Controlled Amphiphilicity and Thermo-Responsiveness of Functional Copolymers Based on Oligo(Ethylene Glycol) Methyl Ether Methacrylates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of POEGMA Homopolymers
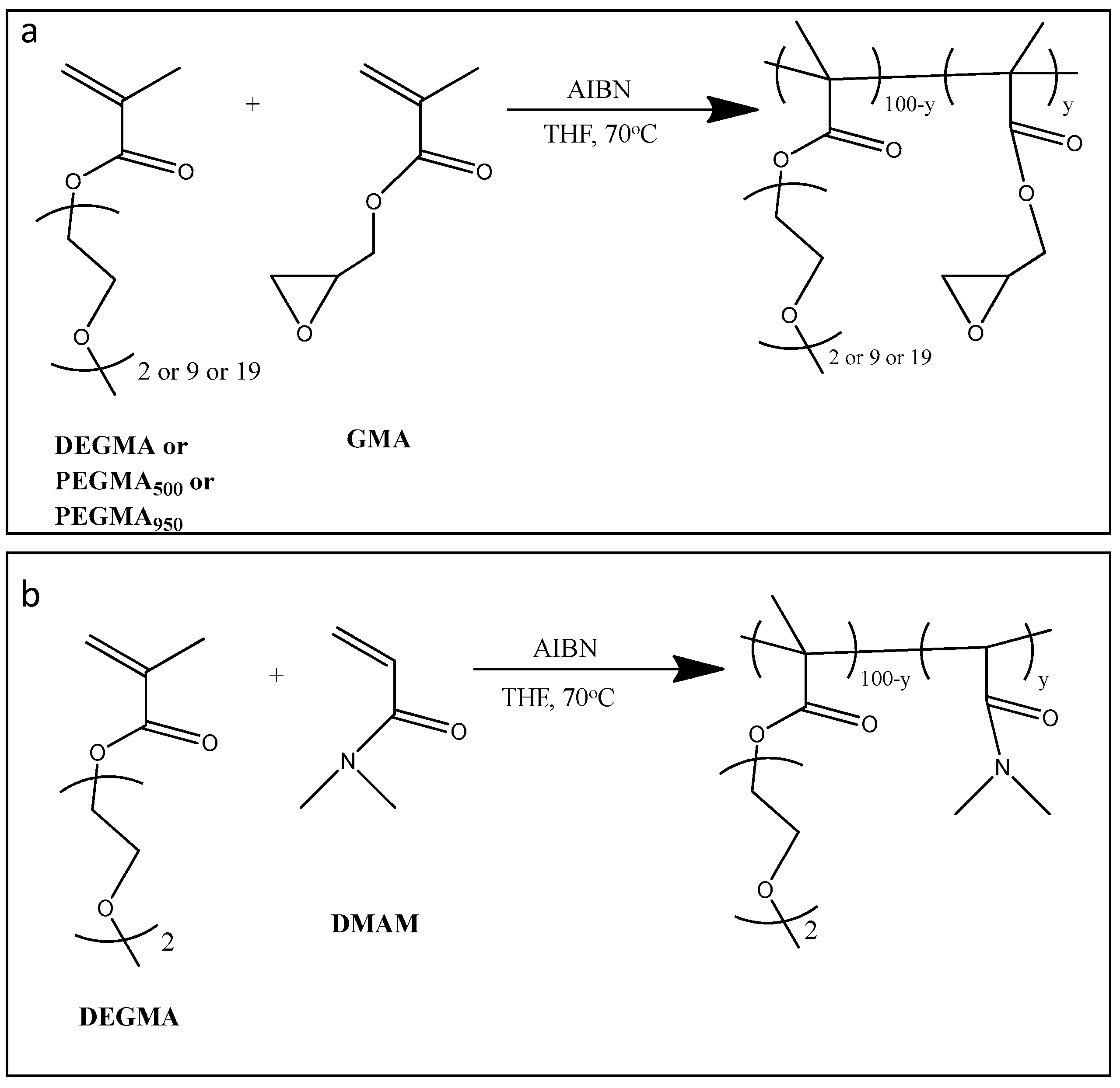
2.3. Synthesis of P(OEGMA-co-GMA) Copolymers
2.4. Synthesis of P(DEGMA-co-DMAM) Copolymers
2.5. Characterization of Copolymers
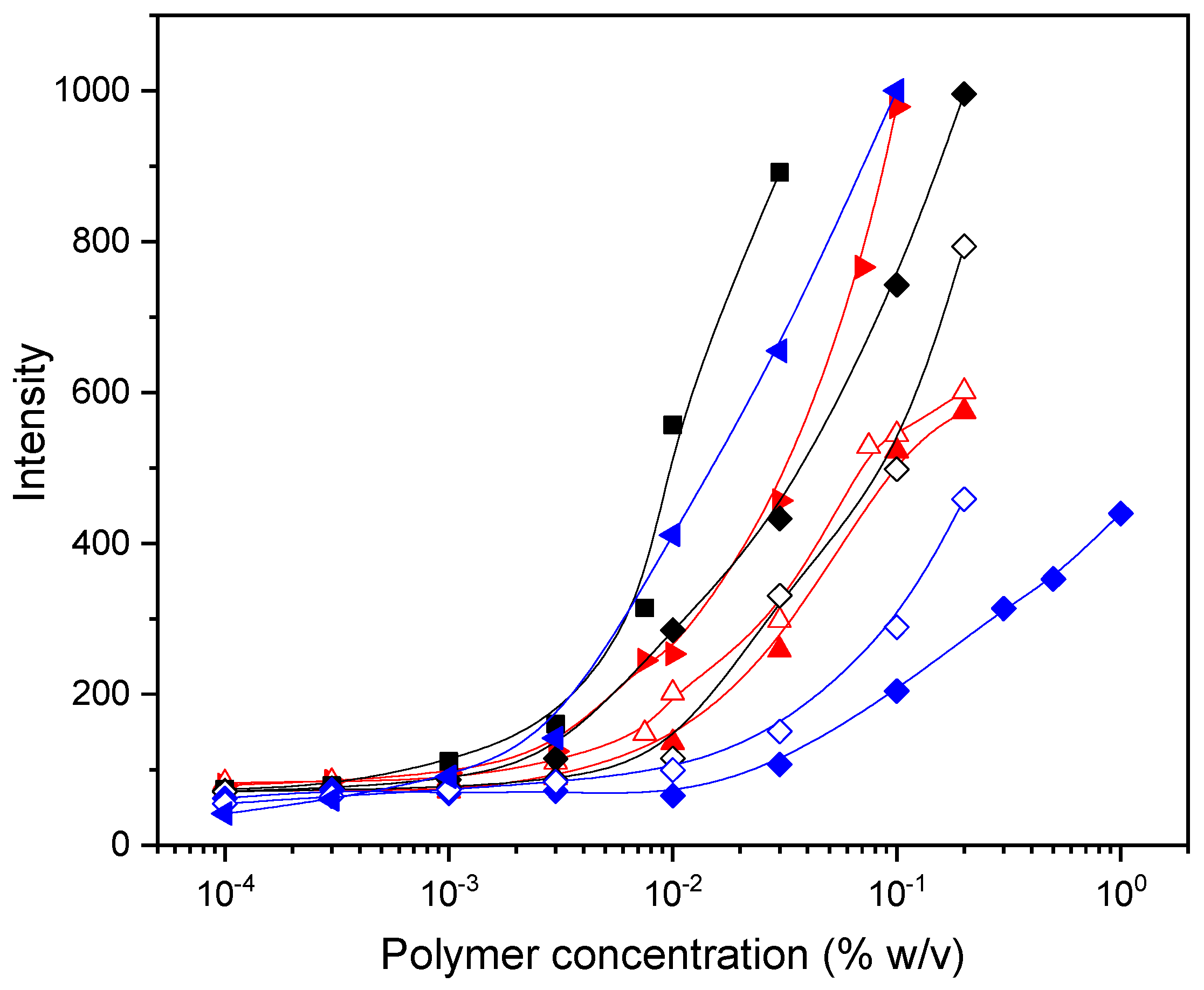
2.6. Determination of Critical Aggregation Concentration (CAC)
2.7. Thermo-Responsive Behavior
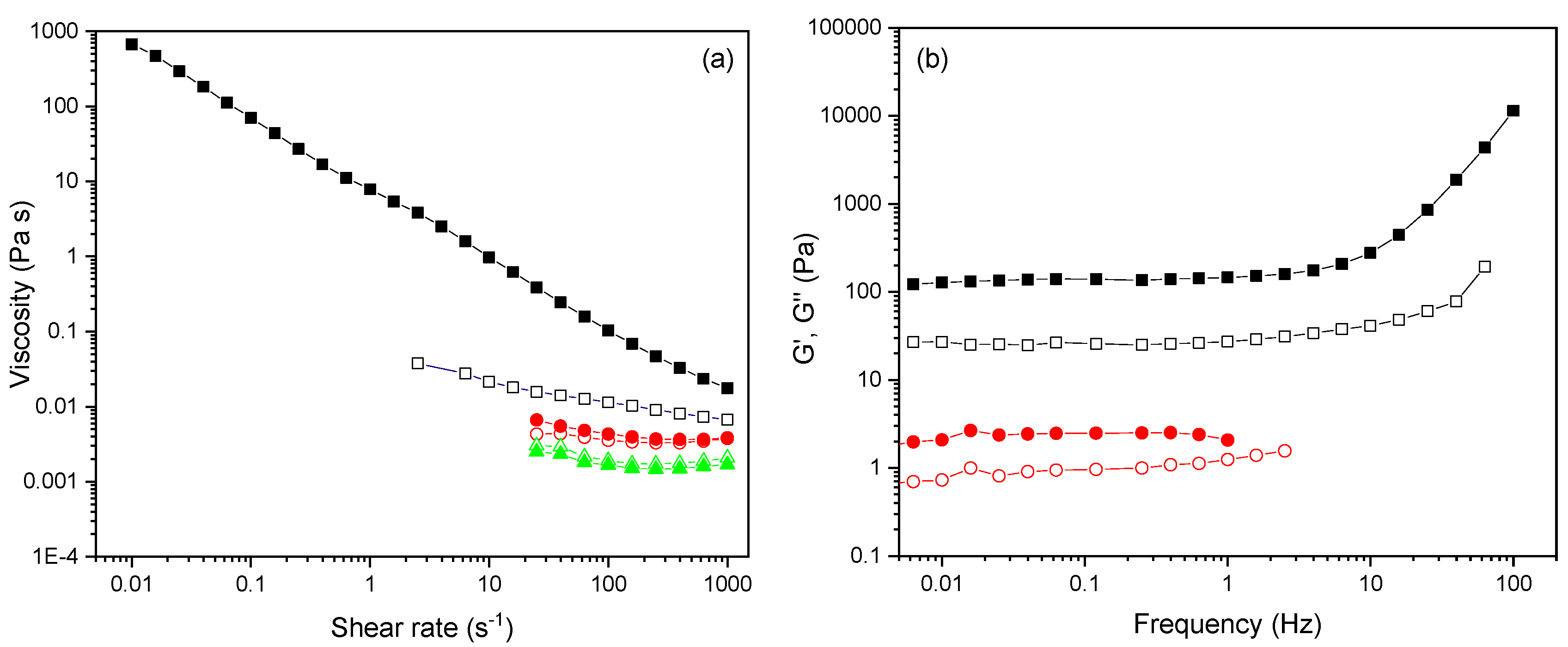
2.8. Rheology Study
3. Results and Discussion
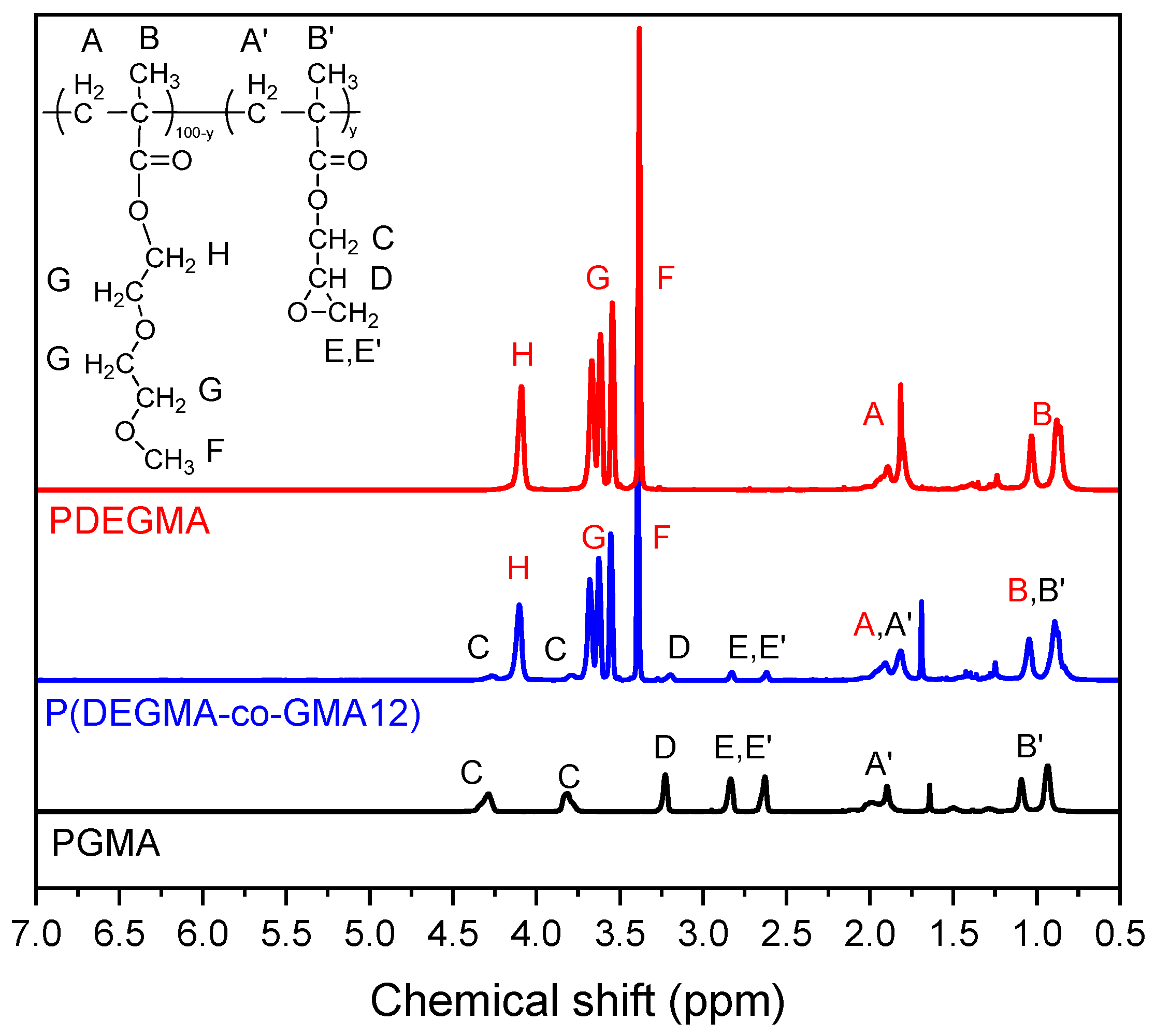
3.1. Synthesis and Characterization of Copolymers
3.2. Self-Assembly of Polymers in Aqueous Solution
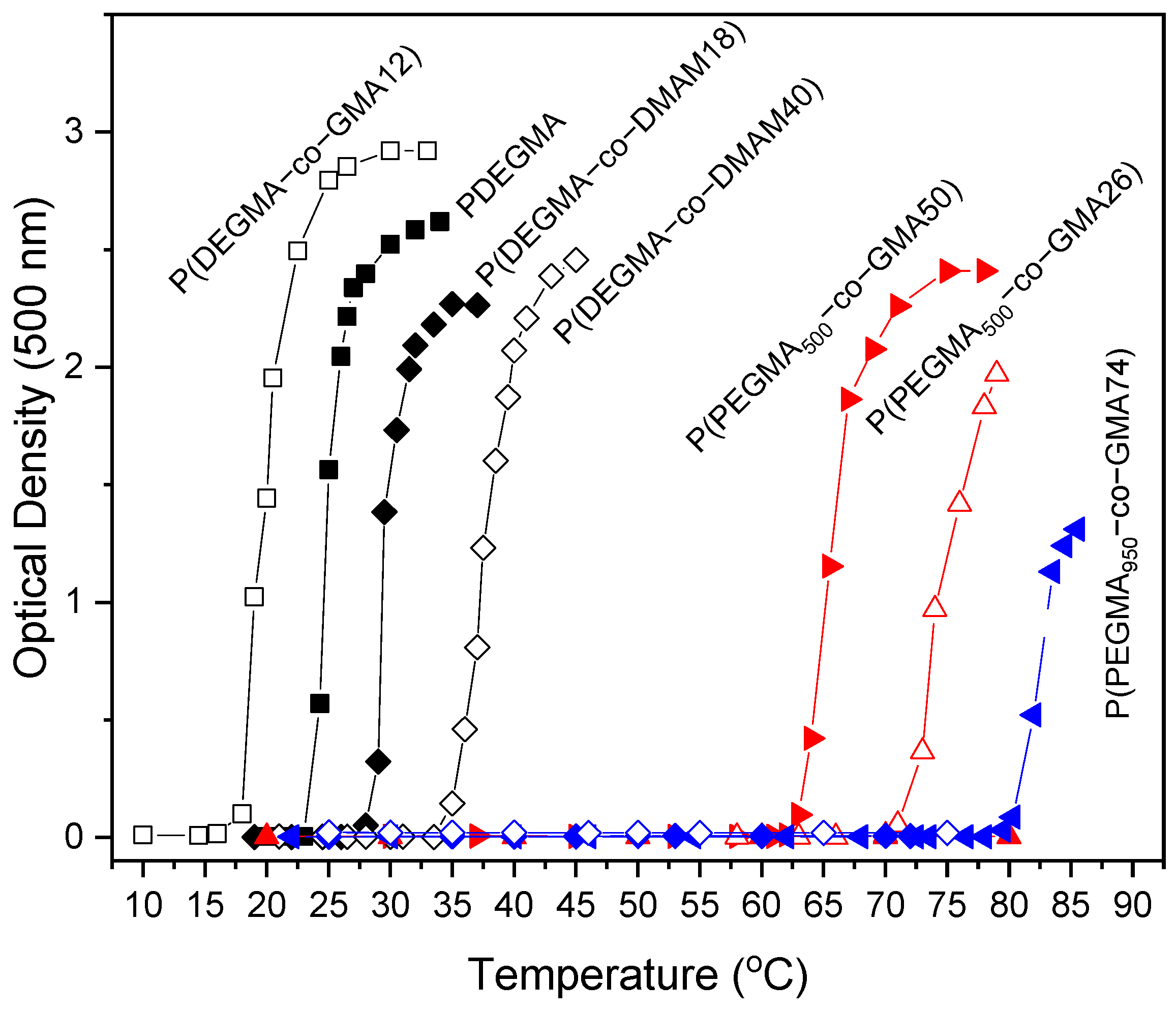
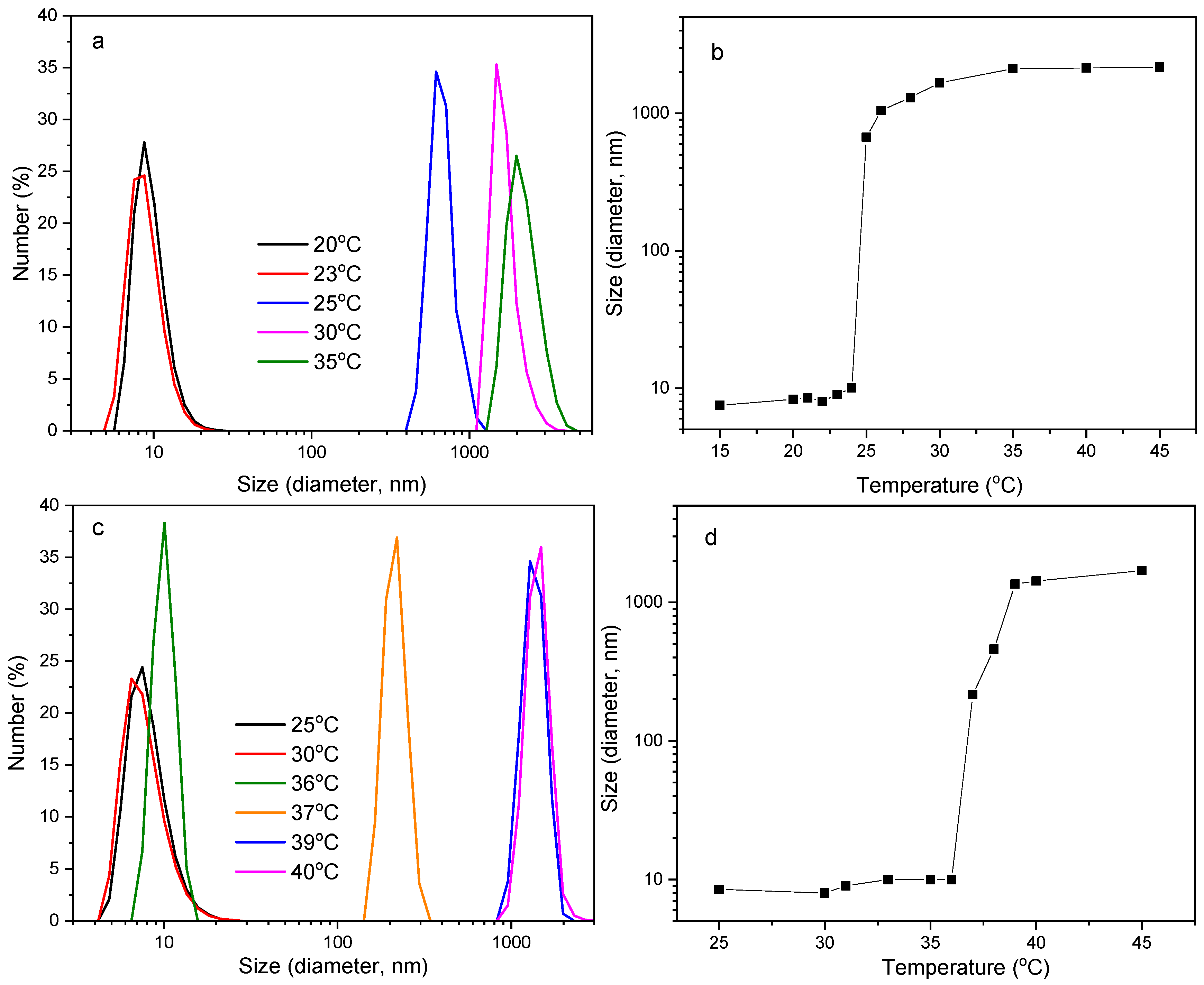
3.3. Thermo-Responsiveness in Aqueous Solution
3.4. Effect of Salts on the Thermo-Sensitive Behavior
3.5. Functional Character of P(PEGMA-co-GMA) Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Z.Q.; Wang, G.J. Multi-Stimuli Responsive Polymer Materials: Particles, Films, and Bulk Gels. Chem. Rec. 2016, 16, 1398–1435. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- García, M.C. Ionic-strength-responsive polymers for drug delivery applications Volume 2: Advanced Nanocarriers for Therapeutics. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 393–409. [Google Scholar]
- Kwak, G.; Lee, W.E.; Jeong, H.; Sakaguchi, T.; Fujiki, M. Swelling-induced emission enhancement in substituted acetylene polymer film with large fractional free volume: Fluorescence response to organic solvent stimuli. Macromolecules 2009, 42, 20–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST Polymers: Thermoresponsive Nanostructured Assemblies towards Bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Light-Responsive Polymer Membranes. Adv. Opt. Mater. 2019, 7, 1900252. [Google Scholar] [CrossRef]
