Bacteria Contaminants Detected by Organic Inverter-Based Biosensors
Abstract
1. Introduction
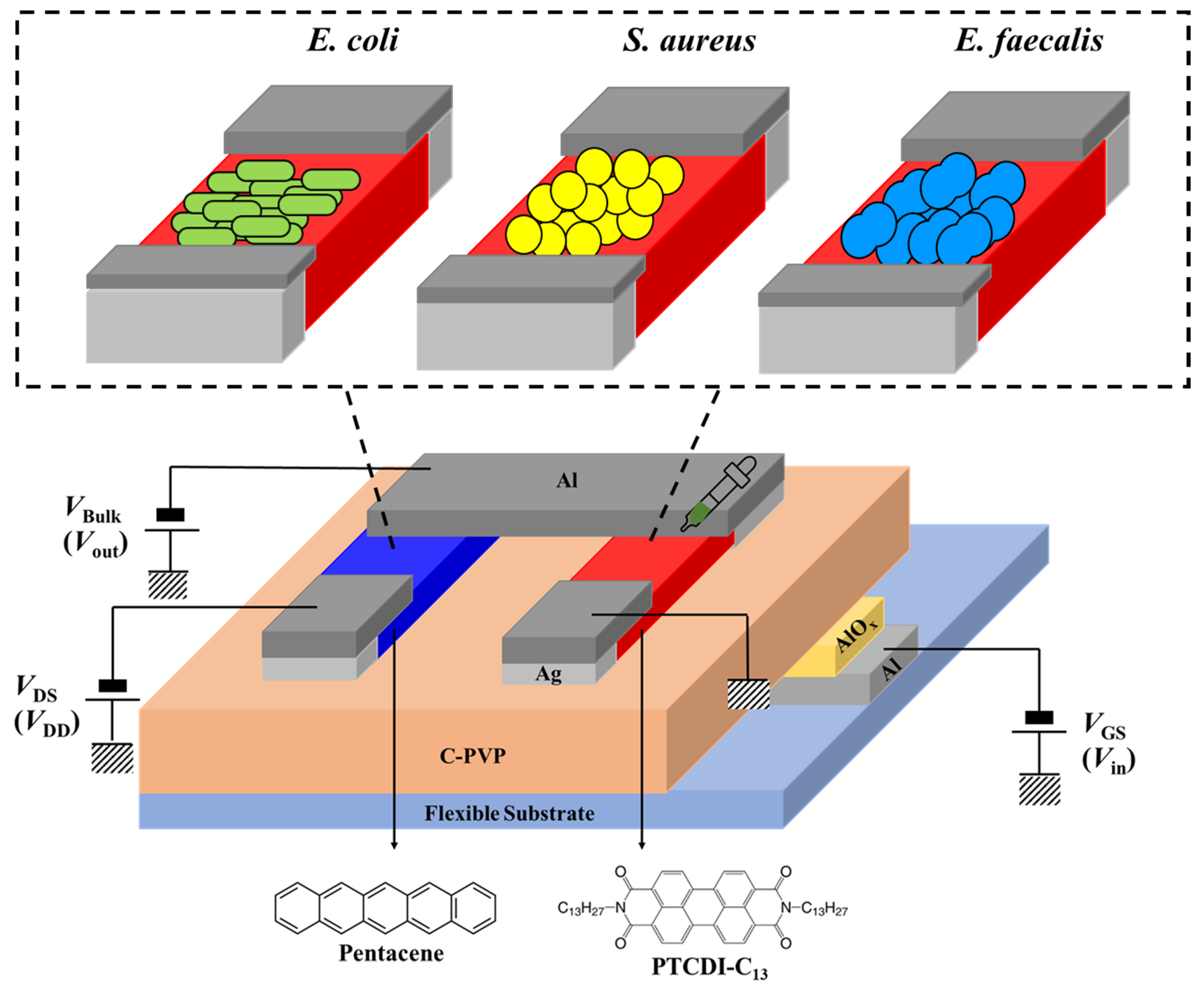
2. Materials and Methods
2.1. Device Fabrication and Characterization
2.2. Bacterial Cultivation and Preparation
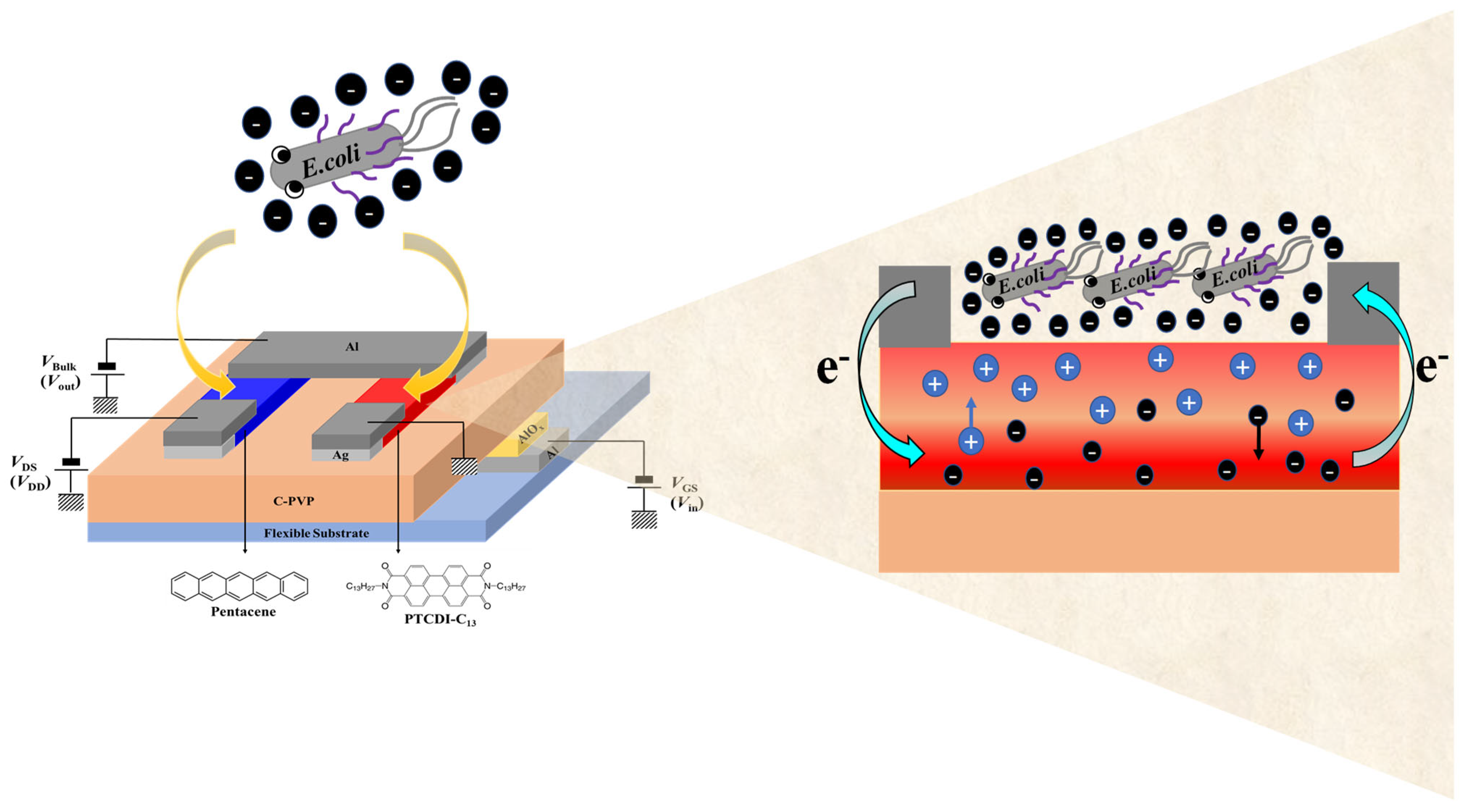
3. Results and Discussion
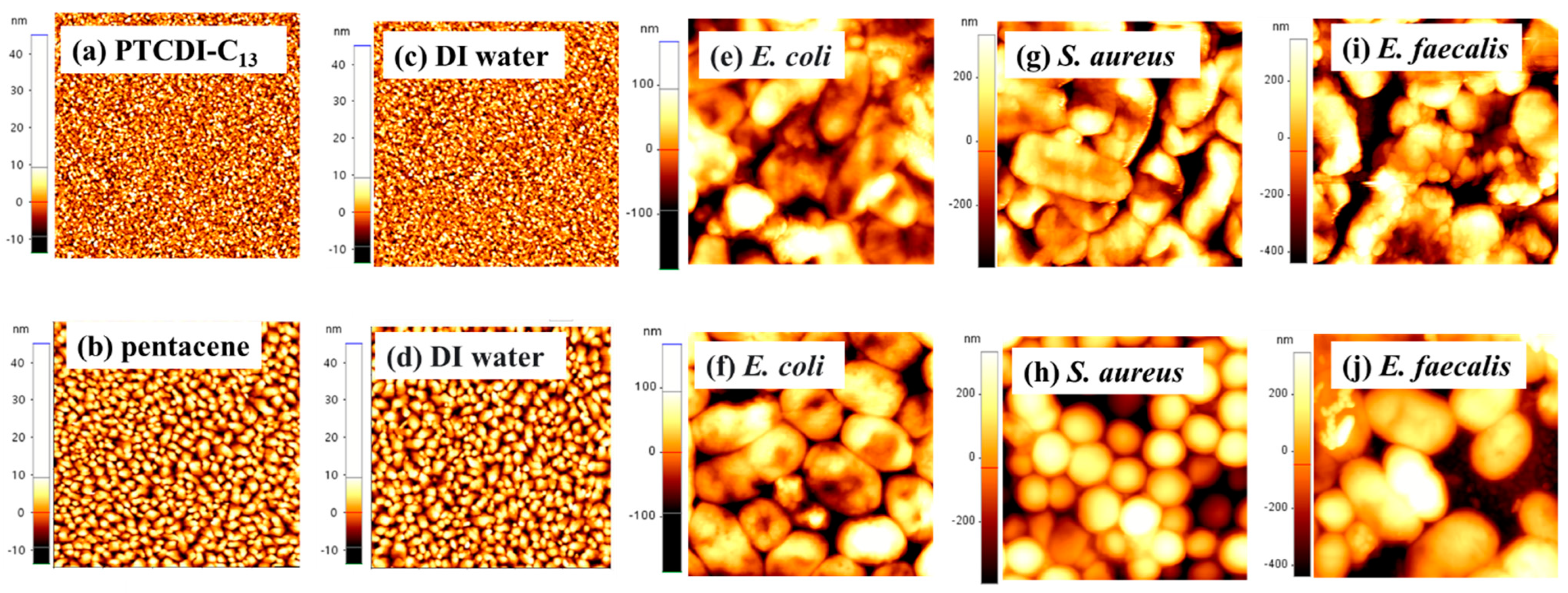
3.1. Surface Morphology
3.2. Electrical Measurements of OFETs
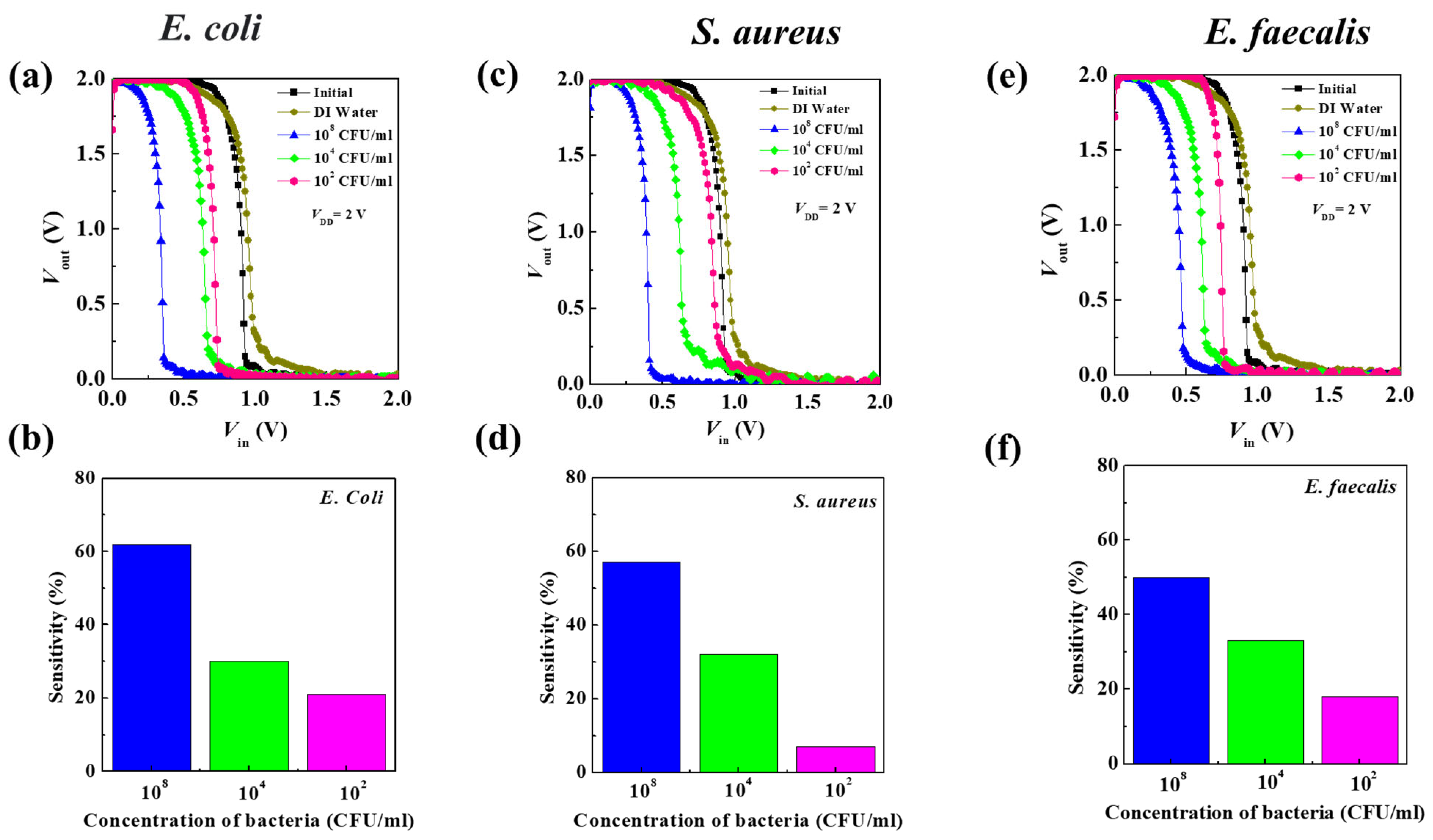
3.3. Determination of Bacteria Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Suthar, S.; Chhimpa, V.; Singh, S. Bacterial contamination in drinking water: A case study in rural areas of northern Rajasthan, India. Environ. Monit. Assess. 2009, 159, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowska, A.C.; Trawińska, B.; Tymczyna, L.; Wencel, H.B.; Matuszewski, Ł. Microbial contamination of the air in livestock buildings as a threat to human and animal health—A review. Ann. Anim. Sci. 2021, 21, 417–431. [Google Scholar] [CrossRef]
- Waldman, A.J.; Balskus, E.P. The Human Microbiota, Infectious Disease, and Global Health: Challenges and Opportunities. ACS Infect. Dis. 2018, 4, 14–26. [Google Scholar] [CrossRef]
- Lacroix, J.S.; Ricchetti, A.; Lew, D.; Delhumeau, C.; Morabia, A.; Stalder, H.; Terrier, F.; Kaiser, L. Symptoms and clinical and radiological signs predicting the presence of pathogenic bacteria in acute rhinosinusitis. Acta Otolaryngol. 2002, 122, 192–196. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting pathogenic bacteria on two fronts: Phages and antibiotics as combined strategy. Front. Cell. Infect. Microb. 2019, 9, 22. [Google Scholar] [CrossRef]
