-AuNC onto CNF Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Akpan, E.I.; Wetzel, B.; Friedrich, K. Eco-friendly and sustainable processing of wood-based materials. Green Chem. 2021, 23, 2198–2232. [Google Scholar] [CrossRef]
- Valdés, A.; Garrigós, M.C. Carbohydrate-Based Advanced Biomaterials for Food Sustainability: A Review. Mater. Sci. Forum 2016, 842, 182–195. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Chen, P.; Lo Re, G.; Berglund, L.A.; Wohlert, J. Surface modification effects on nanocellulose—Molecular dynamics simulations using umbrella sampling and computational alchemy. J. Mater. Chem. A 2020, 8, 23617–23627. [Google Scholar] [CrossRef]
- Walther, A.; Lossada, F.; Benselfelt, T.; Kriechbaum, K.; Berglund, L.; Ikkala, O.; Saito, T.; Wågberg, L.; Bergström, L. Best Practice for Reporting Wet Mechanical Properties of Nanocellulose-Based Materials. Biomacromolecules 2020, 21, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Gandla, K.; Kumar, K.P.; Rajasulochana, P.; Charde, M.S.; Rana, R.; Singh, L.P.; Haque, M.A.; Bakshi, V.; Siddiqui, F.A.; Khan, S.L.; et al. Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels for Biosensing and Drug Delivery Applications. Gels 2023, 9, 669. [Google Scholar] [CrossRef]
- Bai, G.; Tsang, M.K.; Hao, J. Luminescent ions in advanced composite materials for multifunctional applications. Adv. Funct. Mater. 2016, 26, 6330–6350. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Hu, Z.; Xiang, Z.; Song, T.; Lu, F. A Highly Efficient and Durable Fluorescent Paper Produced from Bacterial Cellulose/Eu Complex and Cellulosic Fibers. Nanomaterials 2019, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.M.; Kumar, K.S.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Rajulu, A.V. A comprehensive review of electrospun nanofibers: Food and packaging perspective. Compos. Part B Eng. 2019, 175, 107074. [Google Scholar] [CrossRef]
- Patel, I.; Woodcock, J.; Beams, R.; Stranick, S.J.; Nieuwendaal, R.; Gilman, J.W.; Mulenos, M.R.; Sayes, C.M.; Salari, M.; DeLoid, G.; et al. Fluorescently Labeled Cellulose Nanofibers for Environmental Health and Safety Studies. Nanomaterials 2021, 11, 1015. [Google Scholar] [CrossRef]
- Yu, H.; Fang, D.; Dirican, M.; Wang, R.; Tian, Y.; Chen, L.; Liu, H.; Wang, J.; Tang, F.; Asiri, A.M. Binding conductive ink initiatively and strongly: Transparent and thermally stable cellulose nanopaper as a promising substrate for flexible electronics. ACS Appl. Mater. Interfaces 2019, 11, 20281–20290. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Lu, H.; Li, M.; Chen, C. A New Thioimide-Based Fluorescent ‘Turn-On’and Chromogenic Chemodosimeter in Acetonitrile and Its Nanoparticles in Water for Highly Selective and Sensitive Detection of Hg2+. Chin. J. Chem. 2017, 35, 435–441. [Google Scholar] [CrossRef]
- Li, J.; Song, X.; Guo, Y.; Yang, Q.; Feng, K. The determinants of China’s national and regional energy-related mercury emission changes. J. Environ. Manag. 2019, 246, 505–513. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H. Nanomaterials for the adsorptive treatment of Hg (II) ions from water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; Hu, Z.; Ma, Y.; Duan, Y.; Han, Y.; Chen, X.; Zhou, Y.; Cheng, J.; Wang, W. Particulate mercury in ambient air in Shanghai, China: Size-specific distribution, gas–particle partitioning, and association with carbonaceous composition. Environ. Pollut. 2018, 238, 543–553. [Google Scholar] [CrossRef]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury (II) in aqueous solutions. Angew. Chem. 2004, 116, 4400–4402. [Google Scholar] [CrossRef]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters—A review. Anal. Chim. Acta 2010, 663, 127–138. [Google Scholar] [CrossRef]
