Advancing the Frontiers of Neuroelectrodes: A Paradigm Shift towards Enhanced Biocompatibility and Electrochemical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
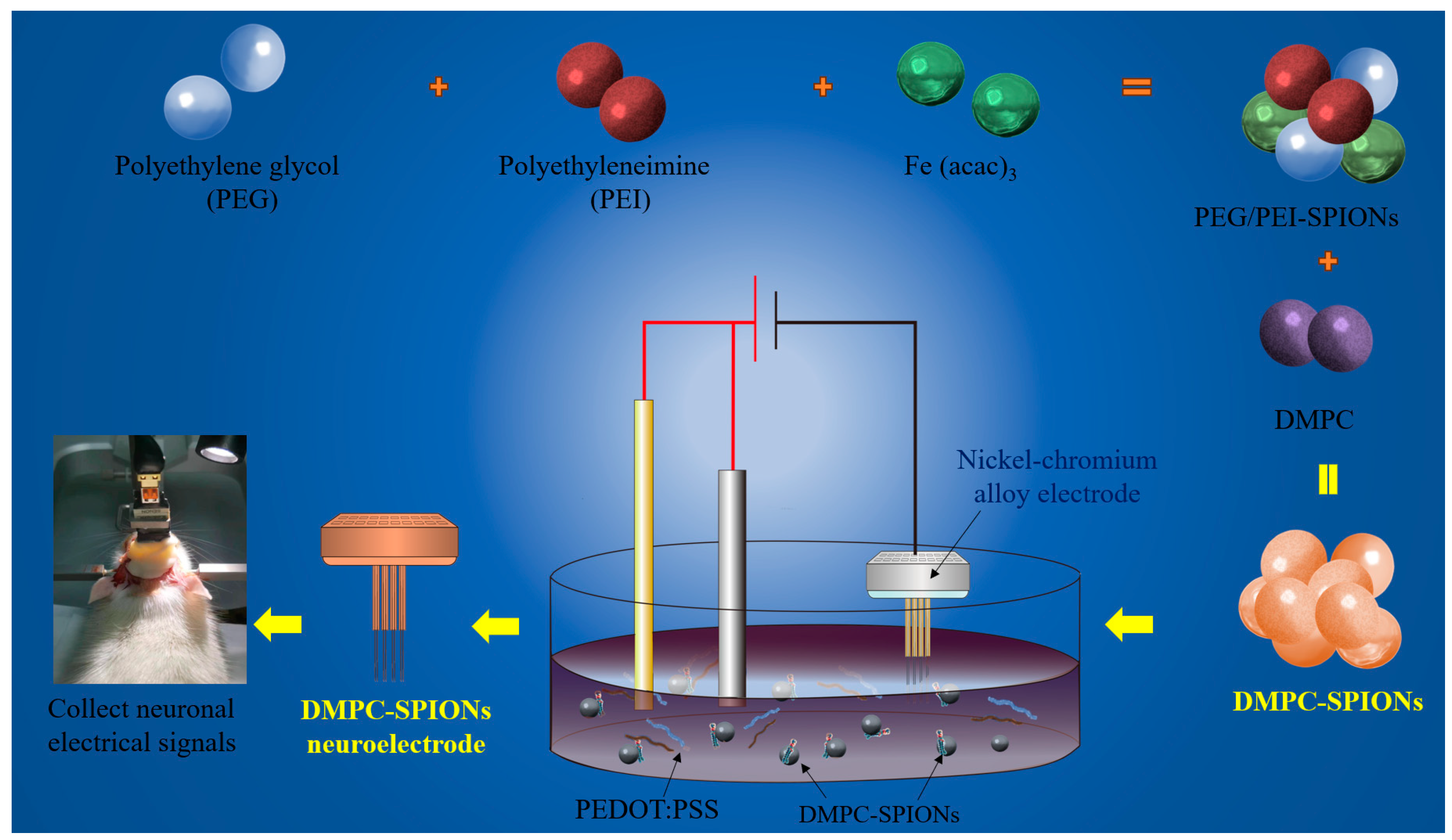
2.2. Preparation of DMPC-SPIONs for Neuroelectrodes
2.2.1. The Preparation of PEG/PEI-SPIONs
2.2.2. The Preparation of DMPC-SPIONs
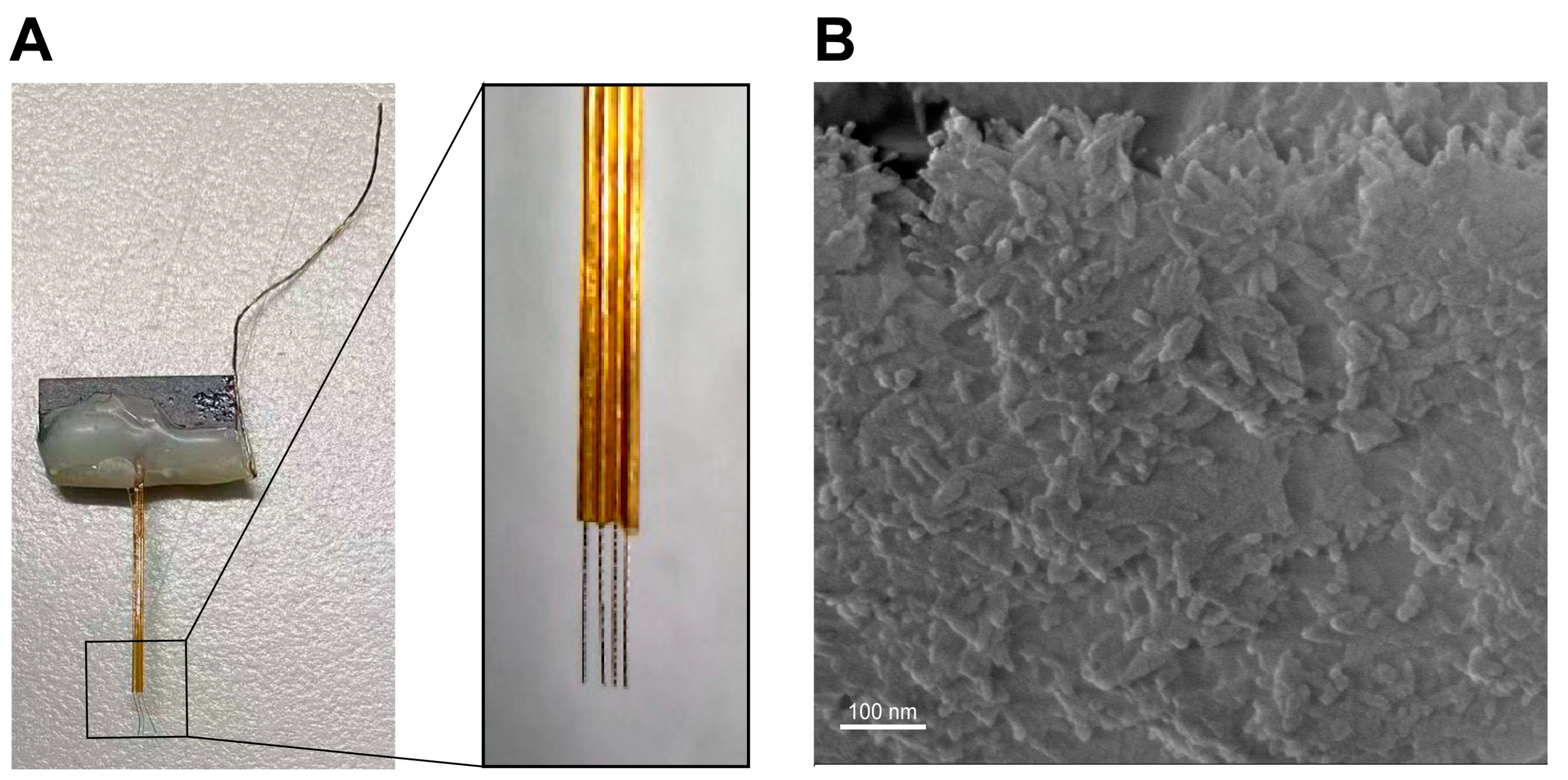
2.2.3. The Preparation of DMPC-SPION Neuroelectrodes
2.3. Performance Testing
2.3.1. Material Performance Testing
2.3.2. Hemolysis Assay
2.3.3. Cellular Cytotoxicity
2.3.4. Experiment of Cellular Uptake
2.3.5. Cell TEM Preparation
2.3.6. Intracellular Fe Content Test
2.3.7. Interfacial Biocompatibility of DMPC-SPION Neuroelectrodes
2.3.8. CV Testing and Electrode EIS Testing of DMPC-SPION Neuroelectrodes
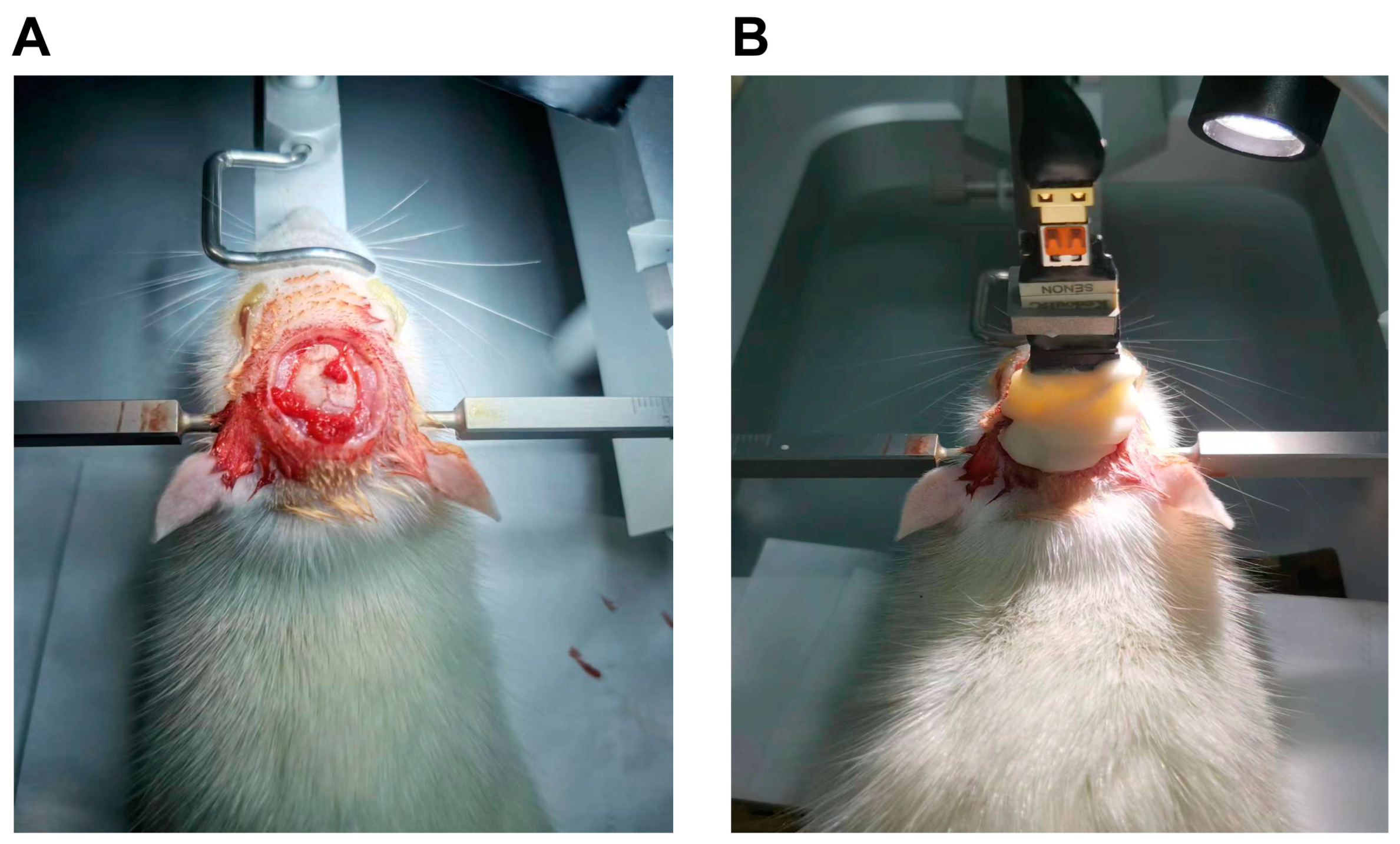
2.3.9. Acquisition of Electrophysiological Signals from DMPC-SPION Neuroelectrodes
2.3.10. Statistical Analysis
3. Results
3.1. Subsection
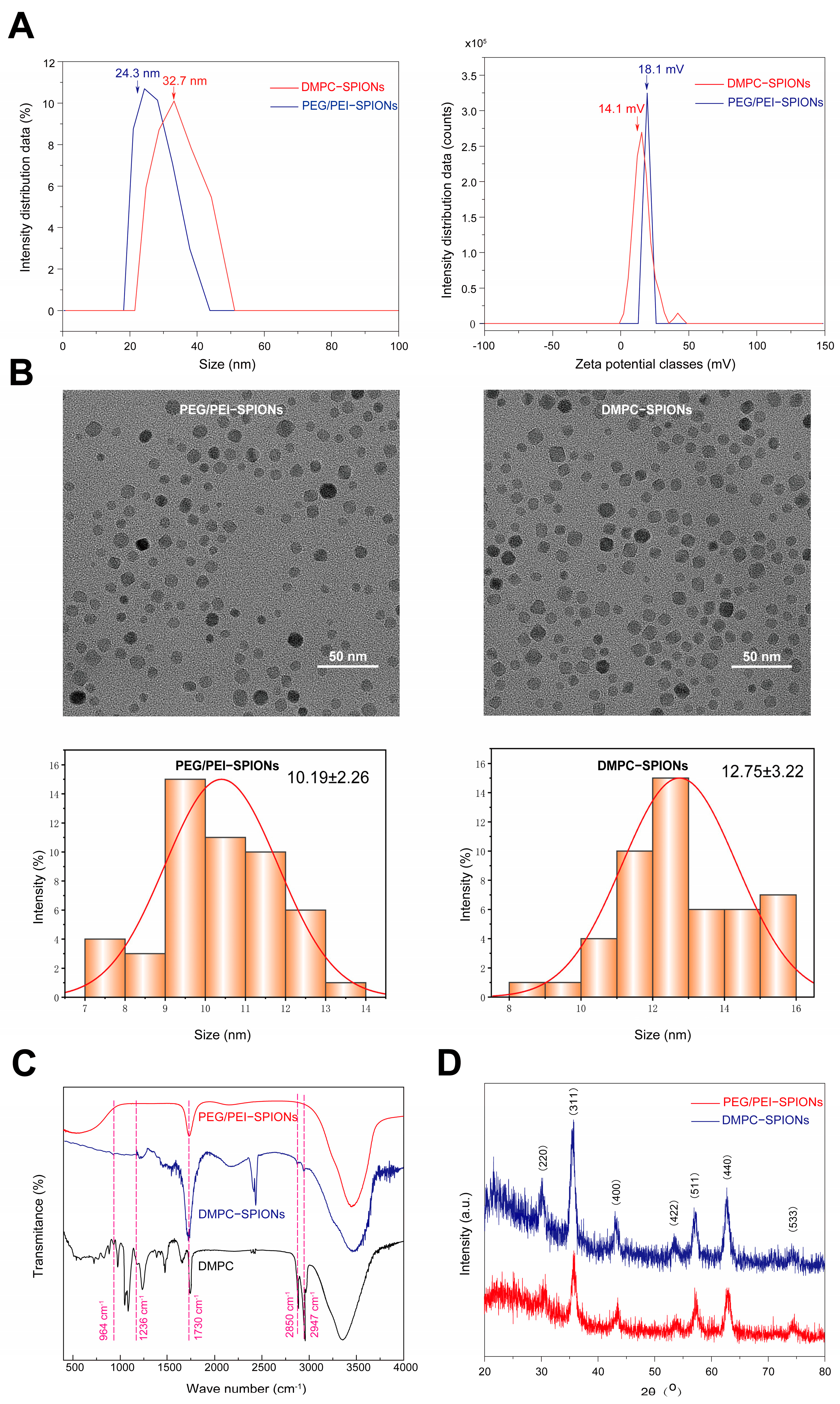
3.1.1. Hydrated Particle Size and Zeta Potential
3.1.2. TEM Test
3.1.3. FTIR Test
3.1.4. XRD Test
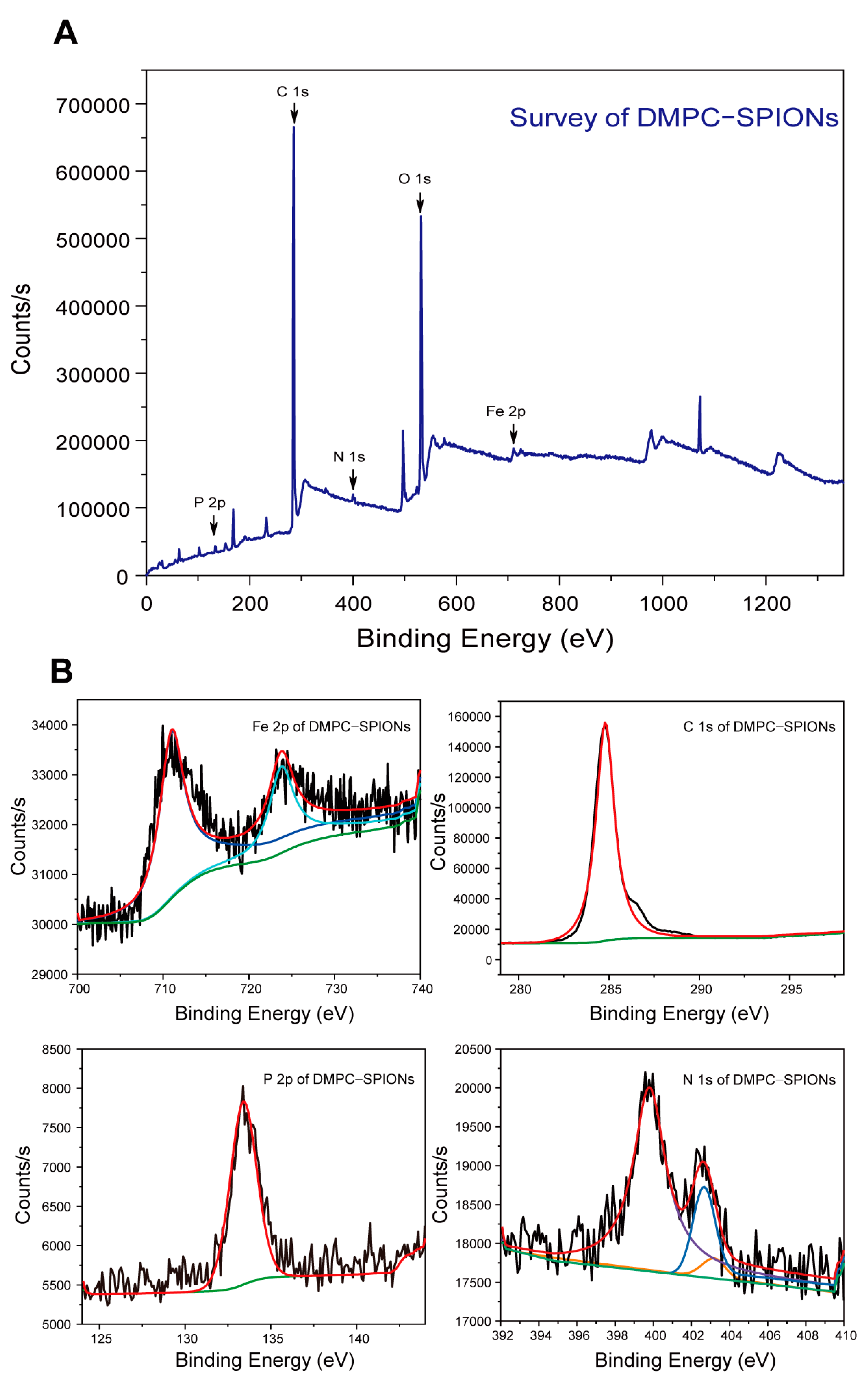
3.1.5. XPS Test
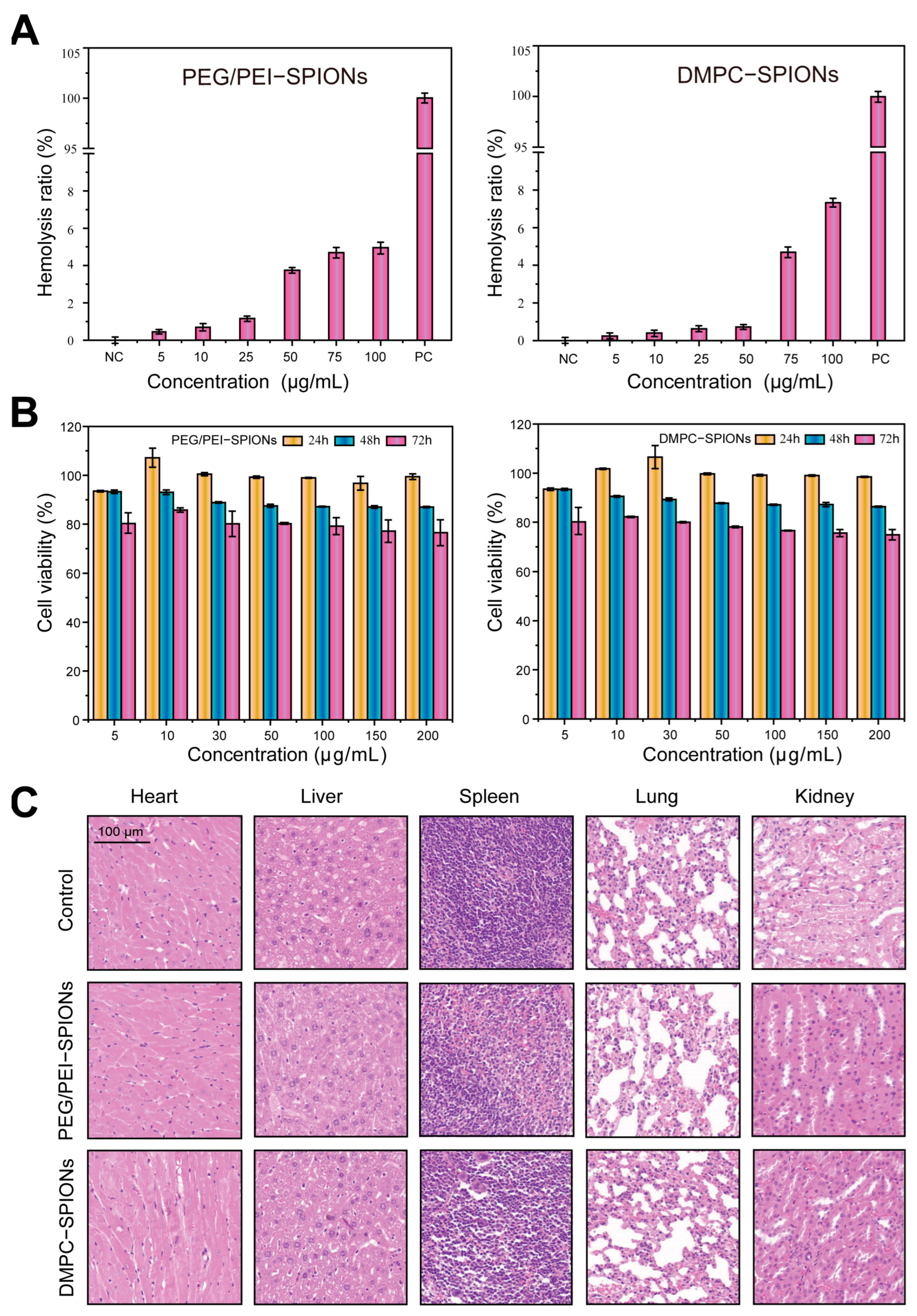
3.2. Hemolysis Assessment
3.3. Cell Toxicity Experiment
3.4. Evaluation of Biocompatibility
3.5. Cellular Uptake
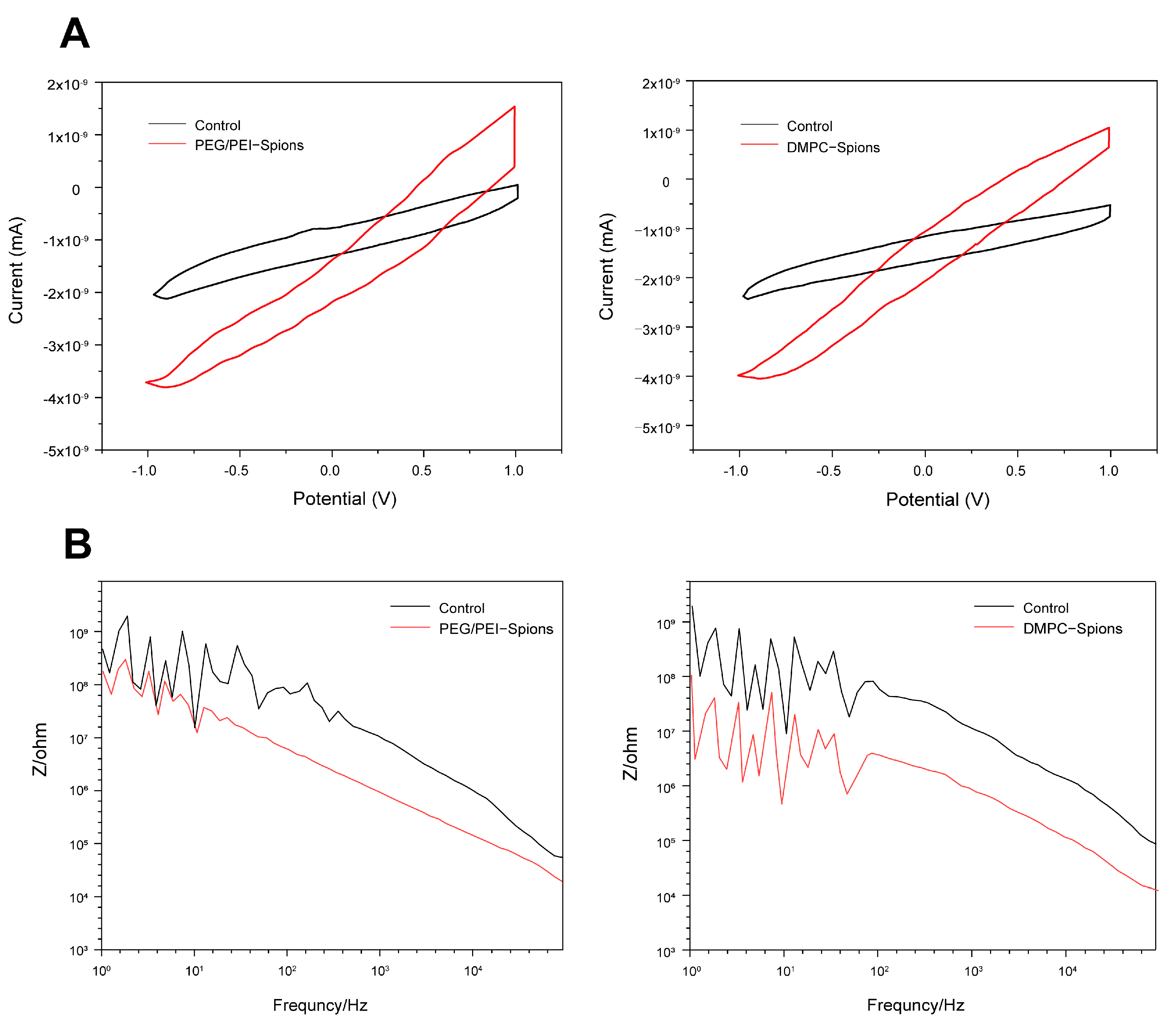
3.6. CV Testing and Electrode EIS Testing of DMPC-SPION Neuroelectrodes
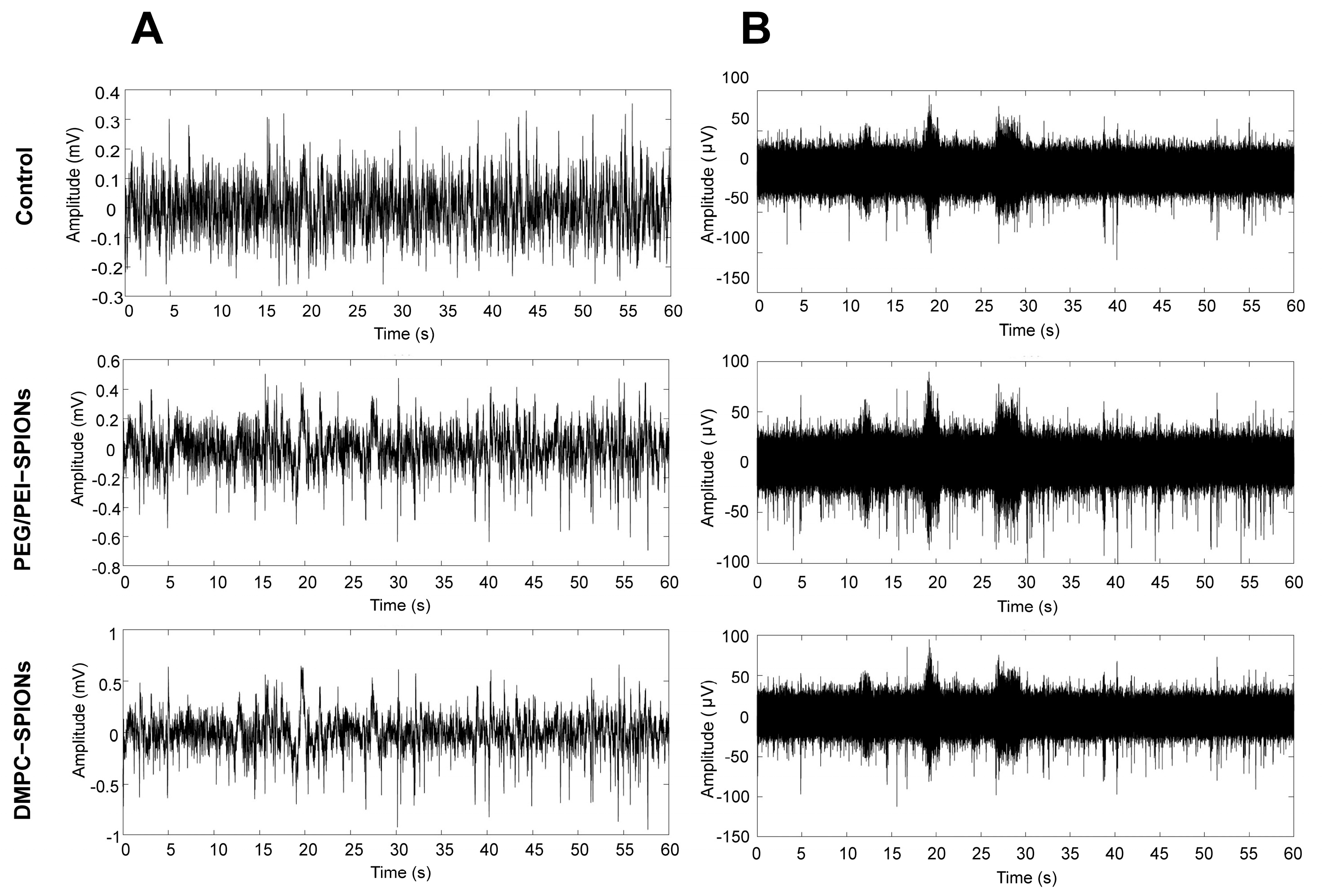
3.7. Acquisition of Electrophysiological Signals from DMPC-SPION Neuroelectrodes