- Lim, S.H.; Mix, S.; Anikst, V.; Budvytiene, I.; Eiden, M.; Churi, Y.; Queralto, N.; Berliner, A.; Martino, R.A.; Rhodesa, P.A.; et al. Bacterial culture detection and identification in blood agar plates with an optoelectronic nose. Analyst 2016, 141, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.X.; Urosevic, N.; Inglis, T.J.J. Accelerated bacterial detection in blood culture by enhanced acoustic flow cytometry (AFC) following peptide nucleic acid fluorescence in situ hybridization (PNA-FISH). PLoS ONE 2019, 14, e0201332. [Google Scholar] [CrossRef]
- Higgins, J.A.; Azad, A.F. Use of polymerase chain reaction to detect bacteria in arthropods: A review. J. Med. Entomol. 1995, 32, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Morrison, S.; Tang, Y.W. Multiplex polymerase chain reaction tests for detection of pathogens associated with gastroenteritis. Clin. Lab. Med. 2015, 35, 461–486. [Google Scholar] [CrossRef]
- Law, J.W.F.; Mutalib, N.S.A.; Chan, K.G.; Lee, L.H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 122692. [Google Scholar] [CrossRef]
- Yadav, N.; Chhillar, A.K.; Rana, J.S. Detection of pathogenic bacteria with special emphasis to biosensors integrated with AuNPs. Sens. Int. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Burlage, R.S.; Tillmann, J. Biosensors of bacterial cells. J. Microbiol. Methods 2017, 138, 2–11. [Google Scholar] [CrossRef]
- Riu, J.; Giussani, B. Electrochemical biosensors for the detection of pathogenic bacteria in food. Trac. Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Hasan, M.R.; Hossain, S.I.; Ahommed, M.S.; Daizy, M. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: State of the art. Biosens. Bioelectron. 2020, 166, 112431. [Google Scholar] [CrossRef]
- Rani, R.; Deep, A.; Mizaikoff, B.; Singh, S. Copper based organic framework modified electrosensor for selective and sensitive detection of ciprofloxacin. Electroanalysis 2020, 32, 2442–2451. [Google Scholar] [CrossRef]