- Karunasagar, D.; Arunachalam, J.; Gangadharan, S. Development of a ‘collect and punch’ cold vapour inductively coupled plasma mass spectrometric method for the direct determination of mercury at nanograms per litre levels. J. Anal. At. Spectrom. 1998, 13, 679–682. [Google Scholar] [CrossRef]
- Mahajan, R.; Kaur, I.; Lobana, T. A mercury (II) ion-selective electrode based on neutral salicylaldehyde thiosemicarbazone. Talanta 2003, 59, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Puk, R.; Weber, J.H. Determination of mercury (II), monomethylmercury cation, dimethylmercury and diethylmercury by hydride generation, cryogenic trapping and atomic absorption spectrometric detection. Anal. Chim. Acta 1994, 292, 175–183. [Google Scholar] [CrossRef]
- Butcher, D.J. Atomic fluorescence spectrometry: A review of advances in instrumentation and novel applications. Appl. Spectrosc. Rev. 2016, 51, 397–416. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Meng, X.; Ragauskas, A.J. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+–Au+ interactions. Chem. Commun. 2010, 46, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-C.; Tan, S.-H.; Hsiao, Y.-C.; Mutalik, C.; Chen, H.-M.; Yougbaré, S.; Kuo, T.-R. Unveiling the Antibacterial Mechanism of Gold Nanoclusters via In Situ Transmission Electron Microscopy. ACS Sustain. Chem. Eng. 2022, 10, 464–471. [Google Scholar] [CrossRef]
- Guo, J.; Filpponen, I.; Su, P.; Laine, J.; Rojas, O.J. Attachment of gold nanoparticles on cellulose nanofibrils via click reactions and electrostatic interactions. Cellulose 2016, 23, 3065–3075. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Lossada, F.; Jiao, D.; Guo, J.; Hoenders, D.; Eckert, A.; Walther, A. Outstanding Synergies in Mechanical Properties of Bioinspired Cellulose Nanofibril Nanocomposites using Self-Cross-Linking Polyurethanes. ACS Appl. Polym. Mater. 2019, 1, 3334–3342. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Wu, W.; Xiao, H. Methods and applications of nanocellulose loaded with inorganic nanomaterials: A review. Carbohydr. Polym. 2020, 229, 115454. [Google Scholar] [CrossRef] [PubMed]
- Borah, R.; Kumar, A. Fluorescence enhancement of glutaraldehyde functionalized polyaniline nanofibers in the presence of aromatic amino acids. Mater. Sci. Eng. C 2016, 61, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, G.-Q.; Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Kamat, P.V. Size-Dependent Excited State Behavior of Glutathione-Capped Gold Clusters and Their Light-Harvesting Capacity. J. Am. Chem. Soc. 2014, 136, 11093–11099. [Google Scholar] [CrossRef]
- Peponi, L.; Puglia, D.; Torre, L.; Valentini, L.; Kenny, J.M. Processing of nanostructured polymers and advanced polymeric based nanocomposites. Mater. Sci. Eng. R Rep. 2014, 85, 1–46. [Google Scholar] [CrossRef]
- Van Rie, J.; Thielemans, W. Cellulose–gold nanoparticle hybrid materials. Nanoscale 2017, 9, 8525–8554. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-B.; Yao, J.-N. Size effects on the optical properties of organic nanoparticles. J. Am. Chem. Soc. 2001, 123, 1434–1439. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuo, P.; Yin, H.; Fan, Y.; Zhang, J.; Liu, X.; Chen, Z. Solid-state fluorescent carbon dots with aggregation-induced yellow emission for white light-emitting diodes with high luminous efficiencies. ACS Appl. Mater. Interfaces 2019, 11, 24395–24403. [Google Scholar] [CrossRef]
- Duan, J.-J.; Zhang, L.-N. Robust and smart hydrogels based on natural polymers. Chin. J. Polym. Sci. 2017, 35, 1165–1180. [Google Scholar] [CrossRef]