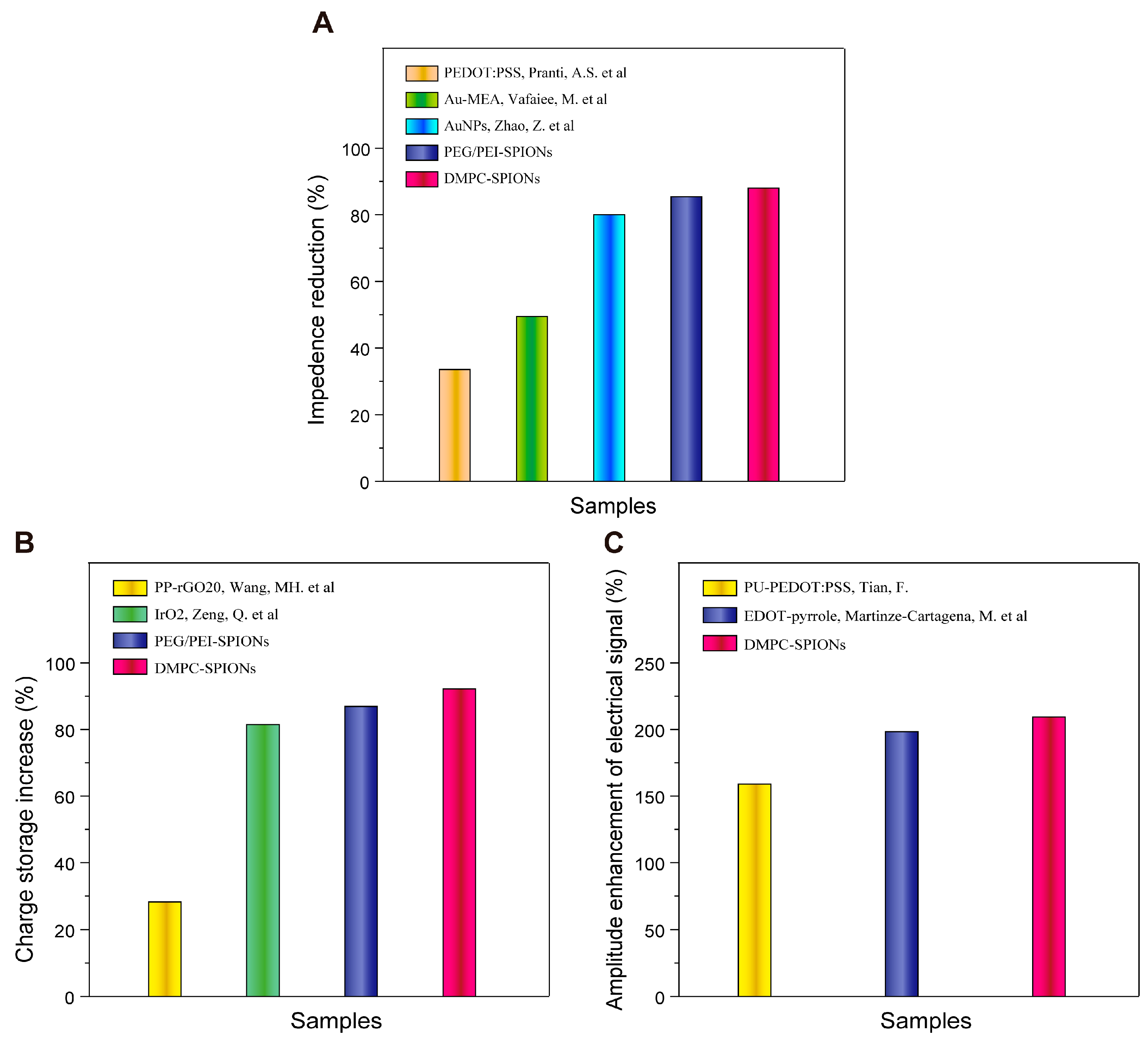
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Wang, Y.; Chen, X.; Gao, S. Interface, interaction, and intelligence in generalized brain–computer interfaces. Trends Cogn. Sci. 2021, 25, 671–684. [Google Scholar] [CrossRef]
- Allison, B.; Graimann, B.; Pfurtscheller, G. Brain-Computer Interfaces: Revolutionizing Human-Computer Interaction; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Shi, J.; Fang, Y. Flexible and implantable microelectrodes for chronically stable neural interfaces. Adv. Mater. 2019, 31, 1804895. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Li, J.; Won, S.M.; Bai, W.; Rogers, J.A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 2020, 19, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. and Its Application in Flexible Bioelectronic Devic Conductive Polymers for Nerve Electrode Modification. Master’s Thesis, Huazhong University of Science & Technology, Wuhan, China, 2020. [Google Scholar]
- Wang, M.-H.; Ji, B.-W.; Gu, X.-W.; Tian, H.-C.; Kang, X.-Y.; Yang, B.; Wang, X.-L.; Chen, X.; Li, C.-Y.; Liu, J.-Q. Direct electrodeposition of Graphene enhanced conductive polymer on microelectrode for biosensing application. Biosens. Bioelectron. 2018, 99, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Y.; Ni, G.; Li, S.; Liu, W.; Gao, Z.; Zhang, X.; Zhang, Q.; Wang, C.; Zhou, J. On-demand electrically controlled melatonin release from PEDOT/SNP composite improves quality of chronic neural recording. Front. Bioeng. Biotechnol. 2023, 11, 1284927. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, P.; Gurke, J.; Malliaras, G.G. Stability of Thin Film Neuromodulation Electrodes under Accelerated Aging Conditions. Adv. Funct. Mater. 2023, 33, 2208881. [Google Scholar] [CrossRef]
