Polymer Composite Hydrogel Based on Polyvinyl Alcohol/Polyacrylamide/Polybenzoxazine Carbon for Use in Flexible Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
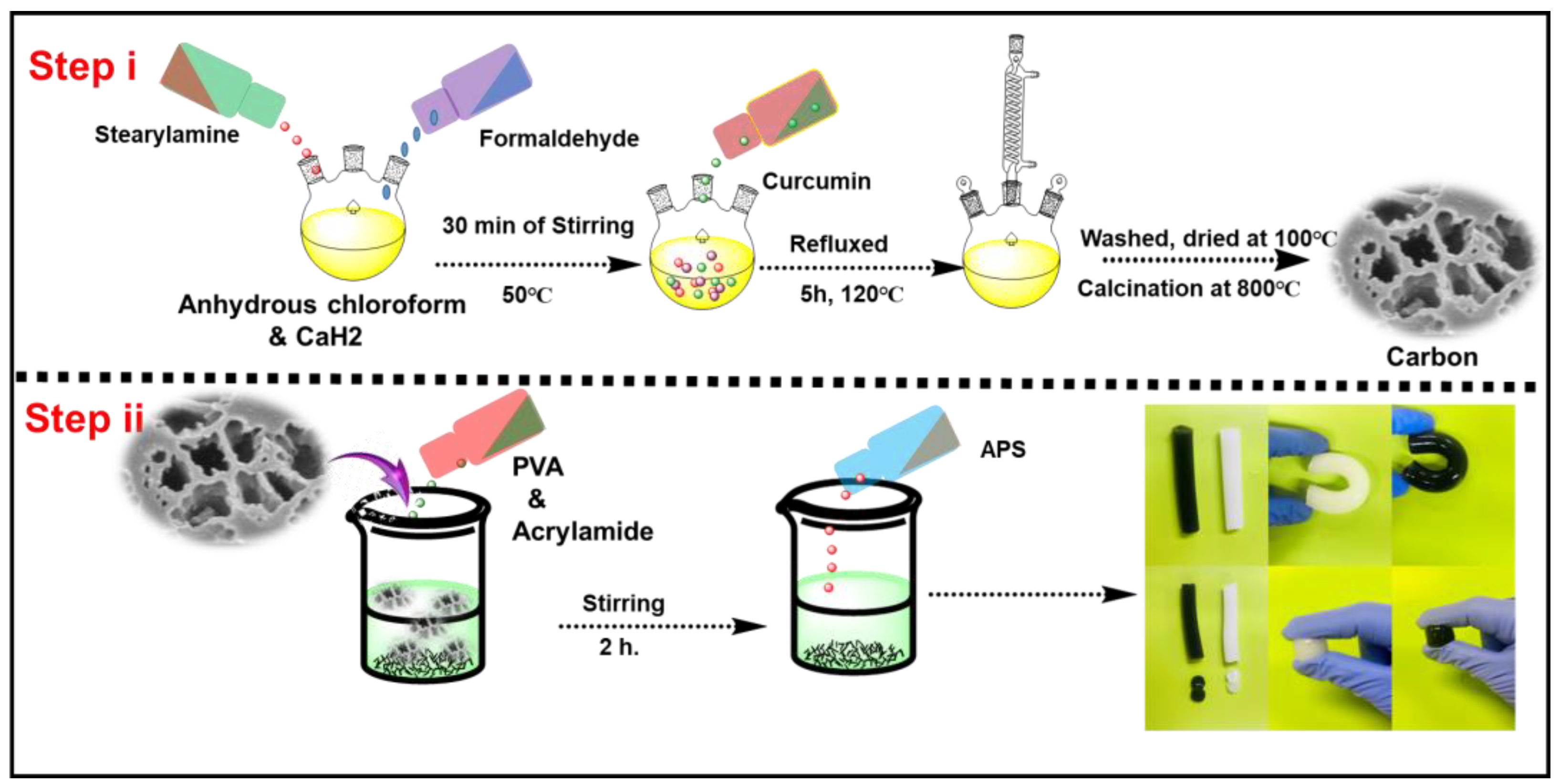
2.2. Synthesis of Polybenzoxazine-Based Carbon (PBzC)
2.3. PVA/Poly (AAm)/PBzC Hydrogel Preparation
2.4. Instrumentation
2.5. Electrochemical Measurements
3. Results and Discussion
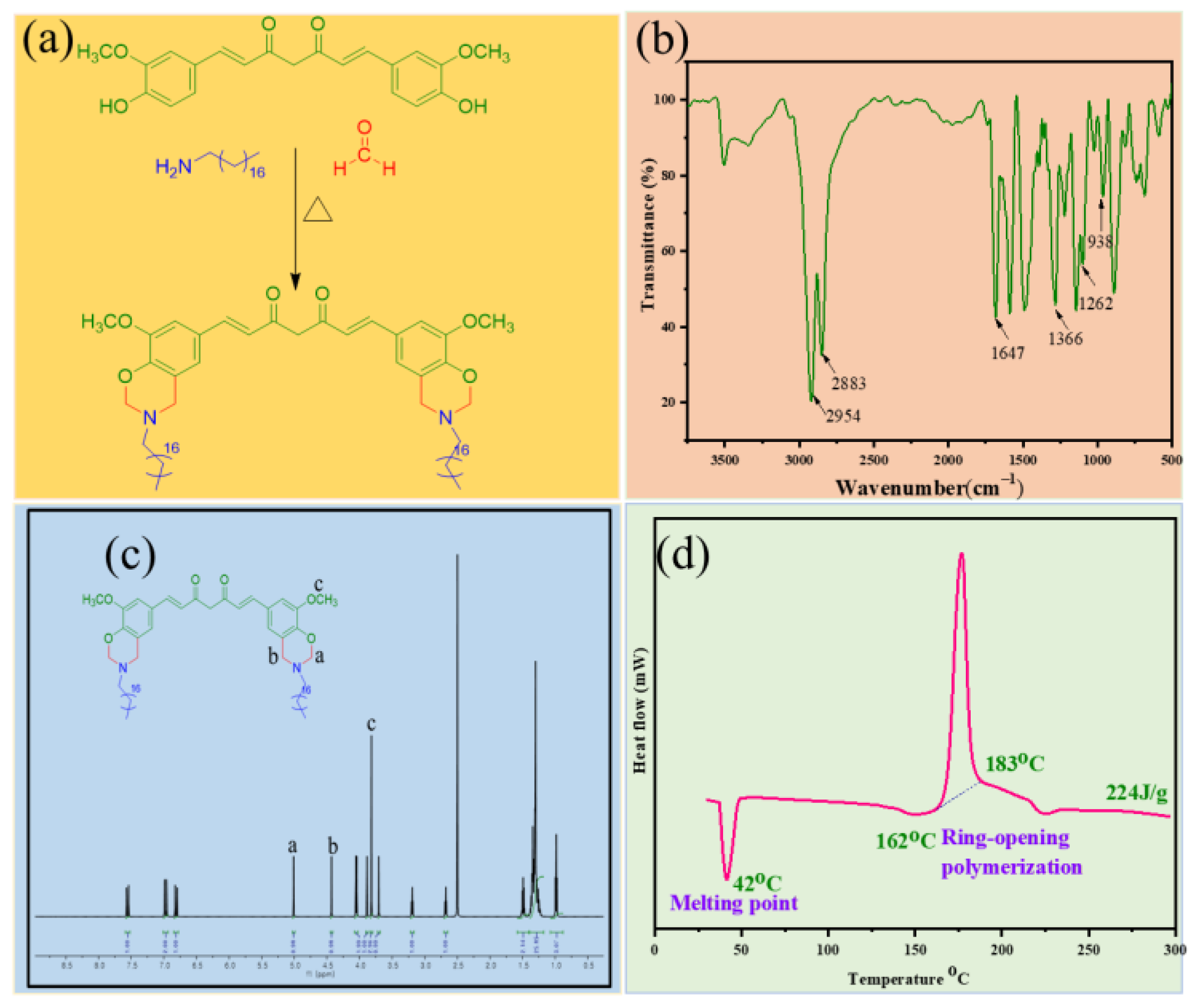
3.1. Structural Characterization of Benzoxazine Monomer (C-st)
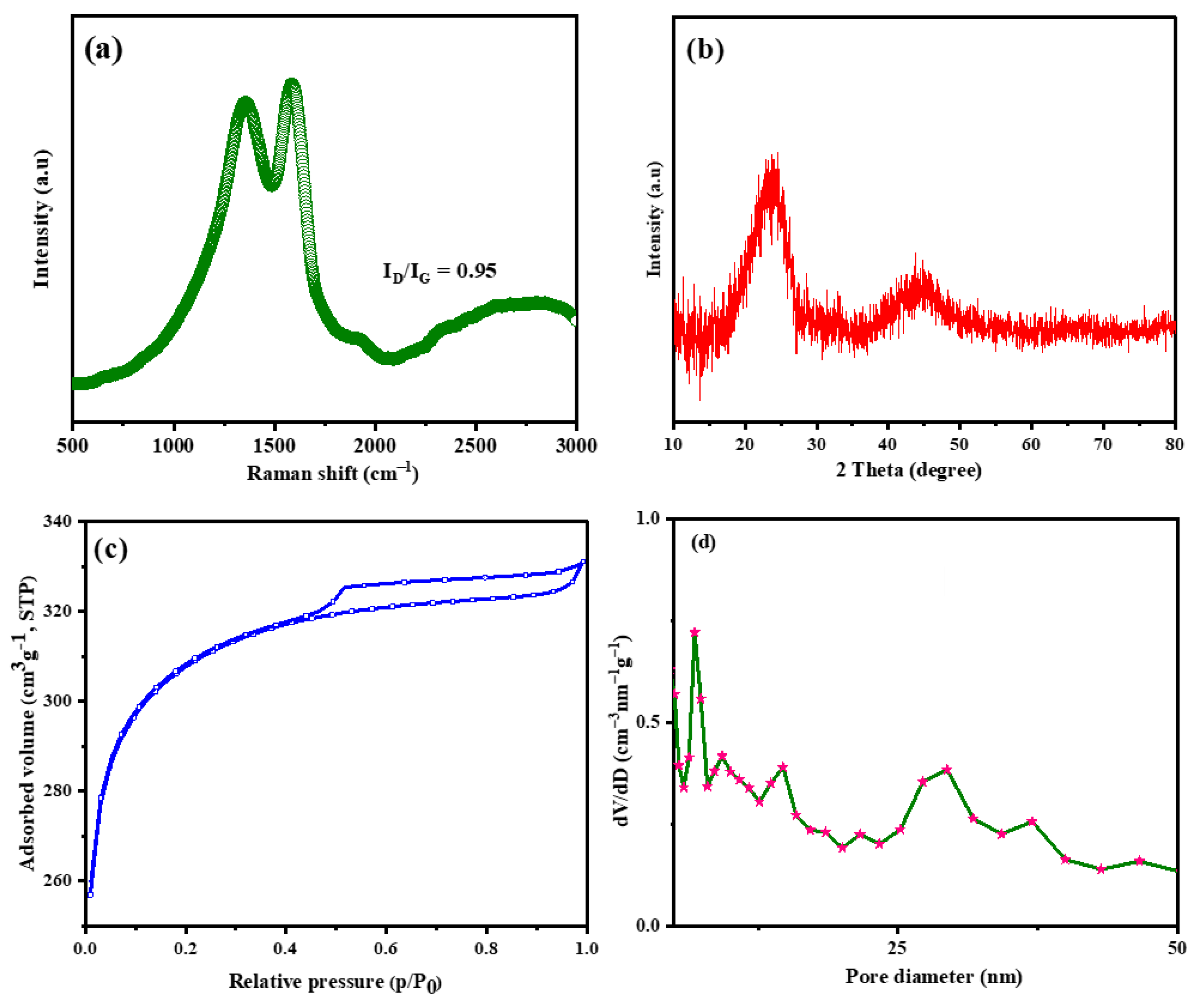
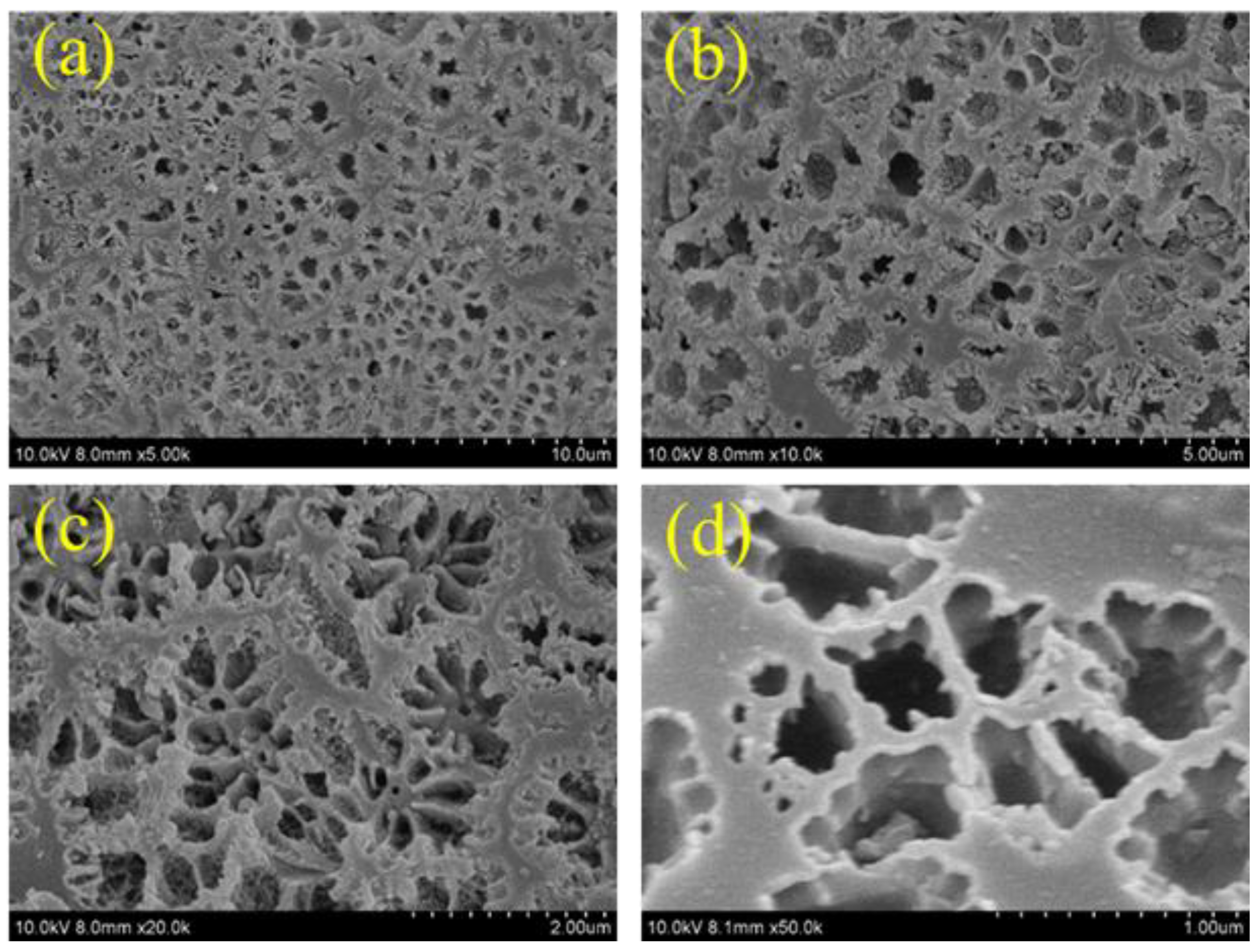
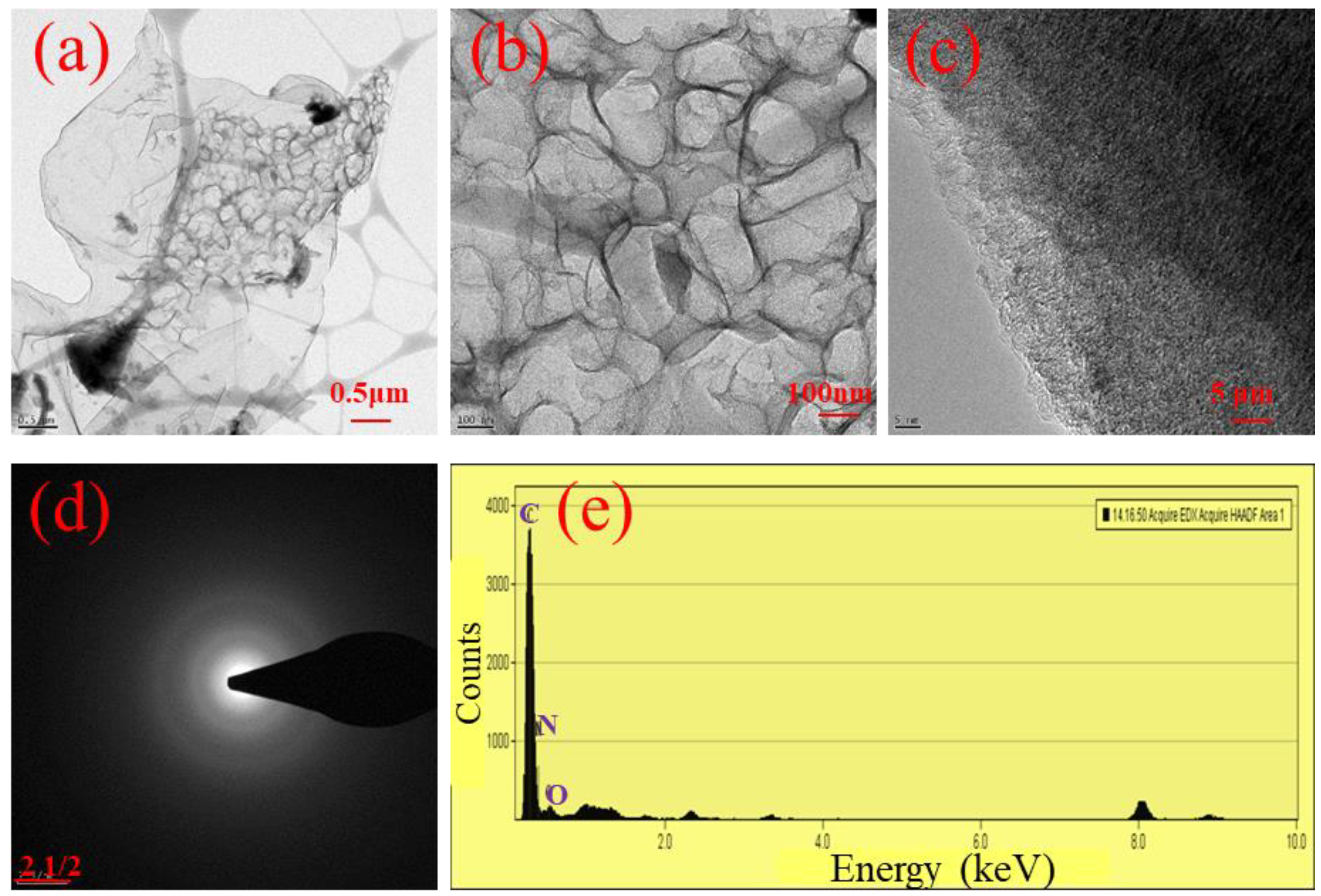
3.2. Structural and Morphological Analyses of PBzC
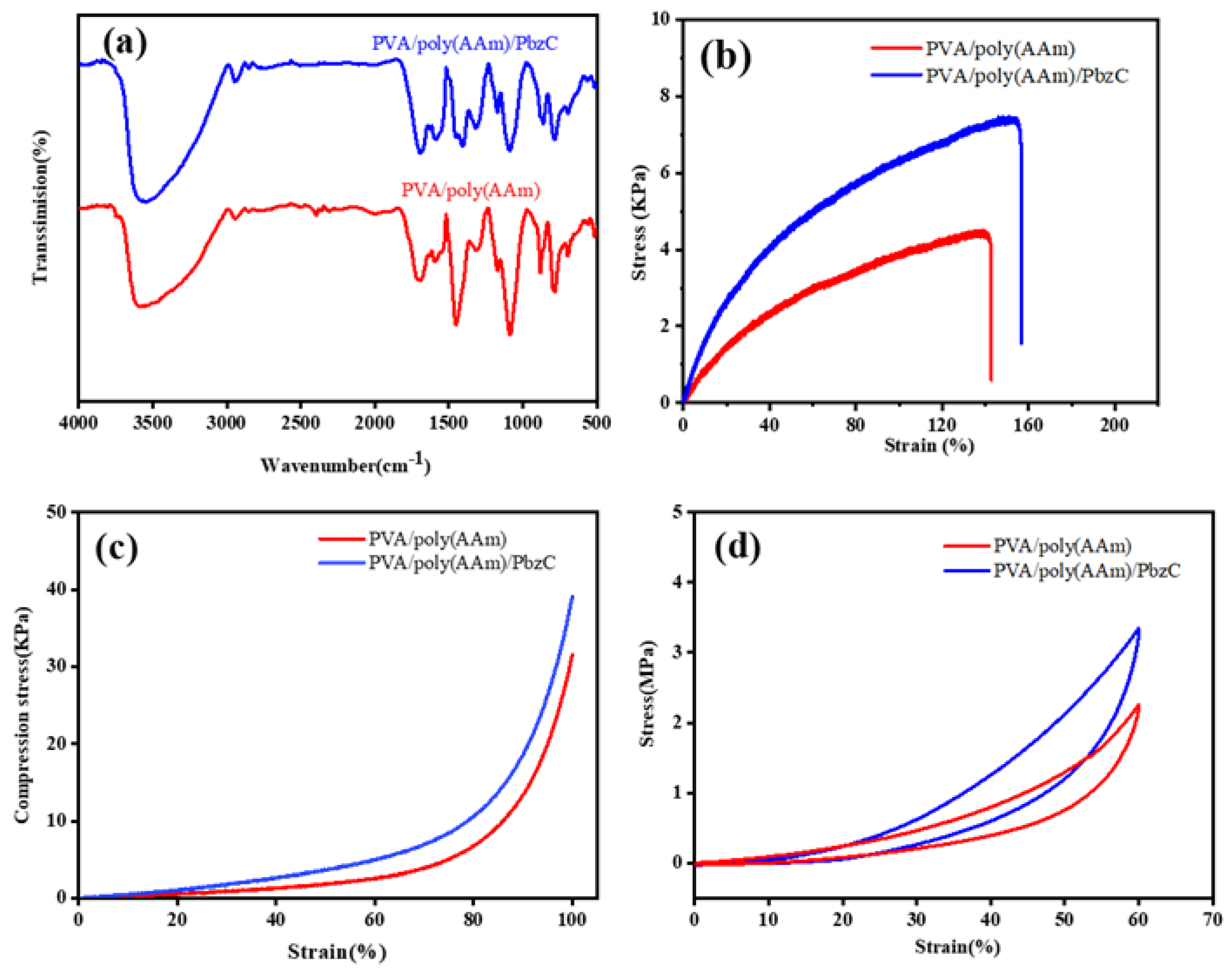
3.3. Characteristic Analysis of PVA/Poly(AAm) and PVA/Poly(AAm)/PBzC Hydrogels
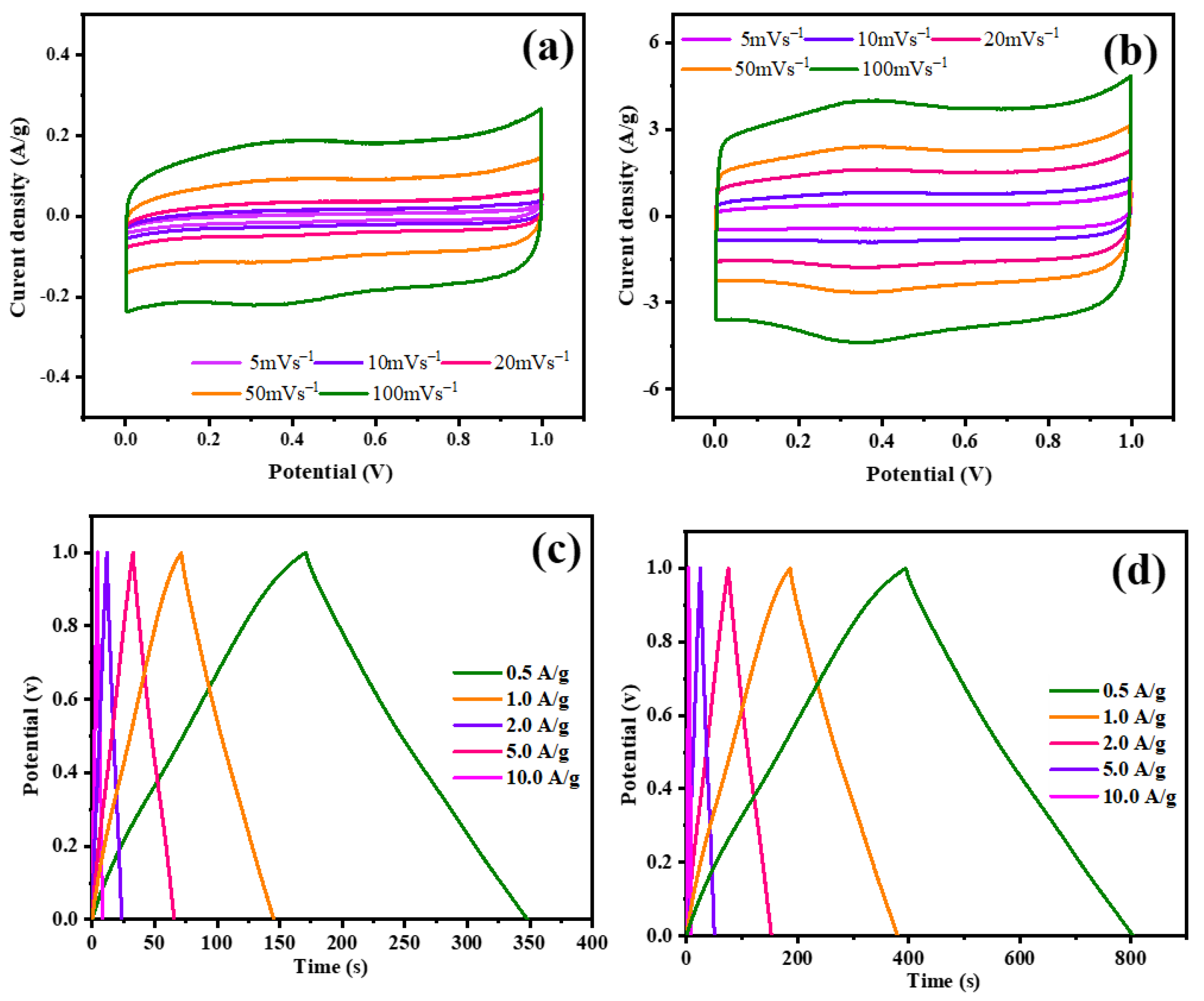
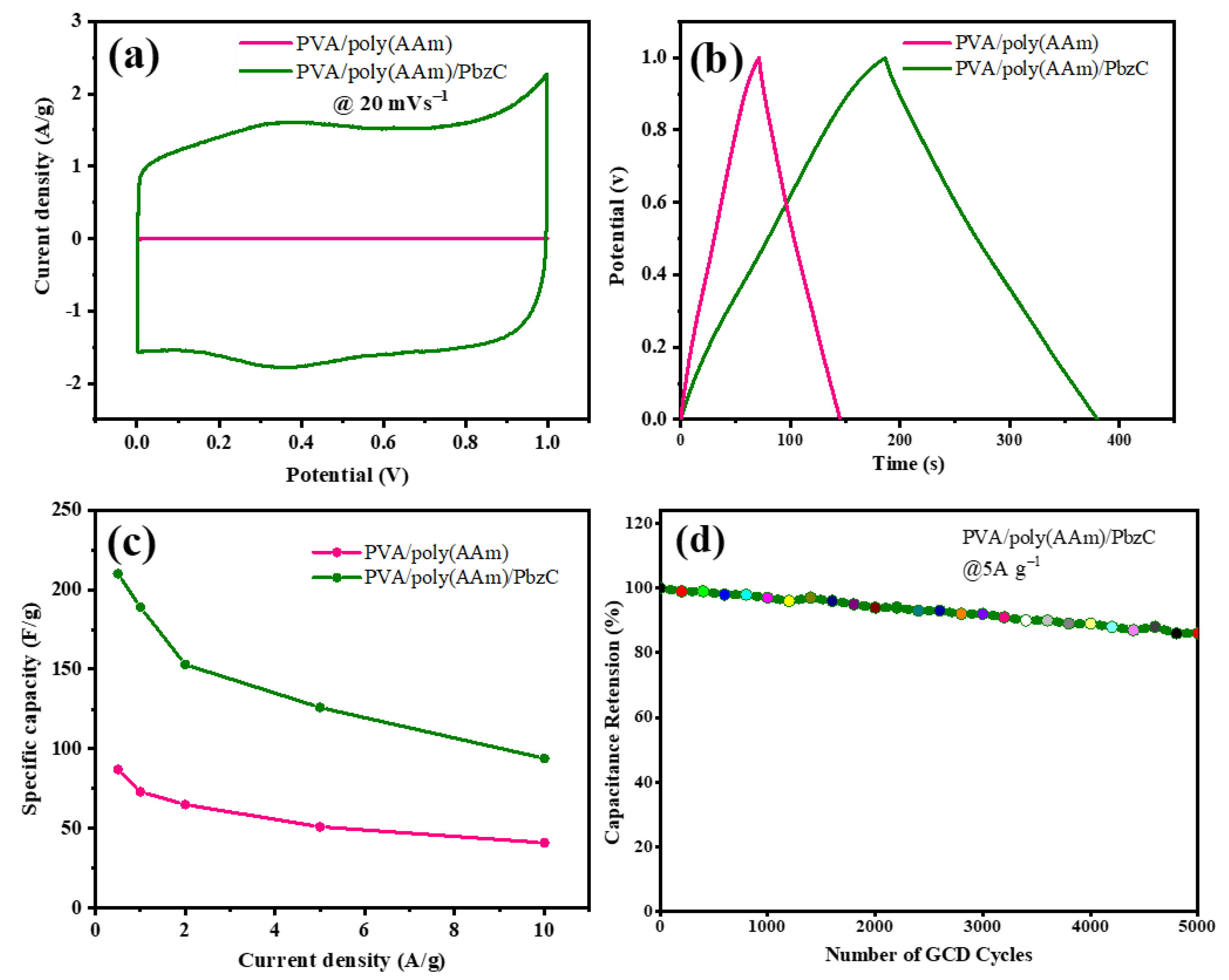
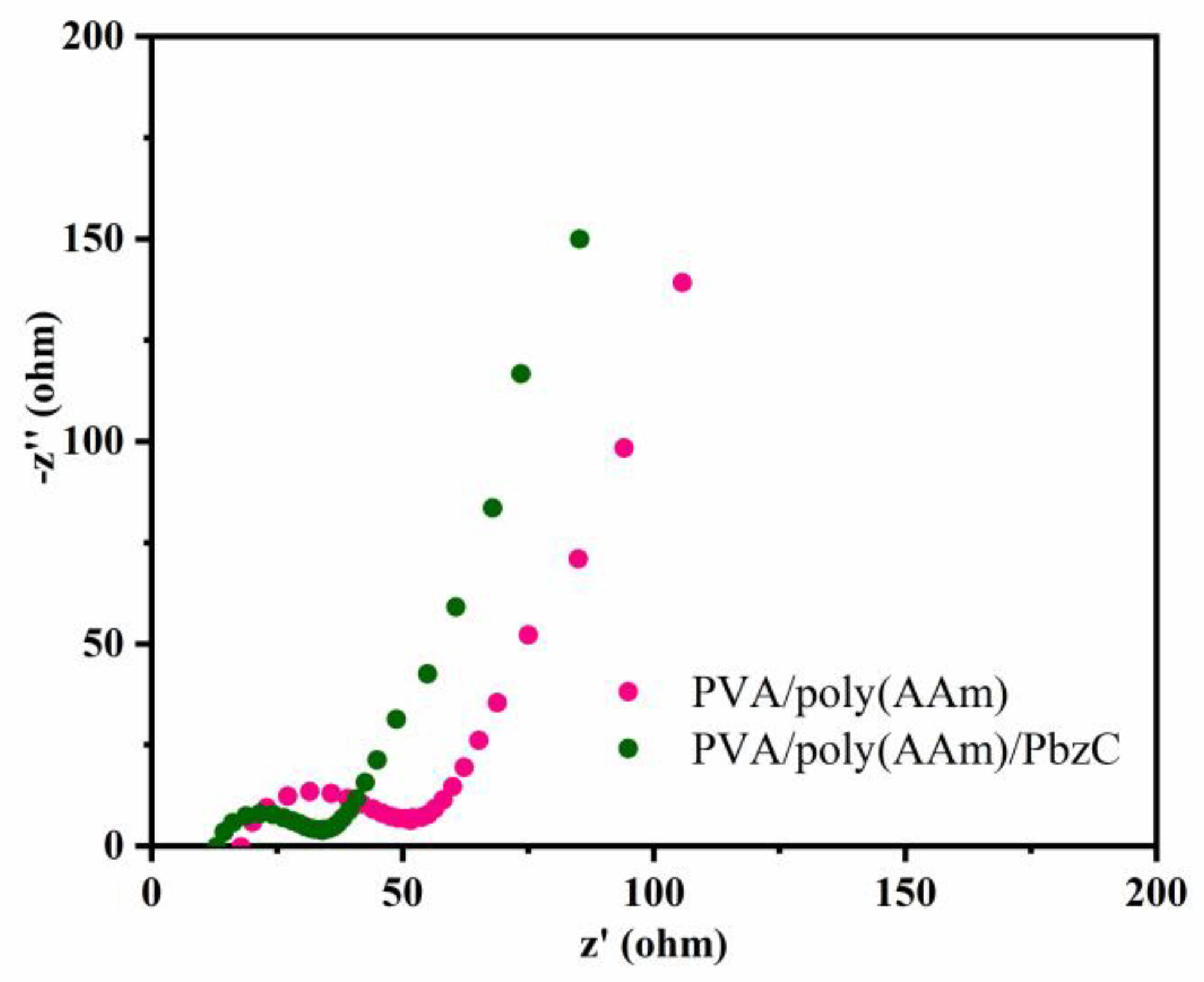
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Zhou, C.G.; Yan, X.H.; Cao, Y.; Gao, H.L.; Luo, H.W.; Gao, K.Z.; Xue, S.C.; Jing, X. Recent Advances and Perspectives on Graphene-Based Gels for Superior Flexible All-Solid-State Supercapacitors. J. Power Sources 2023, 565, 232916. [Google Scholar] [CrossRef]
- Jin, X.; Sun, G.; Zhang, G.; Yang, H.; Xiao, Y.; Gao, J.; Zhang, Z.; Qu, L. A Cross-Linked Polyacrylamide Electrolyte with High Ionic Conductivity for Compressible Supercapacitors with Wide Temperature Tolerance. Nano Res. 2019, 12, 1199–1206. [Google Scholar] [CrossRef]
