Abstract
Non-isocyanate polyurethane (NIPU) networks physically modified with octa(3-hydroxy-3-methylbutyldimethylsiloxy)POSS (8OHPOSS, 0–10 wt%) were conditioned in environments of different relative humidities (up to 97%) to study water–polymer interactions. The equilibrium sorption isotherms are of Brunauer type III in a water activity range of 0–0.97 and are discussed in terms of the Guggenheim (GAB) sorption model. The study shows that the introduction of 8OHPOSS, even in a large amount (10 wt%), does not hinder the water affinity of the NIPU network despite the hydrophobic nature of POSS; this is attributable to the homogenous dispersion of POSS in the polymer matrix. The shift in the urethane-derived carbonyl bands toward lower wavenumbers with a simultaneous shift in the urethane N-H bending bands toward higher wavenumbers exposes the breakage of polymer–polymer hydrogen bonds upon water uptake due to the formation of stronger water–polymer hydrogen bonds. Upon water absorption, a notable decrease in the glass transition temperature () is observed for all studied materials. The progressive reduction in with water uptake is driven by plasticization and slaving mechanisms. POSS moieties are thought to impact slaving indirectly by slightly affecting water uptake at very high hydration levels.
1. Introduction
Non-isocyanate polyurethanes (NIPUs) have attracted a great deal of attention in recent years, and numerous studies have been conducted on the development of new synthesis routes [1,2,3]. The most promising and the most widely applied is the aminolysis of cyclic carbonate, which leads to the formation of polyhydroxyurethanes (PHUs). One of the most important differences between PUs and PHUs is the formation of additional hydroxyl groups in close vicinity to the urethane moiety (Figure 1). Such hydroxyurethane groups strongly affect the interaction between PHUs and organic solvents and cause a relatively high affinity of PHUs towards water [4]. Thus, PHUs, if not modified by the introduction of other, less polar moieties, such as amide groups [5], exhibit significantly higher water absorption than PUs [6,7,8,9,10,11]. This makes polyhydroxyurethanes suitable for applications as hydrogels [12,13] and absorbents [14].
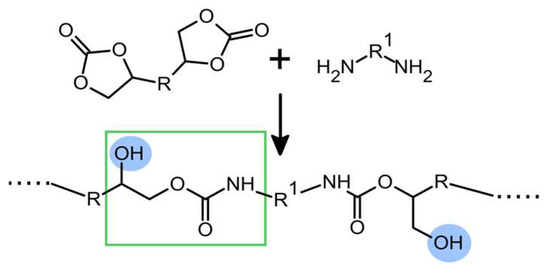
Figure 1.
Scheme of polyhydroxyurethane formation by aminolysis of cyclic carbonate. Hydroxyl groups are highlighted in blue and hydroxyurethane moiety is shown in the green rectangle.
As the modification of polyurethanes leads to advantageous results, the impact of different types of additives on the properties of NIPUs has been studied [15,16,17,18,19], including polyhedral oligomeric silsesquioxanes (POSS) [20,21,22,23,24]. POSS consists of a group of hybrid (organic/inorganic) molecules that are characterized by a well-defined silica cage core that is usually functionalized by reactive or non-reactive substituents (Figure 2) [25].
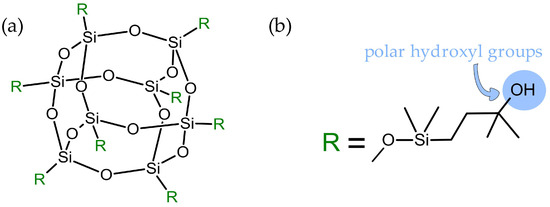
Figure 2.
General structure of closed-cage substituents (R) might be of the same chemical nature or they can vary (a), and structure of the substituents in the octa(3-hydroxy-3-methylbutyldimethylsiloxy) POSS used in this study (b).
The characteristics of the substituents affect the type of interaction between POSS and the polymer matrix [26,27,28]. POSS may be introduced chemically or physically into the polymer structure [29,30,31,32]. Chemical introduction is feasible if substituents attached to the POSS cage are equipped with reactive groups (in the given system) that would allow for covalent bonding with the chain [33,34]. The physical introduction of POSS occurs when POSS-bearing inert groups are introduced into the polymer matrix to form a (nano)composite [35]. Generally, the physical blending of POSS in a polymer matrix is associated with partial agglomeration of POSS since there is a high surface free energy and a high affinity of nanoparticles [35,36,37].
Subsequently, the type of POSS and the method of its introduction into the polymer matrix will influence the properties of the resultant hybrid composites. POSS may positively influence biocompatibility [38,39], flame retardancy [40,41], mechanical properties [37,38,42], etc. However, to achieve the desired effect, proper selection of POSS and the polymer matrix is crucial [28].
Recent studies on NIPU modification with POSS focus mainly on systems where POSS is introduced covalently into the polymer chain [3,20,21,22,23,24,43,44,45,46,47,48,49]. The impact of chemically incorporated POSS in such systems on mechanical and thermal properties has been widely studied. However, little attention has been paid to NIPU/POSS physical blends, and even less attention has been paid to determining the impact of POSS on the hydration properties of NIPUs, even though the high water absorption seems to be a dominant feature for this kind of material. Taking into account the biocompatibility of POSS [50,51] and the possible application of NIPUs in biomedical fields (e.g., in wound treatment) [52,53], shedding light on the influence of POSS on water–polymer interactions in such systems is interesting from the point of view of polymer and material science. It should be noted that although water–polymer interactions attract a lot of attention due to their obvious significance in biotechnology [54,55], more often than not, the studies are performed in solutions, i.e., in an environment of abundant water. Less attention has been paid to the initial stages of hydration and the interaction of the polymer with humid environments, as opposed to direct interaction with water in a solution. However, this low-hydration regime is interesting from the fundamental science point of view as well as from the application side, i.e., in the case of mucosal drug delivery.
In a previous article, we studied hydrophilic NIPU networks physically modified with octa(3-hydroxy-3-methylbutyldimethylsiloxy)POSS (8OHPOSS) with respect to their morphology, hydrogen bonding, and glass transition in a dry state [56]. We found in this previous work that POSS disperse excellently in the NIPU network, possibly thanks to the affinity of the hydroxyl groups on POSS vertices with the polymeric chain. Changes in the glass transition were attributed to competition between mechanisms accelerating or decelerating mobility. Moreover, we studied the interaction of the systems with water in abundance and observed that POSS enhances water absorption in a saline solution.
Since our previous study showed that POSS/NIPU networks may act as hydrogels, we now focus our attention on studying polymer–water interactions in such systems in detail. POSS is a promising additive in biomaterials [57] and, therefore, its influence on water–polymer interactions is paramount from the point of view of applications. In the work at hand, we extend the research on the very same materials [56] to their hydration properties in humid environments. Polymer–water interactions are studied by conditioning materials in a range of progressively increasing relative humidity environments. Materials are studied with respect to their water uptake and its influence on hydrogen bonding and molecular mobility. The GAB sorption model is used to determine the monolayer capacity and interaction parameters.
2. Materials and Methods
2.1. Materials
The materials studied in this work, i.e., a series of NIPUs networks physically modified with octa(3-hydroxy-3-methylbutyldimethylsiloxy)POSS (8OHPOSS), are the same as those described in Ref. [56]. Their synthesis, structure, and micromorphology are described in detail in this previous publication. Briefly, poly(ethylene oxide)-based cyclic carbonate (PEO-CC, Specific Polymers, Castries, France) of Mw~700 g/mol was mixed with hyperbranched polyethyleneimine (PEI, Sigma-Aldrich, Darmstadt, Germany) of Mw~650 g/mol to form a polyhydroxyurethane network. The reaction was carried out at 50 °C in dimethylacetamide (DMAc, Pol-Aura, Zabrze, Poland) using 1,5,7-Triazabicyclodec-5-ene (TBD, Sigma-Aldrich, Darmstadt, Germany) as a catalyst. 8OHPOSS (Hybrid Plastics, Hattiesburg, MI, USA) was added at the beginning of the reaction, along with all other components.