- Lim, J.; Lee, S.; Kim, J.; Hong, J.; Lim, S.; Kim, K.; Kim, J.; Yang, S.; Yang, S.; Ahn, J.H. Hybrid graphene electrode for the diagnosis and treatment of epilepsy in free-moving animal models. NPG Asia Mater. 2023, 15, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X. Carbon-based Nanomaterials for Neural Electrode Technology. Acta Phys. Chim. Sin. 2020, 36, 95–107. [Google Scholar] [CrossRef]
- Cui, X.T.; Zhou, D.D. Poly (3, 4-ethylenedioxythiophene) for chronic neural stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 502–508. [Google Scholar] [CrossRef]
- Su, P.; Zhou, Y.; Wu, J.; Shao, J.; Shen, L.; Bao, N. Flexible Silicon/Titanium Dioxide/Reduced Graphene Oxide Self-Standing Electrode with High Performance and High Stability for Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2024, 63, 1422–1431. [Google Scholar] [CrossRef]
- Heim, M.; Yvert, B.; Kuhn, A. Nanostructuration strategies to enhance microelectrode array (MEA) performance for neuronal recording and stimulation. J. Physiol. 2012, 106, 137–145. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Zhao, X.; Zhang, J.; Cheng, X.; Zhu, N. Recent advances of superparamagnetic iron oxide nanoparticles and its applications in neuroscience under external magnetic field. Appl. Nanosci. 2023, 13, 5489–5500. [Google Scholar] [CrossRef]
