The Effect of Polyvinyl Alcohol Addition on the Optical Properties and Oxygen Detection Performance of Titanium Dioxide and Methylene Blue Nanocomposite Colorimetric Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2/MB Nanocomposite Suspension
2.3. Preparation of TiO2/MB Nanocomposite Films
2.4. Characterization
3. Results and Discussion
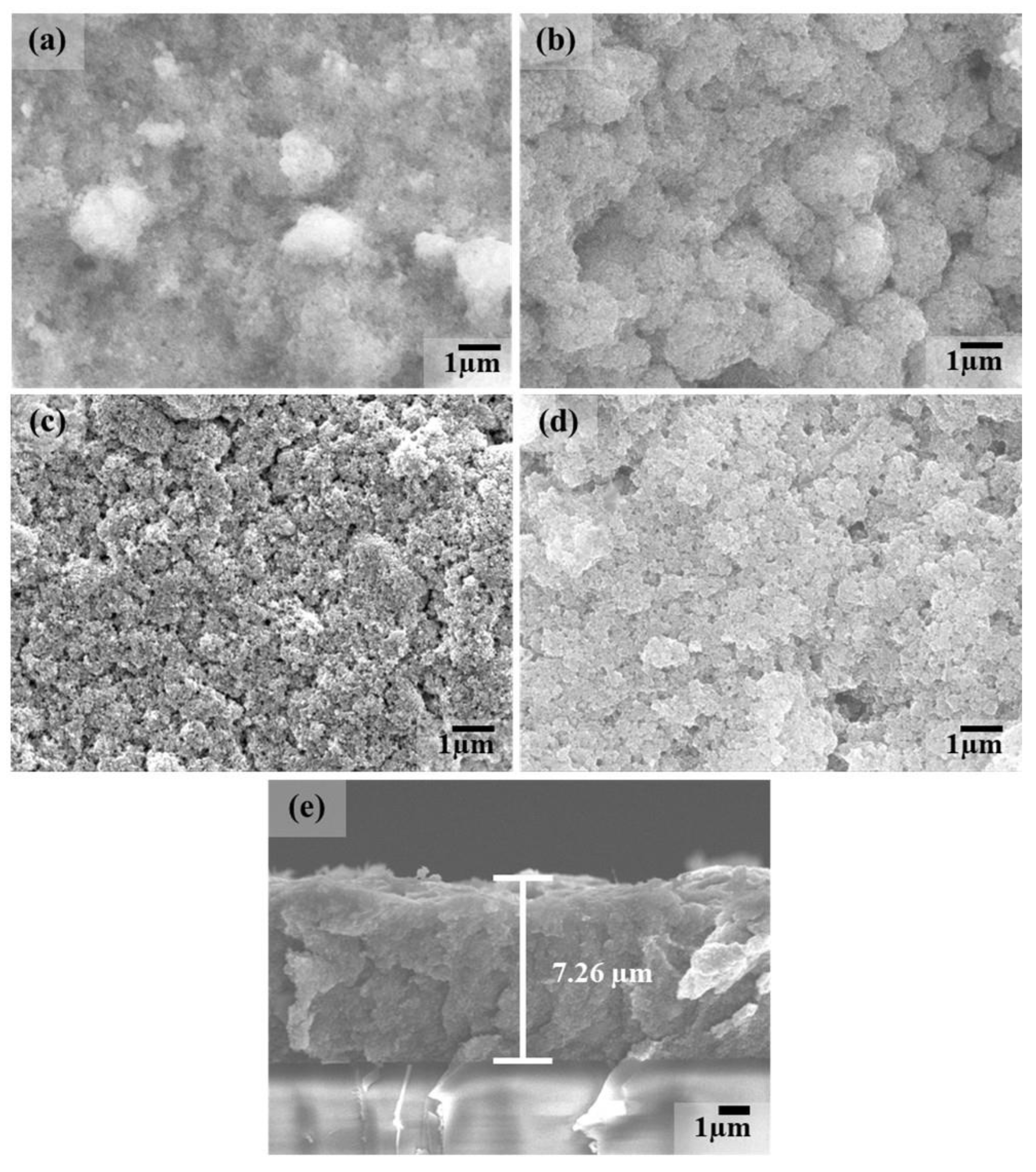
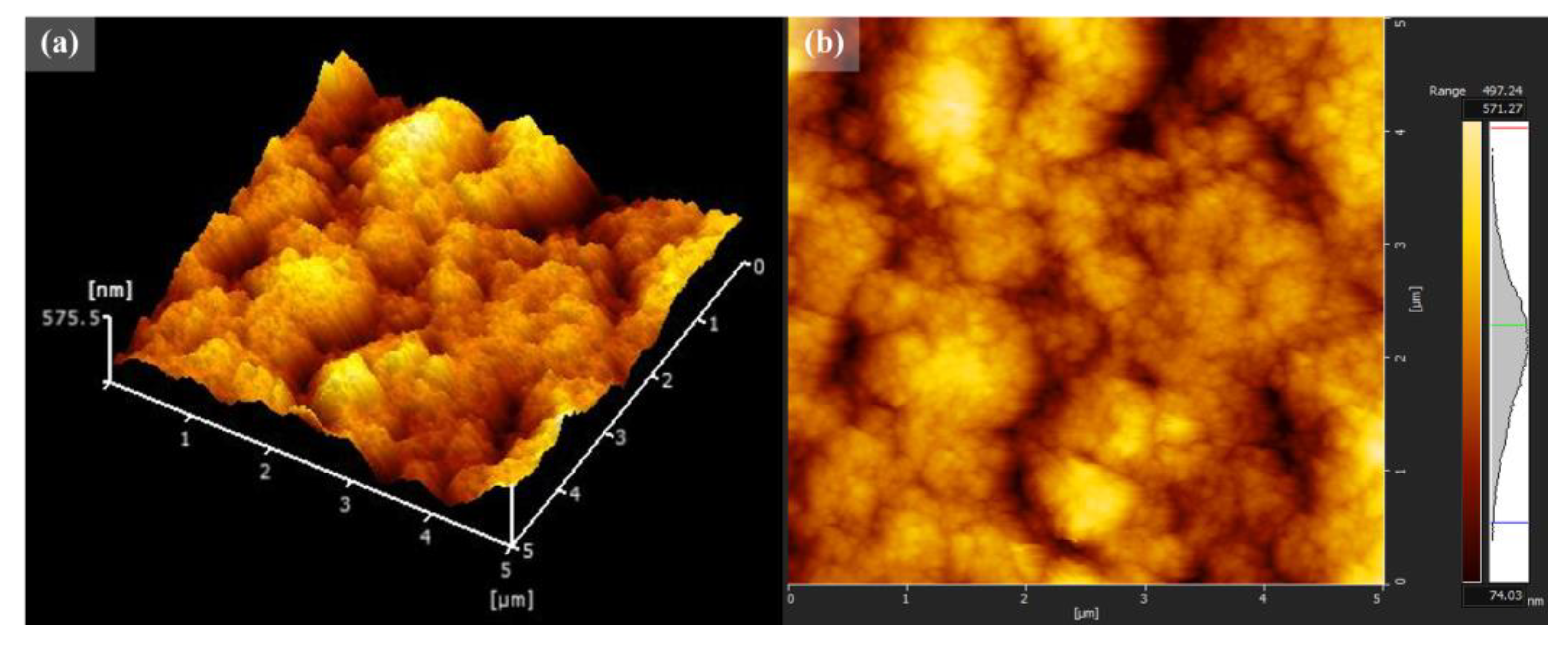
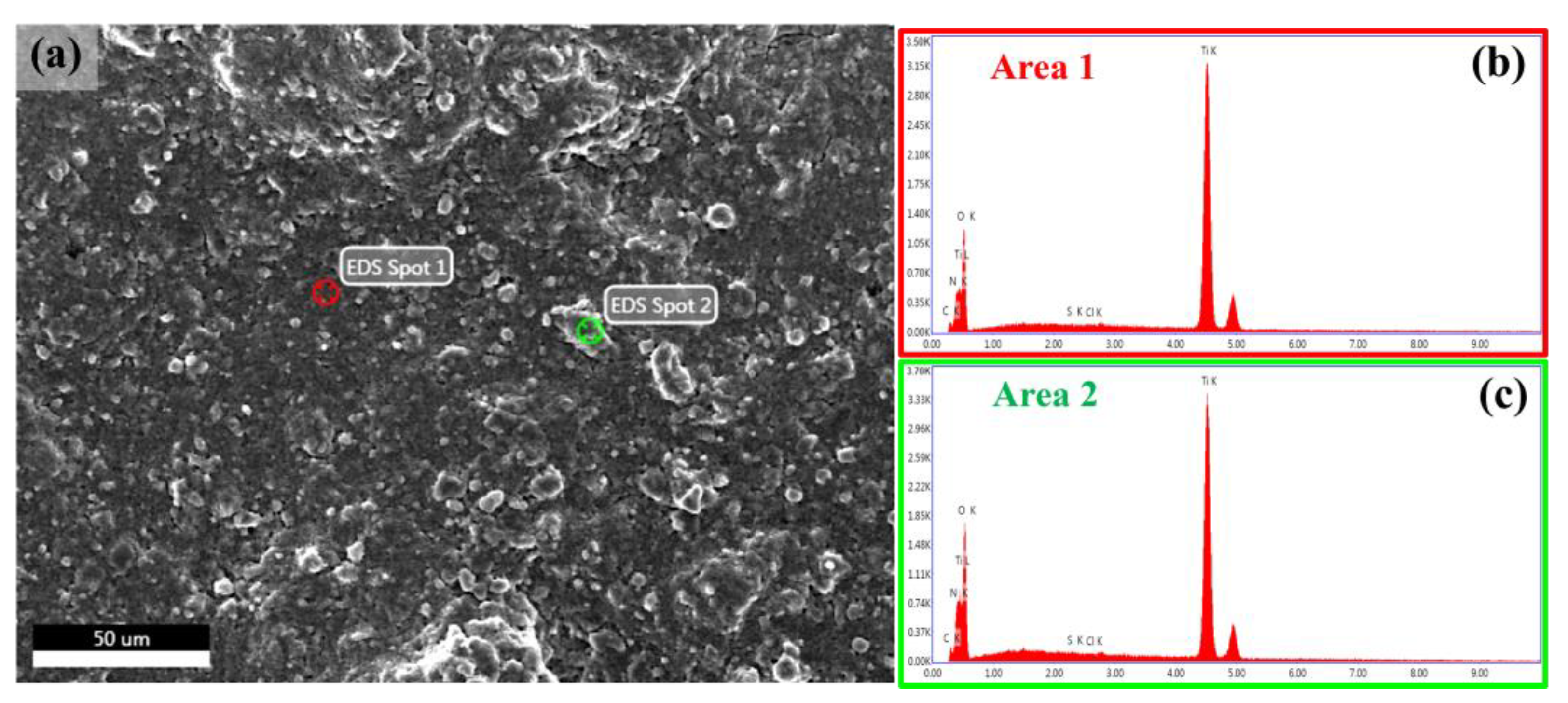
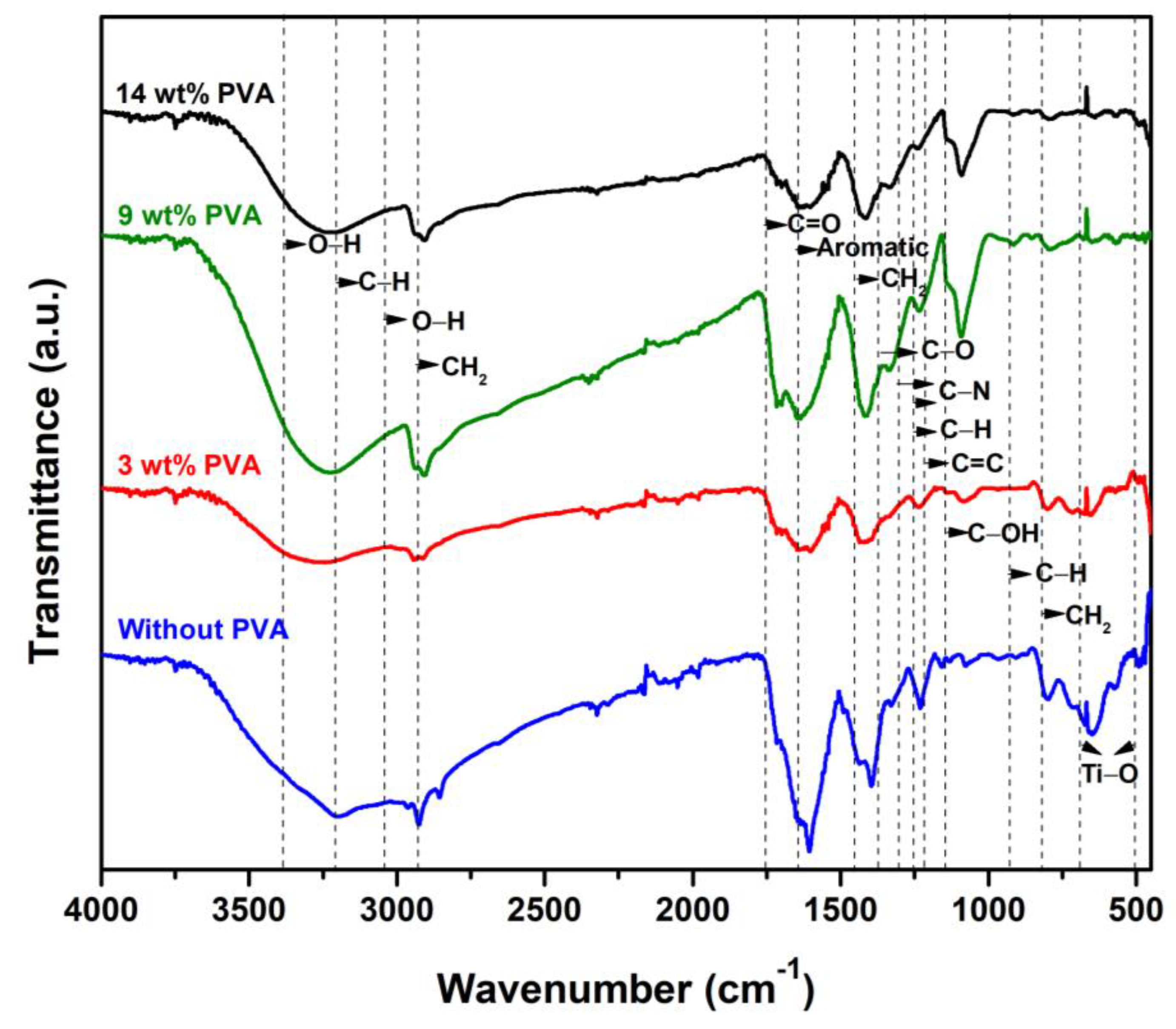
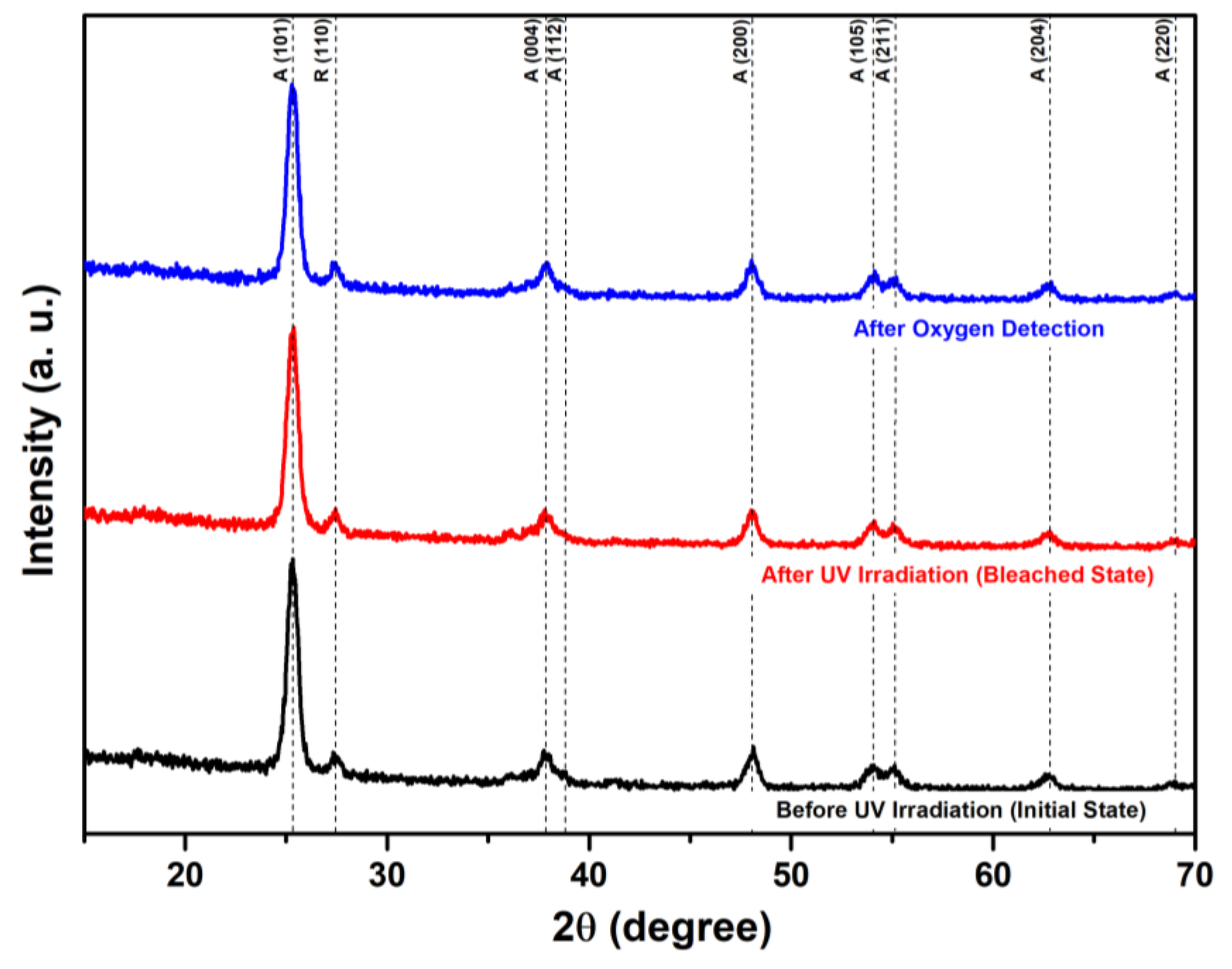
3.1. Structural and Chemical Properties of TiO2/MB Nanocomposite Films
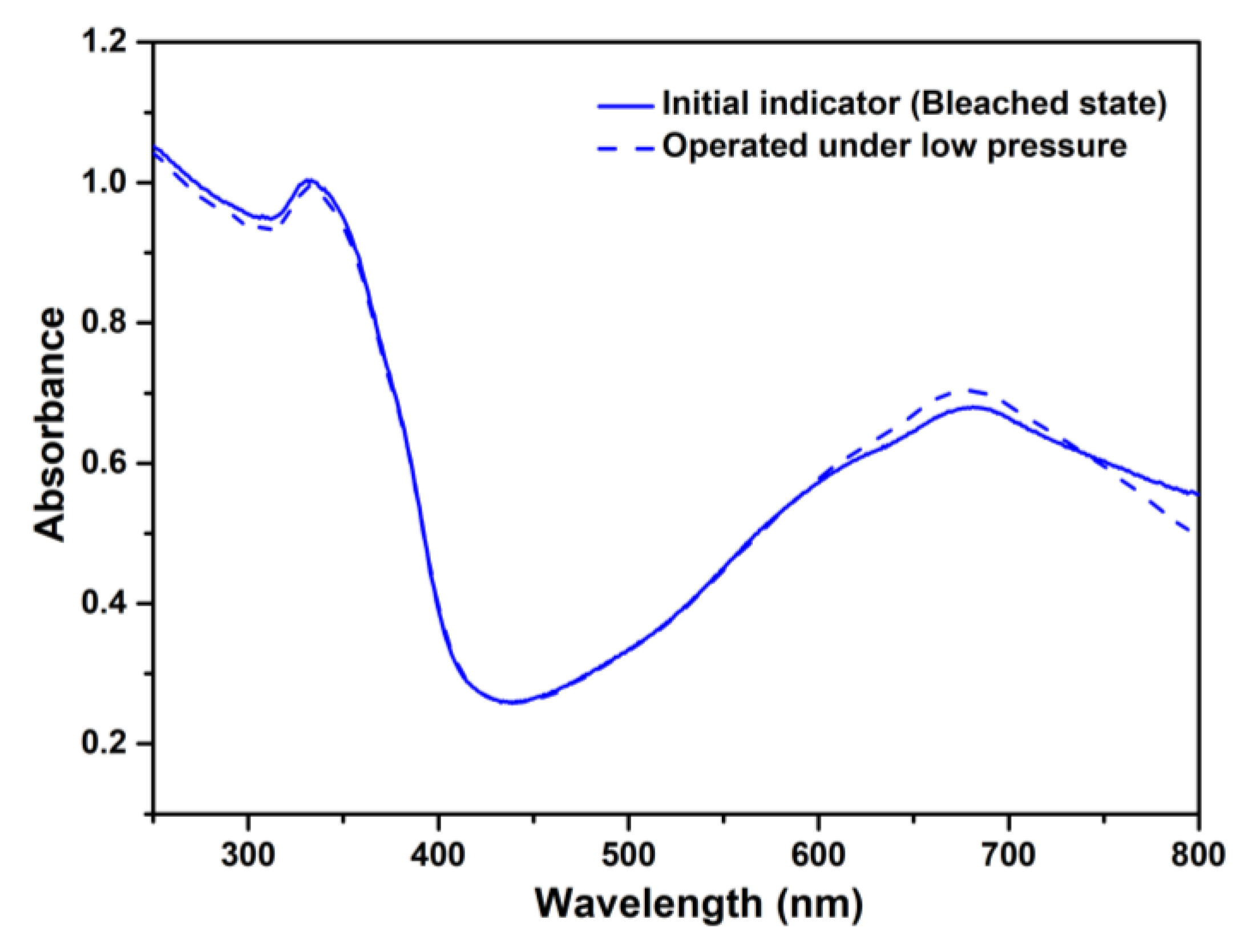
