Preparation and Performance of a Novel ZnO/TM/PET Composite Negative Ion Functional Fiber
Abstract
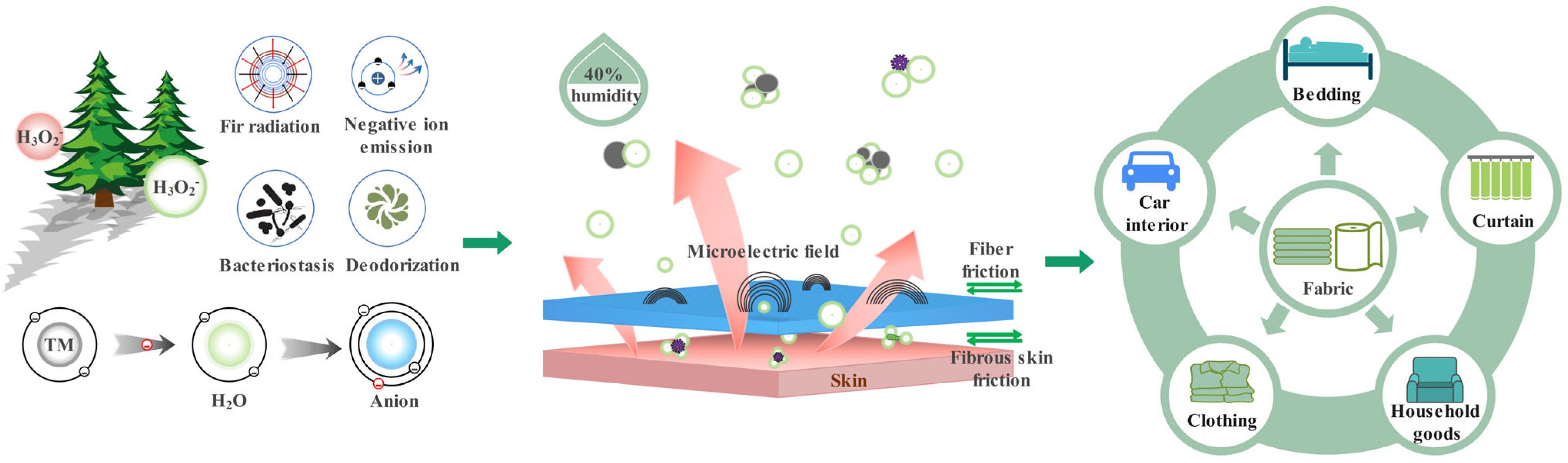
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
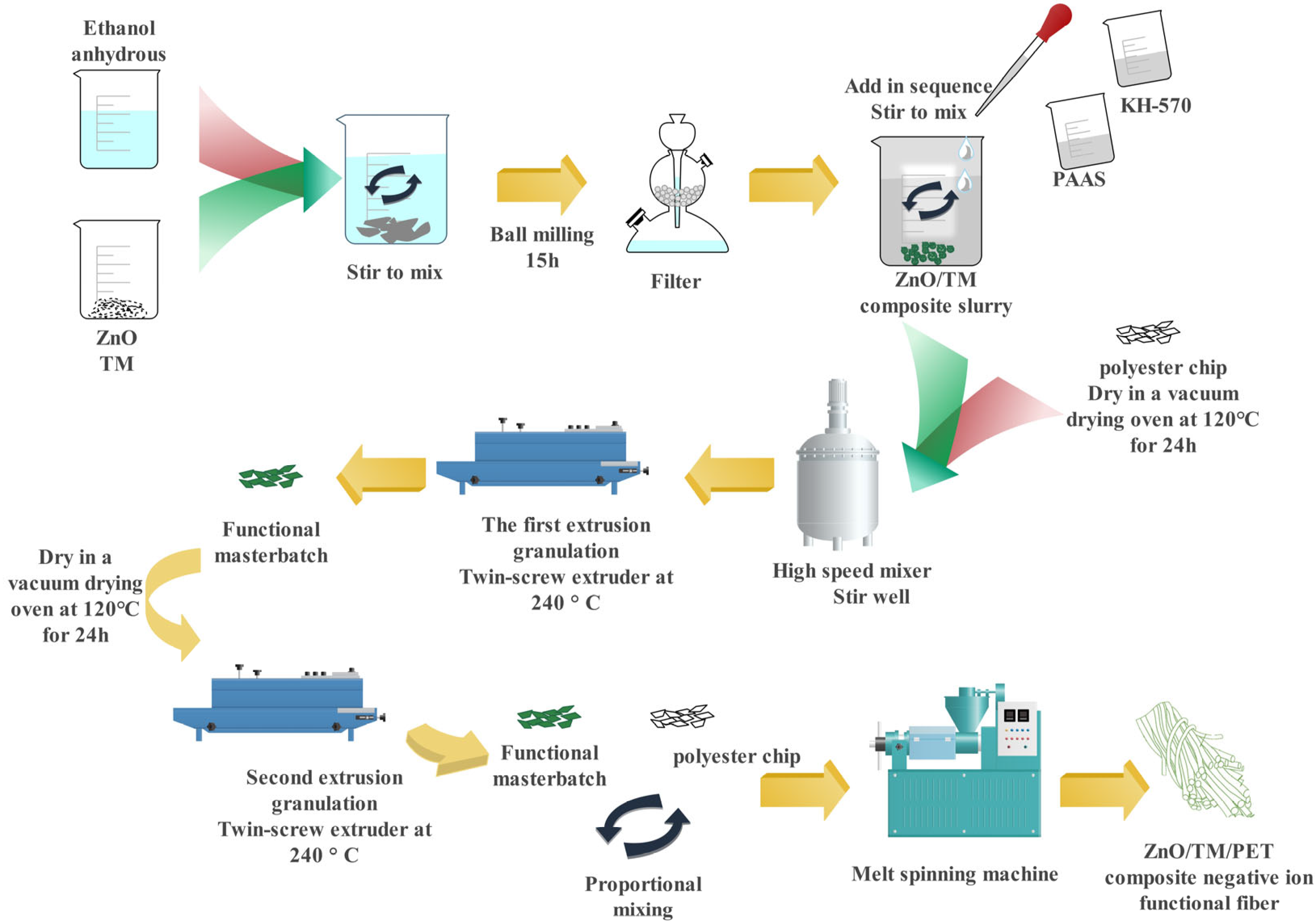
2.3. Sample Preparation
2.3.1. Preparation of ZnO/TM Composite Powder
2.3.2. Preparation of Functional Polyester Cuttings
2.3.3. Preparation of ZnO/TM/PET Negative Ion Functional Fiber
2.4. Testing Method
2.4.1. Particle Size Testing
2.4.2. Rheological Performance Testing
2.4.3. SEM
2.4.4. Measurement of Fiber Fineness
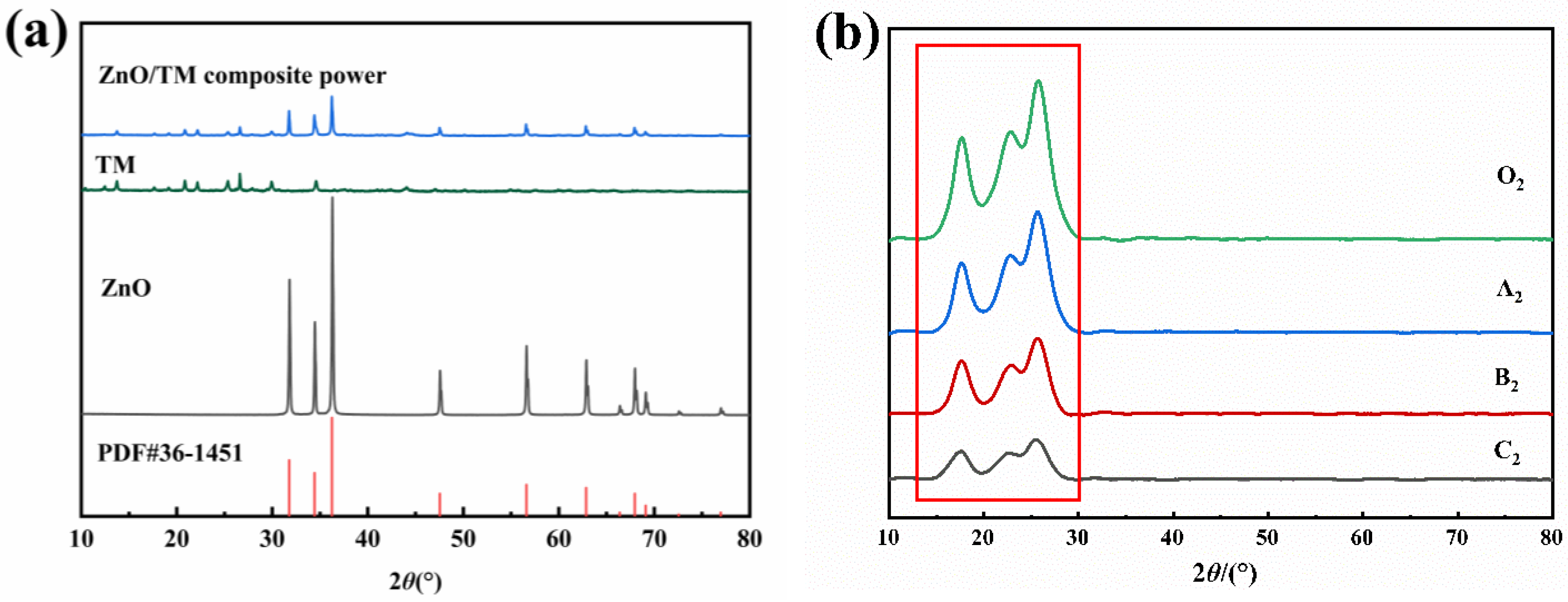
2.4.5. XRD
2.4.6. FTIR
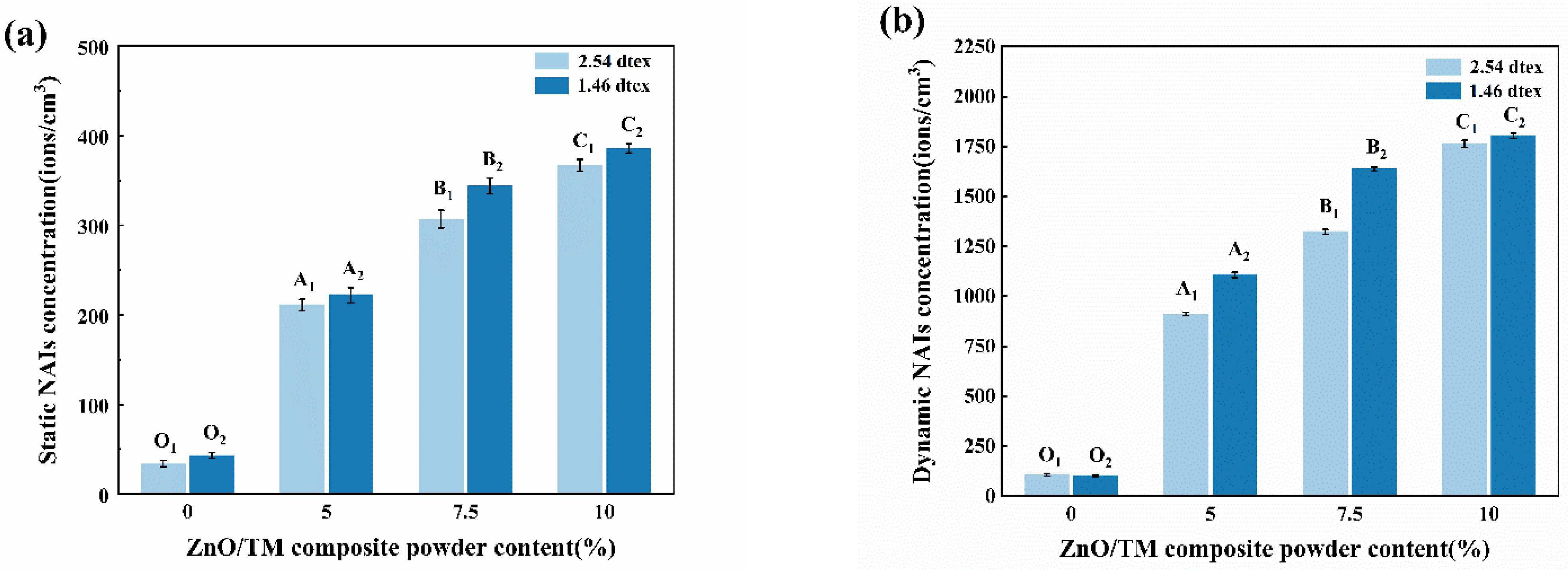
2.4.7. Negative Ion Release Performance Testing
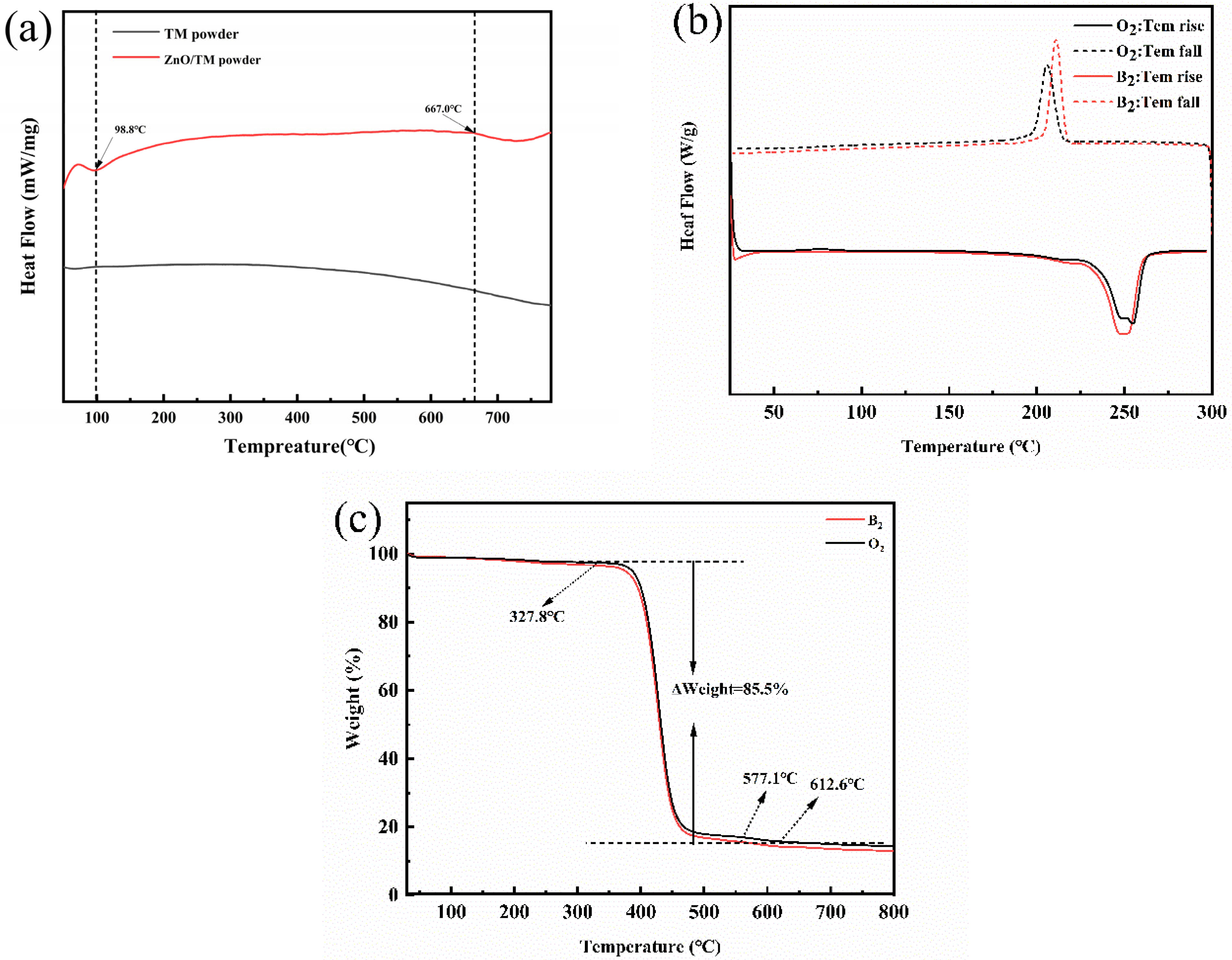
2.4.8. Thermal Performance Testing
2.4.9. Heat Shrinkability Testing
2.4.10. Mechanics Performance Testing
3. Results and Discussion
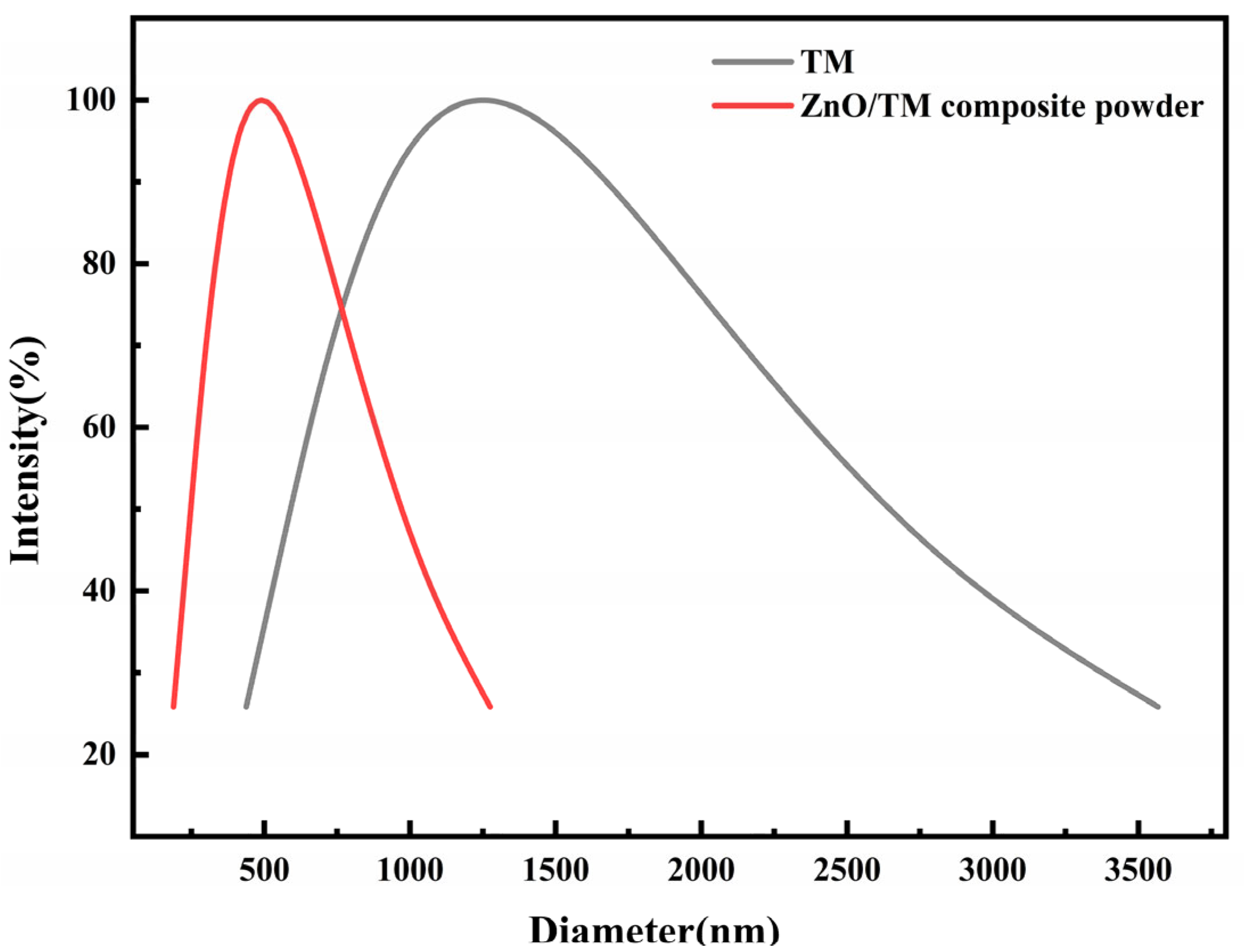