- Pajorova, J.; Skogberg, A.; Hadraba, D.; Broz, A.; Travnickova, M.; Zikmundova, M.; Honkanen, M.; Hannula, M.; Lahtinen, P.; Tomkova, M. Cellulose mesh with charged nanocellulose coatings as a promising carrier of skin and stem cells for regenerative applications. Biomacromolecules 2020, 21, 4857–4870. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Chen, R.; Zhao, J.; Jiang, Z.; Men, Y. Cavitation in isotactic polypropylene at large strains during tensile deformation at elevated temperatures. Macromolecules 2015, 48, 5799–5806. [Google Scholar] [CrossRef]
- Hughes, J.; Thomas, R.; Byun, Y.; Whiteside, S. Improved flexibility of thermally stable poly-lactic acid (PLA). Carbohydr. Polym. 2012, 88, 165–172. [Google Scholar] [CrossRef]
- López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Pascual, D.; Lasanta, T. Amalgamating at the molecular level. A study of the strong closed-shell Au (i)…Hg (ii) interaction. Chem. Commun. 2011, 47, 6795–6797. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Albers, A.E.; Wong, A.P.; Chang, C.J. Screening mercury levels in fish with a selective fluorescent chemosensor. J. Am. Chem. Soc. 2005, 127, 16030–16031. [Google Scholar] [CrossRef]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Feng, S.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef]
- Chansuvarn, W.; Imyim, A. Visual and colorimetric detection of mercury (II) ion using gold nanoparticles stabilized with a dithia-diaza ligand. Microchimica Acta 2012, 176, 57–64. [Google Scholar] [CrossRef]
- Long, Y.J.; Li, Y.F.; Liu, Y.; Zheng, J.J.; Tang, J.; Huang, C.Z. Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem. Commun. 2011, 47, 11939–11941. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Jiao, K.; Yang, X. Colorimetric detection of mercury ion (Hg2+) based on DNA oligonucleotides and unmodified gold nanoparticles sensing system with a tunable detection range. Biosens. Bioelectron. 2009, 24, 3153–3158. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, M.S.; Mirkin, C.A. Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew. Chem.-Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Jin, J.; Yang, R. Gold nanoparticle-based colorimetric and “turn-on” fluorescent probe for mercury (II) ions in aqueous solution. Anal. Chem. 2008, 80, 9021–9028. [Google Scholar] [CrossRef] [PubMed]

| Samples | Sensing Materials | Methods | Linear Range/Limit of Detection | References |
|---|---|---|---|---|
| Water | AuNPs stabilized with dithia-diaza ligands | UV–vis absorbance at 680 nm | 0–9 μM/35 nM | Woravith et al. [47] |
| Water | AuNPs | UV–vis absorbance at 652 nm | 1–600 nM/0.3 nM | Long et al. [48] |
| Water | DNA and unmodified AuNPs | UV–vis absorbance (A700 nm/A520 nm) | 0–5 mM/0.5 mM | Xu et al. [49] |
| Water | DNA-Functionalized AuNPs | Melting temperature | 0–2 mM/100 nM | Lee et al. [50] |
| Water | DNA-functioned gold nanoparticles | Fluorescent analysis method AFS | 96–6400 nM/40 nM | Wang et al. [51] |
| Water | BSA-Au clusters | UV–vis absorbance at 630 nm | 25 μM/1 nM | Present method |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Fang, W.; Liza, A.A.; Gao, R.; Song, J.; Guo, J.; Rojas, O.J. Photoluminescent Nanocellulosic Film for Selective Hg2+ Ion Detection. Polymers 2024, 16, 1583. https://doi.org/10.3390/polym16111583
Sun J, Fang W, Liza AA, Gao R, Song J, Guo J, Rojas OJ. Photoluminescent Nanocellulosic Film for Selective Hg2+ Ion Detection. Polymers. 2024; 16(11):1583. https://doi.org/10.3390/polym16111583
Chicago/Turabian StyleSun, Jing, Wenwen Fang, Afroza Akter Liza, Rui Gao, Junlong Song, Jiaqi Guo, and Orlando J. Rojas. 2024. "Photoluminescent Nanocellulosic Film for Selective Hg2+ Ion Detection" Polymers 16, no. 11: 1583. https://doi.org/10.3390/polym16111583
APA StyleSun, J., Fang, W., Liza, A. A., Gao, R., Song, J., Guo, J., & Rojas, O. J. (2024). Photoluminescent Nanocellulosic Film for Selective Hg2+ Ion Detection. Polymers, 16(11), 1583. https://doi.org/10.3390/polym16111583