3.2. Colorimetric Indicator Efficiency of TiO2/MB Nanocomposite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yılmaz, M.; Altan, A. Optimization of functionalized electrospun fibers for the development of colorimetric oxygen indicator as an intelligent food packaging system. Food Packag. Shelf Life 2021, 28, 100651. [Google Scholar] [CrossRef]
- Imran, M.; Yousaf, A.B.; Zhou, X.; Liang, K.; Jiang, Y.F.; Xu, A.W. Oxygen-deficient TiO2−x/methylene blue colloids: Highly efficient photoreversible intelligent ink. Langmuir 2016, 32, 8980–8987. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, X.; Bhandari, B.; Fang, Z.; Chen, H. Recent application of modified atmosphere packaging (MAP) in fresh and fresh-cut foods. Food Rev. Int. 2015, 31, 172–193. [Google Scholar] [CrossRef]
- Li, H.; Wei, J.; Jin, M.; Yu, Y.; Bu, J.; Chen, Q.; Hao, Z. Colorimetric analysis through electrospun WO3/PAN membrane for indication of oxygen in food packaging using a smartphone. Compos. Commun. 2022, 35, 101321. [Google Scholar] [CrossRef]
- Xie, S.; Liu, X.; Li, H.; Huang, C. The application of oxygen indicator in food packaging. Adv. Mater. Res. 2014, 945–949, 2037–2042. [Google Scholar] [CrossRef]
- Won, S.; Won, K. Self-powered flexible oxygen sensors for intelligent food packaging. Food Packag. Shelf Life 2021, 29, 100713. [Google Scholar] [CrossRef]