3.1. Powder Particle Size analysis
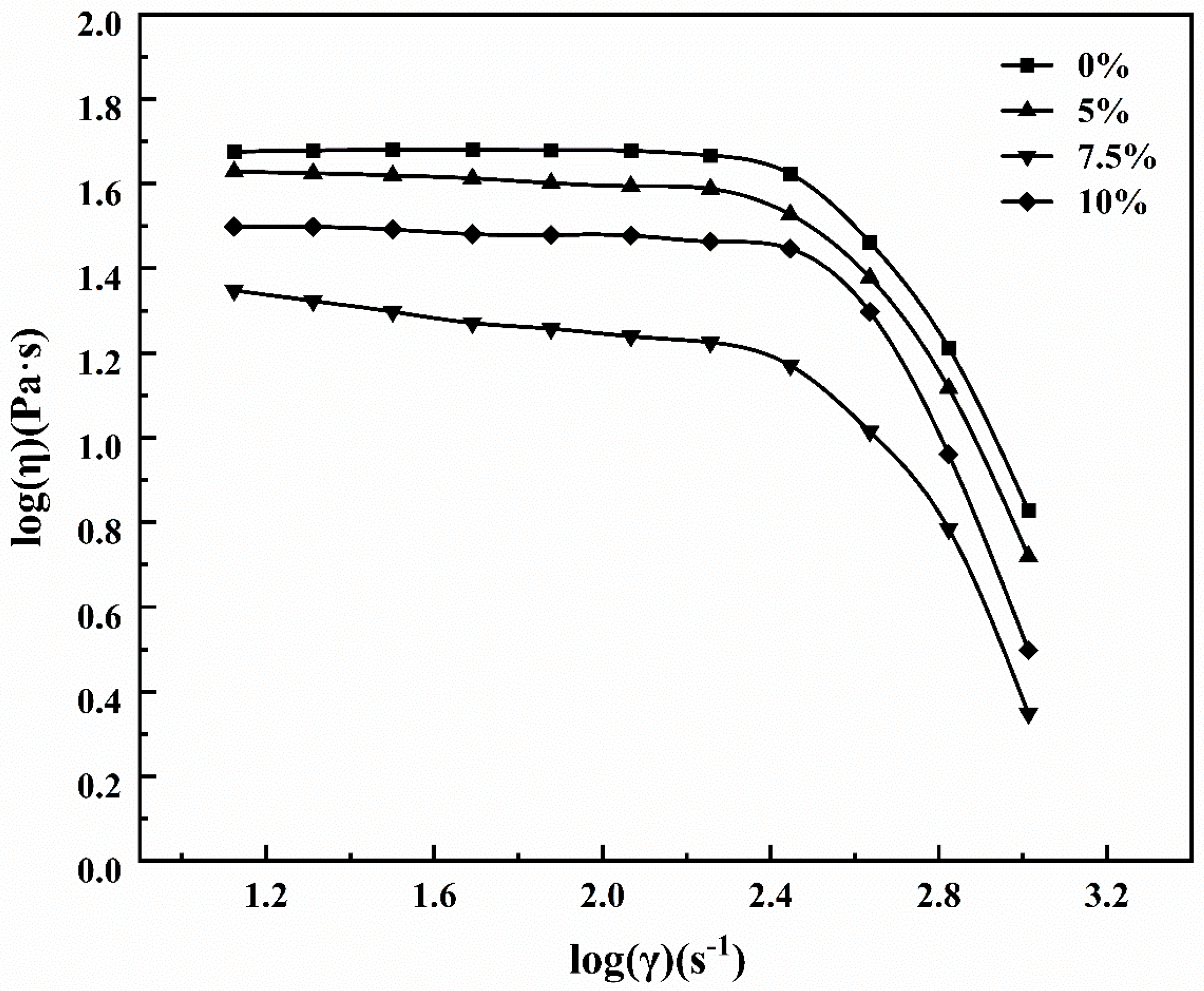
3.2. Effect of Powder Content on Rheological Properties of Fiber Masterbatch
3.3. SEM Analysis
3.4. XRD
3.5. FTIR
3.6. Effect of Powder Content on Mechanical Properties
3.7. Thermal Performance Testing
3.8. Thermal Shrinkage Performance Testing
3.9. Negative Ion Release Performance Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.Y.; Wu, Z.N.; Wang, C.; Li, H.F.; Li, Z.H.; Lin, J.M. Hydrated negative air ions generated by air–water collision with TiO2 photocatalytic materials. RSC Adv. 2020, 10, 43420–43424. [Google Scholar] [CrossRef]
- Li, Q.S.; Zhang, K.J.; Luo, J.Q.; Li, J.A.; Jiang, J.; Liang, Q.Q.; Jin, Y.X.; Liu, B. The research of far infrared flame retardant polyester staple fiber. IOP Conf. Ser. Mater. Sci. Eng. 2017, 167, 012002. [Google Scholar] [CrossRef]