- Niu, Y.; Qin, Z.; Zhang, Y.; Chen, C.; Liu, S.; Chen, H. Expanding the potential of biosensors: A review on organic field effect transistor (OFET) and organic electrochemical transistor (OECT) biosensors. Mater. Futures 2023, 2, 042401. [Google Scholar] [CrossRef]
- Kudo, K. Organic light emitting transistors. Curr. Appl. Phys. 2005, 5, 337–340. [Google Scholar] [CrossRef]
- Daniso, E.; Maroh, B.; Feldbacher, S.; Mühlbacher, I.; Schl, S.; Melpignano, P. Tailoring the chemical functionalization of a transparent polyethylene foil for its application in an OLED-based DNA biosensor. Appl. Surf. Sci. 2021, 552, 149408. [Google Scholar] [CrossRef]
- Xu, W.; Yang, W.K.; Guo, H.K.; Ge, L.Y.; Tu, J.C.; Zhen, C. Constructing a TiO2/PDA core/shell nanorod array electrode as a highly sensitive and stable photoelectrochemical glucose biosensor. RSC Adv. 2020, 10, 10017–10022. [Google Scholar] [CrossRef]
- Lungenschmied, C.; Dennler, G.; Neugebauer, H.; Sariciftci, S.N.; Glatthaar, M.; Meyer, T.; Meyer, A. Flexible, long-lived, large-area, organic solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 379–384. [Google Scholar] [CrossRef]
- Tao, J.W.; Sun, W.B.; Lu, L.H. Organic small molecule semiconductor materials for OFET-based biosensors. Biosens. Bioelectron. 2022, 216, 114667. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.F.; Yue, Y.F.; Li, L.L.; Ji, J.L.; Zhang, Q.; Ding, L.F.; Sang, S.B.; Li, Q. P3HT-based organic field effect transistor for low-cost, label-free detection of immunoglobulin G. J. Biotechnol. 2022, 359, 75–81. [Google Scholar]
- Luo, L.; Liu, Z. Recent progress in organic field-effect transistor-based chem/bio-sensors. View 2022, 3, 20200115. [Google Scholar] [CrossRef]
- Basiricò, L.; Mattana, G.; Mas-Torrent, M. Editorial: Organic Electronics: Future Trends in Materials, Fabrication Techniques and Applications. Front. Phys. 2022, 10, 888155. [Google Scholar] [CrossRef]