- Ast, C.; Schmälzlin, E.; Löhmannsröben, H.G.; Dongen, J.T.V. Optical oxygen micro- and nanosensors for plant applications. Sensors 2012, 12, 7015–7032. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, L.; Tian, Z.; Feng, Z.; Jiang, B.; Wang, W. Fast-response flexible photochromic gels for self-erasing rewritable media and colorimetric oxygen indicator applications. ACS Appl. Mater. Interfaces 2018, 10, 33423–33433. [Google Scholar] [CrossRef]
- Wen, J.; Huang, S.; Jia, L.; Ding, F.; Li, H.; Chen, L.; Liu, X. Visible colorimetric oxygen indicator based on Ag-loaded TiO2 nanotubes for quick response and real-time monitoring of the integrity of modified atmosphere packaging. Adv. Mater. Technol. 2019, 4, 1900121. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Oxygen detection using UV-activated electrospun poly(ethylene oxide) fibers encapsulated with TiO2 nanoparticles. J. Mater. Sci. 2013, 48, 5489–5498. [Google Scholar] [CrossRef]
- Du, W.; Ren, X.; Pei, Z.; Ma, C. Ceramic binder jetting additive manufacturing: A literature review on density. J. Manuf. Sci. Eng. 2020, 142, 040801. [Google Scholar] [CrossRef]
- Environment-Friendly Polymeric Binders. Available online: https://www.pcimag.com/articles/87142-environment-friendly-polymeric-binders (accessed on 1 May 2024).
