Preparation of Preformed Submicron Crosslinked Polymer Coils for Conformance Control in Low-Permeability Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
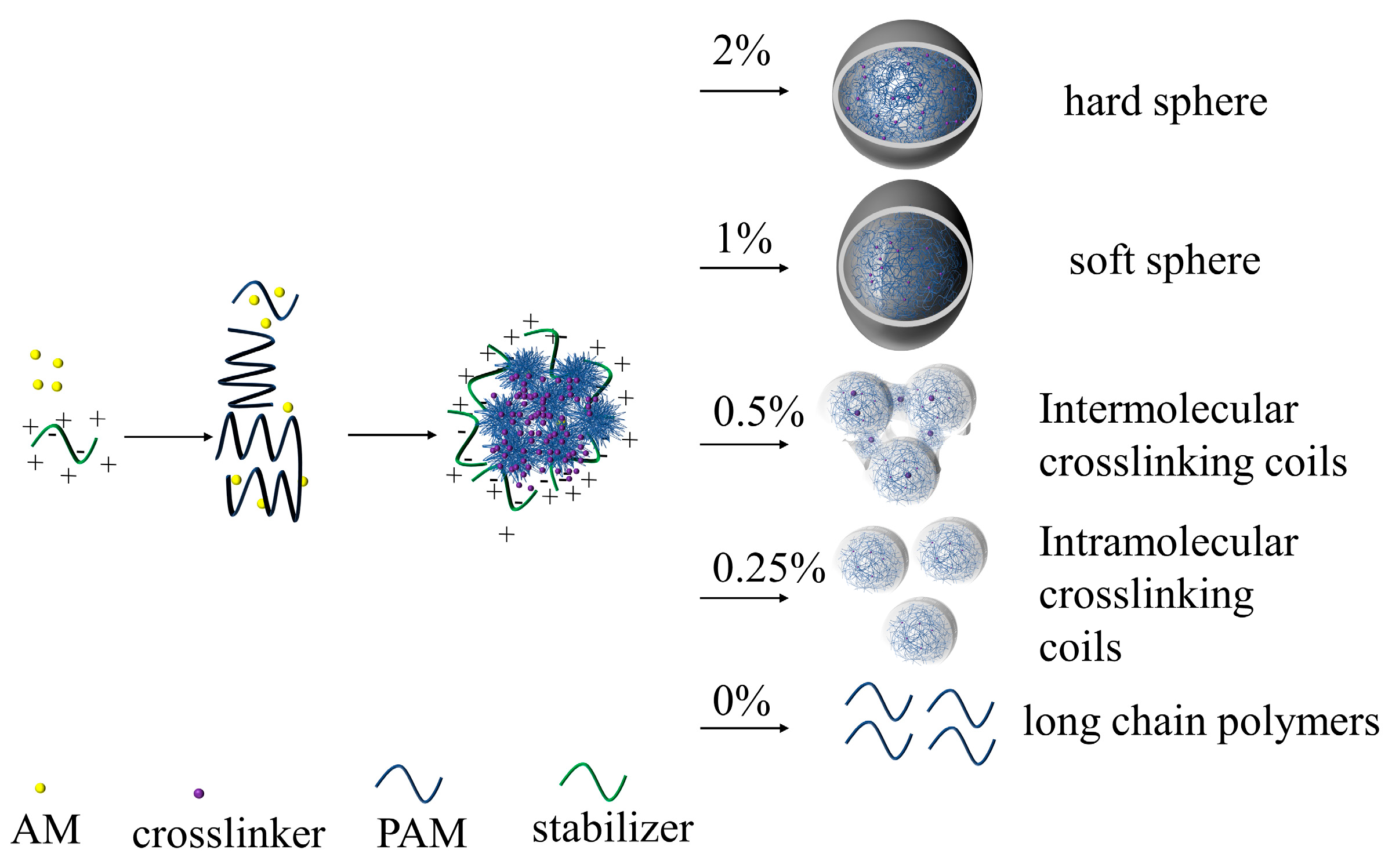
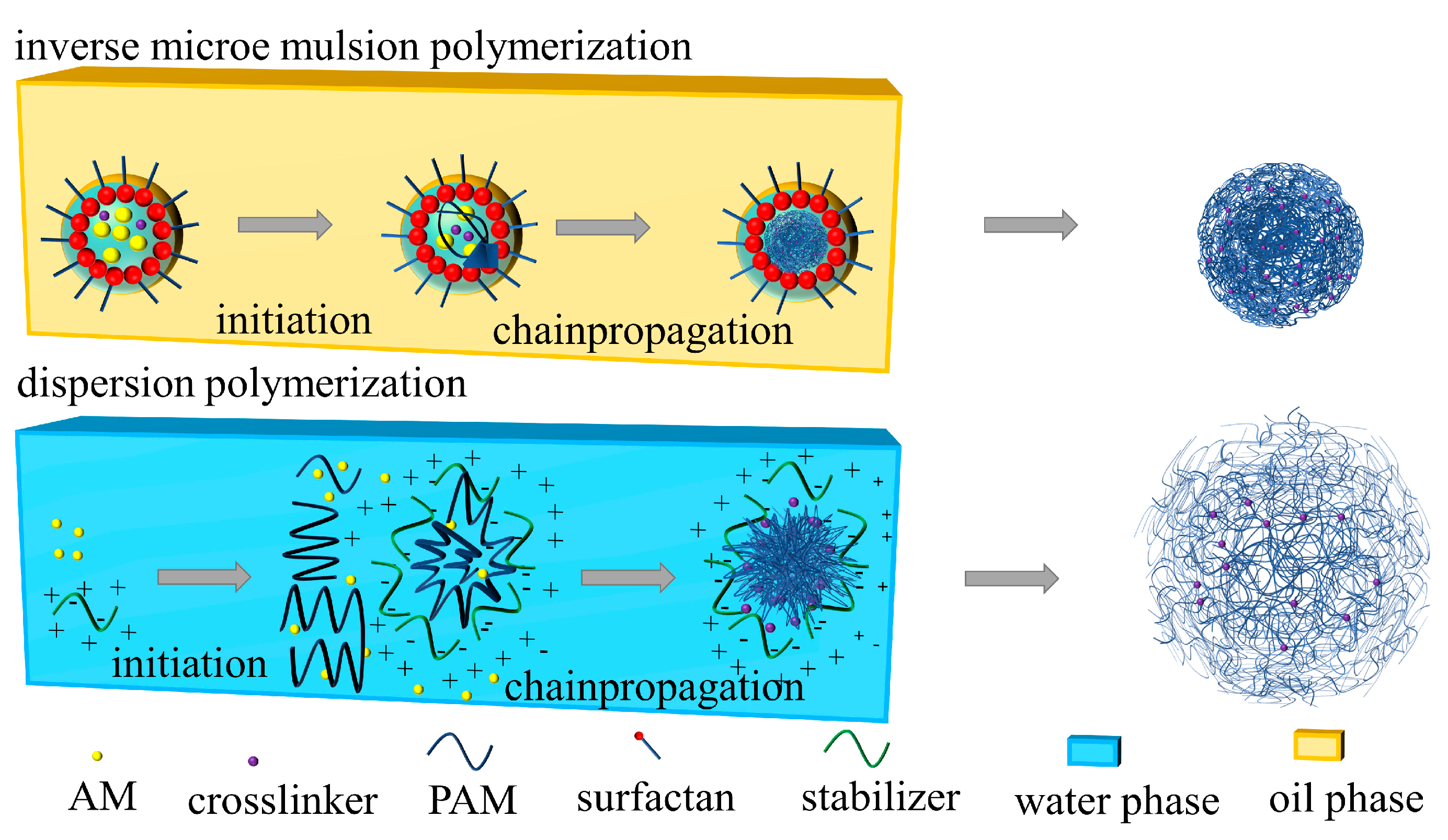
2.2. Preparation of Coils
2.3. Characterization
2.4. Particle Size Distribution and Zeta Potential Measurements
2.5. Rheology Measurements
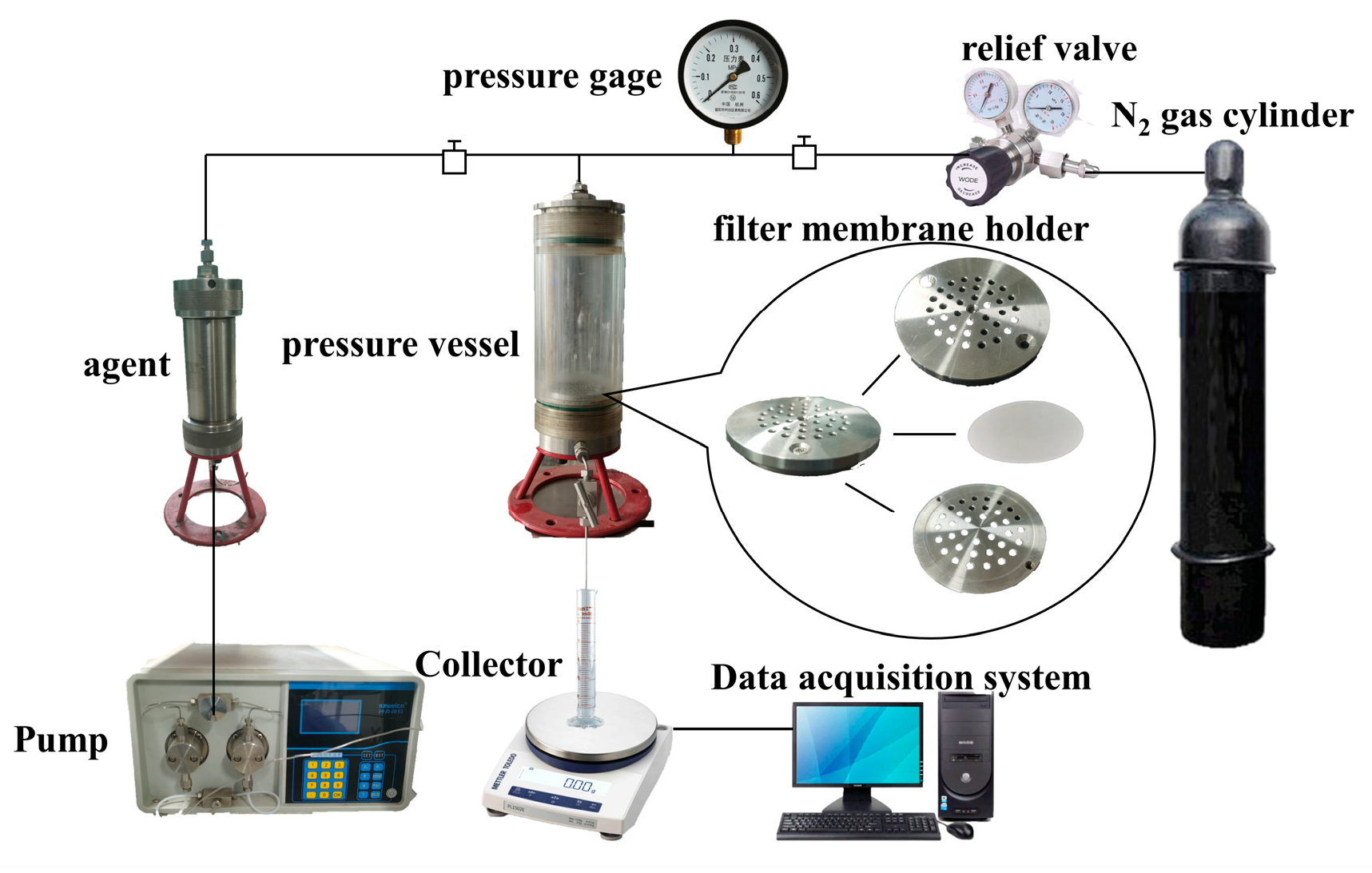
2.6. Microporous Membrane Filtration Experiment
2.7. Core Flooding
3. Results and Discussion
3.1. Characterizations of SCPCs
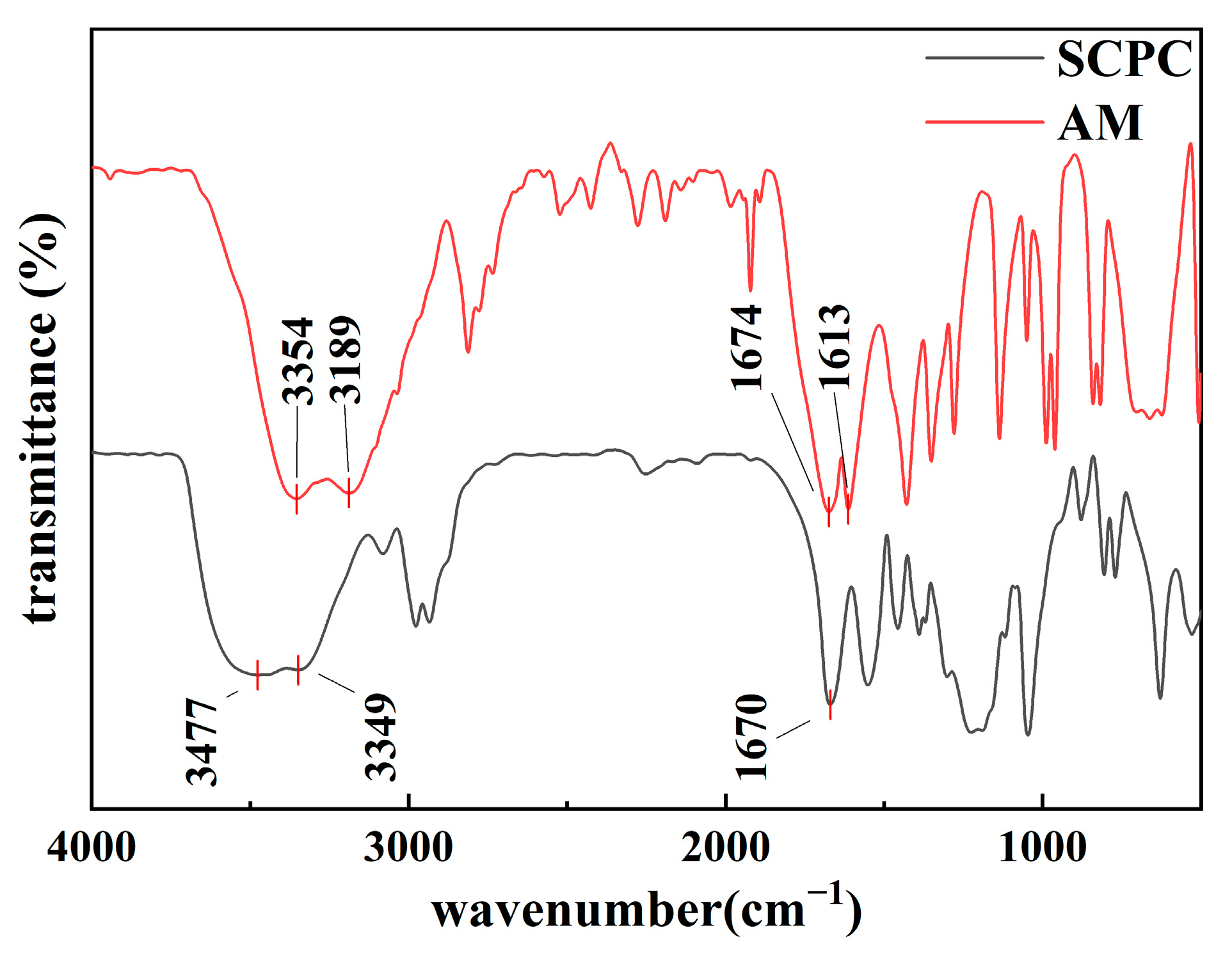
3.1.1. Chemical Structure of Polymer Coils
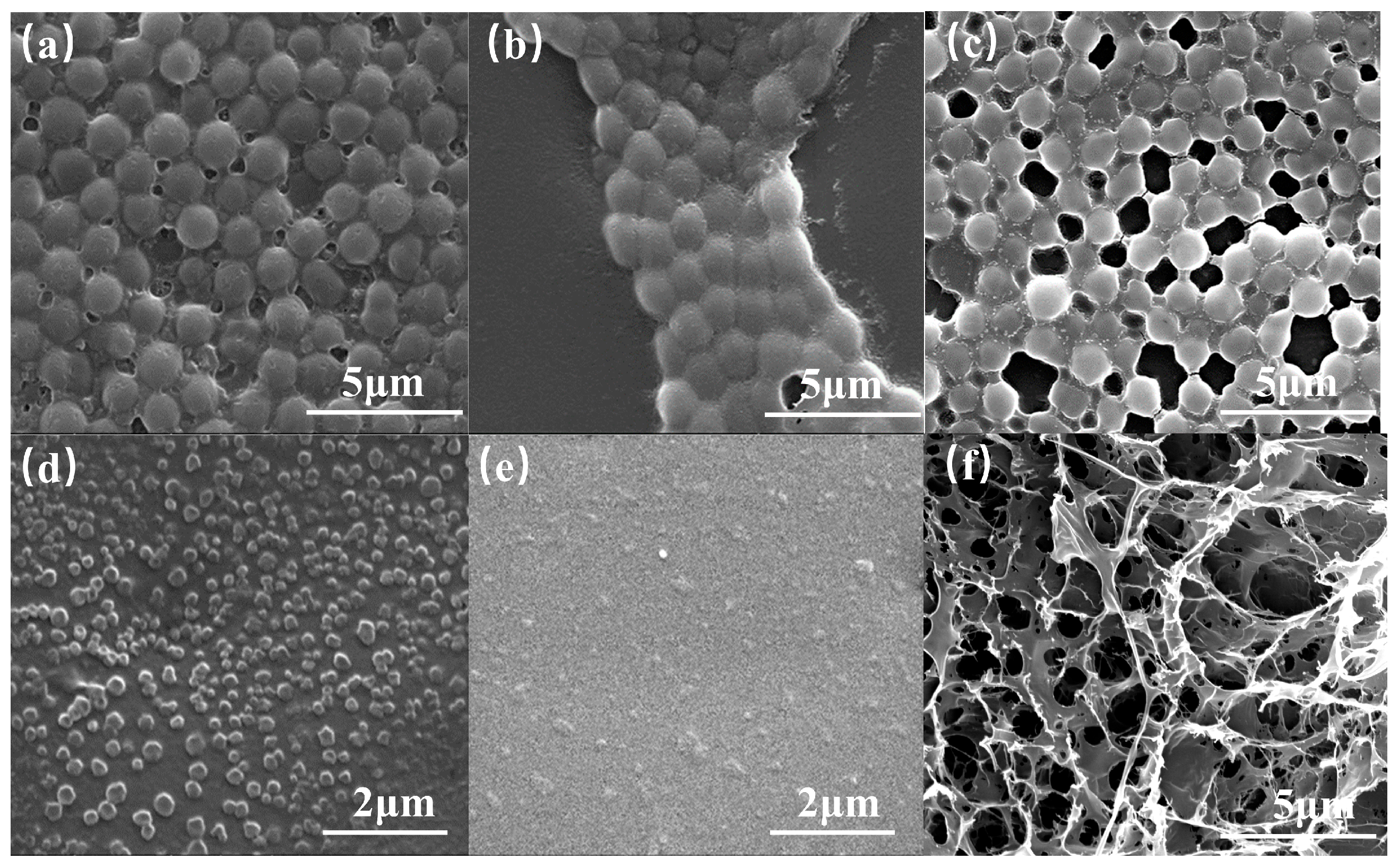
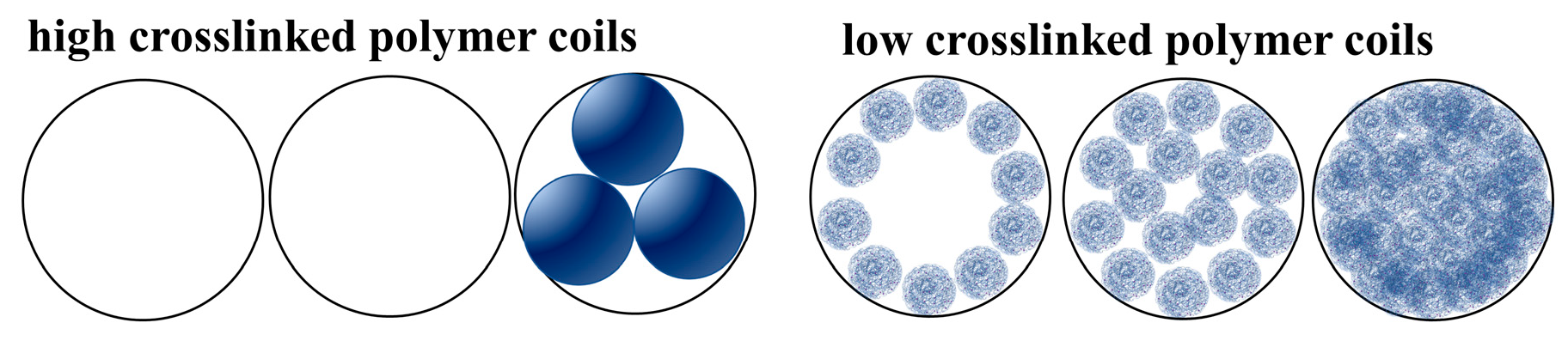
3.1.2. Morphology of Polymer Coils
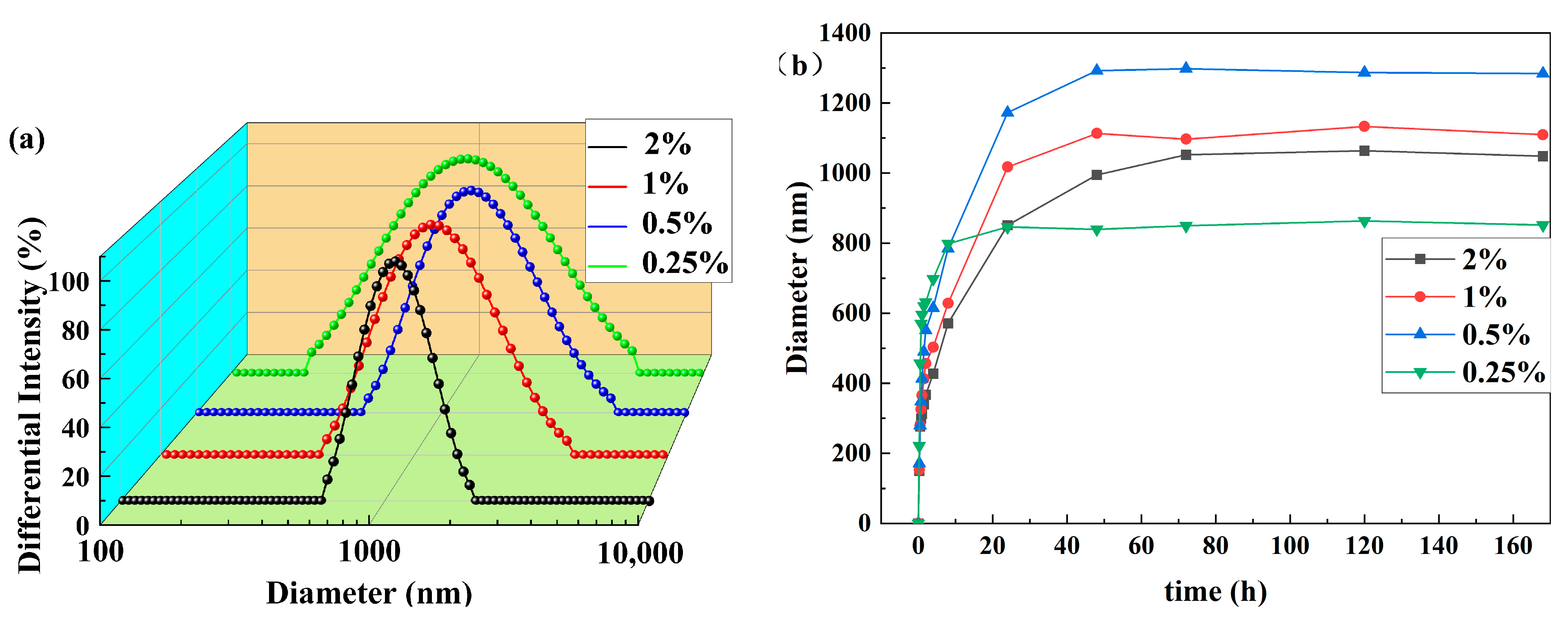
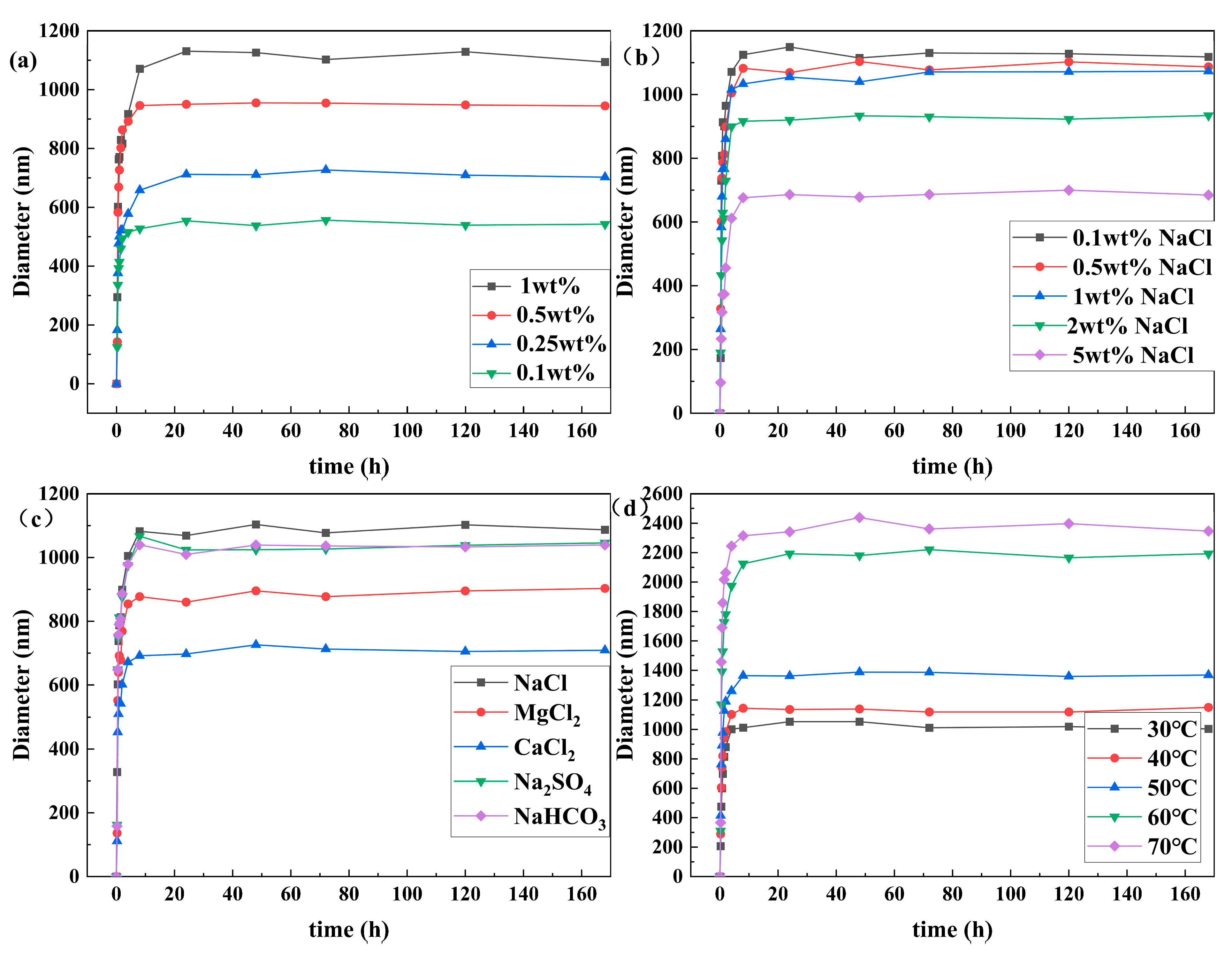
3.2. Particle Size and Zeta Potential Analysis
3.3. Rheology