- Weder, C. Polymers react to stress. Nature 2009, 459, 45–46. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef]
- El-Mohtadi, F.; d’Arcy, R.; Tirelli, N. Oxidation-Responsive Materials: Biological Rationale, State of the Art, Multiple Responsiveness, and Open Issues. Macromol. Rapid Commun. 2019, 40, 1800699. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. Part A Chem. 1969, 2, 1441–1455. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.L.; Jochems, M.J.H.C.; van Lankvelt, B.M.; Fijten, M.W.M.; Schubert, U.S. Tuning the LCST of poly(2-oxazoline)s by varying composition and molecular weight: Alternatives to poly(N-isopropylacrylamide)? Chem. Commun. 2008, 44, 5758–5760. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Liu, M.; Leroux, J.-C.; Gauthier, M.A. Conformation–function relationships for the comb-shaped polymer pOEGMA. Prog. Polym. Sci. 2015, 48, 111–121. [Google Scholar] [CrossRef]
- Badi, N. Non-linear PEG-based thermoresponsive polymer systems. Prog. Polym. Sci. 2017, 66, 54–79. [Google Scholar] [CrossRef]
- Konefał, R.; Spěváček, J.; Mužíková, G.; Laga, R. Thermoresponsive behavior of poly(DEGMA)-based copolymers. NMR and dynamic light scattering study of aqueous solutions. Eur. Polym. J. 2020, 124, 109488. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, W.; Zhou, S. Engineering oligo(ethylene glycol)-based thermosensitive microgels for drug delivery applications. Polymer 2010, 51, 3926–3933. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Peng, J.; Liu, Y.; Liu, F.; Ma, W.; Ma, L.; Yu, C.-Y.; Wei, H. Facile construction of stabilized, pH-sensitive micelles based on cyclic statistical copolymers of poly(oligo(ethylene glycol)methyl ether methacrylate-st-N,N-dimethylaminoethyl methacrylate) for in vitro anticancer drug delivery. Polym. Chem. 2020, 11, 6139–6148. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.-N.; Zhang, P.; Yang, W.-F.; Zhang, C.-Y.; Yin, Y.-L. The Preparation of Novel P(OEGMA-co-MEO2MA) Microgels-Based Thermosensitive Hydrogel and Its Application in Three-Dimensional Cell Scaffold. Gels 2022, 8, 313. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Andrieu, J.; Üzgün, S.; Rudolph, C.; Agarwal, S. Biocompatible, Thermoresponsive, and Biodegradable: Simple Preparation of “All-in-One” Biorelevant Polymers. Macromolecules 2007, 40, 8540–8543. [Google Scholar] [CrossRef]
- Stadler, V.; Kirmse, R.; Beyer, M.; Breitling, F.; Ludwig, T.; Bischoff, F.R. PEGMA/MMA Copolymer Graftings: Generation, Protein Resistance, and a Hydrophobic Domain. Langmuir 2008, 24, 8151–8157. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Georgiou, T.K. Thermoresponsive Gels based on ABC Triblock Copolymers: Effect of the Length of the PEG Side Group. Polym. Chem. 2016, 7, 2045–2056. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Lan, T.; Carroll, D.R.; Georgiou, T.K. Tricomponent thermoresponsive polymers based on an amine-containing monomer with tuneable hydrophobicity: Effect of composition. Eur. Polym. J. 2020, 130, 109655. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Chen, F.; Constantinou, A.P.; Georgiou, T.K. Thermoresponsive oligo(ethylene glycol) methyl ether methacrylate based copolymers: Composition and comonomer effect. Polym. Chem. 2022, 13, 2506–2518. [Google Scholar] [CrossRef]