- Liu, X.; Zou, S.; Liu, K.; Lv, C.; Wu, Z.; Yin, Y.; Liang, T.; Xie, Z. Highly Compressible Three-Dimensional Graphene Hydrogel for Foldable All-Solid-State Supercapacitor. J. Power Sources 2018, 384, 214–222. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Zhong, X.; Huang, X.; Weiss, N.O.; Huang, Y.; Duan, X. Holey Graphene Frameworks for Highly Efficient Capacitive Energy Storage. Nat. Commun. 2014, 5, 4554. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, R.; Chelazzi, D.; Poggi, G.; Fratini, E.; Buemi, L.P.; Petruzzellis, M.L.; Baglioni, P. Twin-Chain Polymer Hydrogels Based on Poly(Vinyl Alcohol) as New Advanced Tool for the Cleaning of Modern and Contemporary Art. Proc. Natl. Acad. Sci. USA 2020, 117, 7011–7020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiao, Y.; Liu, S.; Li, Z.; Liu, X.; Zhang, S.; Li, Z. Boric Acid-Regulated Gelation and Ethanol-Assisted Preparation of Polybenzoxazine Aerogels. Chem. Eng. J. 2024, 486, 150228. [Google Scholar] [CrossRef]
- Kim, J.R.; Woo, S.H.; Son, Y.L.; Kim, J.R.; Kasi, R.M.; Kim, S.C. Ultra-Tough and Super-Swelling Poly(Vinyl Alcohol)/Poly(AAm- Co-AA Sodium Salts) Double Network Hydrogels. Macromolecules 2021, 54, 2439–2448. [Google Scholar] [CrossRef]
- Katanyoota, P.; Chaisuwan, T.; Wongchaisuwat, A.; Wongkasemjit, S. Novel Polybenzoxazine-Based Carbon Aerogel Electrode for Supercapacitors. Mater. Sci. Eng. B 2010, 167, 36–42. [Google Scholar] [CrossRef]
- Nandi, A.K.; Chatterjee, D.P. Hybrid Polymer Gels for Energy Applications. J. Mater. Chem. A 2023, 11, 12593–12642. [Google Scholar] [CrossRef]
- Chen, C.R.; Qin, H.; Cong, H.P.; Yu, S.H. A Highly Stretchable and Real-Time Healable Supercapacitor. Adv. Mater. 2019, 31, 19. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards Flexible Solid-state Supercapacitors for Smart and Wearable Electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Q.; Ran, F. All-natural Hydrogel Electrolytes Prepared by A Universal Strategy for Supercapacitors. New J. Chem. 2022, 46, 19523–19533. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2017, 8, 7. [Google Scholar] [CrossRef]