- Xie, M.; Wang, J.; Zhao, H. A PVA film for detecting lipid oxidation intended for food application. Sens. Actuat. B Chem. 2018, 273, 260–263. [Google Scholar] [CrossRef]
- Park, H.K.; Kong, B.S.; Oh, E.S. Effect of high adhesive polyvinyl alcohol binder on the anodes of lithium ion batteries. Electrochem. Commun. 2011, 13, 1051–1053. [Google Scholar] [CrossRef]
- Lim, W.S.; Kim, M.H.; Park, H.J.; Lee, M.H. Characterization of polyvinyl alcohol (PVA)/polyacrylic acid (PAA) composite film-forming solutions and resulting films as affected by beeswax content. Polymers 2024, 16, 310. [Google Scholar] [CrossRef]
- Songpanit, M.; Mekprasart, W.; Ishihara, K.N. Colorimetric oxygen indicator from titanium dioxide nanocomposite by mechanical milling process. Thai J. Nanosci. Nanotechnol. 2021, 6, 8–15. [Google Scholar]
- Songpanit, M.; Boonyarattanakalin, K.; Limwichean, S.; Lertvanithphol, T.; Horprathum, M.; Pecharapa, W.; Mekprasart, W. Self-cleaning SiO2/TiO2/PMMA nanocomposite films fabricated by spin coating technique: Effect of different spin speed and film layers. J. Mater. Sci. Appl. Energy. 2023, 12, 252043. [Google Scholar] [CrossRef]
- Pimpang, P.; Sumang, R.; Choopun, S. Effect of concentration of citric acid on size and optical properties of fluorescence graphene quantum dots prepared by tuning carbonization degree. Chiang Mai J. Sci. 2018, 45, 2005–2014. [Google Scholar]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS-PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Evaluation of polyvinyl alcohol (PVA) loading in the PVA/titanium dioxide (TiO2) thin film coating on polyvinylidene fluoride (PVDF) membrane for the removal of textile dyes. Chemosphere 2020, 257, 127144. [Google Scholar] [CrossRef]