- Akar, I.; Keogh, R.; Blackman, L.D.; Foster, J.C.; Mathers, R.T.; O’Reilly, R.K. Grafting Density Governs the Thermoresponsive Behavior of P(OEGMA-co-RMA) Statistical Copolymers. ACS Macro Lett. 2020, 9, 1149–1154. [Google Scholar] [CrossRef]
- Kavaliauskaite, M.; Steponaviciute, M.; Kievisaite, J.; Katelnikovas, A.; Klimkevicius, V. Synthesis and Study of Thermoresponsive Amphiphilic Copolymers via RAFT Polymerization. Polymers 2022, 14, 229. [Google Scholar] [CrossRef]
- De, P.; Sumerlin, B.S. Precision Control of Temperature Response by Copolymerization of Di(Ethylene Glycol) Acrylate and an Acrylamide Comonomer. Macromol. Chem. Phys. 2013, 214, 272–279. [Google Scholar] [CrossRef]
- Alejo, T.; Prieto, M.; García-Juan, H.; Andreu, V.; Mendoza, G.; Sebastián, V.; Arruebo, M. A facile method for the controlled polymerization of biocompatible and thermoresponsive oligo(ethylene glycol) methyl ether methacrylate copolymers. Polym. J. 2018, 50, 203–211. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Gónzalez-Ayón, M.A. Tunable Thermo-Responsive Copolymers from DEGMA and OEGMA Synthesized by RAFT Polymerization and the Effect of the Concentration and Saline Phosphate Buffer on Its Phase Transition. Polymers 2019, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Bozorg, M.; Hankiewicz, B.; Abetz, V. Solubility behaviour of random and gradient copolymers of di- and oligo(ethylene oxide) methacrylate in water: Effect of various additives. Soft Matter 2020, 16, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Alves, S.P.C.; Carvalhão, G.; Correia, N.T.; Viciosa, M.T.; Farinha, J.P.S. Phase diagrams of temperature-responsive copolymers p(MEO2MA-co-OEGMA) in water. Polymer 2021, 228, 123858. [Google Scholar] [CrossRef]