- Vasimalla, S.; Subbarao, N.V.V.; Iyer, P.K. Low voltage, low cost, flexible and balanced ambipolar OFETs based on Br2PTCDI-C18/CuPc fabricated on an Al foil gate substrate with good ambient stability. J. Mater. Chem. C 2016, 4, 7102–7109. [Google Scholar] [CrossRef]
- Shi, W.; Guo, Y.; Liu, Y. When Flexible Organic Field-Effect Transistors Meet Biomimetics: A Prospective View of the Internet of Things. Adv. Mater. 2020, 32, 1901493. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Di, C.A.; Zhu, D. Organic transistor for bioelectronic applications. Sci. China Chem. 2017, 60, 437–449. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Duba, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Han, G.; Liu, J.; Zhang, J.; Li, C.; Liu, J.; Yi, Y.; Li, T.; Gao, X.; et al. Monolayer Two-dimensional Molecular Crystals for an Ultrasensitive OFET-based Chemical Sensor. Angew. Chem. Int. Ed. 2020, 59, 4380–4384. [Google Scholar] [CrossRef]
- Ohayon, D.; Inal, S. Organic Bioelectronics: From Functional Materials to Next-Generation Devices and Power Sources. Adv. Mater. 2020, 32, 2001439. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, F. Organic Thin-Film Transistors for Chemical and Biological Sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef]
- Magliulo, M.; Mallardi, A.; Gristina, R.; Ridi, F.; Sabbatini, L.; Cioffi, N.; Palazzo, G.; Torsi, L. Part per Trillion Label-Free Electronic Bioanalytical Detection. Anal. Chem. 2013, 85, 3849–3857. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W.C. Detection of bacterial metabolic volatile indole using a graphene-based field-effect transistor biosensor. Nanomaterials 2021, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.B.; Yang, M.Y.; Zhu, L.; Gunathilaka, G.; Zhou, Z.X.; Zhang, Y.F.; Cheng, M.M.C. Ultrasensitive and Selective Bacteria Sensors Based on Functionalized Graphene Transistors. IEEE Sens. J. 2022, 22, 5514–5520. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, S.J.; Park, C.S.; Seo, S.E.; Lee, J.; Kim, J.Y.; Lee, S.H.; Lee, S.H.; Kim, J.S.; Ryu, C.M.; et al. High-performance portable graphene field-effect transistor device for detecting Gram-positive and -negative bacteria. Biosens. Bioelectron. 2020, 167, 112514. [Google Scholar] [CrossRef]
- Dey, A.; Singh, A.; Dutta, D.; Ghosh, S.S.; Iyer, P.K. Rapid and label-free bacteria detection using a hybrid tri-layer dielectric integrated n-type organic field effect transistor. J. Mater. Chem. A 2019, 7, 18330–18337. [Google Scholar] [CrossRef]
- Jia, D.Y.; Jiang, L.; Cai, X.Z.; Dong, H.L.; Meng, Q.; Tian, G.F.; Wu, D.Z.; Li, J.Z.; Hu, W.P. Large scale, flexible organic transistor arrays and circuits based on polyimide materials. Org. Electron. 2013, 14, 2528–2533. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Guo, T.; Zhou, L.; Offenhäusser, A.; Mayer, D. Label-Free Split Aptamer Sensor for Femtomolar Detection of Dopamine by Means of Flexible Organic Electrochemical Transistors. Materials 2020, 13, 2577. [Google Scholar] [CrossRef]
- Griggs, S.; Marks, A.; Bristowa, H.; McCullochab, I. n-Type organic semiconducting polymers: Stability limitations, design considerations and applications. J. Mater. Chem. C 2021, 9, 8099. [Google Scholar] [CrossRef]
- Hwang, D.K.; Fuentes-Hernandez, C.; Fenoll, M.; Yun, M.; Park, J.; Shim, J.W.; Knauer, K.A.; Dindar, A.; Kim, H.; Kim, Y.; et al. Systematic Reliability Study of Top-Gate p- and n-Channel Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2014, 6, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Zschieschang, U.; Amsharov, K.; Jansen, M.; Kern, K.; Klauk, H.; Weitz, R.T. Separating the impact of oxygen and water on the long-term stability of n-channel perylene diimide thin-film transistors. Org. Electron. 2015, 26, 340–344. [Google Scholar] [CrossRef]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 957. [Google Scholar] [CrossRef]