Phosphorus pentoxide (P4O10) and all salts listed in Table 1 were supplied by Chempur (Piekary Śląskie, Poland). Salts were added to distilled water to prepare saturated solutions to control the humidity.

Table 1.
Salts used to prepare solutions for conditioning materials and corresponding relative humidity levels of their saturated solutions [2].
2.2. Specimen Conditioning
Specimens of ~250 mg were first dried at 80 °C for 48 h under vacuum and subsequently conditioned in a desiccator over P4O10 in order to remove any residual water [58]. The mass values obtained after the specimens reached equilibrium in P4O10 were considered the masses of dry materials (). Subsequently, the samples were conditioned at progressively increasing levels of relative humidity (rh) (Figure 3) provided by saturated aqueous salt solutions (Table 1) [59]. The procedure was carried out at 25 °C. The equilibrium state was assumed when no further change in specimen mass was recorded between measurements with a two-day time interval. The time needed for reaching the equilibrium state was approximately 2–3 weeks per hydration level.
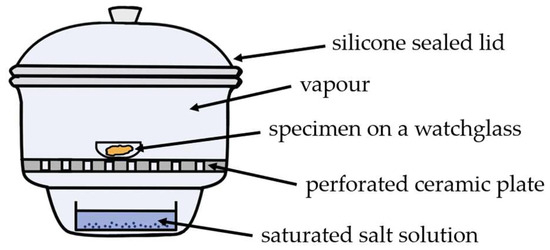
Figure 3.
Schematic representation of specimen conditioning in a desiccator over saturated salt solutions.
After mass equilibrium was reached at the given rh level, the specimens were weighed on an analytical scale with a precision of 0.1 mg. Water uptake (WU) was calculated as in Equation (1):
where is the mass of dry specimens (after conditioning over phosphorus pentoxide) and is a mass of hydrated specimen at a given rh level.
Alongside these samples, smaller pieces (~5–6 mg) were placed in perforated aluminum pans for DSC experiments (Section 3.3) and pieces of mass of ~300 mg, which were used for the FTIR experiments (Section 3.2).
2.3. Analysis of Equilibrium Sorption Isotherms
To describe equilibrium sorption isotherms, the Guggenheim–Anderson–de Boer (GAB) model was fitted to the data. The GAB model constitutes a generalization of the BET model describing the sorption of the materials based on the theory of the formation of monolayers and further layers, the so-called multilayers. The GAB model (Equation (2)) takes into consideration the difference in interactions between monolayers and multilayers, as well as that between multilayers and “bulk” water. Therefore, the GAB model introduces a third parameter that is associated with the energy of the multilayer–bulk water interaction, while the parameter in the GAB model is a constant correlated with the monolayer–multilayer interaction energy [60,61].
—equilibrium water content in relation to dry mass
—monolayer (first sorption layer) moisture content
—energy constant logarithmically related to the difference in water molecule potential in the monolayer and multilayer
—energy constant logarithmically related to the difference in water molecule potential in the multilayer and in the “bulk”
—water activity
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
Fourier transform infrared (FTIR) spectroscopy analysis was carried out using a Thermo Scientific Nicolet iS5 spectrometer equipped with a diamond prism iD7 ATR Accessory (Waltham, MA, USA). Spectra were recorded in the wavenumber range of 4000–400 cm−1 with a data gap of 0.428 cm−1 and a scanning resolution of 4 cm−1.
2.5. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry measurements were conducted with an argon-purged differential scanning calorimeter Mettler Toledo 823e (Columbus, OH, USA), cooled by liquid nitrogen. The DSC curves were recorded in the temperature range of −100 °C to 30 °C with a 10 K/min heating/cooling rate. The glass transition temperature () values were calculated at the midpoint of the endothermic step. Due to the absorption of water, the masses varied through hydration levels and stayed in the general range of 5–9 mg. Samples for DSC measurements (one sample per material) were prepared in an aluminum pan with a perforated lid. DSC samples were conditioned alongside those for water vapor absorption measurements. The DSC measurements were conducted after the equilibrium state was reached.
3. Results and Discussion
NIPU networks at hand upon immersion in an aqueous medium exhibit water absorption in the range typical for hydrogels, and we have observed this kind of behavior in our previous work [56]. To study polymer–water interactions in such a system in detail, the changes in physicochemical properties of the NIPUs were monitored as a function of the environmental relative humidity (water vapor absorption).
3.1. Water Uptake and Equilibrium Sorption Isotherms
Figure 4 shows the water uptake as a function of POSS, recorded at all rh levels. With increasing relative humidity, water uptake differences between rh levels become more pronounced, showing that already-absorbed water increases the materials’ affinity towards water vapor. At low rh levels, no differences in the water absorption of specimens with different POSS contents are observed. At high relative humidities (>68%), slight differences emerge; however, their nature is not monotonous, and the observed differences in water uptake are rather small. Therefore, it can be concluded that 8OHPOSS does not hinder the water absorption of the studied materials, even at high POSS contents (10 wt%). This might originate from hydration sites still being accessible to water molecules, even at high POSS loadings. The addition of POSS of course reduces the number of NIPU-originating hydration points per unit mass; however, the effect is small (~10%) and likely compensated to some extent by the introduction of hydroxyl groups residing on the vertex groups of POSS. We will further follow this point with a formal analysis of equilibrium sorption isotherms.
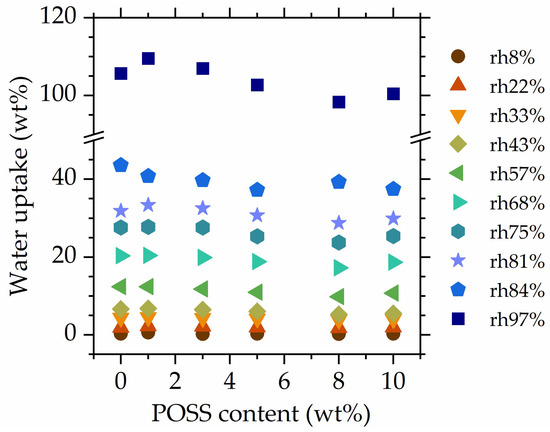
Figure 4.
Water uptake as a function of the POSS content at all studied rh levels.
Equilibrium sorption isotherms (ESI) (Figure 5a) show a Brunauer type III form [62], which is observed mainly for systems such as biopolymers, microporous polymeric adsorbents, and water-soluble solids [63,64,65], and suggests sorption through the capillary condensation mechanism [66]. Interestingly, ESI of type III is often observed for conventional and non-isocyanate polyurethanes [5,67,68]. Slight differences in water uptake (Figure 5a) vanish when water absorption is normalized to the polymer mass (Figure 5b), which supports the previous statement regarding the lack of POSS influence on the water absorption, despite the fact that silica fillers could decrease water absorption (g water/g polymer) in composite materials [69].
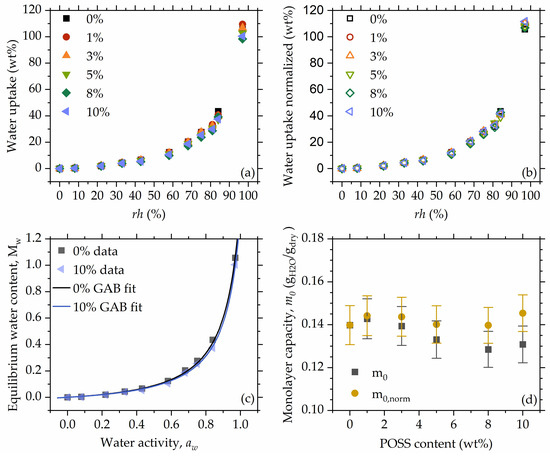
Figure 5.
Equilibrium sorption isotherms (a), sorption isotherms with water uptake normalized to polymer mass (b), fitted curves shown for 0% and 10% as examples (c), monolayer capacity obtained from the model fitting as calculated (), and normalized to polymer content () (d).
