Revisit of Polyaniline as a High-Capacity Organic Cathode Material for Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
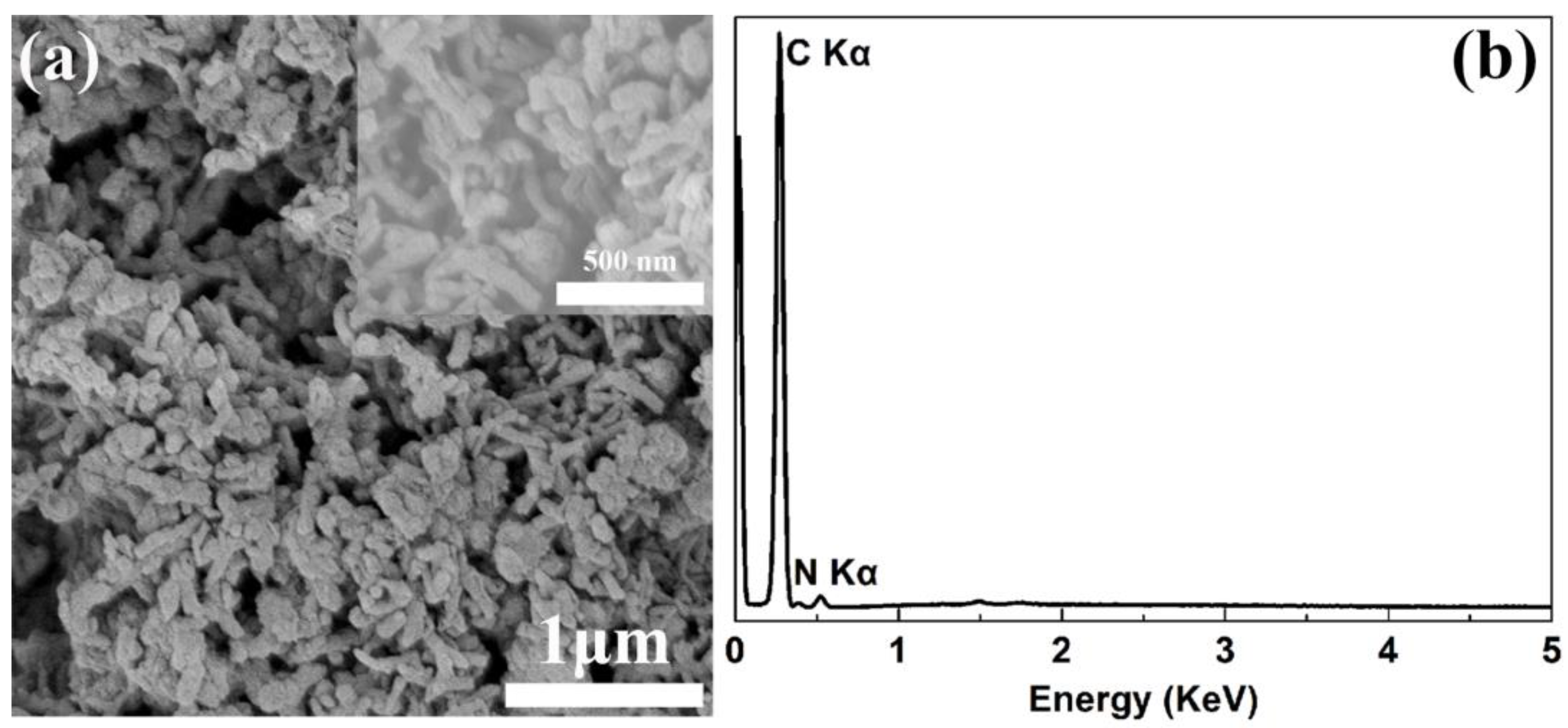
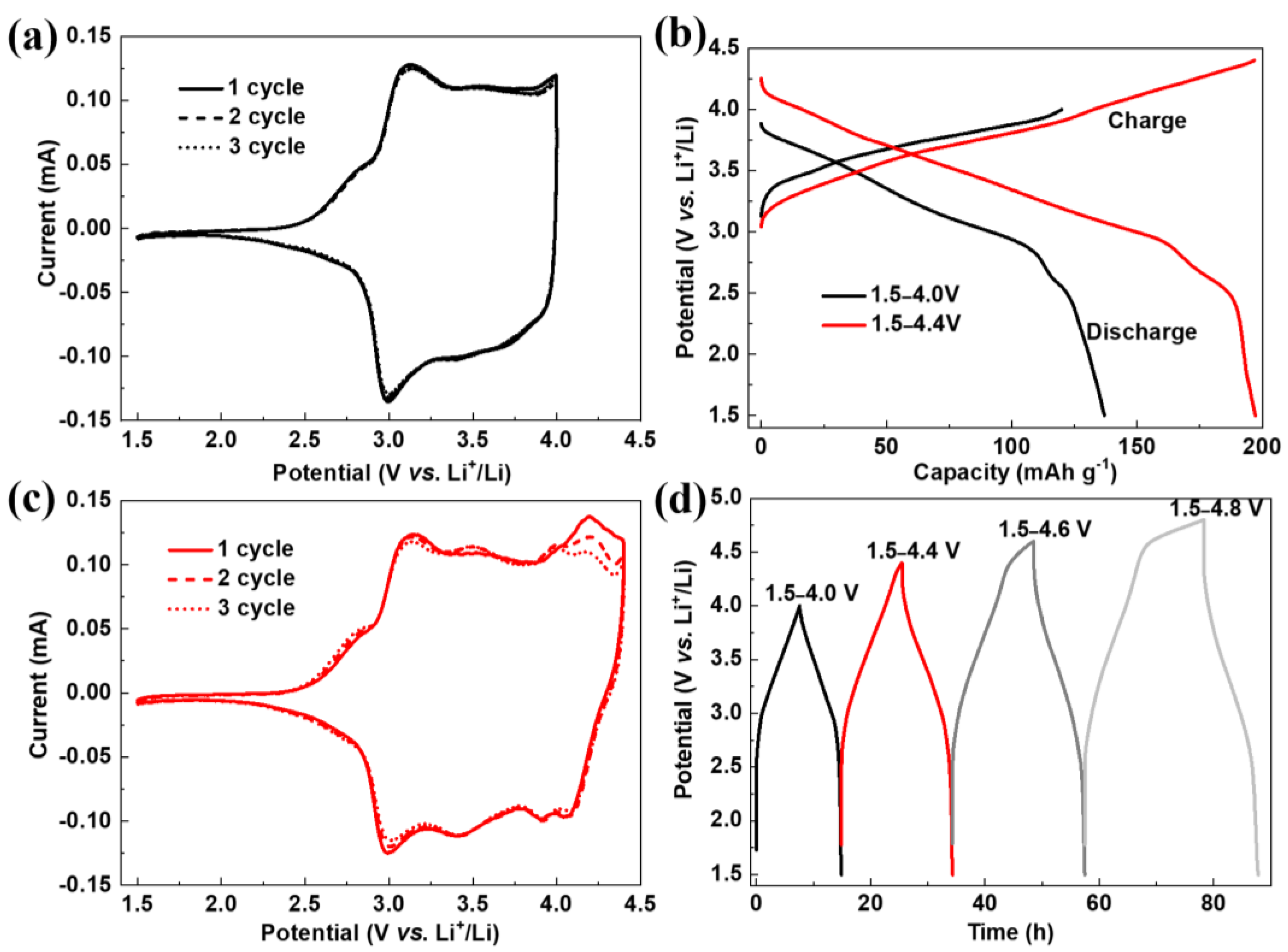
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kyuyun, S.; Young, H.; Hong, S. High-density positive electrodes containing carbon nanotubes for use in Li-ion cells. J. Power Sources 2006, 158, 421–425. [Google Scholar]
- Shchegolkov, A.; Komarov, F.; Lipkin, M.; Milchanin, O.; Parfimovich, I.; Shchegolkov, A.; Nokhaeva, V. Synthesis and study of cathode materials based on carbon nanotubes for lithium-ion batteries. Inorg. Mater. Appl. Res. 2021, 12, 1281–1287. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J. Prospects of Organic Electrode Materials for Practical Lithium Batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yao, Y. Positioning Organic Electrode Materials in the Battery Landscape. Joule 2018, 2, 1690–1706. [Google Scholar] [CrossRef]
- Deng, W.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Graphene-Wrapped Na2C12H6H4 Nanoflowers as High Performance Anodes for Sodium-Ion Batteries. Small 2016, 12, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, X.; Yu, N.; Yang, W.; Zhou, Y.; Shi, Y.; Wang, Y.; Dong, L.; Di, J.; Li, Q. A Polypyrrole-Coated MnO2/Carbon Nanotube Film Cathode for Rechargeable Aqueous Zn-Ion Batteries. Acta Phys. Chim. Sin. 2022, 38, 2006059–2006067. [Google Scholar]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Muller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically Active Polymers for Rechargeable Batteries. Chem. Rev. 1997, 97, 207–282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Lv, L.; Zhu, G.; Qu, Q.; Zheng, H. Electroactive Organics as Promising Anode Materials for Rechargeable Lithium Ion and sodium Ion Batteries. Energy Mater. 2022, 2, 200014–200040. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef] [PubMed]
