Direct Observations of Ordered Nanoporosity in Deprotonated 2-(Acetoacetoxy)ethyl Methacrylate (AAEMA–) Polymer: Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
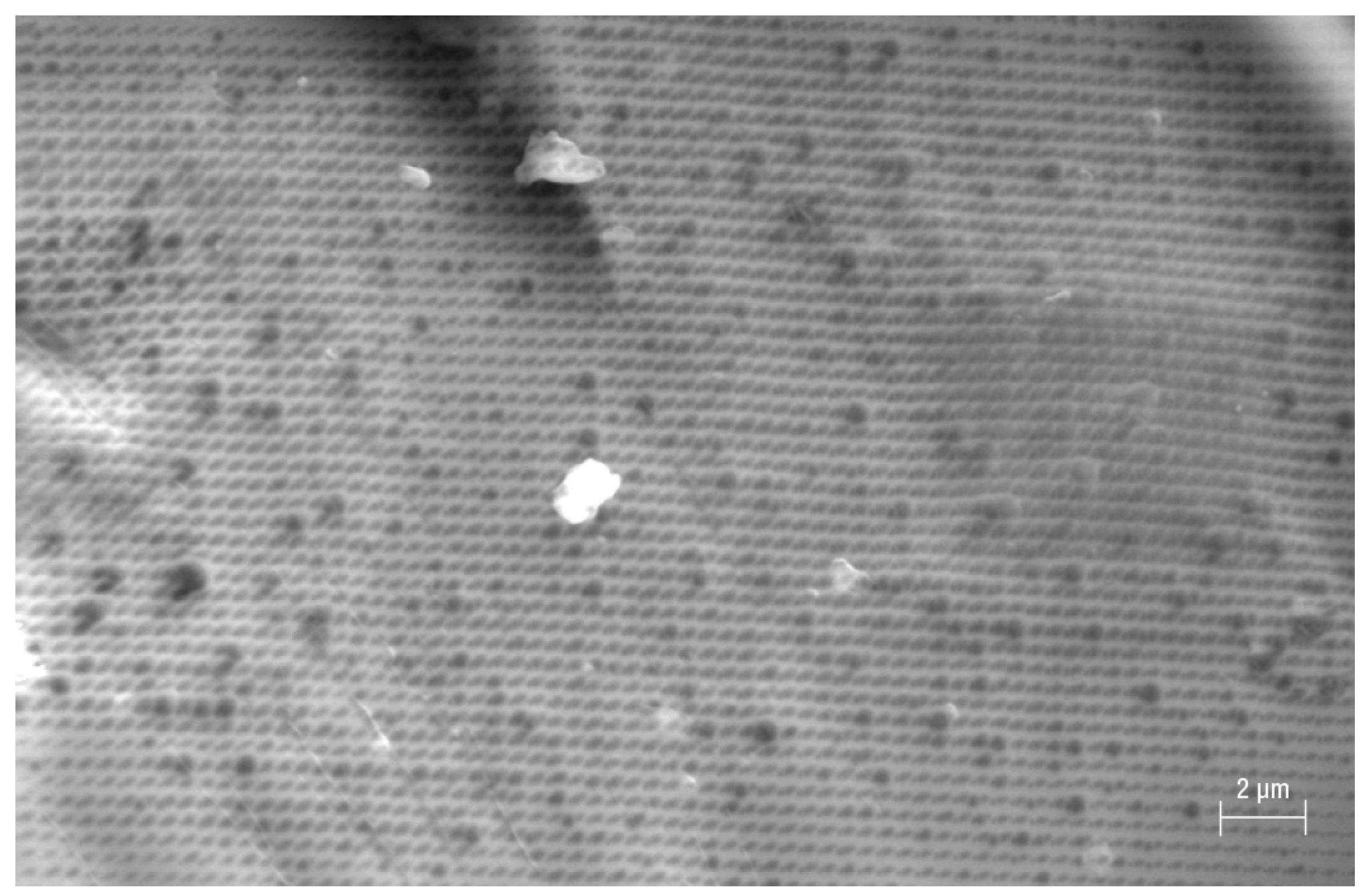
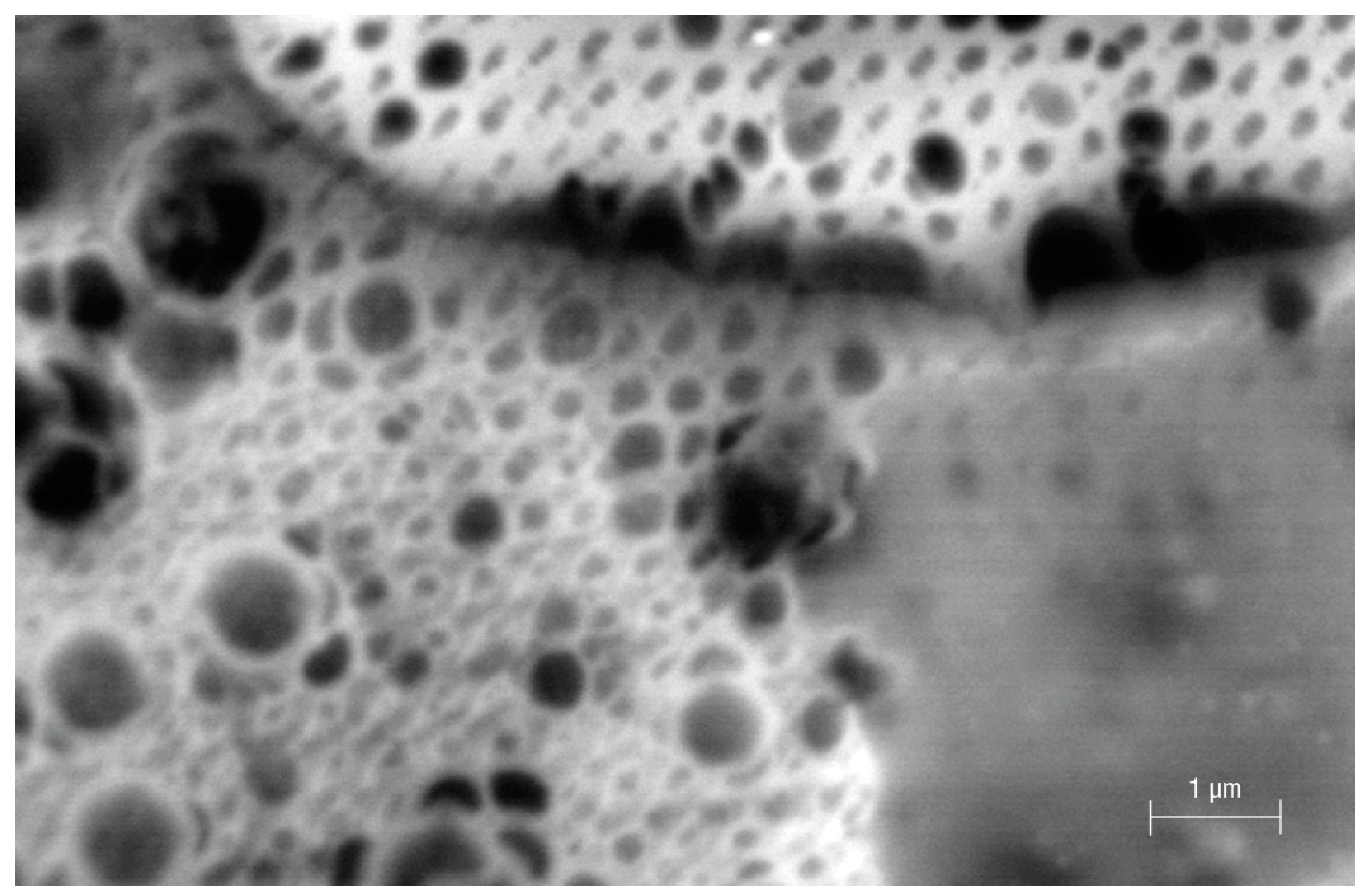
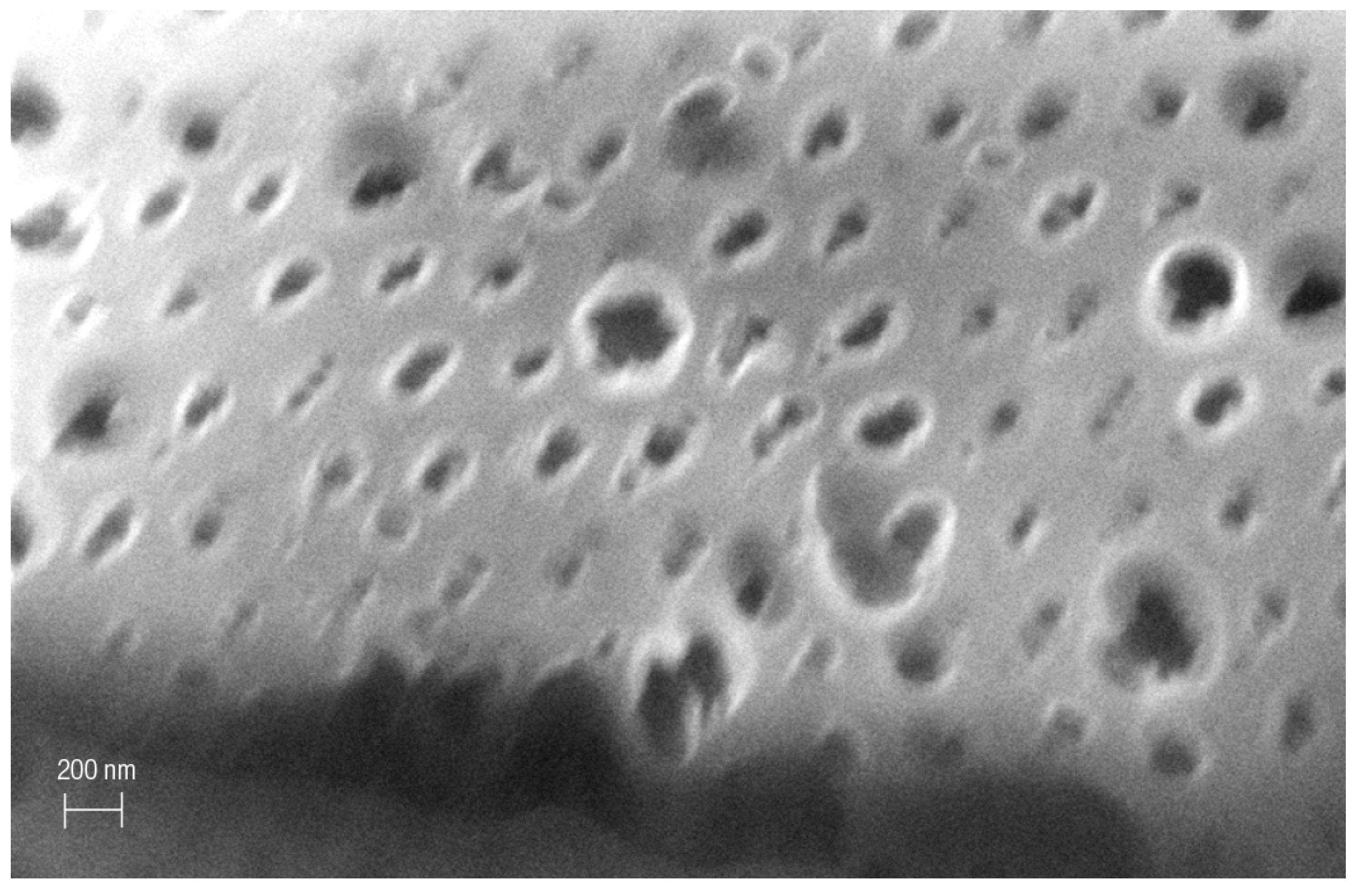
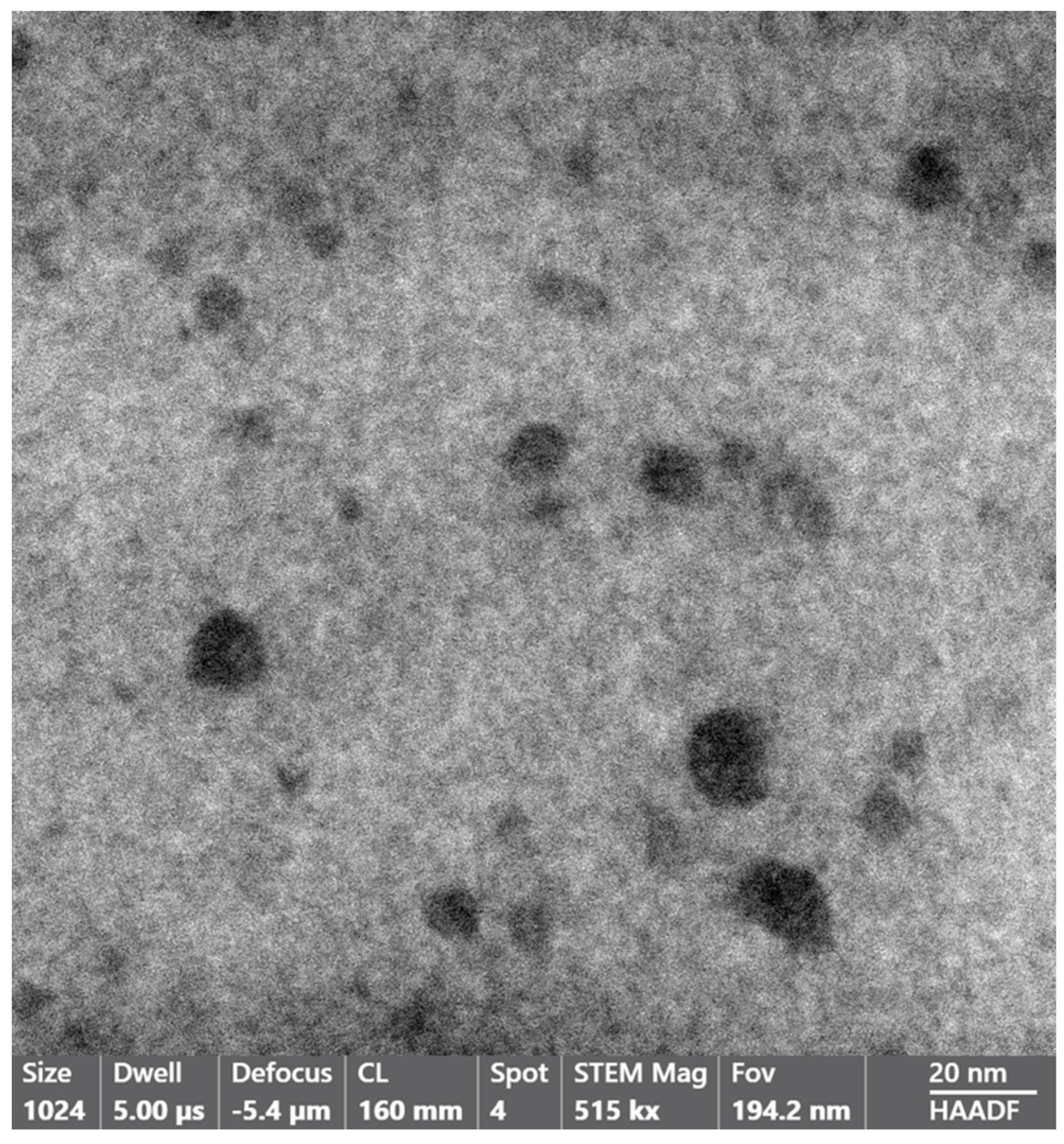
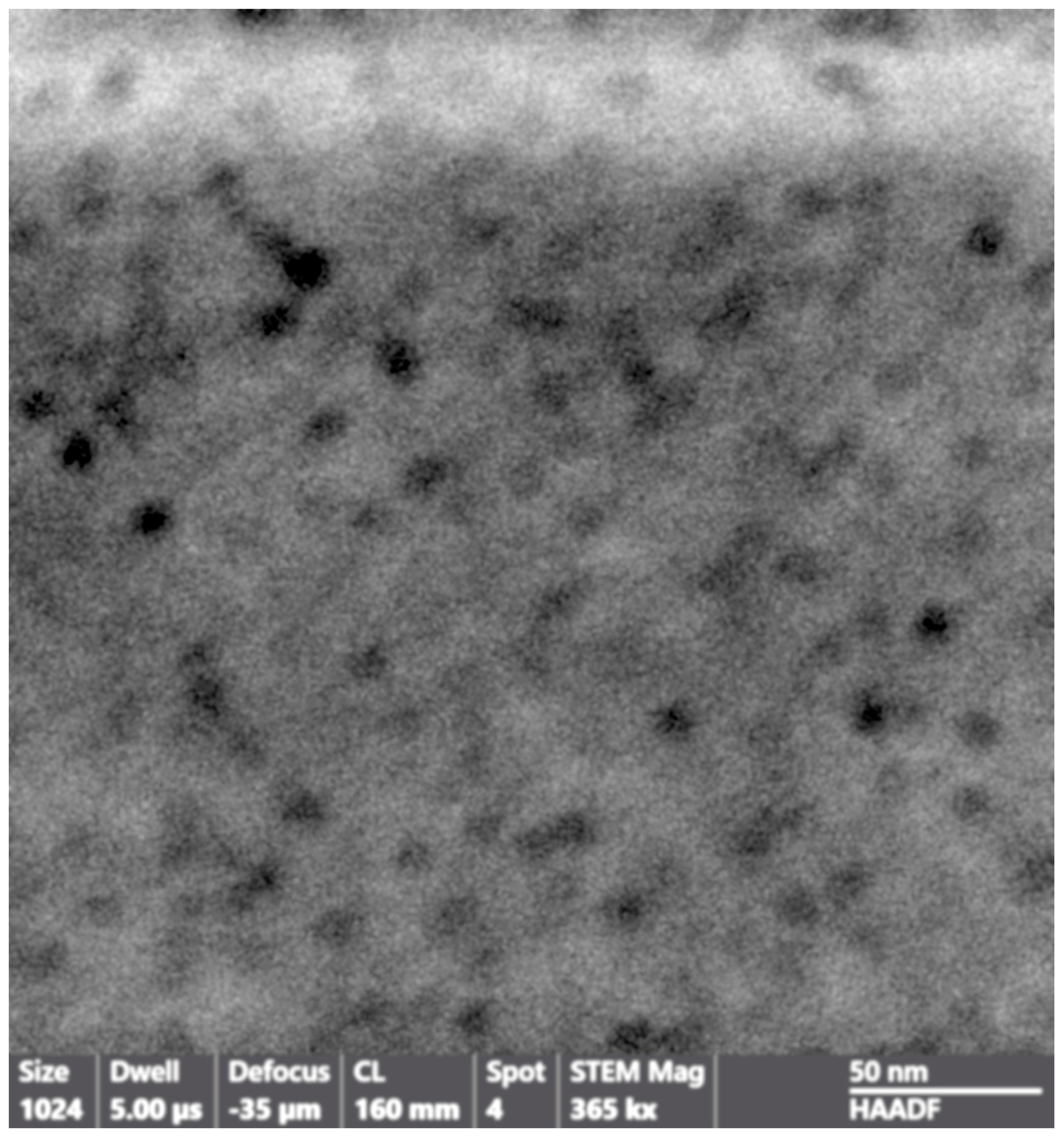
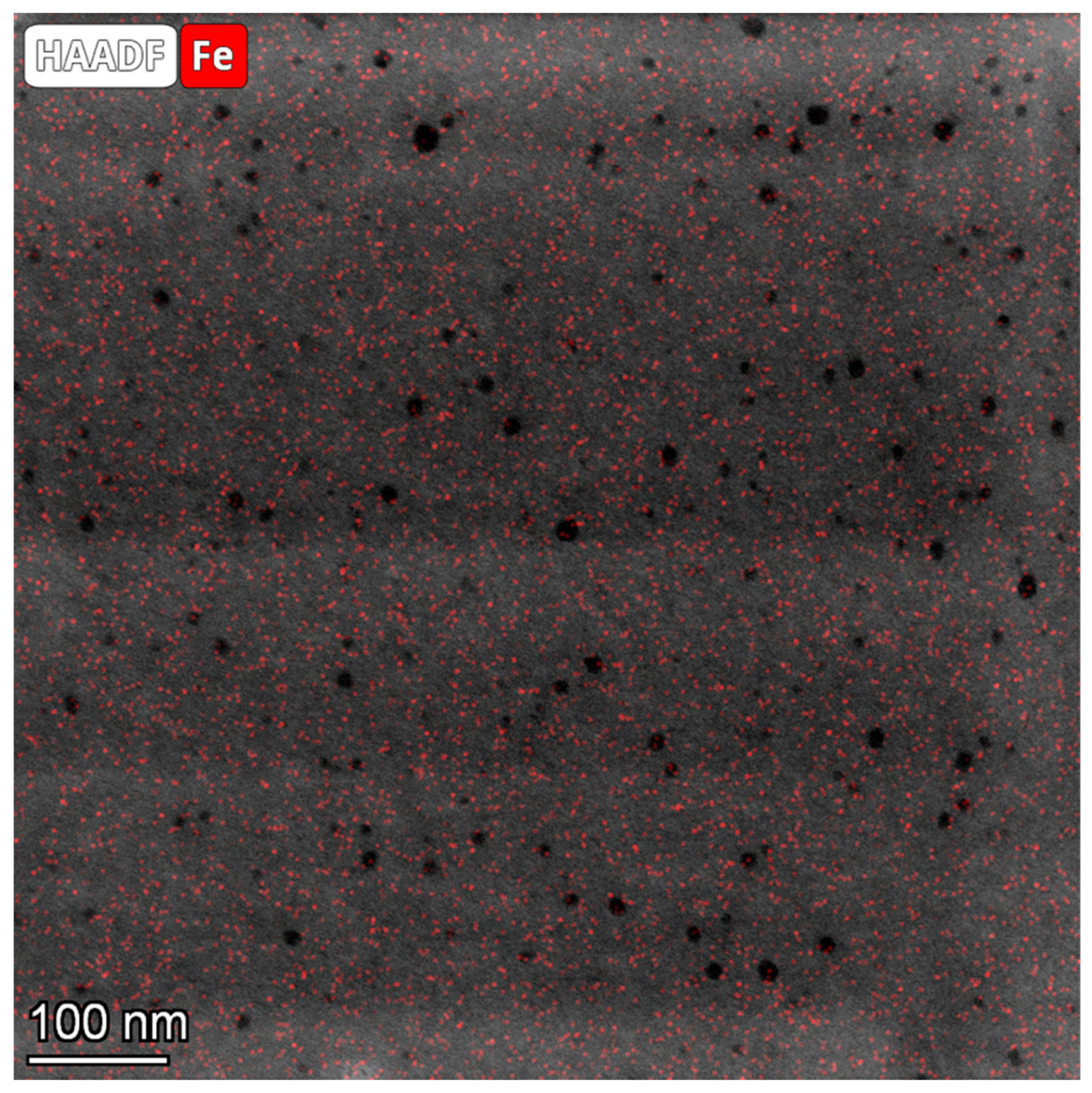
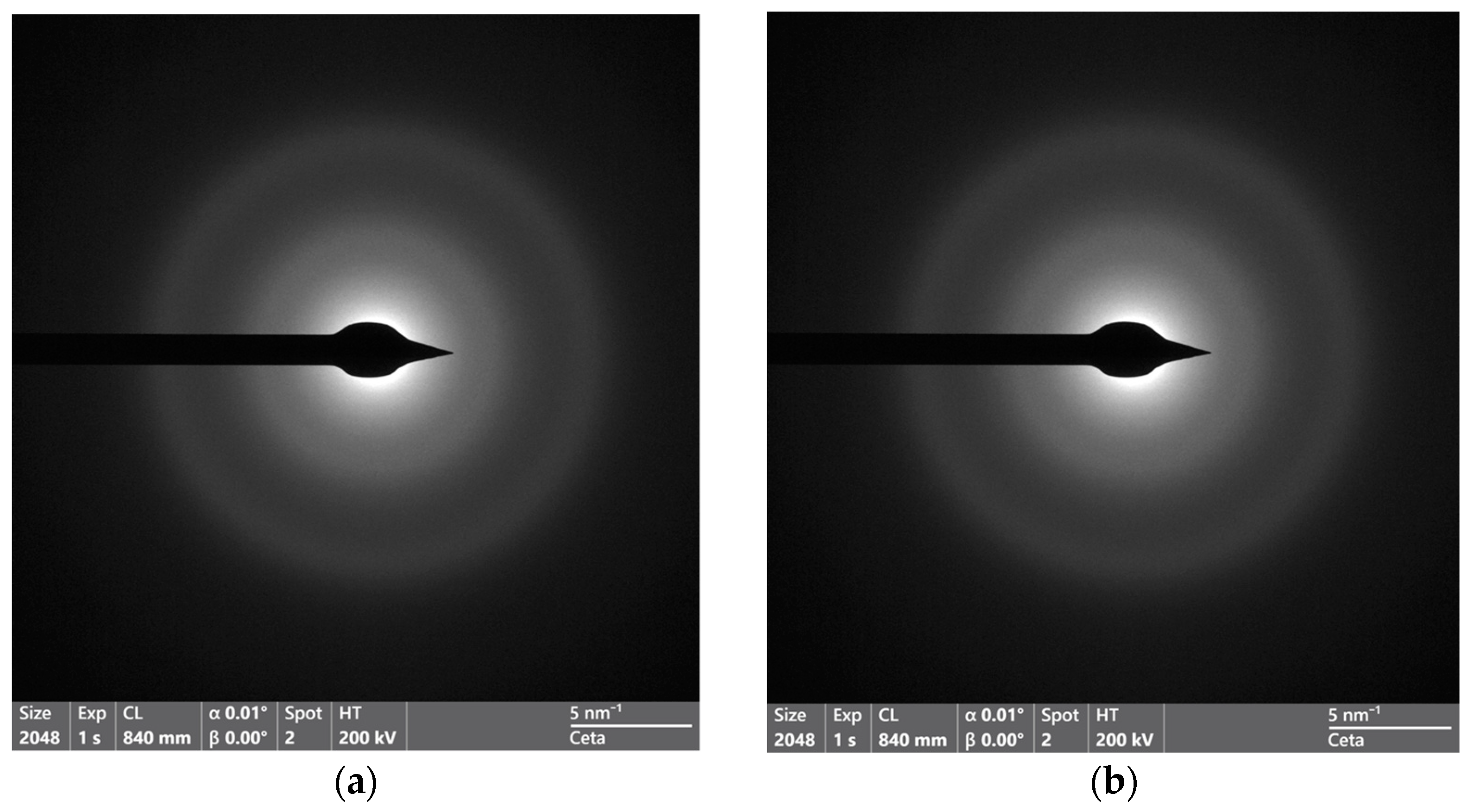
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.X.; Trewin, A.; Su, F.; Wood, C.D.; Niu, H.; Jones, J.T.A.; Khimyak, Y.Z.; Cooper, A.I. Microporous poly(tri(4-ethynylphenyl)amine) networks: Synthesis, properties, and atomistic simulation. Macromolecules 2009, 42, 2658–2666. [Google Scholar] [CrossRef]