To characterize materials in terms of monolayer capacity, the Guggenheim–Anderson–de Boer (GAB) model was fitted (Figure 5c). Preliminary fits did not show any significant differences in the C and K values, while significant covariances were observed. This stability suggests that the apparent interaction strength between the polymer chain and water situated in the monolayer is constant through the whole composition range, i.e., POSS does not influence polymer–monolayer water interactions. To increase the statistical significance of the monolayer capacity and interaction parameters, a second fit was performed simultaneously for all sorption datasets, assuming and values as constant throughout the POSS content range. The resulting values for and were 0.63 ± 0.071 and 0.918 ± 0.004, respectively. The parameter exhibits a rather low value compared to the values reported in the literature for similar systems [67,68]; however, it remains in the range characteristic for Brunauer type III isotherms [70]. The parameter is close to 1.0 and of the value is usually reported for the materials exhibiting Brunauer type III behavior [67,68]. The closer the parameter is to unity, the more similar the behavior of the formed multilayers is to the behavior of liquid water [71].
Monolayer capacity () showed a slightly decreasing trend with increasing POSS content; however, after normalizing it to the polymer mass in the material, the values are stable throughout the entire composition range (Figure 5d). This points to the assumption that the absorbed water is located on the polymer chain, especially at lower water uptakes, and not around POSS molecules. Taking also into account that there is no significant change in the energetic parameters and it may be concluded that 8OHPOSS does not hinder access of water to hydration sites and it does not “block” any of them. Although in [56] we observed some attachment of POSS on the polymer chain by hydrogen bonds, this does not hinder the attachment of the more mobile and more polar water molecules to access the hydration sites. The obtained values of monolayer capacity are around 14 g per 100 g of dry material, which is more than 25 times higher than in cellulose-based hydrogels studied by Filip et al. [72], 3.5 times higher than for acrylate hydrogels reported by Ferrer et al. [73], and 1.5 times the value reported for polyacrylamide-based superporous hydrogel composites reported by Mittal et al. [74].
3.2. Water Influence on Carbonyl Region—FTIR
Hydroxyurethane moieties are the most polar sites in the chains of studied NIPUs, and therefore they consist of the strongest (primary) hydration sites. Thus, one should expect that absorbed water molecules are most likely to attach to those moieties, prioritizing carbonyl as the most polar region of the hydroxyurethane group. The carbonyl stretching region is known to be sensitive to hydrogen bonding and usually consists of the region most commonly studied in terms of quantitative and/or qualitative analysis of HBs in polypeptides [75,76,77], polyureas [78,79], polyamides [80,81,82], conventional polyurethanes [83,84,85], and non-isocyanate polyurethanes [58,86,87]. Figure 6 shows, for all materials under investigation, FTIR spectra in the region corresponding to the stretching vibration of urethane-derived carbonyl (1630–1730 cm−1) and the bending vibration of urethane-derived NH groups (1500–1580 cm−1) at all studied hydrations. The spectra were recorded after equilibrium at a given hydration level was reached.
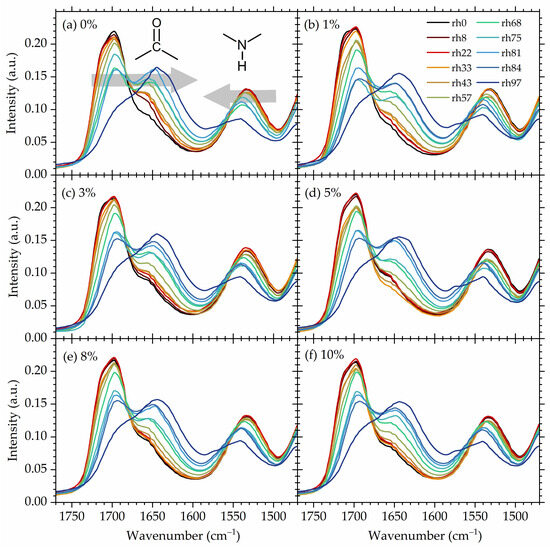
Figure 6.
FTIR spectra in the urethane region of all studied NIPUs at all hydration conditions. Spectra of 0% are taken from Ref. [56]. (a–f) spectra for different specimen as indicated in the plot. Arrows indicate the shift of the band with increasing hydration.
The carbonyl stretching region exhibits a complex nature with three main component bands overlapping, as is also usually observed for conventional polyurethanes [88,89,90]. Here, the components in the carbonyl stretching region are as follows (locations given in relation to the spectra of dry materials): 1720 cm−1 corresponding to the free carbonyls (i.e., non H-bonded), 1700 cm−1 corresponding to the weakly H-bonded ones, and 1660 cm−1 corresponding to the strongly H-bonded carbonyls (e.g., forming two or more hydrogen bonds) [58].
Upon water uptake (upon an increase in the rh level), the free C=O component gradually vanishes, while two other components shift towards a lower wavenumber (redshift). The shift towards a lower wavenumber of stretching modes indicates the formation of new hydrogen bonds and/or the strengthening of the already existing HBs [91,92,93]. In this study, the red shift in C=O stretching modes upon water absorption is related to the formation of water–polymer hydrogen bonds that either replace the polymer–polymer HBs (breakage of C=O⋯H-N hydrogen bonds and formation of C=O⋯H2O resulting from competition between water O-H and amide N-H for C=O acceptor sides [94]) or are formed as additional hydrogen bonds. In both cases, the resulting hydrogen bonding of C=O becomes stronger since hydroxy moieties form with carbonyl stronger hydrogen bonds in comparison to NH groups. The stronger hydrogen bonding of C=O⋯H-O compared to C=O⋯H-N results from the higher polarity of the OH bond compared to the NH bond [95]. The strength of hydrogen bonds influences the strength—the bigger the shift, the stronger the hydrogen bonding [96,97,98,99].
Simultaneously, the band associated with urethane NH bending gradually shifts towards higher wavenumbers (blue shift) [100], suggesting that polymer NH groups are also engaged in new, stronger interactions with water [5,101]. It is commonly observed that upon the formation of hydrogen bonds and/or the strengthening of existing ones, bands corresponding to the bending vibrations shift in the direction opposite to that of the stretching bands, i.e., towards higher wavenumbers (blue shift) [102,103,104,105,106,107].
The above originates from the inversely proportional relation between the stretching mode frequency and the bending mode frequency, that is, the lower the stretching mode frequency, the higher the bending mode frequency [105]. Upon the formation of X-H⋯Y hydrogen bonds, the X-H bonds undergo elongation (usually explained by electrostatic interaction or hyperconjugation), which causes stronger polarization of the X-H bond and leads to increased interaction with H⋯Y [95]. This elongation results in the weakening of the X-H bond due to the strengthening of H⋯Y [108]. The weakening of X-H enables stretching of this bond at a lower energy cost, and thus the shift in stretching modes towards lower wavenumbers is observed. Simultaneously, in the formed X-H⋯Y, stabilized complex forces of the X-H bond and H⋯Y interaction seem to promote the positioning of the X-H bond in the plane and therefore out-of-plane bending modes are more energetically expensive. Since the wavenumber is directly proportional to the energy [109], a shift toward a higher wavenumber is observed for the bending modes upon hydrogen bond formation. The opposing behavior of the stretching and bending modes upon HB formation is shown schematically in Figure 7. The above is consistent with the reported blue shift of the water bending modes upon the formation of a more dense hydrogen-bond network [110].
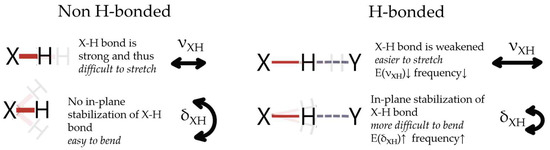
Figure 7.
Schematic representation of the opposite shift-wise response of stretching and bending modes to the formation/strengthening of hydrogen bonds. Details in text.