- Yuan, B.; Xing, L.-L.; Zhang, Y.-D.; Lu, Y.; Mai, Z.-H.; Li, M. Self-assembly of highly oriented lamellar nanoparticle-phospholipid nanocomposites on solid surfaces. J. Am. Chem. Soc. 2007, 129, 11332–11333. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chung, M.-C.; Wang, C.-C.; Huang, C.-H.; Liang, H.-J.; Jan, T.-R. Iron oxide nanoparticles suppress the production of IL-1beta via the secretory lysosomal pathway in murine microglial cells. Part. Fibre Toxicol. 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Cai, Q.; Huang, H.; Zhou, P. Liver targeting and anti-HBV activity of reconstituted HDL–acyclovir palmitate complex. Eur. J. Pharm. Biopharm. 2008, 68, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Veiseh, O.; Bhattarai, N.; Fang, C.; Gunn, J.W.; Lee, D.; Ellenbogen, R.G.; Olson, J.M.; Zhang, M. PEI–PEG–chitosan-copolymer-coated iron oxide nanoparticles for safe gene delivery: Synthesis, complexation, and transfection. Adv. Funct. Mater. 2009, 19, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Park, I.-K.; Cho, C.-S. Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnol. Adv. 2013, 31, 1224–1236. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Lu, Y.-M.; Wang, H.; Liu, J.; Liao, M.-H.; Hong, L.-J.; Tao, R.-R.; Ahmed, M.M.; Liu, P.; Liu, S.-S. The effect of lipid nanoparticle PEGylation on neuroinflammatory response in mouse brain. Biomaterials 2013, 34, 7960–7970. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, B.; Wang, J.; Tu, Z. Synthesis and properties of magnetite nanoparticles coated with poly (ethylene glycol) and poly (ethylene imine). J. Nanosci. Nanotechnol. 2013, 13, 6793–6797. [Google Scholar] [CrossRef]