- Venancio, E.C.; Motheo, A.J.; Amaral, F.A.; Bocchi, N. Performance of Polyaniline Electrosynthesized in the Presence of Trichloroacetic Acid as a Battery Cathode. J. Power Sources 2001, 94, 36–39. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, T.; Miao, Y.; Zhao, X. Chloride Ion-Doped Polyaniline/Carbon Nanotube Nanocomposite Materials as New Cathodes for Chloride Ion Battery. Electrochim. Acta 2018, 270, 30–36. [Google Scholar] [CrossRef]
- Desilvestro, J.; Scheifele, W.; Haas, O. In Situ Determination of Gravimetric and Volumetric Charge Densities of Battery Electrodes: Polyaniline in Aqueous and Nonaqueous Electrolytes. J. Electrochem. Soc. 1992, 139, 2727. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (Pani) Based Electrode Materials for Energy Storage and Conversion. J. Sci.—Adv. Mater. Dev. 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Cheng, F.; Tang, W.; Li, C.; Chen, J.; Liu, H.; Shen, P.; Dou, S. Conducting Poly(Aniline) Nanotubes and Nanofibers: Controlled Synthesis and Application in Lithium/Poly(Aniline) Rechargeable Batteries. Chem. Eur. J. 2006, 12, 3082–3088. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Hua, K.; Li, S.; Fang, D.; Luo, Z.; Bao, R.; Fan, X.; Yi, J. Vertically Aligned Polyaniline Nanowire Arrays for Lithium-Ion Battery. Colloid Polym. Sci. 2018, 296, 1395–1400. [Google Scholar] [CrossRef]
- Cao, X.; Liu, J.; Zhu, L.; Xie, L. Polymer Electrode Materials for High-Performance Lithium/Sodium-Ion Batteries: A Review. Energy Technol. 2019, 7, 1800759. [Google Scholar] [CrossRef]