Turning our attention to the influence of POSS on hydrogen bonding (Figure 8), it is interesting to observe that at low hydrations ( 8%), POSS has some influence on the strength of the component of the carbonyl band assigned to strongly bonded carbonyls. This is on par with what was observed for dry materials [56]. However, at high hydrations, spectra of all materials coincide, indicating that POSS now does not play any significant role in hydrogen bonding of the carbonyl bands, presumably because water now dominates the behavior of the system.
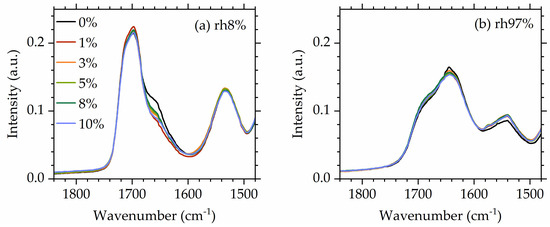
Figure 8.
Comparative FTIR spectra in the urethane region for all studied NIPUs at (a) a low humidity (8%) and (b) a high humidity (97%).
As shown in Section 3.3, the replacement of polymer–polymer C=O⋯H-N hydrogen bonds with polymer–water C=O⋯H-O bonds increases the system’s mobility despite the increased strength of hydrogen bonding since now polymer forms HBs with small and mobile water molecules; as a result, a decrease in values upon water uptake is observed.
3.3. Influence of Absorbed Water on Glass Transition Temperature
The DSC profiles recorded for the matrix after conditioning at progressively increasing hydration levels (Figure 9a) up to rh 84% contain only a glass transition step and show a gradual decrease in values. This is a very common phenomenon and results from increasing water uptake—water acts as a plasticizing agent that increases molecular mobility of the system and, therefore, reduces . However, the mechanisms that drive it are more complicated. In the following, we will discuss them in detail for the system at hand. It is interesting to note that XRD experiments showed no signs of crystallinity in the composites upon hydration. Hence, we believe that the system is still homogenous at high hydrations. It is possible for physically introduced fillers to agglomerate when the mobility of the polymer increases. Here, however, we have no indication of such behavior, which might be attributed to molecules of absorbed water acting as a barrier that prevents POSS agglomeration.
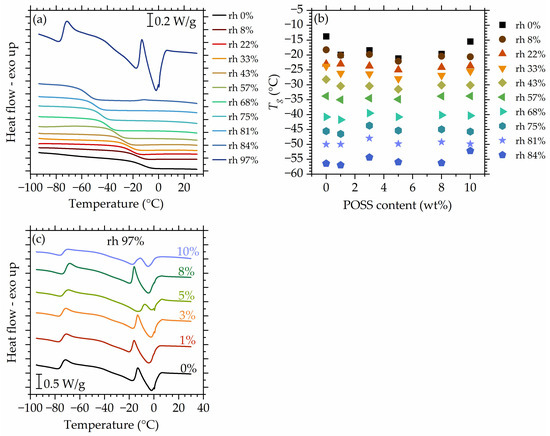
Figure 9.
DSC curves recorded for the matrix at all studied hydrations. (a) Glass transition temperature as a function of the POSS content for materials studied at values up to 84% (b), DSC curves recorded for all studied materials after conditioning at 97% (c). Data for dry materials are taken from Ref. [56].
Figure 9b shows values as a function of POSS content for all studied hydrations. With increasing hydration, a slight deviation of observed in the studied materials at a dry/low hydration state vanishes in the range of medium hydrations, as is expected, since POSS does not hinder water uptake or water–polymer interactions as shown earlier in this work. At rh 84%, a slight deviation in values is observed. This will be explained further in the text.
Figure 9c shows the DSC curves recorded for the materials after conditioning at rh 97%. At this rh level, the effects of ‘free’ water appear, which were not visible on the curves recorded for lower hydrations (Figure 9a). At −15 °C to −10 °C, cold crystallization [111] occurs and is followed by a melting just below 0 °C. The temperature of cold crystallization varies slightly and monotonously between materials. Interestingly, another endothermic event is present at around −70 °C, in the temperature range where the glass transition of the system would be expected. The origin of this event is unclear at this point. A plausible hypothesis is that it reflects a low-temperature cold crystallization of water, triggered by the glass transition of the system, now masked by the exothermic event.
Plotting versus water uptake (Figure 10a) and normalized water uptake to the polymer mass (Figure 10b) show that the differences between mobility in the studied materials occur rather at the dry/low hydrated state. The highest deviations are visible at rh 87%. Moreover, at the initial hydration stages (at low water uptakes), a very steep decrease in is observed, despite the low water content in the materials.
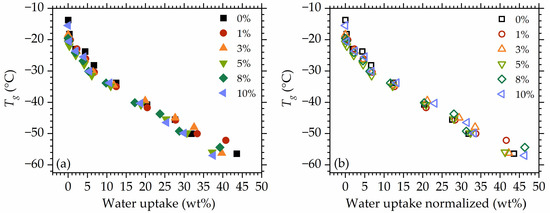
Figure 10.
as a function of water uptake (a) and water uptake normalized to the polymer mass (b).
Looking closely at the change in values with water uptake (Figure 11), it can be concluded that at least two mechanisms drive the reduction in , as two main different changes in slope might be distinguished. reduction is often described as plasticization; however, the latest studies use the term plasticization to describe only one of the mechanisms that drive the decrease in in hydrated polymers [56,57]. Therefore, to avoid terminology conflict, the decrease in the glass transition will be referred to as reduction, while plasticization will be used to describe the water-driven increase in polymer mobility that occurs due to the increase in the flexibility of the polymer chain [112].
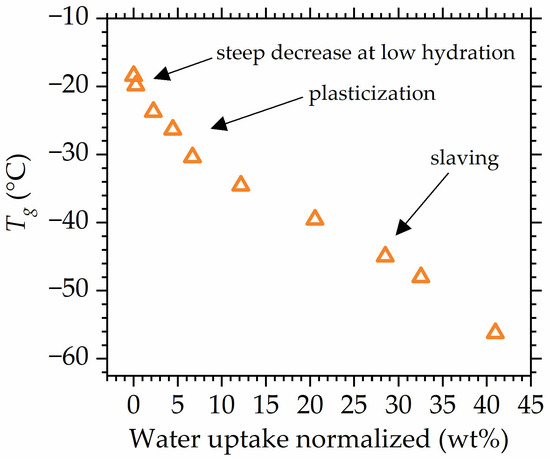
Figure 11.
as a function of water uptake with changes in slope assigned to mechanisms (plasticization, slaving) driving decrease. Data for composite with 3 wt% POSS are shown as an example, all other studied materials exhibit the same behavior.
Plasticization is observed in low to medium hydrations; however, at high relative humidities, another effect seems to impact the mobility of polymer–water systems. At high hydrations, when the amount of absorbed water is high enough for bulk water to be formed, the water gains its own mobility. As a result, water mobility now determines polymer mobility in a sense that the dynamics of bulk water and dynamics of polymer are now coupled and the observed glass transition of the systems is a result of cooperative motion between two components that possess their own dynamics [113]. Therefore, water possessing it own mobility drives reduction in a different manner than in the case of plasticization, which is seen as a pronounced change in slope in the data points (Figure 11). The above is referred to as slaving, since polymer mobility is “slaved” by bulk water [112,114,115,116,117].
The fact that in the range of the medium hydration levels, the dependency is the same for all studied materials shows that POSS does not influence the plasticization of the polymer matrix. However, at high hydrations, where slaving is the dominant phenomenon, the deviation between specimens’ behavior becomes more pronounced, suggesting that POSS slightly impacts the slaving mechanism. This impact might result from the hindering of water mobility by the dispersed POSS molecules that possess hydrophobic silica cages. Therefore, as shown in Figure 6, weak differences between values of the specimens appear at high hydrations. Those deviations exhibit a different nature from the ones spotted for dry/low-hydrated materials, which suggests that they are of a different origin. Thus, the most plausible explanation is the disclosure of POSS–water interactions at high hydrations (high water uptakes) that hinder water mobility. The above might be related to the fact that at very high water uptake, most of the free volume in the material is already hydrated, and now water molecules attach to the hydroxy moieties at the ends of POSS substituents.