- Bokias, G.; Staikos, G.; Iliopoulos, I. Solution properties and phase behaviour of copolymers of acrylic acid with N-isopropylacrylamide: The importance of the intrachain hydrogen bonding. Polymer 2000, 41, 7399–7405. [Google Scholar] [CrossRef]
- Iatridi, Z.; Bokias, G. Temperature-Sensitive Water-Soluble Hybrid Organic/Inorganic Nanoparticles Formed through Complexation of Cu2+ Ions with Poly(sodium acrylate)-g-poly(N-isopropylacrylamide) Comb-Type Copolymers in Aqueous Solution. Langmuir 2009, 25, 7695–7703. [Google Scholar] [CrossRef] [PubMed]
- Iatridi, Z.; Vamvakidis, K.; Tsougos, I.; Vasiou, K.; Dendrinou-Samara, C.; Bokias, G. Multifunctional polymeric platform of magnetic ferrite colloidal superparticles for luminescent, imaging and hyperthermia applications. ACS Appl. Mater. Interfaces 2016, 8, 35059–35070. [Google Scholar] [CrossRef] [PubMed]
- Tzoumani, I.; Beobide, A.S.; Iatridi, Z.; Voyiatzis, G.A.; Bokias, G.; Kallitsis, J.K. Glycidyl Methacrylate-Based Copolymers as Healing Agents of Waterborne Polyurethanes. Int. J. Mol. Sci. 2022, 23, 8118. [Google Scholar] [CrossRef]
- Du, Z.; Sun, X.; Tai, X.; Wang, G.; Liu, X. Synthesis of hybrid silica nanoparticles grafted with thermoresponsive poly(ethylene glycol) methyl ether methacrylate via AGET-ATRP. RSC Adv. 2015, 5, 17194. [Google Scholar] [CrossRef]
- Huang, H.; Feng, Y.; Qu, J. Preparation and Performance of Silica-di-Block Polymer Hybrids for BSA-Resistance Coatings. Materials 2020, 13, 3478. [Google Scholar] [CrossRef]