- Lai, C.C.; Jen, C.W.; Chang, Y.S.; Huang, K.S. Preparation and properties of multifunctional nylon 6 composite material. J. Compos. Mater. 2011, 45, 2707–2715. [Google Scholar]
- Zhi, C.; Huizhen, K.; Wang, J.; Li, Y.G.; Jia, H.; Wei, Q.F. Novel germanium-polyamide6 fibers with negative air ions release and far-infrared radiation as well as antibacterial property. Text. Res. J. 2022, 92, 1739–1747. [Google Scholar]
- Kutlu, B.; Schröttner, P.; Leuteritz, A.; Boldt, R.; Jacobs, E.; Heinrich, G. Preparation of melt-spun antimicrobially modified LDH/polyolefin nanocomposite fibers. Mater. Sci. Eng. C 2014, 41, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bosi, F. Tourmaline crystal chemistry. Am. Mineral. 2018, 103, 298–306. [Google Scholar] [CrossRef]
- Liang, Y.F.; Tang, X.J.; Zhu, Q.; Han, J.H.; Wang, C.P. A review: Application of tourmaline in environmental fields. Chemosphere 2021, 281, 130780. [Google Scholar] [CrossRef] [PubMed]
- Chena, K.R.; Gai, X.H.; Zhou, G.J.; Shan, Y.; Zhao, C.C.; Shen, K.; Fan, Z.J. Study on a new type of pyroelectric materials with structure of tourmaline. Ceram. Int. 2019, 45, 10684–10690. [Google Scholar] [CrossRef]
- Ding, H.H.; Jiang, X.F.; Wang, W.; Zhang, J.S. Preparation and characterization of ultrafine tourmaline powder suitable for textile finishing application. Wool Text. J. 2020, 48, 24–27. [Google Scholar]
- Dong, X.W.; Xie, W.B.; Du, J.; Luo, Y. Sol-gel deodorizing finishing of cotton stocks and their deodorant effect verification by germiculture. Text. Aux. 2012, 29, 19–22. [Google Scholar]
- Zhao, X.L.; Li, Y.Y.; Hua, T.; Jiang, P.; Yin, X.; Yu, J.Y.; Ding, B. Low-Resistance Dual-Purpose Air Filter Releasing Negative Ions and Effectively Capturing PM2.5. ACS Appl. Mater. Interfaces 2017, 9, 12054–12063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Hu, J.L. Robust