4. Conclusions
POSS-modified NIPU networks exhibit a high affinity for absorbing moisture from the air that allows for water uptake typical of hydrogels even when the materials are not immersed in water (water uptake up to 115 wt% in rh 97%). POSS, despite its heavy hydrophobic silica cage, does not hinder water absorption capacity, even at high loadings (10 wt%)—it shows that 8OHPOSS, due to its endcapping OH polar groups, is able to disperse homogenously in the matrix and that this homogeneity is retained upon water absorption. The above shows that 8OHPOSS might be successfully used as filler for the modification of other properties, without a negative influence on materials’ affinity towards moisture/water absorption—normalizing water uptake to the polymer mass yields the same values for all studied composites. Moreover, according to the fitted GAB model, water absorption capacity is not inhibited by POSS.
Absorbed water causes cleavage of polymer–polymer and polymer–POSS hydrogen bonds due to the formation of stronger water–polymer bonds, as indicated by a red shift in the urethane-derived C=O band and the simultaneous blue-shift in urethane NH bending modes. The above results in a reduction in values upon hydration. The reduction occurs according to two mechanisms—plasticization and slaving. Plasticization occurs at low to medium hydrations, where absorbed water promotes the elasticity of the polymer chain. Slaving is observed at higher hydrations, where the amount of absorbed water is high enough to cause the formation of bulk water that possesses its own dynamics.
The 8OHPOSS does not influence the changes in as a function of water uptake or relative humidity, which is to be expected since the introduced POSS does not hinder the water absorption capacity and polymer–water affinity in the entire composition range.
The DSC curves recorded for the specimens conditioned at the highest studied relative humidity level (rh 97%) show effects related to the so-called “free” water, mainly cold crystallization around −15 °C followed by melting slightly below 0 °C, and the third effect at −70 °C of yet unclarified origin. To explain the origin of this third phenomenon, further studies focused on the mobility of water in confinement are required.
Author Contributions
Conceptualization I.Ł. and A.B.; methodology, I.Ł., A.B. and K.N.R.; formal analysis, I.Ł. and K.N.R.; investigation, I.Ł.; visualization, I.Ł.; resources, K.P.; data curation, I.Ł.; writing—original draft preparation, I.Ł.; writing—review and editing, A.B., K.N.R. and K.P.; supervision, K.P. and K.N.R.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Center in Poland (Narodowe Centrum Nauki, NCN) under contract number 2017/27/B/ST8/01584.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Acknowledgments
We express our gratitude to Apostolos Kyritsis from the National Technical University of Athens for insightful discussions. Data analysis was carried out with software Grafity grafitylabs.com (accessed on 21 March 2019).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomez-Lopez, A.; Elizalde, F.; Calvo, I.; Sardon, H. Trends in Non-Isocyanate Polyurethane (NIPU) Development. Chem. Commun. 2021, 57, 12254–12265. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A Review on the Production, Properties and Applications of Non-Isocyanate Polyurethane: A Greener Perspective. Prog. Org. Coatings 2021, 154, 106124. [Google Scholar] [CrossRef]
- Stachak, P.; Łukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of Non-Isocyanate Polyurethane-Based Composite Materials. Materials 2021, 14, 3497. [Google Scholar] [CrossRef] [PubMed]
- Gennen, S.; Grignard, B.; Thomassin, J.M.; Gilbert, B.; Vertruyen, B.; Jerome, C.; Detrembleur, C. Polyhydroxyurethane Hydrogels: Synthesis and Characterizations. Eur. Polym. J. 2016, 84, 849–862. [Google Scholar] [CrossRef]
- Zhang, K.; Nelson, A.M.; Talley, S.J.; Chen, M.; Margaretta, E.; Hudson, A.G.; Moore, R.B.; Long, T.E. Non-Isocyanate Poly(Amide-Hydroxyurethane)s from Sustainable Resources. Green Chem. 2016, 18, 4667–4681. [Google Scholar] [CrossRef]
- Radzi, A.M.; Sapuan, S.M.; Jawaid, M.; Mansor, M.R. Water Absorption, Thickness Swelling and Thermal Properties of Roselle/Sugar Palm Fibre Reinforced Thermoplastic Polyurethane Hybrid Composites. J. Mater. Res. Technol. 2019, 8, 3988–3994. [Google Scholar] [CrossRef]
- Atiqah, A.; Jawaid, M.; Ishak, M.R.; Sapuan, S.M. Moisture Absorption and Thickness Swelling Behaviour of Sugar Palm Fibre Reinforced Thermoplastic Polyurethane. Procedia Eng. 2017, 184, 581–586. [Google Scholar] [CrossRef]
- Xu, D.H.; Liu, F.; Pan, G.; Zhao, Z.G.; Yang, X.; Shi, H.C.; Luan, S.F. Softening and Hardening of Thermal Plastic Polyurethane Blends by Water Absorbed. Polymer 2021, 218, 123498. [Google Scholar] [CrossRef]
- Guan, J.; Song, Y.; Lin, Y.; Yin, X.; Zuo, M.; Zhao, Y.; Tao, X.; Zheng, Q. Progress in Study of Non-Isocyanate Polyurethane. Ind. Eng. Chem. Res. 2011, 50, 6517–6527. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-Isocyanate Polyurethanes: From Chemistry to Applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Wang, X.; Soucek, M.D. Investigation of Non-Isocyanate Urethane Dimethacrylate Reactive Diluents for UV-Curable Polyurethane Coatings. Prog. Org. Coatings 2013, 76, 1057–1067. [Google Scholar] [CrossRef]
- Bourguignon, M.; Thomassin, J.; Grignard, B.; Vertruyen, B.; Detrembleur, C. Water-Borne Isocyanate-Free Polyurethane Hydrogels with Adaptable Functionality and Behavior. Macromol. Rapid Commun. 2021, 42, 2000482. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Mosteirín, N.; Aguirresarobe, R.; Sadaba, N.; Larrañaga, A.; Marin, E.; Martin, J.; Ramos-Gomez, N.; Arno, M.C.; Sardon, H.; Dove, A.P. Crystallization-Induced Gelling as a Method to 4D Print Low-Water-Content Non-Isocyanate Polyurethane Hydrogels. Chem. Mater. 2021, 33, 7194–7202. [Google Scholar] [CrossRef] [PubMed]
- Aduba, D.C.; Zhang, K.; Kanitkar, A.; Sirrine, J.M.; Verbridge, S.S.; Long, T.E. Electrospinning of Plant Oil-Based, Non-Isocyanate Polyurethanes for Biomedical Applications. J. Appl. Polym. Sci. 2018, 135, 46464. [Google Scholar] [CrossRef]
- Hogör, Z.; Kayaman-Apohan, N.; Karata, S.; Mencelolu, Y.; Güngör, A. Preparation and Characterization of Phosphine Oxide Based Polyurethane/Silica Nanocomposite via Non-Isocyanate Route. Prog. Org. Coatings 2010, 69, 366–375. [Google Scholar] [CrossRef]
- Santos, J.J.; Lopes, J.H.; de Aguiar, K.M.F.R.; Simões, M.B.; M.´Peko, J.-C.; Jasinevicius, R.G.; Cavalheiro, E.T.; Imasato, H.; Rodrigues-Filho, U.P. Hybrid Bisphenol A Non-Isocyanate Polyurethane Composite with Mica Powder: A New Insulating Material. J. CO2 Util. 2023, 67, 102303. [Google Scholar] [CrossRef]
- Fleischer, M.; Blattmann, H.; Mülhaupt, R. Glycerol-, Pentaerythritol- and Trimethylolpropane-Based Polyurethanes and Their Cellulose Carbonate Composites Prepared via the Non-Isocyanate Route with Catalytic Carbon Dioxide Fixation. Green Chem. 2013, 15, 934–942. [Google Scholar] [CrossRef]