- Li, L.; Raghupathi, K.; Song, C.; Prasad, P.; Thayumanavan, S. Self-assembly of random copolymers. Chem. Commun. 2014, 50, 13417. [Google Scholar] [CrossRef]
- Terashima, T. Controlled self-assembly of amphiphilic random copolymers into folded micelles and nanostructure materials. J. Oleo Sci. 2020, 69, 529. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Loh, X.J. Design of a micellized a-cyclodextrin based supramolecular hydrogel system. Soft Matter 2015, 11, 5425. [Google Scholar] [CrossRef] [PubMed]
- Maksym-Bębenek, P.; Neugebauer, D. Study on Self-Assembled Well-Defined PEG Graft Copolymers as Efficient Drug-Loaded Nanoparticles for Anti-Inflammatory Therapy. Macromol. Biosci. 2015, 15, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Mya, K.Y.; He, C. Self-Assembly of Brush-Like Poly[poly(ethylene glycol) methyl ether methacrylate] Synthesized via Aqueous Atom Transfer Radical Polymerization. Langmuir 2008, 24, 13279–13286. [Google Scholar] [CrossRef] [PubMed]
- Becer, C.R.; Hahn, S.; Fitjen, M.W.N.; Thijs, H.M.L.; Hoogenboom, R.; Schubert, U.S. Libraries of Methacrylic Acid and Oligo(ethylene glycol) Methacrylate Copolymers with LCST Behavior. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7138–7147. [Google Scholar] [CrossRef]
- Hishida, M.; Kanno, R.; Terashima, T. Hydration State on Poly(ethylene glycol)-Bearing Homopolymers and Random Copolymer Micelles: In Relation to the Thermoresponsive Property and Micellar Structure. Macromolecules 2023, 56, 7587–7596. [Google Scholar] [CrossRef]
- Weaver, L.G.; Stockmann, R.; Postma, A.; Thang, S.H. Multi-responsive (diethylene glycol)methyl ether methacrylate (DEGMA)-based copolymer systems. RSC Adv. 2016, 6, 90923–90933. [Google Scholar] [CrossRef]