- Evanko, B.; Boettcher, S.W.; Yoo, S.J.; Stucky, G.D. Redox-Enhanced Electrochemical Capacitors: Status, Opportunity, and Best Practices for Performance Evaluation. ACS Energy Lett. 2017, 2, 2581–2590. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Sang, M.; Wang, X.; Zuo, D.; Xu, J.; Zhang, H. A Supramolecular Hydrogel Electrolyte for High-performance Supercapacitors. J. Energy Storage 2021, 33, 101931. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Huang, Y.; Zhu, M.; Pei, Z.; Wang, Z.; Xue, Q.; Xie, X.; Zhi, C. A Self-healable and Highly Stretchable Supercapacitor Based on A Dual Crosslinked Polyelectrolyte. Nat. Commun. 2015, 6, 10310. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Lee, S.-H.; Ruh, H.; Yu, K.-M. Development of A Thickness Meter for Conductive Thin Films Using Four-Point Probe Method. J. Electr. Eng. Technol. 2021, 16, 2265–2273. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Y.; Que, M. A Facile in Situ Approach to Ion Gel Based Polymer Electrolytes for Flexible Lithium Batteries. RSC Adv. 2017, 7, 54391. [Google Scholar] [CrossRef]
- Kamarulazam, F.; Bashir, S.; Hina, M.; Kumar, S.S.A.; Gunalan, S.; Ramesh, S.; Ramesh, K. Effect of Electrode Substrate and Poly(acrylamide) Hydrogel Electrolytes on the Electrochemical Performance of Supercapacitors. Ionics 2021, 27, 4507–4519. [Google Scholar] [CrossRef]
