Molecular Effects of Li+-Coordinating Binders and Negatively Charged Binders on the Li+ Local Mobility near the Electrolyte/LiFePO4 Cathode Interface within Lithium-Ion Batteries
Abstract
1. Introduction
2. Methods
2.1. Electrolyte/Cathode Systems
2.2. Simulation Details
3. Results and Discussions
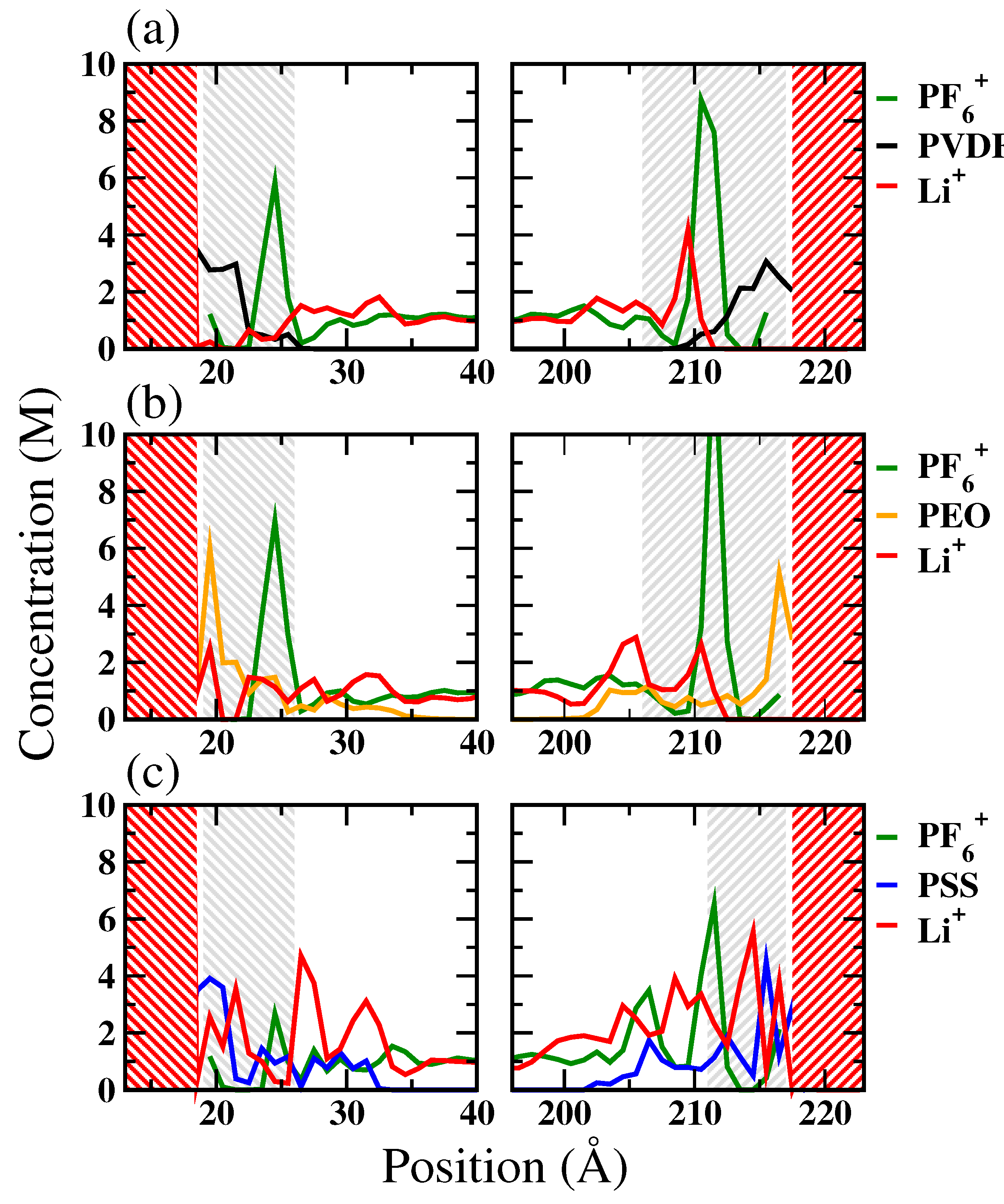
3.1. Li+ Distribution and Diffusivity near Liquid Electrolyte/Cathode Interface
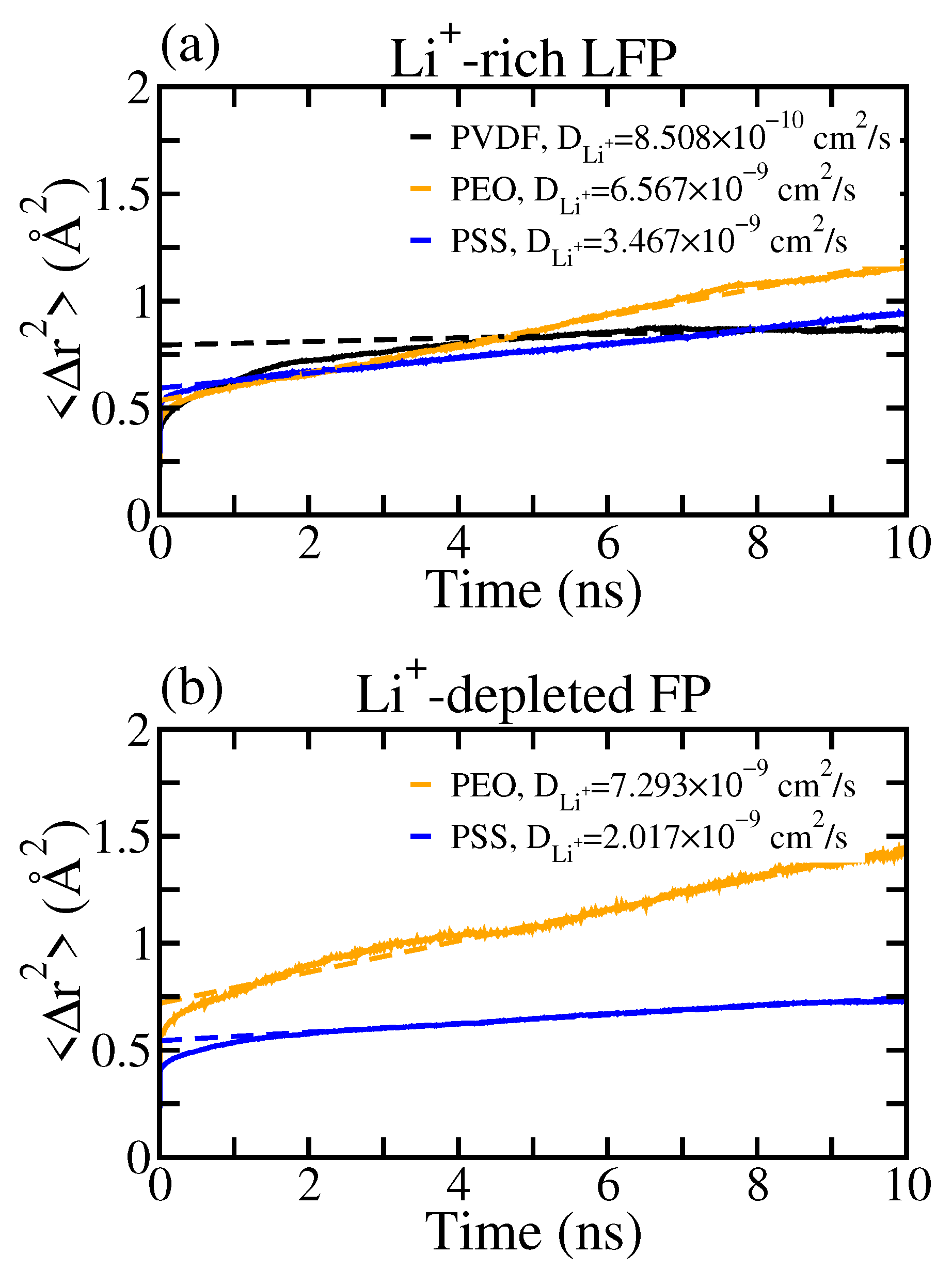
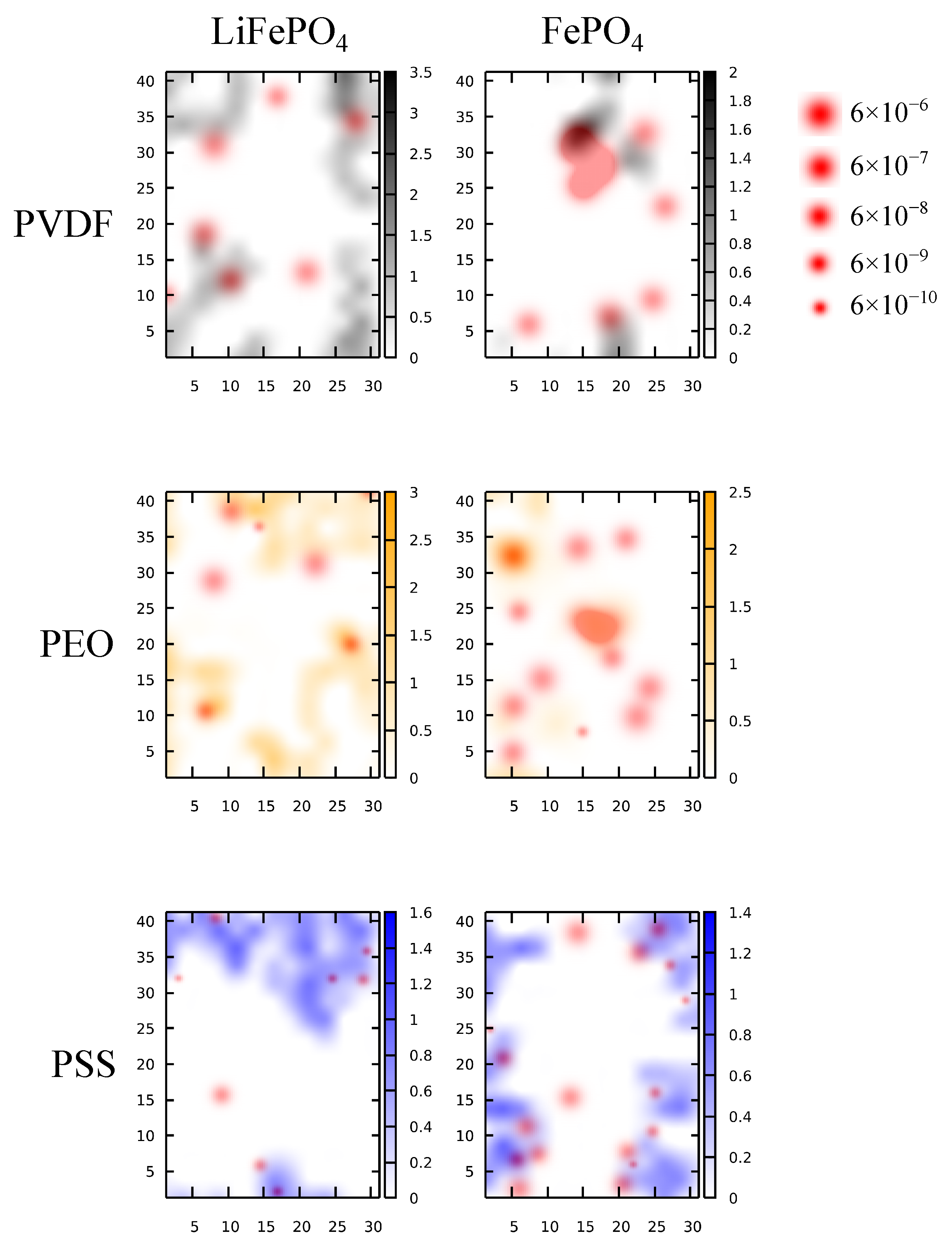
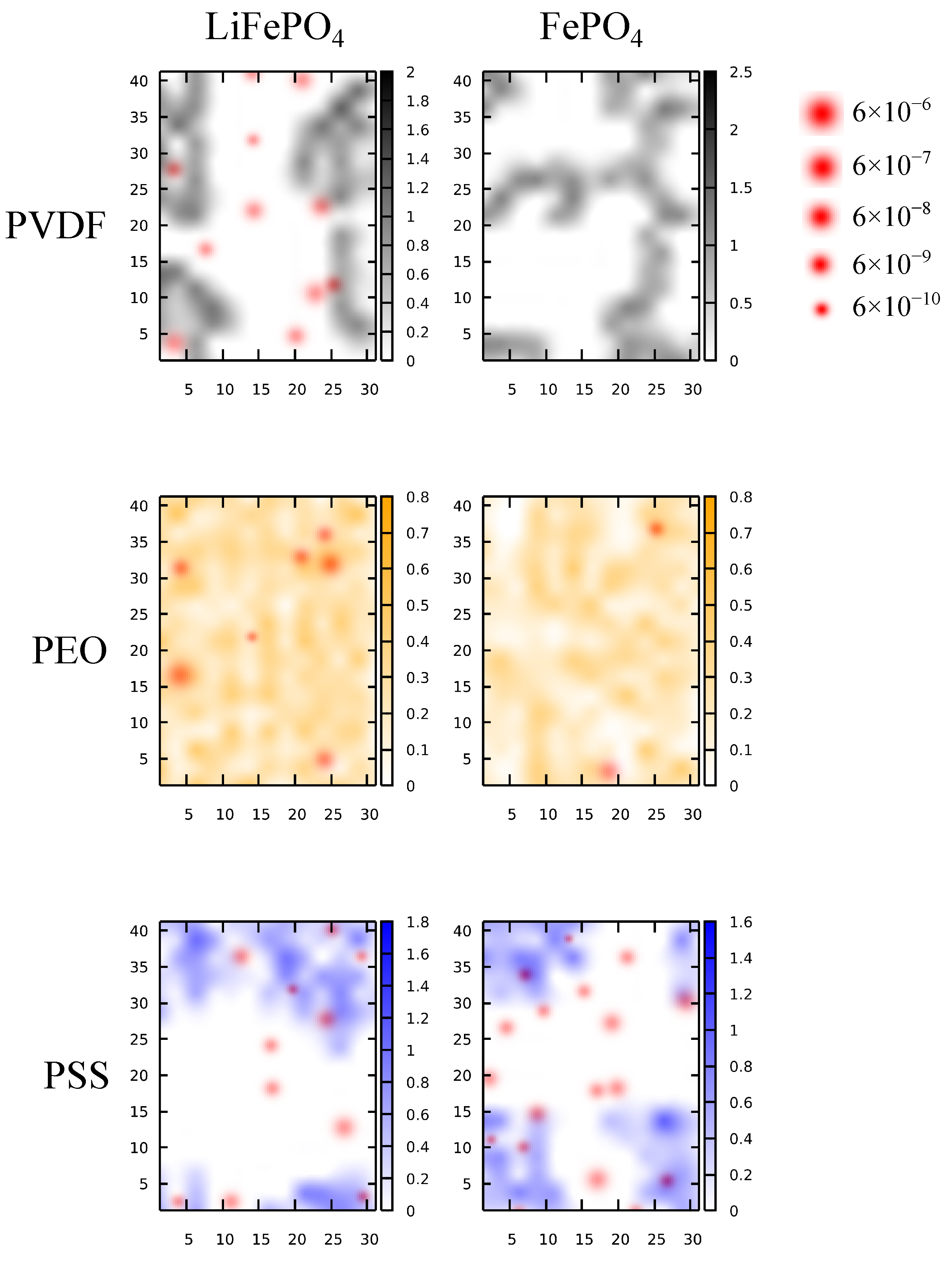
3.2. Li+ Distribution and Diffusivity near Solid Polymer Electrolyte/Cathode Interface
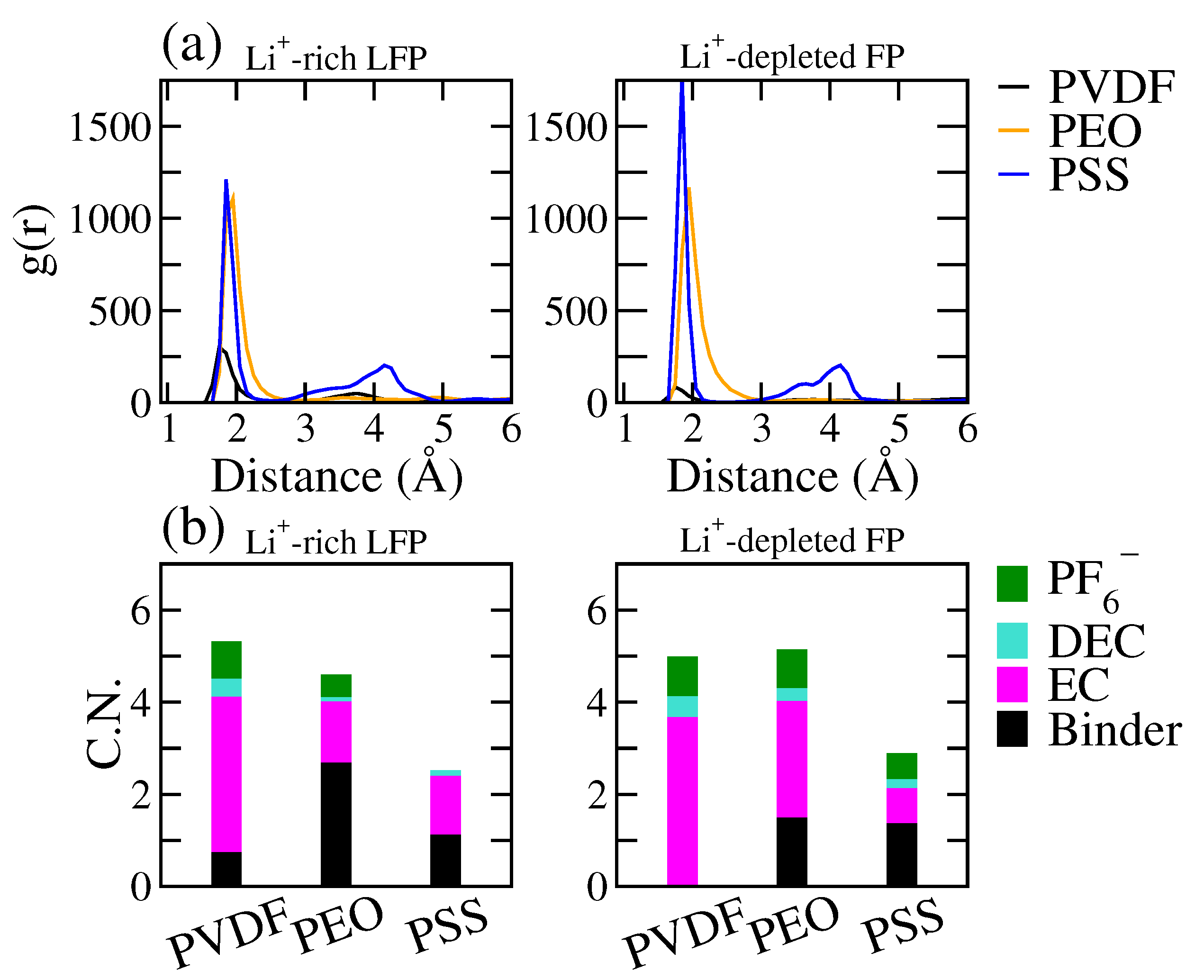
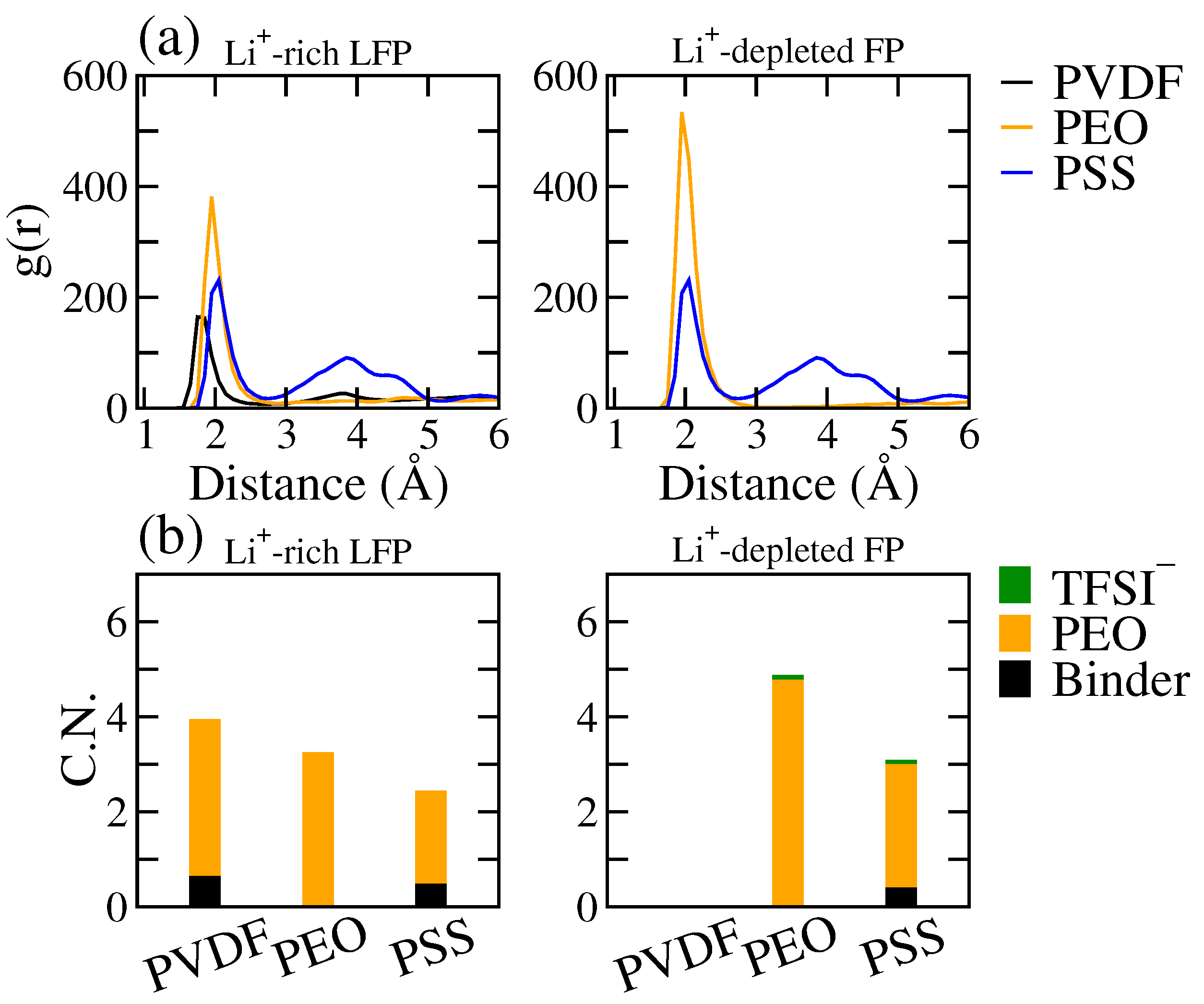
3.3. The Effects of Binders on Li+ Local Diffusivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LIB | lithium-ion battery |
| MD | molecular dynamics |
| LFP | lithium iron phosphate |
| FP | iron phosphate |
| EC | ethylene carbonate |
| DEC | diethyl carbonate |
| PVDF | polyvinylidene fluoride |
| PEO | polyethylene oxide |
| PSS | polystyrene sulfonate |
| LE | liquid electrolyte |
| SPE | solid polymer electrolyte |
References