- Kim, J.K.; Yang, S.Y.; Lee, Y.; Kim, Y. Functional nanomaterials based on block copolymer self-assembly. Prog. Polym. Sci. 2010, 35, 1325–1349. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Fritsch, D. Polymers of Intrinsic Microporosity Derived from Bis(phenazyl) Monomers. Macromolecules 2008, 41, 1640. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Thomas, S.; Guiver, M.D. Polymers of Intrinsic Microporosity Containing Trifluoromethyl and Phenylsulfone Groups as Materials for Membrane Gas Separation. Macromolecules 2008, 41, 9656–9662. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Dorney, B.; White, D.; Kirklin, S.; Zapol, P.; Yu, L.; Liu, D.J. Microporous polyphenylenes with tunable pore size for hydrogenstorage. Chem. Commun. 2010, 46, 4547–4549. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Adams, D.J.; Cooper, A.I. Chemical tuning of CO2 sorption in robust nanoporous organic polymers. Chem. Sci. 2011, 2, 1173–1177. [Google Scholar] [CrossRef]
- Schwab, M.G.; Lennert, A.; Pahnke, J.; Jonschker, G.; Koch, M.; Senkovska, I.; Rehahn, M.; Kaskel, S. Nanoporous copolymer networks through multiple Friedel-Crafts-alkylation-studies on hydrogen and methane storage. J. Mater. Chem. 2011, 21, 2131–2135. [Google Scholar] [CrossRef]
- Martín, C.F.; Stöckel, E.; Clowes, R.; Adams, D.J.; Cooper, A.I.; Pis, J.J.; Rubiera, F.; Pevida, C. Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. J. Mater. Chem. 2011, 21, 5475–5483. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Huang, B.; Bai, F.; Yang, X. Synthesis of pH-sensitive hollow polymer microspheres and their application as drug carriers. Polymer 2009, 50, 3556–3563. [Google Scholar] [CrossRef]