- Li, L.; Lou, Z.; Chen, D.; Jiang, K.; Han, W.; Shen, G. Recent Advances in Flexible/Stretchable Supercapacitors for Wearable Electronics. Small 2017, 14, 43. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Wang, L.; Wu, D. Highly Elastic and Ultratough Hybrid Ionic−Covalent Hydrogels with Tunable Structures and Mechanics. Adv. Mater. 2018, 30, 1707071. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-M.; Yang, S.-J. Studying the Miscibility and Thermal Behavior of Polybenzoxazine/Poly(3-caprolactone) Blends Using DSC, DMA, and Solid State 13C NMR Spectroscopy. Polymer 2005, 46, 8068–8078. [Google Scholar] [CrossRef]
- Rimdusit, S.; Pirstpindvong, S.; Tanthapanichakoon, W.; Damrongsakkul, S. Toughening of Polybenzoxazine by Alloying with Urethane Prepolymer and Flexible Epoxy: A Comparative Study. Polym. Eng. Sci. 2005, 45, 288–296. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Shakila Parveen, A.; Lee, Y.R.; Kim, S.-C. The synthesis of mechanically stable polybenzoxazine-based porous carbon and its application as high-performance supercapacitor electrodes. New J. Chem. 2021, 45, 8738. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Kim, S.-C. Nitrogen-Rich Porous Carbon/NiMn Hybrids as Electrode Materials for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 15605–15614. [Google Scholar]
- Shakila Parveen, A.; Thirukumaran, P.; Kim, S.-C. Enhanced electrochemical performance of HC/NiCo@ 800 C//HC using redox-active electrolytes showing increased energy density. J. Alloys Compd. 2024, 972, 172753. [Google Scholar]
- Rimdusit, S.; Mongkhonsi, T.; Kamonchaivanich, P.; Sujirote, K.; Thiptipakorn, S. Effects of Polyol Molecular Weight on Properties of Benzoxazine–Urethane Polymer Alloys. Polym. Eng. Sci. 2008, 48, 2238–2246. [Google Scholar] [CrossRef]