- Zhao, R.R.; Zhu, L.M.; Cao, Y.L.; Ai, X.P.; Yang, H.X. An Aniline-Nitroaniline Copolymer as a High Capacity Cathode for Na-Ion Batteries. Electrochem. Commun. 2012, 21, 36–38. [Google Scholar] [CrossRef]
- Jiménez, P.; Levillain, E.; Alévêque, O.; Guyomard, D.; Lestriez, B.; Gaubicher, J. Lithium N-Doped Polyaniline as a High-Performance Electroactive Material for Rechargeable Batteries. Angew. Chem. Int. Ed. 2017, 56, 1553–1556. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Tollan, C.; Yang, F.; Beltrán, M.; Qin, Y.; Knez, M. Conductive Polymer–Inorganic Hybrid Materials through Synergistic Mutual Doping of the Constituents. ACS Appl. Mater. Interfaces 2017, 9, 27964–27971. [Google Scholar] [CrossRef]
- Guo, Z.H.; Wang, J.X.; Yu, P.; Li, M.L.; Huang, L.; Hu, Z.J.; Wang, Y.; Song, Z. Toward Full Utilization and Stable Cycling of Polyaniline Cathode for Nonaqueous Rechargeable Batteries. Adv. Energy Mater. 2023, 13, 2301520–2301531. [Google Scholar] [CrossRef]
- Ryu, K.S.; Jeong, S.K.; Joo, J.; Kim, K.M. Polyaniline Doped with Dimethyl Sulfate as a Nucleophilic Dopant and Its Electrochemical Properties as an Electrode in a Lithium Secondary Battery and a Redox Supercapacitor. J. Phys. Chem. B 2007, 111, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Andriianova, A.N.; Biglova, Y.N.; Mustafin, A.G. Effect of structural factors on the physicochemical properties of functionalized polyanilines. RSC Adv. 2020, 10, 7468–7491. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xia, Z.; Sun, R.; An, H.; Qi, F.; Wang, S.; Liu, Q.; Sun, G. A Self-Charging Hybrid Electric Power Device with High Specific Energy and Power. ACS Energy Lett. 2018, 3, 2425–2432. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, A.; Wang, J.; Wu, J.; Bai, H. Degradation-Induced Capacitance: A New Insight into the Superior Capacitive Performance of Polyaniline/Graphene Composites. Energy Environ. Sci. 2017, 10, 2372–2382. [Google Scholar] [CrossRef]
- Skaarup, S.; Gunaratne, L.; West, K.; Zachau-Christiansen, B. Polyaniline: Influence of Polymerization Current Density. MRS Online Proc. Libr. Arch. 1994, 369, 565. [Google Scholar] [CrossRef]
- Lu, W.; Fadeev, A.G.; Qi, B.; Smela, E.; Mattes, B.R.; Ding, J.; Spinks, G.M.; Mazurkiewicz, J.; Zhou, D.; Wallace, G.G.; et al. Use of Ionic Liquids for Π-Conjugated Polymer Electrochemical Devices. Science 2002, 297, 983–987. [Google Scholar] [CrossRef]
- Jeon, J.-W.; Ma, Y.; Mike, J.F.; Shao, L.; Balbuena, P.B.; Lutkenhaus, J.L. Oxidatively Stable Polyaniline:Polyacid Electrodes for Electrochemical Energy Storage. Phys. Chem. Chem. Phys. 2013, 15, 9654–9662. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gao, X.; Ci, L.; Xiong, S. A Novel Bifunctional Additive for 5 V-Class, High-Voltage Lithium Ion Batteries. RSC Adv. 2016, 6, 7224–7228. [Google Scholar] [CrossRef]
- Xia, L.; Xia, Y.; Wang, C.; Hu, H.; Lee, S.; Yu, Q.; Chen, H.; Liu, Z. 5 V-Class Electrolytes Based on Fluorinated Solvents for Li-Ion Batteries with Excellent Cyclability. ChemElectroChem 2015, 2, 1707–1712. [Google Scholar] [CrossRef]
- Arsov, L.D.; Plieth, W.; Koßmehl, G. Electrochemical and Raman Spectroscopic Study of Polyaniline; Influence of the Potential on the Degradation of Polyaniline. J. Solid State Electr. 1998, 2, 355–361. [Google Scholar] [CrossRef]