3.4. Filtration Curves
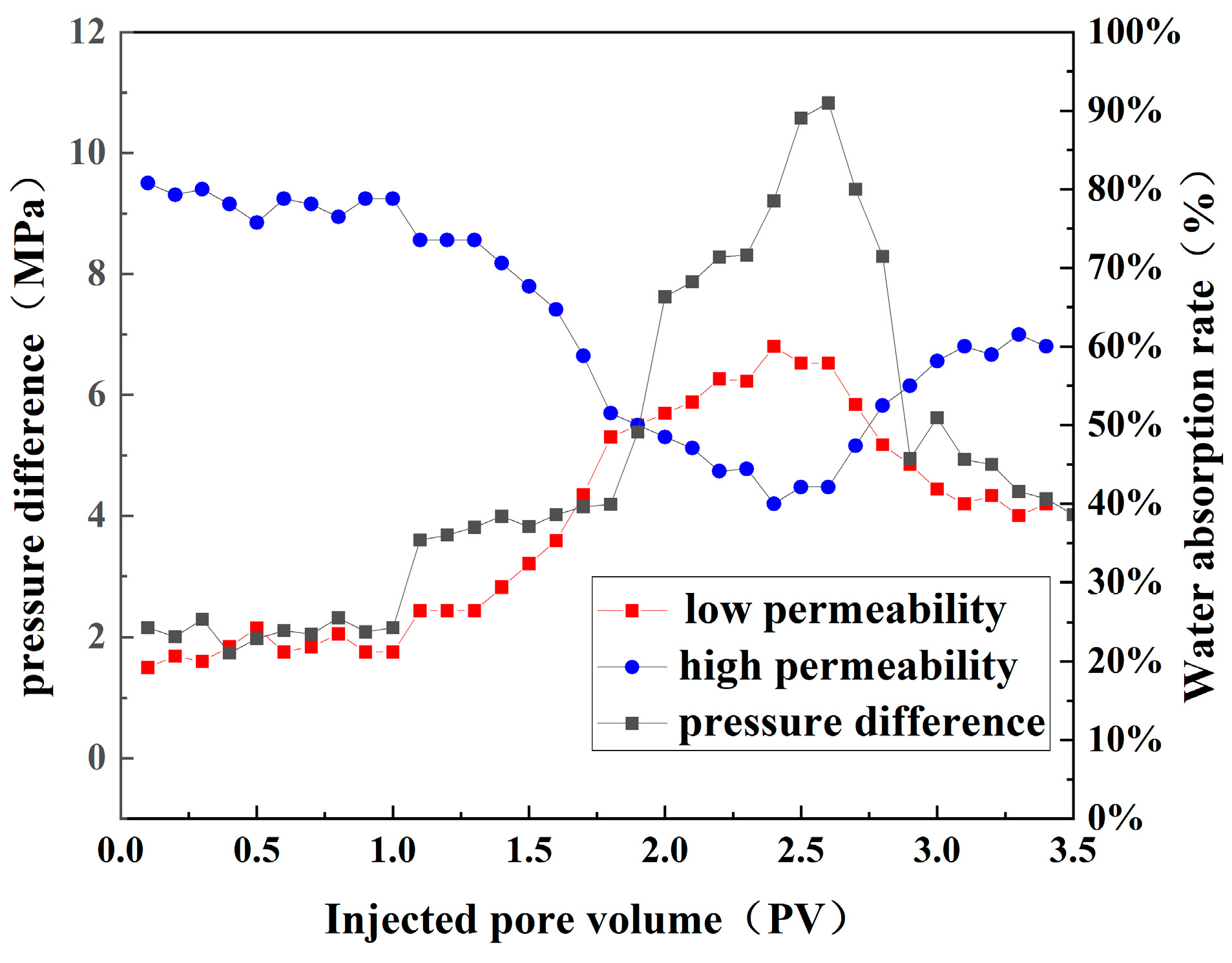
3.5. Displacement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, W.; Zhou, B.; Issakhov, M.; Gabdullin, M. Advances in enhanced oil recovery technologies for low permeability reservoirs. Pet. Sci. 2022, 19, 1622–1640. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; Li, H.; Liu, X.; Tan, Y.; Chen, S.; Cai, J. A brief review of dynamic capillarity effect and its characteristics in low permeability and tight reservoirs. J. Pet. Sci. Eng. 2020, 189, 106959. [Google Scholar] [CrossRef]
- Zou, J.; Yue, X.; Dong, J.; Shao, M.; Wang, L.; Gu, J.; An, W. Novel in-depth profile control agent based on in-situ polymeric microspheres in low permeability reservoir. J. Dispers. Sci. Technol. 2020, 41, 1254–1264. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yang, H.; Yang, H.; Wu, T.; Wu, H.; Wang, H.; Yu, F.; Jiang, H. Compatibility evaluation of in-depth profile control agents in dominant channels of low-permeability reservoirs. J. Pet. Sci. Eng. 2020, 194, 107529. [Google Scholar] [CrossRef]
- Rahimi, R.; Dehaghani, A.H.S. An experimental study comparing the stability of colloidal dispersion gels with normal polymeric solutions for enhanced-oil-recovery purposes. Can. J. Chem. Eng. 2021, 99, 1116–1124. [Google Scholar] [CrossRef]
- García, R.H.C.; Toro, G.A.M.; Muñoz, J.E.S.; Paternina, L.M.C. Colloidal Dispersion Gels (CDG) to Improve Volumetric Sweep Efficiency in Waterflooding Processes. CT F Cienc. Tecnol. Y Futuro 2013, 5, 61–78. [Google Scholar] [CrossRef]
- Li, M.; Dong, Z.; Lin, M.; Wu, Z.-L. A study on the size and conformation of linked polymer coils. J. Pet. Sci. Eng. 2004, 41, 213–219. [Google Scholar] [CrossRef]
- Shiran, B.S.; Skauge, A. Similarities and Differences of Low Salinity Polymer and Low Salinity LPS (Linked Polymer Solutions) for Enhanced Oil Recovery. J. Dispers. Sci. Technol. 2014, 35, 1656–1664. [Google Scholar] [CrossRef]
- Li, W.; Wei, F.; Xiong, C.; Ouyang, J.; Zhao, G.; Shao, L.; Dai, M. A novel binary compound flooding system based on DPG particles for enhancing oil recovery. Arab. J. Geosci. 2019, 12, 256. [Google Scholar] [CrossRef]
- Dai, C.; Liu, Y.; Zou, C.; You, Q.; Yang, S.; Zhao, M.; Zhao, G.; Wu, Y.; Sun, Y. Investigation on matching relationship between dispersed particle gel (DPG) and reservoir pore-throats for in-depth profile control. Fuel 2017, 207, 109–120. [Google Scholar] [CrossRef]
- Du, L.; Xiao, Y.; Jiang, Z.; Zeng, H.; Li, H. Towards in-depth profile control using dispersed particle gels (DPGs). Fuel 2023, 354, 129419. [Google Scholar] [CrossRef]