- Ardhyananta, H.; Wahid, M.H.; Sasaki, M.; Agag, T.; Kawauchi, T.; Ismail, H.; Takeichi, T. Performance Enhancement of Polybenzoxazine by Hybridization with Polysiloxane. Polymer 2008, 49, 4585–4591. [Google Scholar] [CrossRef]
- Kumar, K.S.S.; Nair, C.P.R.; Ninan, K.N. Investigations on the Cure Chemistry and Polymer Properties of Benzoxazine–Cyanate Ester Blends. Eur. Polym. J. 2009, 45, 494–502. [Google Scholar] [CrossRef]
- Liu, Y.; He, K.; Chen, G.; Leow, W.R.; Chen, X. Nature-Inspired Structural Materials for Flexible Electronic Devices. Chem. Rev. 2017, 117, 12893–12941. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Vellacheri, R.; Lei, Y. Recent Advances in Designing and Fabricating Self-Supported Nanoelectrodes for Supercapacitors. Adv. Sci. 2017, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 48. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and Stress in Electrode Materials for Li-ion Batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J. Toward Emerging Sodium-Based Energy Storage Technologies: From Performance to Sustainability. Adv. Energy Mater. 2022, 12, 29. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, T.; Li, H.-N.; Cong, H.-P.; Antonietti, M.; Yu, S.-H. Dynamic Au-Thiolate Interaction Induced Rapid Self-Healing Nanocomposite Hydrogels with Remarkable Mechanical Behaviors. Chem 2017, 3, 691–705. [Google Scholar] [CrossRef]
- Miccoli, I.; Edler, F.; Pfnür, H.; Tegenkamp, C. The 100th Anniversary of the Four-point Probe Technique: The Role of Probe Geometries in Isotropic and Anisotropic Systems. J. Phys. Condens. Matter 2015, 27, 223201. [Google Scholar] [CrossRef]