- Pud, A.A. Stability and Degradation of Conducting Polymers in Electrochemical Systems. Synth. Met. 1994, 66, 1–18. [Google Scholar] [CrossRef]
- Fu, X.; Wang, S.; Xia, Z.; Li, Y.; Jiang, L.; Sun, G. Aligned Polyaniline Nanorods in Situ Grown on Gas Diffusion Layer and Their Application in Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2016, 41, 3655–3663. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. Ftir Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Kang, S.-G.; Lee, G.J.; Joo, J.; Chang, S.H. Electrochemical and physical characterization of lithium ionic salt doped polyaniline as a polymer electrode of lithium secondary battery. Synth. Met. 2000, 110, 213–217. [Google Scholar] [CrossRef]
- Yang, H.; Song, T.; Liu, L.; Devadoss, A.; Xia, F.; Han, H.; Park, H.; Sigmund, W.; Kwon, K.; Paik, U.J. Polyaniline/polyoxometalate hybrid nanofibers as cathode for lithium ion batteries with improved lithium storage capacity. Phys. Chem. C 2013, 117, 17376–17381. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Kozarenko, O.A.; Dyadyun, V.S.; Koshechko, V.G.; Pokhodenko, V.D. Electrochemical performance of mechanochemically prepared polyaniline doped with lithium salt. Synth. Met. 2012, 162, 2206–2211. [Google Scholar] [CrossRef]
- Manuel, J.; Raghavan, P.; Shin, C.; Heo, M.-Y.; Ahn, J.-H.; Noh, J.-P.; Cho, G.-B.; Ryu, H.-S.; Ahn, H.-J. Electrosprayed polyaniline as cathode material for lithium secondary batteries. Mater. Res. Bull. 2010, 45, 265–268. [Google Scholar] [CrossRef]
- Liu, P.; Han, J.-J.; Jiang, L.-F.; Li, Z.-Y.; Cheng, J.-N. Polyaniline/multi-walled carbon nanotubes composite with core-shell structures as a cathode material for rechargeable lithium-polymer cells. Appl. Surf. Sci. 2017, 400, 446–452. [Google Scholar] [CrossRef]