- Farasat, A.; Younesian-Farid, H.; Sadeghnejad, S. Conformance control study of preformed particle gels (PPGs) in mature waterflooded reservoirs: Numerical and experimental investigations. J. Pet. Sci. Eng. 2021, 203, 108575. [Google Scholar] [CrossRef]
- Saghafi, H.R. Retention characteristics of enhanced preformed particle gels (PPGs) in porous media: Conformance control implications. J. Pet. Sci. Eng. 2018, 166, 962–968. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Jin, S.; Yang, Z.; Dong, Z.; Zhang, J. Combined flooding systems with polymer microspheres and nonionic surfactant for enhanced water sweep and oil displacement efficiency in heterogeneous reservoirs. J. Dispers. Sci. Technol. 2020, 41, 267–276. [Google Scholar] [CrossRef]
- Lin, M.; Zhao, Q.; Dang, S.; Yang, Z.; Dong, Z.; Zhang, J. Temperature resistance of AM/AMPS/NVP copolymer microspheres. Iran. Polym. J. 2020, 29, 445–453. [Google Scholar] [CrossRef]
- Li, J.; Niu, L.; Wu, W.; Sun, M. The Reservoir Adaptability and Oil Displacement Mechanism of Polymer Microspheres. Polymers 2020, 12, 885. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, M.; Xin, J.; Li, M.-Y. Influence of dissolved oxygen content on oxidative stability of linked polymer solution. Pet. Sci. 2009, 6, 421–425. [Google Scholar] [CrossRef]
- He, J.; Shao, M.; Yue, X.; Yue, T. Fabrication and characteristics of self-aggregation nanoparticles used for conformance control treatment. Polym. Adv. Technol. 2021, 32, 190–201. [Google Scholar] [CrossRef]
- Li, X.; Yue, X.; Yue, T.; Yan, R.; Olaleye, O.E.; Shao, M. Novel chemical flood combination of CSA particles and strong emulsifying surfactant in heterogeneous reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126838. [Google Scholar] [CrossRef]