- Ruano, G.; Iribarren, J.I.; Pérez-Madrigal, M.M.; Torras, J.; Alemán, C. Electrical and Capacitive Response of Hydrogel Solid-Like Electrolytes for Supercapacitors. Polymers 2021, 13, 1337. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Hamid, N.A.A.; Rahiman, W.; Mohamad, A.A. Electrode Polymer Binders for Supercapacitor Applications: A review. J. Mater. Res. Technol. 2023, 23, 3470–3491. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38, 9. [Google Scholar] [CrossRef]
- Weng, W.; Chen, P.; He, S.; Sun, X.; Peng, H. Smart Electronic Textiles. Angew. Chem. Int. Ed. 2016, 55, 6140–6169. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and Challenges in Electrochemical Energy Storage Devices: Fabrication, Electrode Material, and Economic Aspects. Chem. Eng. J. 2023, 468, 1385–8947. [Google Scholar] [CrossRef]
- Yang, H.; Ji, X.; Tan, Y.; Liu, Y.; Ran, F. Modified Supramolecular Carboxylated Chitosan as Hydrogel Electrolyte for Quasi-solidstate Supercapacitors. J. Power Sources 2019, 441, 227174. [Google Scholar] [CrossRef]
- Yu, D.; Li, X.; Xu, J. Safety Regulation of Gel Electrolytes in Electrochemical Energy Storage Devices. Sci. China Mater. 2019, 62, 1556–1573. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel Ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Shin, S.J.; Gittins, J.W.; Balhatchet, C.J.; Walsh, A.; Forse, A.C. Metal–Organic Framework Supercapacitors: Challenges and Opportunities. Adv. Funct. Mater. 2023, 23, 11. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.; Yu, X.; Zhang, W. Recent advances of PVA-based hydrogels in cartilage repair application. J. Mater. Res. Technol. 2023, 24, 2279–2298. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Shui, T.; Pan, M.; Li, A.; Fan, H.; Wu, J.; Liu, Q.; Zeng, H. Poly(vinyl Alcohol) (PVA)-Based Hydrogel Scaffold with Isotropic Ultratoughness Enabled by Dynamic Amine−Catechol Interactions. Chem. Mater. 