- Rauhala, T.; Davodi, F.; Sainio, J.; Sorsa, O.; Kallio, T. On the stability of polyaniline/carbon nanotube composites as binder-free positive electrodes for electrochemical energy storage. Electrochim. Acta 2020, 336, 135735. [Google Scholar] [CrossRef]
- Manuel, J.; Kim, J.-K.; Matic, A.; Jacobsson, P.; Chauhan, G.S.; Ha, J.K.; Cho, K.-K.; Ahn, J.-H. Electrochemical properties of lithium polymer batteries with doped polyaniline as cathode material. Mater. Res. Bull. 2012, 47, 2815–2818. [Google Scholar] [CrossRef]
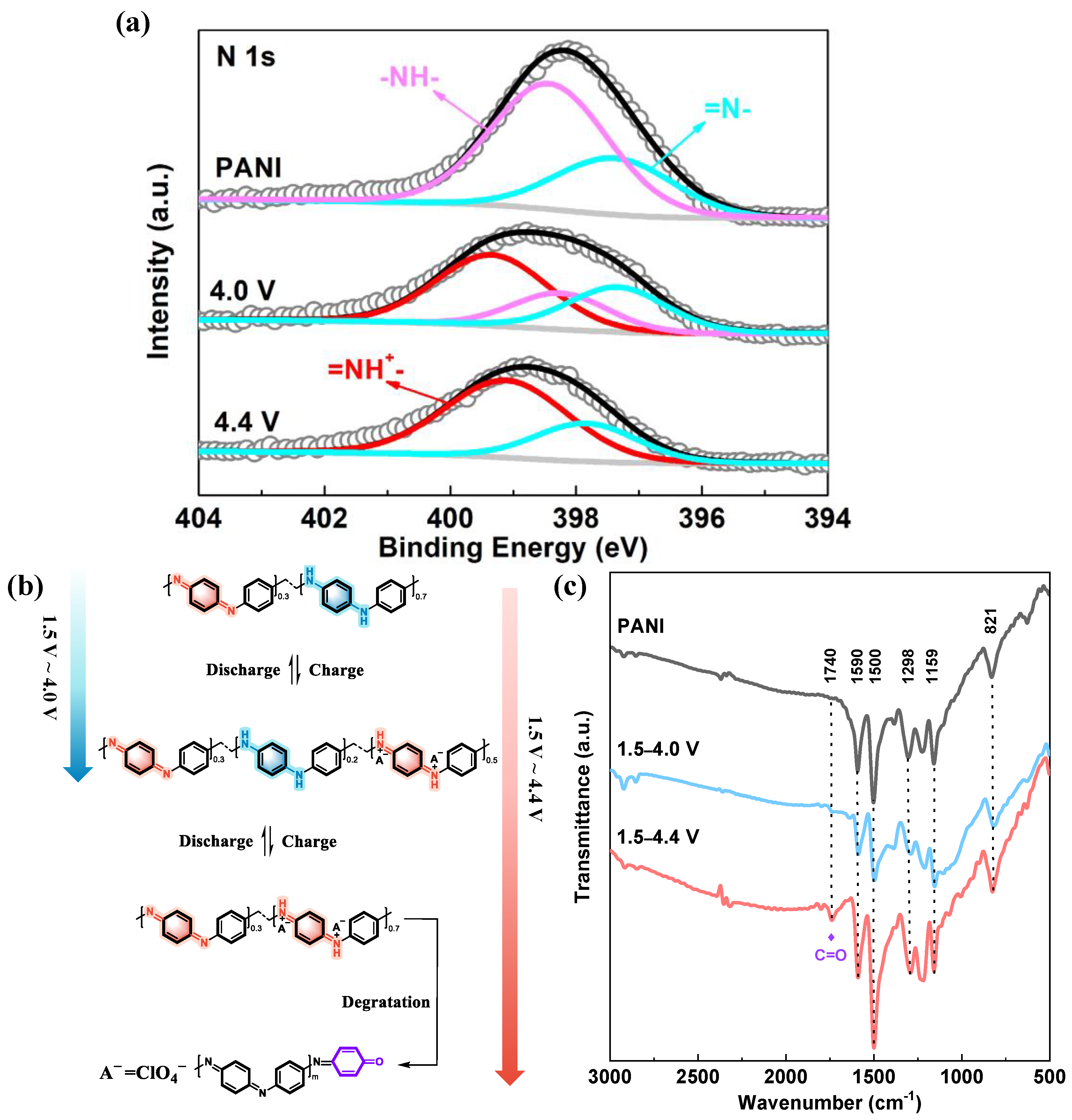

| =N- | -NH- | =NH+- | |
|---|---|---|---|
| PANI | 30.02% | 69.98% | - |
| 4.0 V | 28.90% | 21.70% | 49.39% |
| 4.4 V | 29.06% | - | 70.94% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Chang, Z.; Fu, X.; Xu, M.; Ai, X.; Qian, J. Revisit of Polyaniline as a High-Capacity Organic Cathode Material for Li-Ion Batteries. Polymers 2024, 16, 1401. https://doi.org/10.3390/polym16101401
Zhao R, Chang Z, Fu X, Xu M, Ai X, Qian J. Revisit of Polyaniline as a High-Capacity Organic Cathode Material for Li-Ion Batteries. Polymers. 2024; 16(10):1401. https://doi.org/10.3390/polym16101401
Chicago/Turabian StyleZhao, Ruirui, Zu Chang, Xudong Fu, Mingli Xu, Xinping Ai, and Jiangfeng Qian. 2024. "Revisit of Polyaniline as a High-Capacity Organic Cathode Material for Li-Ion Batteries" Polymers 16, no. 10: 1401. https://doi.org/10.3390/polym16101401
APA StyleZhao, R., Chang, Z., Fu, X., Xu, M., Ai, X., & Qian, J. (2024). Revisit of Polyaniline as a High-Capacity Organic Cathode Material for Li-Ion Batteries. Polymers, 16(10), 1401. https://doi.org/10.3390/polym16101401