- Seiti, M.; Giuri, A.; Corcione, C.E.; Ferraris, E. Advancements in tailoring PEDOT: PSS properties for bioelectronic applications: A comprehensive review. Biomater. Adv. 2023, 154, 213655. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Zhang, H.; Dong, M.; Wang, J.; Jia, P.; Bu, T.; Wang, X.; Wang, L. Near-Infrared Light-Regulated Drug-Food Homologous Bioactive Molecules and Photothermal Collaborative Precise Antibacterial Therapy Nanoplatform with Controlled Release Property. Adv. Healthc. Mater. 2021, 10, 2100546. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, B.; Su, L.; Huang, Y.; Fan, Z.; Zhao, Y. Cellular Distribution of 1,2-dimyristoyl-sn-glycero-3-phosphocholine Modified Iron Oxide Nanoparticles. Mater. Rep. 2019, 33, 1047–1051. [Google Scholar]
- Orendorff, C.J.; Alam, T.M.; Sasaki, D.Y.; Bunker, B.C.; Voigt, J.A. Phospholipid—Gold nanorod composites. ACS Nano 2009, 3, 971–983. [Google Scholar] [CrossRef]
- Jiang, C.; Gamarnik, A.; Tripp, C.P. Identification of lipid aggregate structures on TiO2 surface using headgroup IR bands. J. Phys. Chem. B 2005, 109, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Thakurta, S.G.; Bellare, J.; Nigam, A.K.; Bahadur, D. Preparation and characterization of phospholipid stabilized uniform sized magnetite nanoparticles. J. Magn. Magn. Mater. 2005, 293, 62–68. [Google Scholar] [CrossRef]
- Zhang, B.; Tu, Z.; Zhao, F.; Wang, J. Superparamagnetic iron oxide nanoparticles prepared by using an improved polyol method. Appl. Surf. Sci. 2013, 266, 375–379. [Google Scholar] [CrossRef]