- Wilhelm, M.J.; Gh, M.S.; Wu, T.; Li, Y.; Chang, C.M.; Ma, J.Q.; Dai, H.L. Determination of bacterial surface charge density via saturation of adsorbed ions. Biophys. J. 2021, 120, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.V.; Gunsolus, I.L.; Qiu, T.A.; Hurley, K.R.; Nyberg, L.H.; Frew, H.; Johnson, K.P.; Vartanian, A.M.; Jacob, L.M.; Lohse, S.E.; et al. Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria. Chem. Sci. 2015, 6, 5186–5196. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Conde, A.; Garcı, F.J.; Liou, J.J.; Cerdeira, A.; Estrada, M.; Yue, Y. A review of recent MOSFET threshold voltage extraction methods. Microelectron. Reliab. 2002, 42, 583–596. [Google Scholar] [CrossRef]
- Park, J.S.; Maeng, W.J.; Kim, H.S.; Park, J.S. Review of recent developments in amorphous oxide semiconductor thin-film transistor devices. Thin Solid Films 2012, 520, 1679–1693. [Google Scholar] [CrossRef]
- Chou, W.Y.; Peng, S.K.; Wu, F.C.; Sheu, H.S.; Wang, Y.F.; Huang, P.K.; Cheng, H.L. Memory characteristics of organic field-effect memory transistors modulated by nano-p–n junctions. J. Mater. Chem. C 2020, 8, 7501–7508. [Google Scholar] [CrossRef]
- Fang, P.H.; Wu, F.C.; Sheu, H.S.; Lai, J.H.; Cheng, H.L.; Chou, W.Y. Analysis of ultrathin organic inverters by using in situ grazing incidence X-ray diffraction under high bending times and low voltage. Org. Electron. 2021, 88, 106002. [Google Scholar] [CrossRef]

| Organic Semiconductor | Types of Solutions | Vth (V) | Vth (V) (Average Value of Five Devices) | On/Off Current Ratio | S.S. (V/dec) |
|---|---|---|---|---|---|
| PTCDI-C13 | Non solution | 0.38 | 0.31 ± 0.39 | 1.37 × 104 | 0.15 |
| DI water | 0.48 | 0.50 ± 0.38 | 1.27 × 104 | 0.23 | |