2022, 34, 8613–8628. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, C.; Zhang, Y.; Wang, B.; Xiong, Q.; Zhao, M.; Ni, Y. Multi-layer hierarchical cellulose nanofibers/carbon nanotubes/vinasse activated carbon composite materials for supercapacitors and electromagnetic interference shielding. Nano Res. 2024, 17, 904–912. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Qiu, J.; Zhang, K.; Shao, J.; Yan, L. In Situ Formation of a Renewable Cellulose Hydrogel Electrolyte for High-Performance Flexible All-Solid-State Asymmetric Supercapacitors. Sustain. Energy Fuels 2019, 3, 3109–3115. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Yao, F.; Peng, C.; Cao, C.; Feng, Y.; Feng, W. Nitrogen and Fluorine Co-Doped Holey Graphene Hydrogel as a Binder-Free Electrode Material for Flexible Solid-State Supercapacitors. Sustain. Energy Fuels 2019, 3, 2237–2245. [Google Scholar] [CrossRef]
- Feng, H.; Xie, P.; Xue, S.; Li, L.; Hou, X.; Liu, Z.; Wu, D.; Wang, L.; Chu, P.K. Synthesis of Three-Dimensional Porous Reduced Graphene Oxide Hydrogel/Carbon Dots for High-Performance Supercapacitor. J. Electroanal. Chem. 2018, 808, 321–328. [Google Scholar] [CrossRef]
- Gao, H.; Hao, C.; Qi, Y.; Li, J.; Wang, X.; Zhou, S.; Huang, C. In Situ Hydrothermal Construction of Hydrogel Composites by Anchoring Ni(OH)2 Nanoparticles onto Sulfonated Graphene and Their Application for Functional Supercapacitor Electrode. J. Alloys Compd. 2018, 767, 1048–1056. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-Based Nitrogen Self-Doped Hierarchical Porous Carbon Aerogels Derived from Chitosan for High Performance Supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]