Effects of Graphene Oxide on Polyurethane/Tourmaline Nanocomposite Fiber. Polymers 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, J.; Li, J.; Zhu, Y.N.; Ge, M.Q. Negative air ion release and far infrared emission properties of polyethylene terephthalate/germanium composite fiber. J. Eng. Fibers Fabr. 2017, 12, 59–65. [Google Scholar] [CrossRef]
- Zhang, K.J.; Li, Q.S.; Luo, J.Q. Preparation and characterization of anion functional polyester fiber. J. Funct. Mater. 2017, 48, 9184–9188. [Google Scholar]
- Liu, Y.; Rui, Y.Y.; Yu, B.Y.; Fu, L.H.; Lu, G.; Liu, J. Study on the negative oxygen ion release behavior and mechanism of tourmaline composites. Mater. Chem. Phys. 2023, 313, 128779. [Google Scholar] [CrossRef]
- Helian, Y.; Cui, S.; Ma, X. The Effect of Tourmaline on SCR Denitrification Activity of MnOx/TiO2 at Low Temperature. Catalysts 2020, 10, 1020. [Google Scholar] [CrossRef]
- Yu, C.Q.; Tong, Z.; Li, S.H.; Yin, Y.H. Enhancing the photocatalytic activity of ZnO by using tourmal-ine. Mater. Lett. 2019, 240, 161–164. [Google Scholar] [CrossRef]
- Luo, Y.L.; Chen, Q.; Wang, C.H.; Guo, T.T. Preparation and improved negative ion release of graphene/tourmaline composite. Mater. Res. Express 2019, 6, 055507. [Google Scholar] [CrossRef]
- Wang, X.J.; Hong, S.; Lian, H.L. Photocatalytic degradation of surface-coated tourmaline-titanium dioxide for self-cleaning of formaldehyde emitted from furniture. J. Hazard. Mater. 2021, 420, 126565. [Google Scholar] [CrossRef]
- Wang, C.H.; Chen, Q.; Guo, T.T.; Li, Q. Environmental effects and enhancement mechanism of graphene/tourmaline composites. J. Clean. Prod. 2020, 262, 121313. [Google Scholar] [CrossRef]
- Cui, K.; Hu, Y.M.; Wu, S.S.; Guo, S.F. Functional modification and characterization of polyester fibers for negative ion release. N. Chem. Mater. 2024, 52, 151–154. [Google Scholar]
- Ding, H.H.; Jiang, X.F.; Zhang, J.S. Research progress of preparation process and test method of anion functional fiber. N. Chem. Mater. 2020, 48, 46–49. [Google Scholar]
- Hu, Y.M.; Yang, X. The surface organic modification of tourmaline powder by span-60 and its composite. Appl. Surf. Sci. 2012, 258, 7540–7545. [Google Scholar] [CrossRef]
- Huang, G.; Cui, Z.; Zhu, P. Modification of nano tourmaline surface treatment agent and its performance on negative ion release. Comput. Mater. Contin. 2018, 57, 145–150. [Google Scholar] [CrossRef]
- GB/T 30128-2013; Detection and Evaluation of the Amount of Negative Ions in Textiles. China Standards Press: Beijing, China, 2014.
- SN/T 2558.2-2011; Inspection Methods for Import and Export Functional Textiles Part II: Negative Ion Content. China Standards Press: Beijing, China, 2011.