- Fischlechner, M.; Zaulig, M.; Meyer, S.; Estrela-Lopis, I.; Cuéllar, L.; Irigoyen, J.; Pescador, P.; Brumen, M.; Messner, P.; Moya, S. Lipid layers on polyelectrolyte multilayer supports. Soft Matter 2008, 4, 2245–2258. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Ershov, D.; Gucht, J.v.d.; Alink, G.M.; Rietjens, I.M.M.; Zuilhof, H.; Marcelis, A.T. Surface charge-specific cytotoxicity and cellular uptake of tri-block copolymer nanoparticles. Nanotoxicology 2013, 7, 71–84. [Google Scholar] [CrossRef]
- Bartczak, D.; Muskens, O.L.; Nitti, S.; Sanchez-Elsner, T.; Millar, T.M.; Kanaras, A.G. Interactions of human endothelial cells with gold nanoparticles of different morphologies. Small 2012, 8, 122–130. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Gallo, J.; Long, N.J.; Aboagye, E.O. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 2013, 42, 7816–7833. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R. Coupling the magnetic and heat dissipative properties of Fe3O4 particles to enable applications in catalysis, drug delivery, tissue destruction and remote biological interfacing. RSC Adv. 2016, 6, 4262–4270. [Google Scholar] [CrossRef]
- Gupta, J.; Juneja, S.; Bhattacharya, J. UV Lithography-assisted fabrication of low-cost copper electrodes modified with gold nanostructures for improved analyte detection. ACS Omega 2020, 5, 3172–3180. [Google Scholar] [CrossRef]