| E. coli | −0.09 | −0.12 ± 0.21 | 7.85 × 106 | 0.06 | |
| S. aureus | 0.06 | 0.19 ± 0.73 | 6.45 × 105 | 0.11 | |
| E. faecalis | 0.28 | 0.07 ± 0.28 | 6.99 × 106 | 0.08 | |
| Pentacene | Non solution | −0.68 | −0.56 ± 0.08 | 1.58 × 104 | 0.37 |
| DI water | −0.44 | −0.30 ± 0.19 | 3.23 × 103 | 0.46 | |
| E. coli | −0.31 | −0.39 ± 0.17 | 1.68 × 103 | 0.69 | |
| S. aureus | −0.37 | −0.25 ± 0.71 | 8.51 × 103 | 0.42 | |
| E. faecalis | −0.43 | −0.28 ± 0.51 | 4.01 × 103 | 0.52 |
| Concentration of Bacteria (CFU/mL) | ΔVS (V) | ||
|---|---|---|---|
| E. coli | S. aureus | E. faecalis | |
| 108 | 0.56 | 0.51 | 0.45 |
| 104 | 0.27 | 0.29 | 0.30 |
| 102 | 0.19 | 0.06 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, P.-H.; Chang, H.-C.; Cheng, H.-L.; Huang, C.-C.; Wang, S.; Teng, C.-H.; Chia, Z.-C.; Chiang, H.-P.; Ruan, J.; Shih, W.-A.; et al. Bacteria Contaminants Detected by Organic Inverter-Based Biosensors. Polymers 2024, 16, 1462. https://doi.org/10.3390/polym16111462
Fang P-H, Chang H-C, Cheng H-L, Huang C-C, Wang S, Teng C-H, Chia Z-C, Chiang H-P, Ruan J, Shih W-A, et al. Bacteria Contaminants Detected by Organic Inverter-Based Biosensors. Polymers. 2024; 16(11):1462. https://doi.org/10.3390/polym16111462
Chicago/Turabian StyleFang, Po-Hsiang, Han-Chun Chang, Horng-Long Cheng, Chih-Chia Huang, Shuying Wang, Ching-Hao Teng, Zi-Chun Chia, Hai-Pang Chiang, Jrjeng Ruan, Wei-An Shih, and et al. 2024. "Bacteria Contaminants Detected by Organic Inverter-Based Biosensors" Polymers 16, no. 11: 1462. https://doi.org/10.3390/polym16111462
APA StyleFang, P.-H., Chang, H.-C., Cheng, H.-L., Huang, C.-C., Wang, S., Teng, C.-H., Chia, Z.-C., Chiang, H.-P., Ruan, J., Shih, W.-A., & Chou, W.-Y. (2024). Bacteria Contaminants Detected by Organic Inverter-Based Biosensors. Polymers, 16(11), 1462. https://doi.org/10.3390/polym16111462