- El Khezraji, S.; Chaib, M.; Thakur, S.; Raihane, M.; Lopez-Manchado, M.A.; Verdejo, R.; Lahcini, M. Synthesis of Novel Non-Isocyanate Polyurethane/Functionalized Boron Nitride Composites. Polymer 2022, 14, 3934. [Google Scholar] [CrossRef]
- Huang, J.; Shao, Z.; Iswanto, A.H.; Adly, M.; Lubis, R.; Sutiawan, J.; Saifulazry, S.; Al-Edrus, O.; Lee, S.H.; Antov, P.; et al. Latest Advancements in the Development of High-Performance Lignin- and Tannin-Based Non-Isocyanate Polyurethane Adhesive for Wood Composites. Polymer 2023, 15, 3864. [Google Scholar] [CrossRef]
- Liu, G.; Wu, G.; Chen, J.; Huo, S.; Jin, C.; Kong, Z. Synthesis and Properties of POSS-Containing Gallic Acid-Based Non-Isocyanate Polyurethanes Coatings. Polym. Degrad. Stab. 2015, 121, 247–252. [Google Scholar] [CrossRef]
- Blattmann, H.; Mülhaupt, R. Multifunctional POSS Cyclic Carbonates and Non-Isocyanate Polyhydroxyurethane Hybrid Materials. Macromolecules 2016, 49, 742–751. [Google Scholar] [CrossRef]
- Liu, G.; Wu, G.; Chen, J.; Kong, Z. Synthesis, Modification and Properties of Rosin-Based Non-Isocyanate Polyurethanes Coatings. Prog. Org. Coatings 2016, 101, 461–467. [Google Scholar] [CrossRef]
- Liu, W.; Hang, G.; Mei, H.; Li, L.; Zheng, S. Nanocomposites of Polyhydroxyurethane with POSS Microdomains: Synthesis via Non-Isocyanate Approach, Morphologies and Reprocessing Properties. Polymers 2022, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, C.M.; Younes, G.R.; Marić, M. The Effect of Polyhedral Oligomeric Silsesquioxane Fillers in Non-Isocyanate Polyurethane Hybrid Resins. J. Appl. Polym. Sci. 2022, 139, e53225. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Lichtenhan, J.D.; Pielichowski, K.; Blanco, I. POSS-Based Polymers. Polymers 2019, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Chujo, Y. Advanced Functional Materials Based on Polyhedral Oligomeric Silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Milliman, H.W.; Boris, D.; Schiraldi, D.A. Experimental Determination of Hansen Solubility Parameters for Select POSS and Polymer Compounds as a Guide to POSS-Polymer Interaction Potentials. Macromolecules 2012, 45, 1931–1936. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral Oligomeric Silsesquioxanes (POSS)-Containing Nanohybrid Polymers. Adv. Polym. Sci. 2006, 201, 225–296. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, S.B. Polymeric Nanocomposites—Polyhedral Oligomeric Silsesquioxanes (POSS) as Hybrid Nanofiller. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 389. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; He, C. Some Recent Developments of Polyhedral Oligomeric Silsesquioxane (POSS)-Based Polymeric Materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Pielichowski, K. Segmental Dynamics in Hybrid Polymer/POSS Nanomaterials. Prog. Polym. Sci. 2016, 52, 136–187. [Google Scholar] [CrossRef]
- Misra, R.; Alidedeoglu, A.H.; Jarrett, W.L.; Morgan, S.E. Molecular Miscibility and Chain Dynamics in POSS/Polystyrene Blends: Control of POSS Preferential Dispersion States. Polymer 2009, 50, 2906–2918. [Google Scholar] [CrossRef]
- Li, S.; Simon, G.P.; Matisons, J.G. Morphology of Blends Containing High Concentrations of POSS Nanoparticles in Different Polymer Matrices. Polym. Eng. Sci. 2010, 50, 991–999. [Google Scholar] [CrossRef]
- Matějka, L.; Murias, P.; Pleštil, J. Effect of POSS on Thermomechanical Properties of Epoxy–POSS Nanocomposites. Eur. Polym. J. 2012, 48, 260–274. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, D.; Huang, L.; Li, W.; Tian, J.; Lu, L.; Zhou, C. Simultaneous Improvement in Toughness, Strength and Biocompatibility of Poly(Lactic Acid) with Polyhedral Oligomeric Silsesquioxane. Chem. Eng. J. 2018, 346, 649–661. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Q.; Shen, C.; Han, Y.; Tang, J.; Chen, H. Synthesis of MA POSS–PMMA as an Intraocular Lens Material with High Light Transmittance and Good Cytocompatibility. RSC Adv. 2014, 4, 52959–52966. [Google Scholar] [CrossRef]
- Fox, D.M.; Novy, M.; Brown, K.; Zammarano, M.; Harris, R.H.; Murariu, M.; McCarthy, E.D.; Seppala, J.E.; Gilman, J.W. Flame Retarded Poly(Lactic Acid) Using POSS-Modified Cellulose. 2. Effects of Intumescing Flame Retardant Formulations on Polymer Degradation and Composite Physical Properties. Polym. Degrad. Stab. 2014, 106, 54–62. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Camino, G. Metal Functionalized POSS as Fire Retardants in Polypropylene. Polym. Degrad. Stab. 2006, 91, 2275–2281. [Google Scholar] [CrossRef]
- Zhao, Y.; Schiraldi, D.A. Thermal and Mechanical Properties of Polyhedral Oligomeric Silsesquioxane (POSS)/Polycarbonate Composites. Polymer 2005, 46, 11640–11647. [Google Scholar] [CrossRef]
- Bukowczan, A.; Stachak, P.; Łukaszewska, I.; Majka, T.M.; Hebda, E.; Pielichowski, K. Pyrolysis and Thermal Degradation Studies of Non-Isocyanate Polyurethanes Modified by Polyhedral Oligomeric Silsesquioxanes. Thermochim. Acta 2023, 723, 179484. [Google Scholar] [CrossRef]
- Li, H.; Ren, F.Y.; Li, H.R.; He, L.N. Modification of Ricinoleic Acid Based Nonisocyanate Polyurethane Using Polyamine Containing Polyhedral Oligomeric Silsesquioxane. Polym. Eng. Sci. 2023, 63, 1507–1515. [Google Scholar] [CrossRef]
- Ozimek, J.; Pielichowski, K. Recent Advances in Polyurethane/Poss Hybrids for Biomedical Applications. Molecules 2022, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, K.; Wang, L.; Zheng, S. Poly(Hydroxyl Urethane)s with Double Decker Silsesquioxanes in the Main Chains: Synthesis, Shape Recovery, and Reprocessing Properties. Macromolecules 2020, 53, 434–444. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Bin Rusayyis, M.A.; Purwanto, N.S.; Torkelson, J.M. Reprocessable Polyhydroxyurethane Networks Reinforced with Reactive Polyhedral Oligomeric Silsesquioxanes (POSS) and Exhibiting Excellent Elevated Temperature Creep Resistance. Polymer 2022, 252, 124971. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, S.Q.; Li, Q.; Khan, M.R.; Liu, Y.; Lu, P.; Huang, C.X.; Huang, L.J.; Jiang, T. Fabrication and Properties of Waterborne Thermoplastic Polyurethane Nanocomposite Enhanced by the POSS with Low Dielectric Constants. Polymer 2020, 209, 122992. [Google Scholar] [CrossRef]
- Bizet, B.; Grau, E.; Asua, J.M.; Cramail, H. Hybrid Nonisocyanate Polyurethanes (H-NIPUs): A Pathway towards a Broad Range of Novel Materials. Macromol. Chem. Phys. 2022, 223, 2100437. [Google Scholar] [CrossRef]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A Nanocage for Nanomedicine: Polyhedral Oligomeric Silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef]