- Kwon, T.w.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K. Direction for Development of Next-Generation Lithium-Ion Batteries. ACS Energy Lett. 2017, 2, 2694–2695. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zheng, M.; Zhang, W.; Liu, T.; Wang, F.; Wan, G.; Liu, Y.; Tao, X. Review on the Binders for Sustainable High-Energy-Density Lithium Ion Batteries: Status, Solutions, and Prospects. Adv. Funct. Mater. 2023, 33, 2305161. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energy Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Saal, A.; Hagemann, T.; Schubert, U.S. Polymers for Battery Applications—Active Materials, Membranes, and Binders. Adv. Energy Mater. 2020, 121, 2001984. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, J.; Cui, G. Small things make big deal: Powerful binders of lithium batteries and post-lithium batteries. Energy Storage Mater. 2019, 20, 146–175. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 163, 2002508. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Accounts Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, T.; Lai, Y.; Jia, M.; Li, J. A comparative study of different binders and their effects on electrochemical properties of LiMn 2 O 4 cathode in lithium ion batteries. J. Power Sources 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Niketic, S.; Yim, C.H.; Zhou, J.; Wang, J.; Abu-Lebdeh, Y. Revealing the Role of Poly(vinylidene fluoride) Binder in Si/Graphite Composite Anode for Li-Ion Batteries. ACS Omega 2018, 3, 11684–11690. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Paik, U.; Hackley, V.A.; Choi, Y.M. Effect of Carboxymethyl Cellulose on Aqueous Processing of Natural Graphite Negative Electrodes and their Electrochemical Performance for Lithium Batteries. J. Electrochem. Soc. 2005, 152, A1763–A1769. [Google Scholar] [CrossRef]
- Li, J.; Lewis, R.B.; Dahn, J.R. Sodium Carboxymethyl Cellulose: A Potential Binder for Si Negative Electrodes for Li-Ion Batteries. Electrochem. Solid-State Lett. 2007, 10, A17. [Google Scholar] [CrossRef]
- Lux, S.F.; Schappacher, F.; Balducci, A.; Passerini, S.; Winter, M. Low Cost, Environmentally Benign Binders for Lithium-Ion Batteries. J. Electrochem. Soc. 2010, 157, A320–A325. [Google Scholar] [CrossRef]
- Li, J.; Klöpsch, R.; Nowak, S.; Kunze, M.; Winter, M.; Passerini, S. Investigations on cellulose-based high voltage composite cathodes for lithium ion batteries. J. Power Sources 2011, 196, 7687–7691. [Google Scholar] [CrossRef]