- Wen, J.; Huang, S.; Sun, Y.; Chen, Z.; Wang, Y.; Li, H.; Liu, X. Titanium dioxide nanotube-based oxygen indicator for modified atmosphere packaging: Efficiency and accuracy. Materials 2018, 11, 2410. [Google Scholar] [CrossRef] [PubMed]
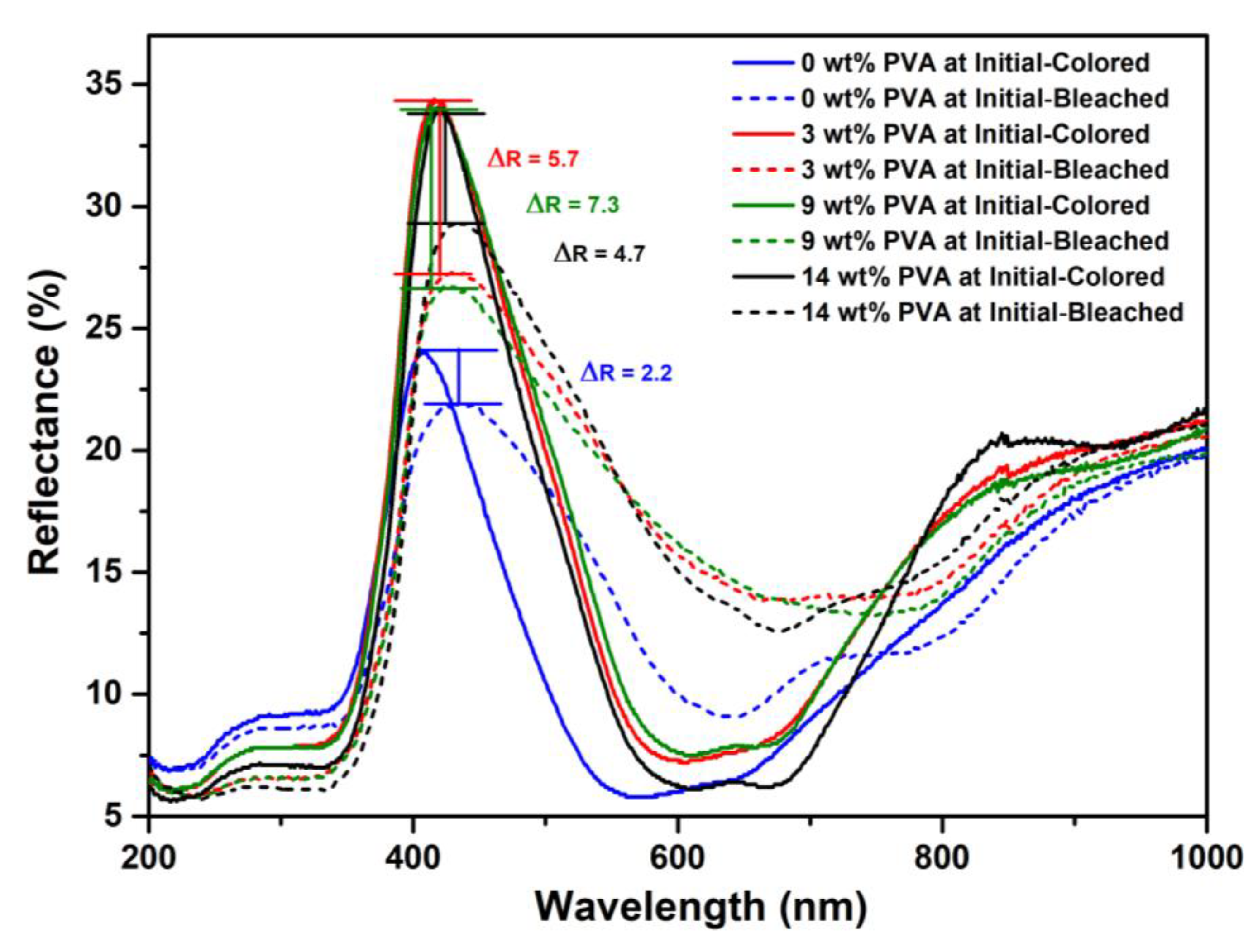
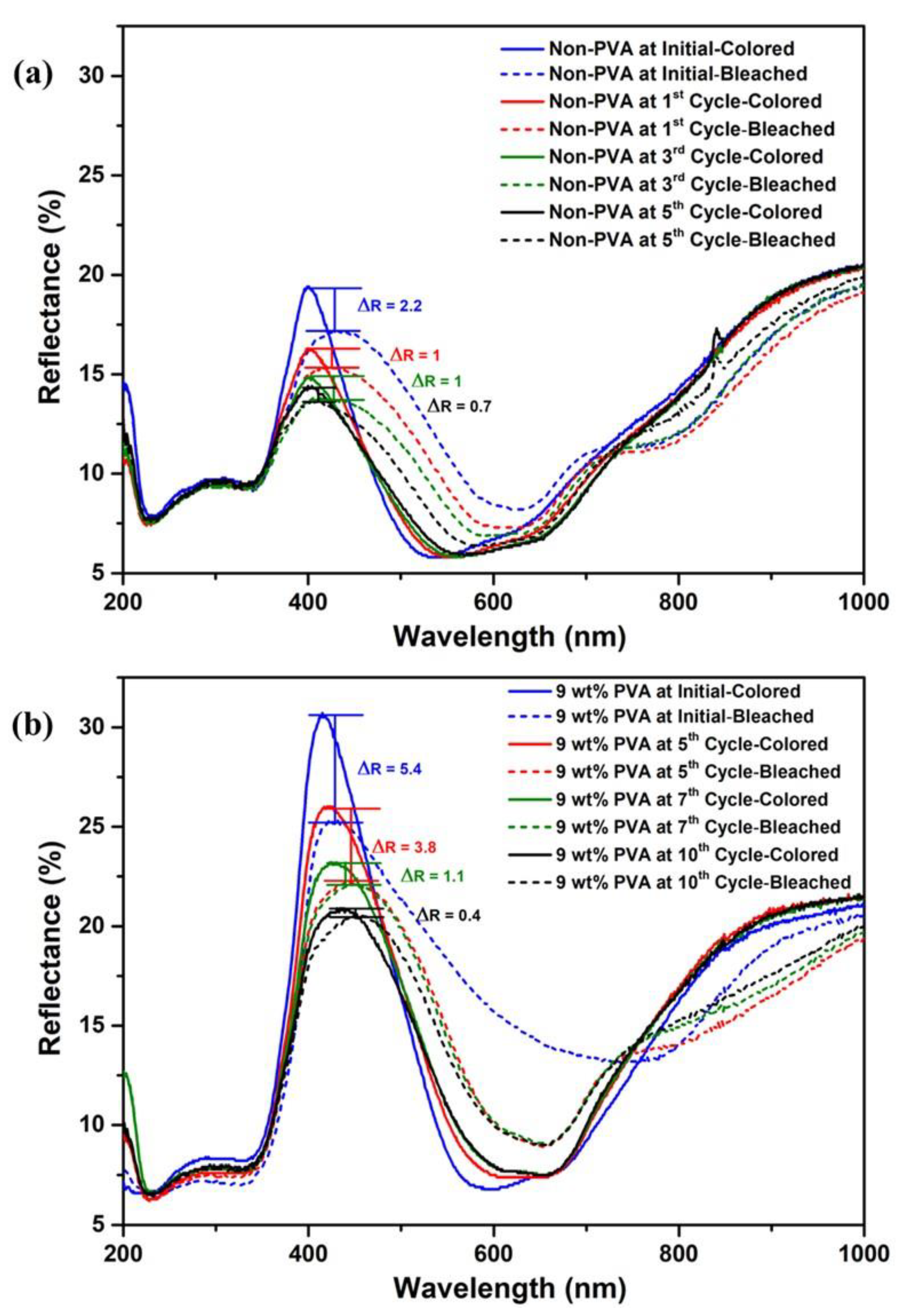
| PVA Content (wt%) | Before UVA Irradiation | After UVA Irradiation | Bleached Time (s) |
|---|---|---|---|
| 0 |  |  | 34 |
| 3 |  |  | 30 |
| 9 |  |  | 28 |
| 14 |  |  | 100 |
| PVA Content (wt%) | Cyan Percentage on the TiO2/MB Film Surface | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial State | After UVA Irradiation | Oxygen Detection Times | |||||||
| 5 min | 30 min | 2 h | 12 h | 24 h | 36 h | 48 h | |||
| 0 | 85 | 46 | 85 | 85 | 85 | 85 | 85 | 85 | 85 |
| 3 | 99 | 39 | 62 | 66 | 78 | 99 | 100 | 100 | 100 |
| 9 | 98 | 38 | 55 | 55 | 66 | 94 | 98 | 100 | 100 |
| 14 | 100 | 47 | 64 | 66 | 79 | 99 | 100 | 100 | 100 |
| Container Volume (mL) | Cyan Percentage on the TiO2/MB Film Surface | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before UVA | Oxygen Detection Times (h) | ||||||||
| 0 | 0.5 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| 250 | 82 | 46 | 55 | 67 | 69 | 72 | 76 | 82 | 82 |
| 590 | 82 | 48 | 57 | 67 | 69 | 72 | 75 | 82 | 82 |
| 1200 | 81 | 47 | 55 | 67 | 69 | 72 | 76 | 81 | 81 |