- Pulko, I.; Wall, J.; Krajnc, P.; Cameron, N.R. Ultra-High Surface Area Functional Porous Polymers by Emulsion Templating and Hypercrosslinking: Efficient Nucleophilic Catalyst Supports. Chem. Eur. J. 2010, 16, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Danish, E.; Cameron, N.R.; Kataky, R. Emulsion-templated porous materials (PolyHIPEs) for selective ion and molecular recognition and transport: Applications in electrochemical sensing. J. Mater. Chem. 2007, 17, 2446–2453. [Google Scholar] [CrossRef]
- Fu, R.; Li, Z.; Liang, Y.; Li, F.; Xu, F.; Wu, D. Hierarchical porous carbons: Design, preparation, and performance in energy storage. New Carbon Mater. 2011, 26, 171–179. [Google Scholar] [CrossRef]
- Fiore, A.M.; Varvaro, G.; Agostinelli, E.; Mangone, A.; De Giglio, E.; Terzano, R.; Allegretta, I.; Dell’Anna, M.M.; Fiore, S.; Mastrorilli, P. Synthesis and Use in Catalysis of Hematite Nanoparticles Obtained from a Polymer Supported Fe(III) Complex. Eur. J. Inorg. Chem. 2022, 2022, e202100943. [Google Scholar] [CrossRef]
- Zhou, W.; Qu, Q.; Yu, W.; An, Z. Single Monomer for Multiple Tasks: Polymerization Induced Self-Assembly, Functionalization and Cross-Linking, and Nanoparticle Loading. ACS Macro Lett. 2014, 3, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U.; Rheinberger, V. Reaction behaviour of monomeric β-ketoesters. Polym. Bull. 1994, 32, 411–417. [Google Scholar] [CrossRef]
- Viljanen, E.K.; Skrifvars, M.; Vallittu, P.K. Dendritic copolymers and particulate filler composites for dental applications: Degree of conversion and thermal properties. Dent. Mater. 2007, 23, 1420–1427. [Google Scholar] [CrossRef]