- Changshi, H.; Yiding, W.; Hongpeng, W.; Shaojing, D.; Bo, L.; Luting, Y. Trace Cu2+ detection based on GH-PEDOT: PSS-Pt NP-modified glassy carbon electrode. J. Mater. Sci. Mater. Electron. 2024, 35, 56. [Google Scholar] [CrossRef]
- Badi, N.; Khasim, S.; Alatawi, A.S.; Pasha, A.; Al-Ghamdi, S.A.; Ignatiev, A. Fabrication and Testing of PEDOT: PSS Wrapped WO2/Au Ternary Nanocomposite Electrodes for High Performance Flexible Supercapacitor Applications. J. Electrochem. Soc. 2021, 168, 040526. [Google Scholar] [CrossRef]
- Pranti, A.S.; Schander, A.; Bödecker, A.; Lang, W. PEDOT: PSS Coating on Gold Microelectrodes with Excellent Stability and High Charge Injection Capacity for Chronic Neural Interfaces. Sens. Actuators B Chem. 2018, B275, 382–393. [Google Scholar] [CrossRef]
- Vafaiee, M.; Mohammadpour, R.; Vossoughi, M.; Asadian, E.; Janahmadi, M.; Sasanpour, P. Carbon nanotube modified microelectrode array for neural interface. Front. Bioeng. Biotechnol. 2021, 8, 582713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Gong, R.; Zheng, L.; Wang, J. In vivo neural recording and electrochemical performance of microelectrode arrays modified by rough-surfaced AuPt alloy nanoparticles with nanoporosity. Sensors 2016, 16, 1851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Xia, K.; Sun, B.; Yin, Y.; Wu, T.; Humayun, M.S. Electrodeposited Iridium Oxide on Platinum Nanocones for Improving Neural Stimulation Microelectrodes. Electrochim. Acta 2017, 237, 152–159. [Google Scholar] [CrossRef]
- Tian, F. Construction of Implantable PEDOT:PSS Conducting Polymer Neural Electrode Interface for Neural Signal Recording. Master’s Thesis, Jiangxi Science and Technology Normal University, Nanchang, China, 2023. [Google Scholar]