- Vardaxi, A.; Pispas, S. Stimuli-Responsive Self-Assembly of Poly(2-(Dimethylamino)ethyl Methacrylate-co-(oligo ethylene glycol)methacrylate) Random Copolymers and Their Modified Derivatives. Polymers 2023, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Eggers, S.; Fischer, B.; Abetz, V. Aqueous Solutions of Poly[2-(N-morpholino)ethyl methacrylate]: Learning about Macromolecular Aggregation Processes from a Peculiar Three-Step Thermoresponsive Behavior. Macromol. Chem. Phys. 2015, 217, 735–747. [Google Scholar] [CrossRef]
- Druvari, D.; Koromilas, N.D.; Lainioti, G.C.; Bokias, G.; Vasilopoulos, G.; Vantarakis, A.; Baras, I.; Dourala, N.; Kallitsis, J.K. Polymeric Quaternary Ammonium-Containing Coatings with Potential Dual Contact-Based and Release-Based Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 35593–35605. [Google Scholar] [CrossRef] [PubMed]
- Druvari, D.; Kyriakopoulou, F.; Lainioti, G.C.; Vlamis-Gardikas, A.; Kallitsis, J.K. Humidity-Responsive Antimicrobial Membranes Based on Cross-Linked Copolymers Functionalized with Ionic Liquid Moieties. ACS Appl. Mater. Interfaces 2023, 15, 11193–11207. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, I.; Bokias, G. Investigation of Cross-Linked Chitosan-Based Membranes as Potential Adsorbents for the Removal of Cu2+ Ions from Aqueous Solutions. Materials 2023, 16, 1926. [Google Scholar] [CrossRef] [PubMed]
- Sanei, M.; Mahdavian, A.R.; Torabi, S.; Salehi-Mobarakeh, H. Efficient modification of nanosilica particles in preparation of anti-scratch transparent polyacrylic films through miniemulsion polymerization. Polym. Bull. 2017, 74, 1879–1898. [Google Scholar] [CrossRef]