- Yahyaei, H.; Mohseni, M.; Ghanbari, H.; Messori, M. Synthesis and Characterization of Polyhedral Oligomeric Titanized Silsesquioxane: A New Biocompatible Cage like Molecule for Biomedical Application. Mater. Sci. Eng. C 2016, 61, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Gholami, H.; Yeganeh, H. Soybean Oil-Derived Non-Isocyanate Polyurethanes Containing Azetidinium Groups as Antibacterial Wound Dressing Membranes. Eur. Polym. J. 2021, 142, 110142. [Google Scholar] [CrossRef]
- Choong, P.S.; Tam, K.W.E.; Chong, N.X.; Seayad, A.M.; Seayad, J.; Jana, S. Biobased, Biodegradable, and Water-Soluble Amine-Functionalized Non-Isocyanate Polyurethanes for Potential Home Care Application. ACS Appl. Polym. Mater. 2023, 5, 5503–5513. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Zhao, F.; Shi, W.; Yu, G.; Zhou, X.; Guo, Y.; Zhao, F.; Shi, W.; Yu Materials Science, G.; et al. Topology-Controlled Hydration of Polymer Network in Hydrogels for Solar-Driven Wastewater Treatment. Adv. Mater. 2020, 32, 2007012. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewska, I.; Bukowczan, A.; Raftopoulos, K.N.; Pielichowski, K. ‘Spider-like’ POSS in NIPU Webs: Enhanced Thermal Stability and Unique Swelling Behavior. J. Polym. Res. 2023, 30, 456. [Google Scholar] [CrossRef]
- Ozimek, J.; Łukaszewska, I.; Pielichowski, K. POSS and SSQ Materials in Dental Applications: Recent Advances and Future Outlooks. Int. J. Mol. Sci. 2023, 24, 4493. [Google Scholar] [CrossRef]
- Łukaszewska, I.; Lalik, S.; Bukowczan, A.; Marzec, M.; Pielichowski, K.; Raftopoulos, K.N. Tailoring the Physical Properties of Non-Isocyanate Polyurethanes by Introducing Secondary Amino Groups along Their Main Chain. J. Mol. Liq. 2023, 391, 123263. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81A, 89–96. [Google Scholar] [CrossRef]
- Timmermann, E.O. Multilayer Sorption Parameters: BET or GAB Values? Colloids Surfaces A Physicochem. Eng. Asp. 2003, 220, 235–260. [Google Scholar] [CrossRef]
- Timmermann, E.O.; Chirife, J.; Iglesias, H.A. Water Sorption Isotherms of Foods and Foodstuffs: BET or GAB Parameters? J. Food Eng. 2001, 48, 19–31. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs Adsorption Isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Blahovec, J.; Yanniotis, S. Modified Classification of Sorption Isotherms. J. Food Eng. 2009, 91, 72–77. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Solid Polymer Desiccants Based on Poly(Acrylic Acid-Co-Acrylamide) and Laponite RD: Adsorption Isotherm and Kinetics Studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124813. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Spray Drying of Nopal Mucilage (Opuntia Ficus-Indica): Effects on Powder Properties and Characterization. Carbohydr. Polym. 2010, 81, 864–870. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M.; Agung Susantyoko, R. Zeolites and Superporous Hydrogels-Based Hybrid Composites as Solid Desiccants to Capture Water Vapors from Humid Air. Microporous Mesoporous Mater. 2022, 342, 112116. [Google Scholar] [CrossRef]
- Dolmaire, N.; Espuche, E.; Méchin, F.; Pascault, J.P. Water Transport Properties of Thermoplastic Polyurethane Films. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 473–492. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Łukaszewska, I.; Bujalance Calduch, C.; Stachak, P.; Lalik, S.; Hebda, E.; Marzec, M.; Pielichowski, K. Hydration and Glass Transition of Hybrid Non-Isocyanate Polyurethanes with POSS Inclusions. Polymer 2022, 253, 125010. [Google Scholar] [CrossRef]
- Pandis, C.; Spanoudaki, A.; Kyritsis, A.; Pissis, P.; Hernández, J.C.R.; Gõmez Ribelles, J.L.; Monleõn Pradas, M. Water Sorption Characteristics of Poly(2-Hydroxyethyl Acrylate)/Silica Nanocomposite Hydrogels. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 657–668. [Google Scholar] [CrossRef]
- Rohitha, P. Prediction of Moisture Adsorption Characteristics of Dehydrated Fruits Using the GAB Isotherm Model. Agric. Crop. Sci. 2018, 3, 1036. [Google Scholar]
- Pérez-Alonso, C.; Beristain, C.I.; Lobato-Calleros, C.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J. Thermodynamic Analysis of the Sorption Isotherms of Pure and Blended Carbohydrate Polymers. J. Food Eng. 2006, 77, 753–760. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Zaltariov, M.F.; Ciubotaru, B.I.; Bargan, A.; Varganici, C.D.; Vasiliu, A.L.; Peptanariu, D.; Balan-Porcarasu, M.; Timofte-Zorila, M.M. Hydroxypropyl Cellulose/Pluronic-Based Composite Hydrogels as Biodegradable Mucoadhesive Scaffolds for Tissue Engineering. Gels 2022, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, G.G.; Pradas, M.M.; Gómez Ribelles, J.L.; Sánchez, M.S. Thermodynamical Analysis of the Hydrogel State in Poly(2-Hydroxyethyl Acrylate). Polymer 2004, 45, 6207–6217. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Adsorption Isotherm and Kinetics of Water Vapors on Novel Superporous Hydrogel Composites. Microporous Mesoporous Mater. 2020, 299, 110106. [Google Scholar] [CrossRef]
- Szyk-Warszy´nska, L.; Warszy´nska, W.; Raszka, K.; Warszy´nski, P.; Warszy´nski, W. Interactions of Casein and Polypeptides in Multilayer Films Studied by FTIR and Molecular Dynamics. Polymers 2019, 11, 920. [Google Scholar] [CrossRef]
- Haris, P.I.; Chapman, D. Analysis of Polypeptide and Protein Structures Using Fourier Transform Infrared Spectroscopy. Methods Mol. Biol. 1994, 22, 183–202. [Google Scholar] [CrossRef]
- Schmidt, P.; Dybal, J.; Rodriguez-Cabello, J.C.; Reboto, V. Role of Water in Structural Changes of Poly(AVGVP) and Poly(GVGVP) Studied by FTIR and Raman Spectroscopy and Ab Initio Calculations. Biomacromolecules 2005, 6, 697–706. [Google Scholar] [CrossRef]
- Li, T.; Zhang, C.; Xie, Z.; Xu, J.; Guo, B.H. A Multi-Scale Investigation on Effects of Hydrogen Bonding on Micro-Structure and Macro-Properties in a Polyurea. Polymer 2018, 145, 261–271. [Google Scholar] [CrossRef]
- Lu, P.; Huang, W.; Shi, H.; Zhu, L. Effect of Curing Temperature on Morphology and Properties of Polyureas Based on Polyaspartic Esters. Mater. Sci. Forum 2010, 650, 33–37. [Google Scholar] [CrossRef]
- Schroeder, L.R.; Cooper, S.L. Hydrogen Bonding in Polyamides. J. Appl. Phys. 1976, 47, 4310–4317. [Google Scholar] [CrossRef]
- Skrovanek, D.J.; Painter, P.C.; Coleman, M.M. Hydrogen Bonding in Polymers. 2. Infrared Temperature Studies of Nylon 11. Macromolecules 1986, 19, 699–705. [Google Scholar] [CrossRef]
- Roberts, M.F.; Jenekhe, S.A. Site-Specific Reversible Scission of Hydrogen Bonds in Polymers. An Investigation of Polyamides and Their Lewis Acid-Base Complexes by Infrared Spectroscopy. Macromolecules 1991, 24, 3142–3146. [Google Scholar] [CrossRef]
- Wang, F.C.; Feve, M.; Lam, T.M.; Pascault, J.-P. FTIR Analysis of Hydrogen Bonding in Amorphous Linear Aromatic Polyurethanes. II. Influence of Styrene Solvent. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1315–1320. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Gancarz, I.; Clarke, T.C. The Effects of Morphological Transitions on Hydrogen Bonding in Polyurethanes: Preliminary Results of Simultaneous DSC–FTIR Experiments. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 2487–2498. [Google Scholar] [CrossRef]