- Meng, J.P.; Jin, W.; Liang, J.S.; Ding, Y.; Gan, K.; Yuan, Y.D. Effects of particle size on far infrared emission properties of tourmaline superfine powders. J. Nanosci. Nanotechnol. 2010, 10, 2083–2087. [Google Scholar] [CrossRef]
- Wu, C.M.; Cheong, S.S.; Chang, T.H. Rheological properties of graphene/nylon 6 nanocomposites prepared by masterbatch melt mixing. J. Polym. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhou, J.L.; Yu, S.L.; Wei, L.F.; Hu, Z.X.; Xiang, H.X.; Zhu, M.F. Melt-spun bio-based PLA-co-PET copolyester fibers with tunable properties: Synergistic effects of chemical structure and drawing process. Int. J. Biol. Macromol. 2023, 226, 670–678. [Google Scholar] [CrossRef] [PubMed]




| Process Condition | 1st Area Screw Temp. | 2nd Area Screw Temp. | 3rd Area Screw Temp. | 4th Area Screw Temp. | Screw Flange Temp. | Metering Pump Temp. | 1st Area Hot Roll Temp. | 2nd Area Hot Roll Temp. | 3rd Area Hot Roll Temp. |
|---|---|---|---|---|---|---|---|---|---|
| SV (Actual temp) (°C) | 202 | 260 | 270 | 270 | 269.9 | 265.2 | 65.1 | 66.3 | 68.2 |
| PV (Set temp) (°C) | 200 | 260 | 270 | 270 | 270.0 | 65.0 | 65.0 | 65.0 | 65.0 |
| Process Condition | Extruder Screw Frequency (Hz) | Extrusion Screw Current (A) | Metering Pump Speed (r/min) | Oiling Speed (r/min) | 1st Area Hot Roll Speed (r/min) | 2nd Area Hot Roll Speed (r/min) | 3rd Area Hot Roll Speed (r/min) | Winding Speed (r/min) | Winding Angle (°) | Theoretical Drawing Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Process parameter | 2.00 | 8.90 | 5.0 | 3.0 | 300.0 | 450.0 | 470.0 | 500.0 | 5.0 | 1.7 |
| 2nd Process parameter | 2.00 | 8.90 | 5.0 | 3.0 | 440.0 | 700.0 | 760.0 | 800.0 | 5.0 | 1.8 |
| Name | O1 | O2 | A1 | A2 | B1 | B2 | C1 | C2 |
|---|---|---|---|---|---|---|---|---|
| ZnO/TM composite powder (wt%) | 0 | 0 | 5 | 5 | 7.5 | 7.5 | 10 | 10 |
| PET (wt%) | 100 | 100 | 95 | 95 | 92.5 | 92.5 | 90 | 90 |
| Theoretical drawing ratio | 1.7 | 1.8 | 1.7 | 1.8 | 1.7 | 1.8 | 1.7 | 1.8 |
| Name | 010 | 110 | 100 | Crystallinity (%) | Average Particle Size D(nm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2θ (°) | FWHM | 2θ (°) | FWHM | 2θ (°) | FWHM | 010 | 110 | 100 | ||
| O2 | 17.509 | 1.585 | 22.412 | 1.467 | 26.016 | 1.704 | 71.88 | 5.06 | 3.96 | 3.42 |
| A2 | 17.572 | 1.641 | 22.452 | 1.458 | 25.961 | 1.725 | 69.01 | 5.04 | 3.96 | 3.43 |
| B2 | 17.306 | 1.965 | 22.215 | 1.475 | 25.739 | 1.948 | 67.26 | 5.12 | 4.00 | 3.46 |
| C2 | 17.286 | 2.014 | 22.155 | 1.418 | 25.771 | 1.968 | 65.27 | 5.13 | 4.01 | 3.45 |
| Name | O1 | O2 | A1 | A2 | B1 | B2 | C1 | C2 |
|---|---|---|---|---|---|---|---|---|
| Fiber thermal shrinkage (%) | 7.63 | 7.32 | 6.89 | 6.23 | 5.92 | 5.54 | 5.01 | 4.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, J.; Lu, X.; Wu, J.; Peng, J.; Wang, W.; Tao, J. Preparation and Performance of a Novel ZnO/TM/PET Composite Negative Ion Functional Fiber. Polymers 2024, 16, 1439. https://doi.org/10.3390/polym16101439
Zhang M, Zhang J, Lu X, Wu J, Peng J, Wang W, Tao J. Preparation and Performance of a Novel ZnO/TM/PET Composite Negative Ion Functional Fiber. Polymers. 2024; 16(10):1439. https://doi.org/10.3390/polym16101439
Chicago/Turabian StyleZhang, Mengxin, Jishu Zhang, Xin Lu, Jianbing Wu, Jiajia Peng, Wei Wang, and Jin Tao. 2024. "Preparation and Performance of a Novel ZnO/TM/PET Composite Negative Ion Functional Fiber" Polymers 16, no. 10: 1439. https://doi.org/10.3390/polym16101439
APA StyleZhang, M., Zhang, J., Lu, X., Wu, J., Peng, J., Wang, W., & Tao, J. (2024). Preparation and Performance of a Novel ZnO/TM/PET Composite Negative Ion Functional Fiber. Polymers, 16(10), 1439. https://doi.org/10.3390/polym16101439