- Martinez-Cartagena, M.; Bernal-Martínez, J.; Roman-Aguirre, M.; Aguilar-Elguezabal, A. Electroconductive copolymer EDOT-pyrrole for intracellular recording experiments in Helix aspersa neurons. Mater. Today Chem. 2023, 30, 101454. [Google Scholar] [CrossRef]

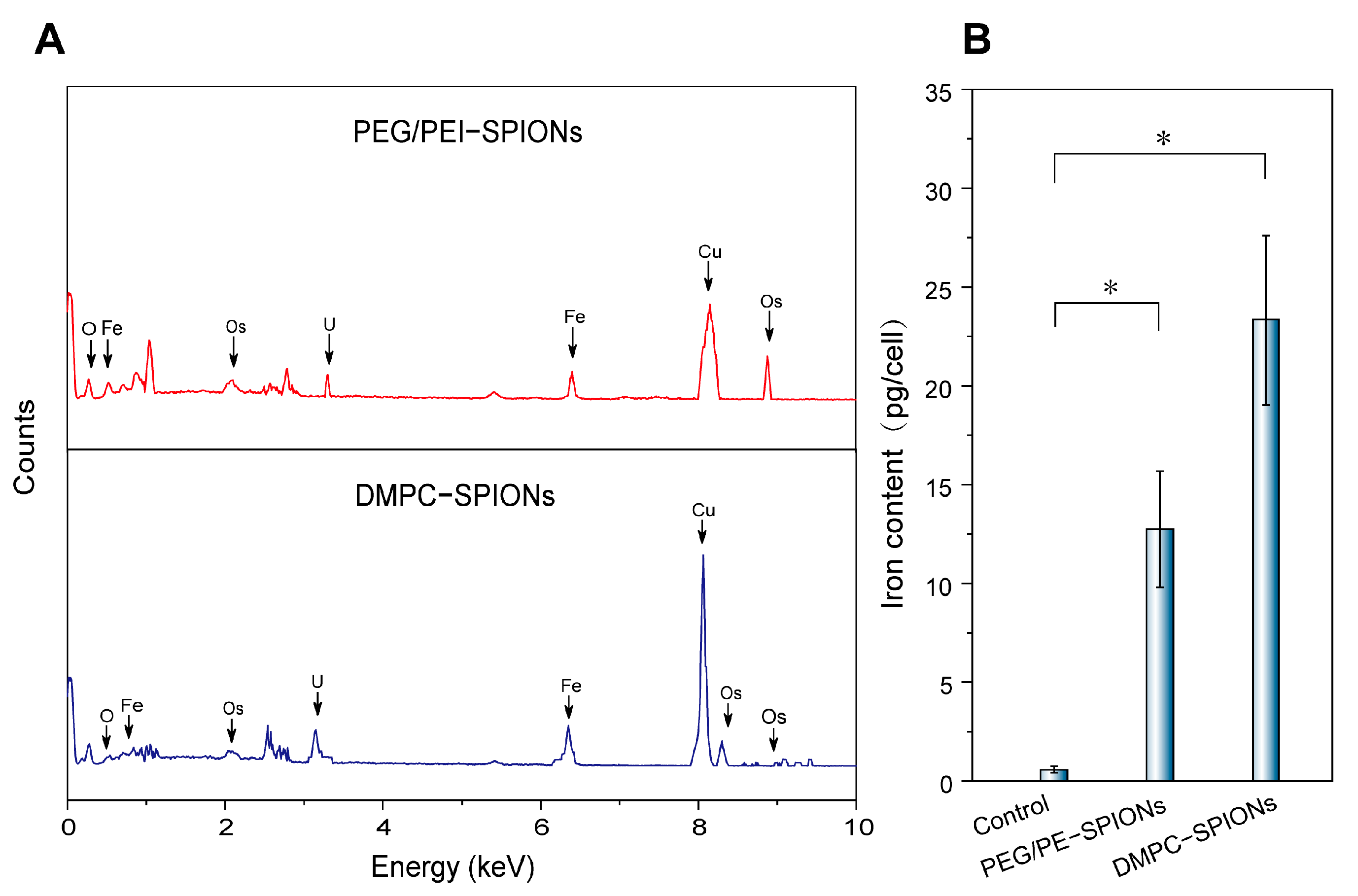

| Group | Iron Content (pg/Cell) |
|---|---|
| Control | 0.58 ± 0.16 |
| PEG/PEI-SPIONs | 12.74 ± 3.14 |
| DMPC-SPIONs | 23.36 ± 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, Y.; Zhang, B.; Dong, J.; Wang, L. Advancing the Frontiers of Neuroelectrodes: A Paradigm Shift towards Enhanced Biocompatibility and Electrochemical Performance. Polymers 2024, 16, 1457. https://doi.org/10.3390/polym16111457
Wang Q, Liu Y, Zhang B, Dong J, Wang L. Advancing the Frontiers of Neuroelectrodes: A Paradigm Shift towards Enhanced Biocompatibility and Electrochemical Performance. Polymers. 2024; 16(11):1457. https://doi.org/10.3390/polym16111457
Chicago/Turabian StyleWang, Qin, Yiyang Liu, Baolin Zhang, Jianghui Dong, and Liping Wang. 2024. "Advancing the Frontiers of Neuroelectrodes: A Paradigm Shift towards Enhanced Biocompatibility and Electrochemical Performance" Polymers 16, no. 11: 1457. https://doi.org/10.3390/polym16111457
APA StyleWang, Q., Liu, Y., Zhang, B., Dong, J., & Wang, L. (2024). Advancing the Frontiers of Neuroelectrodes: A Paradigm Shift towards Enhanced Biocompatibility and Electrochemical Performance. Polymers, 16(11), 1457. https://doi.org/10.3390/polym16111457