| 2680 | 80 | 47 | 53 | 67 | 69 | 72 | 75 | 80 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonyarattanakalin, K.; Rattan, P.; Songpanit, M.; Chutipaijit, S.; Okumura, H.; Ishihara, K.N.; Mekprasart, W.; Pecharapa, W. The Effect of Polyvinyl Alcohol Addition on the Optical Properties and Oxygen Detection Performance of Titanium Dioxide and Methylene Blue Nanocomposite Colorimetric Indicators. Polymers 2024, 16, 1400. https://doi.org/10.3390/polym16101400
Boonyarattanakalin K, Rattan P, Songpanit M, Chutipaijit S, Okumura H, Ishihara KN, Mekprasart W, Pecharapa W. The Effect of Polyvinyl Alcohol Addition on the Optical Properties and Oxygen Detection Performance of Titanium Dioxide and Methylene Blue Nanocomposite Colorimetric Indicators. Polymers. 2024; 16(10):1400. https://doi.org/10.3390/polym16101400
Chicago/Turabian StyleBoonyarattanakalin, Kanokthip, Praphaporn Rattan, Maneerat Songpanit, Sutee Chutipaijit, Hideyuki Okumura, Keiichi N. Ishihara, Wanichaya Mekprasart, and Wisanu Pecharapa. 2024. "The Effect of Polyvinyl Alcohol Addition on the Optical Properties and Oxygen Detection Performance of Titanium Dioxide and Methylene Blue Nanocomposite Colorimetric Indicators" Polymers 16, no. 10: 1400. https://doi.org/10.3390/polym16101400
APA StyleBoonyarattanakalin, K., Rattan, P., Songpanit, M., Chutipaijit, S., Okumura, H., Ishihara, K. N., Mekprasart, W., & Pecharapa, W. (2024). The Effect of Polyvinyl Alcohol Addition on the Optical Properties and Oxygen Detection Performance of Titanium Dioxide and Methylene Blue Nanocomposite Colorimetric Indicators. Polymers, 16(10), 1400. https://doi.org/10.3390/polym16101400