- Sung, C.S.P.; Schneider, N.S. Infrared Studies of Hydrogen Bonding in Toluene Diisocyanate Based Polyurethanes. Macromolecules 1975, 8, 68–73. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Jiang, S.; Liang, C.; Wang, J.; Kang, M.; Li, Q.; Zhao, Y. Promising Approaches to Improve the Performances of Hybrid Non-Isocyanate Polyurethane. Polym. Int. 2019, 68, 651–660. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Brkljaca, R.; Adhikari, B. Assessment of Interfacial Interactions between Starch and Non-Isocyanate Polyurethanes in Their Hybrids. Carbohydr. Polym. 2020, 246, 116656. [Google Scholar] [CrossRef]
- Wolinska-Grabczy, A.; Kaczmarczyk, B.; Jankowski, A. Investigations of Hydrogen Bonding in the Poly(Urethane-Urea)-Based Membrane Materials by Using FTIR Spectroscopy. Pol. J. Chem. Technol. 2008, 10, 53–56. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR Investigation of the Influence of Diisocyanate Symmetry on the Morphology Development in Model Segmented Polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Yildirim, E.; Yurtsever, M.; Yilgör, E.; Yilgör, I.; Wilkes, G.L. Temperature-Dependent Changes in the Hydrogen Bonded Hard Segment Network and Microphase Morphology in a Model Polyurethane: Experimental and Simulation Studies. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 182–192. [Google Scholar] [CrossRef]
- Behera, B.; Das, P.K. Blue- and Red-Shifting Hydrogen Bonding: A Gas Phase FTIR and Ab Initio Study of RR′CO···DCCl3 and RR′S···DCCl3 Complexes. J. Phys. Chem. A 2018, 122, 4481–4489. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sawatari, C. A Fourier Transform Infra-Red Spectroscopic Analysis of the Character of Hydrogen Bonds in Amorphous Cellulose. Polymer 1996, 37, 393–399. [Google Scholar] [CrossRef]
- Black, S.B.; Chang, Y.; Bae, C.; Hickner, M.A. FTIR Characterization of Water-Polymer Interactions in Superacid Polymers. J. Phys. Chem. B 2013, 117, 16266–16274. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Kollman, P.; Rothenberg, S.; McKelvey, J. Hydrogen Bonding Ability of the Amide Group. J. Am. Chem. Soc. 1974, 96, 3794–3800. [Google Scholar] [CrossRef]
- Joseph, J.; Jemmis, E.D. Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef]
- Kuo, S.W.; Huang, C.F.; Chang, F.C. Study of Hydrogen-Bonding Strength in Poly(ϵ-Caprolactone) Blends by DSC and FTIR. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1348–1359. [Google Scholar] [CrossRef]
- Coleman, M.M.; Moskala, E.J. FTi.r. Studies of Polymer Blends Containing the Poly(Hydroxy Ether of Bisphenol A) and Poly(ε-Caprolactone). Polymer 1983, 24, 251–257. [Google Scholar] [CrossRef]
- Guerin, A.C.; Riley, K.; Rupnik, K.; Kuroda, D.G. Determining the Energetics of the Hydrogen Bond through FTIR: A Hands-On Physical Chemistry Lab Experiment. J. Chem. Educ. 2016, 93, 1124–1129. [Google Scholar] [CrossRef]
- Teo, L.S.; Chen, C.Y.; Kuo, J.F. Fourier Transform Infrared Spectroscopy Study on Effects of Temperature on Hydrogen Bonding in Amine-Containing Polyurethanes and Poly(Urethane-Urea)S. Macromolecules 1997, 30, 1793–1799. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, M.S.; Knowles, J.C.; Gong, M.S. Synthesis of Highly Elastic Biocompatible Polyurethanes Based on Bio-Based Isosorbide and Poly(Tetramethylene Glycol) and Their Properties. J. Biomater. Appl. 2014, 29, 454. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Hearon, K.; Wilson, T.S.; Maitland, D.J. The Effect of Moisture Absorption on the Physical Properties of Polyurethane Shape Memory Polymer Foams. Smart Mater. Struct. 2011, 20, 085010. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Nakashima, S. Changes in IR Band Areas and Band Shifts during Water Adsorption to Lecithin and Ceramide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117779. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.P.; Karthick, N.K.; Arivazhagan, G. Hydrogen Bond Interactions in the Binary Solutions of Formamide with Methanol: FTIR Spectroscopic and Theoretical Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117892. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, T.; Burini, D.; Biczysko, M.; Barone, V. Hydrogen-Bonding Effects on Infrared Spectra from Anharmonic Computations: Uracil-Water Complexes and Uracil Dimers. J. Phys. Chem. A 2015, 119, 4224–4236. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Chiang, K.Y.; Yu, C.C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0-471-39362-2. [Google Scholar]
- Coleman, M.M.; Lee, K.H.; Skrovanek, D.J.; Painter, P.C. Hydrogen Bonding in Polymers. 4. Infrared Temperature Studies of a Simple Polyurethane. Macromolecules 1986, 19, 2149–2157. [Google Scholar] [CrossRef]
- Rozenberg, M.; Jung, C.; Shoham, G. Low Temperature FTIR Spectra and Hydrogen Bonds in Polycrystalline Cytidine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chao, J.; Kang, Y. Variations in Amplitudes and Wave Energy along the Energy Dispersion Paths for Rossby Waves in the Quasigeostrophic Barotropic Model. Adv. Atmos. Sci. 2022, 39, 876–888. [Google Scholar] [CrossRef]
- Ni, Y.; Skinner, J.L. IR and SFG Vibrational Spectroscopy of the Water Bend in the Bulk Liquid and at the Liquid-Vapor Interface, Respectively. J. Chem. Phys. 2015, 143, 14502. [Google Scholar] [CrossRef]
- Tanaka, M.; Motomura, T.; Ishii, N.; Shimura, K.; Onishi, M.; Mochizuki, A.; Hatakeyama, T. Cold Crystallization of Water in Hydrated Poly(2-Methoxyethyl Acrylate) (PMEA). Polym. Int. 2020, 49, 1709–1713. [Google Scholar] [CrossRef]
- Combarro Palacios, I.; Olsson, C.; Kamma-Lorger, C.S.; Swenson, J.; Cerveny, S. Motions of Water and Solutes—Slaving versus Plasticization Phenomena. J. Chem. Phys. 2019, 150, 124902. [Google Scholar] [CrossRef] [PubMed]
- Shinyashiki, N.; Yamamoto, W.; Yokoyama, A.; Yoshinari, T.; Yagihara, S.; Kita, R.; Ngai, K.L.; Capaccioli, S. Glass Transitions in Aqueous Solutions of Protein (Bovine Serum Albumin). J. Phys. Chem. B 2009, 113, 14448–14456. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, S.; Swenson, J. Water Dynamics in the Hydration Shells of Biological and Non-Biological Polymers. J. Chem. Phys. 2019, 150, 234904. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, S.; Combarro-Palacios, I.; Swenson, J. Evidence of Coupling between the Motions of Water and Peptides. J. Phys. Chem. Lett. 2016, 7, 4093–4098. [Google Scholar] [CrossRef]
- Fenimore, P.W.; Frauenfelder, H.; McMahon, B.H.; Parak, F.G. Slaving: Solvent Fluctuations Dominate Protein Dynamics and Functions. Proc. Natl. Acad. Sci. USA 2002, 99, 16047–16051. [Google Scholar] [CrossRef]
- Lutz, T.R.; He, Y.; Ediger, M.D.; Pitsikalis, M.; Hadjichristidis, N. Dilute Polymer Blends: Are the Segmental Dynamics of Isolated Polyisoprene Chains Slaved to the Dynamics of the Host Polymer? Macromolecules 2004, 37, 6440–6448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).