- Tsao, C.H.; Hsu, C.H.; Kuo, P.L. Ionic Conducting and Surface Active Binder of Poly (ethylene oxide)-block-poly(acrylonitrile) for High Power Lithium-ion Battery. Electrochim. Acta 2016, 196, 41–47. [Google Scholar] [CrossRef]
- Tsao, C.H.; Wu, E.T.; Lee, W.H.; Chiu, C.c.; Kuo, P.L. Fluorinated Copolymer Functionalized with Ethylene Oxide as Novel Water-Borne Binder for a High-Power Lithium Ion Battery: Synthesis, Mechanism, and Application. ACS Appl. Energy Mater. 2018, 1, 3999–4008. [Google Scholar] [CrossRef]
- Wei, Z.; Xue, L.; Nie, F.; Sheng, J.; Shi, Q.; Zhao, X. Study of sulfonated polyether ether ketone with pendant lithiated fluorinated sulfonic groups as ion conductive binder in lithium-ion batteries. J. Power Sources 2014, 256, 28–31. [Google Scholar] [CrossRef]
- Tsao, C.H.; Yang, T.K.; Chen, K.y.; Fang, C.E.; Ueda, M.; Richter, F.H.; Janek, J.; Chiu, C.c.; Kuo, P.L. Comparing the Ion-Conducting Polymers with Sulfonate and Ether Moieties as Cathode Binders for High-Power Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 9846–9855. [Google Scholar] [CrossRef]