- Xie, L.; Liang, X.; Huang, H.; Yang, L.; Zhang, F.; Li, X.; Luo, Z. Preparation and properties of long chain branched high-density polyethylene based on nano-SiO2 grafted glycidyl methacrylate. RSC Adv. 2019, 9, 1123. [Google Scholar] [CrossRef]




| Polymer | Feed Composition, %mol GMA or DMAM | Composition, %mol GMA or DMAM from 1H-NMR |
|---|---|---|
| PDEGMA | 0 | 0 |
| P(DEGMA-co-GMA12) | 13 | 12 |
| P(DEGMA-co-DMAM18) | 17 | 18 |
| P(DEGMA-co-DMAM40) | 31 | 40 |
| PPEGMA500 | 0 | 0 |
| P(PEGMA500-co-GMA26) | 28 | 26 |
| P(PEGMA500-co-GMA50) | 50 | 50 |
| PPEGMA950 | 0 | 0 |
| P(PEGMA950-co-GMA54) | 50 | 54 |
| P(PEGMA950-co-GMA74) | 70 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulou, A.; Kazamiakis, C.; Iatridi, Z.; Bokias, G. Controlled Amphiphilicity and Thermo-Responsiveness of Functional Copolymers Based on Oligo(Ethylene Glycol) Methyl Ether Methacrylates. Polymers 2024, 16, 1456. https://doi.org/10.3390/polym16111456
Christopoulou A, Kazamiakis C, Iatridi Z, Bokias G. Controlled Amphiphilicity and Thermo-Responsiveness of Functional Copolymers Based on Oligo(Ethylene Glycol) Methyl Ether Methacrylates. Polymers. 2024; 16(11):1456. https://doi.org/10.3390/polym16111456
Chicago/Turabian StyleChristopoulou, Aggeliki, Charalampos Kazamiakis, Zacharoula Iatridi, and Georgios Bokias. 2024. "Controlled Amphiphilicity and Thermo-Responsiveness of Functional Copolymers Based on Oligo(Ethylene Glycol) Methyl Ether Methacrylates" Polymers 16, no. 11: 1456. https://doi.org/10.3390/polym16111456
APA StyleChristopoulou, A., Kazamiakis, C., Iatridi, Z., & Bokias, G. (2024). Controlled Amphiphilicity and Thermo-Responsiveness of Functional Copolymers Based on Oligo(Ethylene Glycol) Methyl Ether Methacrylates. Polymers, 16(11), 1456. https://doi.org/10.3390/polym16111456