| Electrode Material | Electrolyte | Specific Capacitance | References |
|---|---|---|---|
| N-doped graphene hydrogel | PVA/H2SO4 | 61.7 F/g | [52] |
| 3D graphene hydrogel | PVA/H2SO4 | 186.3 F/g | [53] |
| N,F-doped graphene hydrogel | PVA/KOH | 170.2 F/g | [2] |
| GO/PNIPAM hydrogel | PVA/H2SO4 | 292.0 F/g | [54] |
| rGO/carbon hydrogel | PVA/H2SO4 | 264 F/g | [55] |
| N-doped graphene/carbon hydrogel | PVA/KOH | 197 F/g | [56] |
| PVA/poly (AAm)/PBzC | H2SO4 | 210 F/g | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyasamy, T.; Asrafali, S.P.; Islam, M.; Bari, G.A.K.M.R.; Lee, J. Polymer Composite Hydrogel Based on Polyvinyl Alcohol/Polyacrylamide/Polybenzoxazine Carbon for Use in Flexible Supercapacitors. Polymers 2024, 16, 1463. https://doi.org/10.3390/polym16111463
Periyasamy T, Asrafali SP, Islam M, Bari GAKMR, Lee J. Polymer Composite Hydrogel Based on Polyvinyl Alcohol/Polyacrylamide/Polybenzoxazine Carbon for Use in Flexible Supercapacitors. Polymers. 2024; 16(11):1463. https://doi.org/10.3390/polym16111463
Chicago/Turabian StylePeriyasamy, Thirukumaran, Shakila Parveen Asrafali, Mobinul Islam, Gazi A. K. M. Rafiqul Bari, and Jaewoong Lee. 2024. "Polymer Composite Hydrogel Based on Polyvinyl Alcohol/Polyacrylamide/Polybenzoxazine Carbon for Use in Flexible Supercapacitors" Polymers 16, no. 11: 1463. https://doi.org/10.3390/polym16111463
APA StylePeriyasamy, T., Asrafali, S. P., Islam, M., Bari, G. A. K. M. R., & Lee, J. (2024). Polymer Composite Hydrogel Based on Polyvinyl Alcohol/Polyacrylamide/Polybenzoxazine Carbon for Use in Flexible Supercapacitors. Polymers, 16(11), 1463. https://doi.org/10.3390/polym16111463