- Liu, H.S.; Chen, K.y.; Fang, C.E.; Chiu, C.c. Comparing the Effects of Polymer Binders on Li+ Transport Near the Liquid Electrolyte/LiFePO4 Interfaces: A Molecular Dynamics Simulation Study. Electrochim. Acta 2021, 7, 137915. [Google Scholar] [CrossRef]
- Riley, M.W.; Fedkiw, P.S.; Khan, S.A. Lithium Hectorite Clay as the Ionic Conductor in LiCoO2 Cathodes. J. Electrochem. Soc. 2003, 150, A933–A941. [Google Scholar] [CrossRef][Green Version]
- Cheon, S.E.; Cho, J.H.; Ko, K.S.; Kwon, C.W.; Chang, D.R.; Kim, H.T.; Kim, S.W. Structural Factors of Sulfur Cathodes with Poly(ethylene oxide) Binder for Performance of Rechargeable Lithium Sulfur Batteries. J. Electrochem. Soc. 2002, 149, A1437. [Google Scholar] [CrossRef]
- Gong, L.; Nguyen, M.H.T.; Oh, E.S. High polar polyacrylonitrile as a potential binder for negative electrodes in lithium ion batteries. Electrochem. Commun. 2013, 29, 45–47. [Google Scholar] [CrossRef]
- Lee, S.; Kim, E.Y.; Lee, H.; Oh, E.S. Effects of polymeric binders on electrochemical performances of spinel lithium manganese oxide cathodes in lithium ion batteries. J. Power Sources 2014, 269, 418–423. [Google Scholar] [CrossRef]