- Bertolin, M.; Zecca, M.; Faveno, G.; Palma, G.; Lora, S.; Ajo, D.; Corain, B. High-yield γ-ray-induced polymerization of bis-2-acetoacetoxyethyl methacrylate copper. II. J. Appl. Polym. Sci. 1997, 65, 2201–2207. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Pomposo, J.A. Efficient synthesis of single-chain polymer nanoparticles via amide formation. J. Nanomater. 2015, 2015, 723492. [Google Scholar] [CrossRef]
- Fiore, A.M.; Romanazzi, G.; Dell’Anna, M.M.; Latronico, M.; Leonelli, C.; Mali, M.; Rizzuti, A.; Mastrorilli, P. Mild and efficient synthesis of secondary aromatic amines by one-pot stepwise reductive amination of arylaldehydes with nitroarenes promoted by reusable nickel nanoparticles. Mol. Catal. 2019, 476, 110507. [Google Scholar] [CrossRef]
- Romanazzi, G.; Fiore, A.M.; Mali, M.; Rizzuti, A.; Leonelli, C.; Nacci, A.; Mastrorilli, P.; Dell’Anna, M.M. Polymer supported Nickel nanoparticles as recyclable catalyst for the reduction of nitroarenes to anilines in aqueous medium. Mol. Catal. 2018, 446, 31–38. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Nobile, C.F.; Marchese, G. Cobalt(II) and iron(III) complexes with 2-(acetoacetoxy)ethylmethacrylate: Potential precursors of hybrid catalysts. Inorganica Chim. Acta 1995, 233, 65–69. [Google Scholar] [CrossRef]
- Miranda, M.A.R.; Sasaki, J.M. The limit of application of the Scherrer equation. Acta Crystallogr. Sect. A 2018, 74, 54–65. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, A.M.; Fiore, S.; Huertas, F.J.; Mastrorilli, P. Direct Observations of Ordered Nanoporosity in Deprotonated 2-(Acetoacetoxy)ethyl Methacrylate (AAEMA–) Polymer: Preliminary Results. Polymers 2024, 16, 58. https://doi.org/10.3390/polym16010058
Fiore AM, Fiore S, Huertas FJ, Mastrorilli P. Direct Observations of Ordered Nanoporosity in Deprotonated 2-(Acetoacetoxy)ethyl Methacrylate (AAEMA–) Polymer: Preliminary Results. Polymers. 2024; 16(1):58. https://doi.org/10.3390/polym16010058
Chicago/Turabian StyleFiore, Ambra M., Saverio Fiore, F. Javier Huertas, and Piero Mastrorilli. 2024. "Direct Observations of Ordered Nanoporosity in Deprotonated 2-(Acetoacetoxy)ethyl Methacrylate (AAEMA–) Polymer: Preliminary Results" Polymers 16, no. 1: 58. https://doi.org/10.3390/polym16010058
APA StyleFiore, A. M., Fiore, S., Huertas, F. J., & Mastrorilli, P. (2024). Direct Observations of Ordered Nanoporosity in Deprotonated 2-(Acetoacetoxy)ethyl Methacrylate (AAEMA–) Polymer: Preliminary Results. Polymers, 16(1), 58. https://doi.org/10.3390/polym16010058