- Lis, M.; Chudzik, K.; Bakierska, M.; Świętosławski, M.; Gajewska, M.; Rutkowska, M.; Molenda, M. Aqueous Binder for Nanostructured Carbon Anode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A5354–A5361. [Google Scholar] [CrossRef]
- Smith, G.D.; Borodin, O.; Russo, S.P.; Rees, R.J.; Hollenkamp, A.F. A molecular dynamics simulation study of LiFePO4/electrolyte interfaces: Structure and Li+ transport in carbonate and ionic liquid electrolytes. Phys. Chem. Chem. Phys. 2009, 11, 9884. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Sambasivarao, S.V.; Acevedo, O. Development of OPLS-AA Force Field Parameters for 68 Unique Ionic Liquids. J. Chem. Theory Comput. 2009, 5, 1038–1050. [Google Scholar] [CrossRef]
- Lopes, J.N.C.; Pádua, A.A.H. Molecular Force Field for Ionic Liquids Composed of Triflate or Bistriflylimide Anions. J. Phys. Chem. B 2004, 108, 16893–16898. [Google Scholar] [CrossRef]
- Lopes, J.N.C.; Deschamps, J.; Pádua, A.A.H. Modeling Ionic Liquids Using a Systematic All-Atom Force Field. J. Phys. Chem. B 2004, 108, 2038–2047. [Google Scholar] [CrossRef]
- Doherty, B.; Zhong, X.; Gathiaka, S.; Li, B.; Acevedo, O. Revisiting OPLS Force Field Parameters for Ionic Liquid Simulations. J. Chem. Theory Comput. 2017, 13, 6131–6145. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.E.; Tsai, Y.C.; Scheurer, C.; Chiu, C.c. Revised Atomic Charges for OPLS Force Field Model of Poly(Ethylene Oxide): Benchmarks and Applications in Polymer Electrolyte. Polymers 2021, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, H.L.T.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8596. [Google Scholar] [CrossRef]
- Nose, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Nose, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Mogurampelly, S.; Borodin, O.; Ganesan, V. Computer Simulations of Ion Transport in Polymer Electrolyte Membranes. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 349–371. [Google Scholar] [CrossRef]
- Borodin, O.; Smith, G.D. Mechanism of Ion Transport in Amorphous Poly(ethylene oxide)/LiTFSI from Molecular Dynamics Simulations. Macromolecules 2006, 39, 1620–1629. [Google Scholar] [CrossRef]
- Webb, M.A.; Jung, Y.; Pesko, D.M.; Savoie, B.M.; Yamamoto, U.; Coates, G.W.; Balsara, N.P.; Wang, Z.G.; Miller, T.F., III. Systematic Computational and Experimental Investigation of Lithium-Ion Transport Mechanisms in Polyester-Based Polymer Electrolytes. ACS Cent. Sci. 2015, 1, 198–205. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-Y.; Chiu, T.-H.; Chiu, C.-c. Molecular Effects of Li+-Coordinating Binders and Negatively Charged Binders on the Li+ Local Mobility near the Electrolyte/LiFePO4 Cathode Interface within Lithium-Ion Batteries. Polymers 2024, 16, 319. https://doi.org/10.3390/polym16030319
Wang P-Y, Chiu T-H, Chiu C-c. Molecular Effects of Li+-Coordinating Binders and Negatively Charged Binders on the Li+ Local Mobility near the Electrolyte/LiFePO4 Cathode Interface within Lithium-Ion Batteries. Polymers. 2024; 16(3):319. https://doi.org/10.3390/polym16030319
Chicago/Turabian StyleWang, Po-Yuan, Tzu-Heng Chiu, and Chi-cheng Chiu. 2024. "Molecular Effects of Li+-Coordinating Binders and Negatively Charged Binders on the Li+ Local Mobility near the Electrolyte/LiFePO4 Cathode Interface within Lithium-Ion Batteries" Polymers 16, no. 3: 319. https://doi.org/10.3390/polym16030319
APA StyleWang, P.-Y., Chiu, T.-H., & Chiu, C.-c. (2024). Molecular Effects of Li+-Coordinating Binders and Negatively Charged Binders on the Li+ Local Mobility near the Electrolyte/LiFePO4 Cathode Interface within Lithium-Ion Batteries. Polymers, 16(3), 319. https://doi.org/10.3390/polym16030319