- Zou, J.; Yue, X.; Zhang, J.; Fu, J.Y.; He, J.; Kong, B.; Ling, Q. Self-assembled microspheres feasibility study for conformance control in high temperature and high salinity reservoirs. Arab. J. Geosci. 2018, 11, 195. [Google Scholar] [CrossRef]
- Shi, X.; Yue, X. Migration and plugging mechanisms of self-aggregated microspheres as a novel profile control. J. Pet. Sci. Eng. 2020, 184, 106458. [Google Scholar] [CrossRef]
- Luo, M.; Jia, X.; Si, X.; Luo, S.; Zhan, Y. A novel polymer encapsulated silica nanoparticles for water control in development of fossil hydrogen energy—Tight carbonate oil reservoir by acid fracturing. Int. J. Hydrogen Energy 2021, 46, 31191–31201. [Google Scholar] [CrossRef]
- Yang, H.; Kang, W.; Tang, X.; Gao, Y.; Zhu, Z.; Wang, P.; Zhang, X. Gel kinetic characteristics and creep behavior of polymer microspheres based on bulk gel. J. Dispers. Sci. Technol. 2018, 39, 1808–1819. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, B.; Zhu, T.; Wang, P.; Zhang, X.; Wang, T.; Wu, F.; Zhang, L.; Kang, W.; Ketova, Y.A.; et al. Conformance control mechanism of low elastic polymer microspheres in porous medium. J. Pet. Sci. Eng. 2021, 196, 107708. [Google Scholar] [CrossRef]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear resistance performance of low elastic polymer microspheres used for conformance control treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, H.; Wu, Y.; Wang, D.; Zhang, Y. Preparation and Performance Evaluation of Polymeric Microspheres Used for Profile Control of Low-Permeability Reservoirs. J. Chem. 2020, 2020, 5279608. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Wu, T.; Zhao, Y.; Zhou, D.; Liu, S.; Brogi, A. Pore-Scale Investigation on the Plugging Behavior of Submicron-Sized Microspheres for Heterogeneous Porous Media with Higher Permeability. Geofluids 2020, 2020, 8869760. [Google Scholar] [CrossRef]
- Hou, G.; Zhao, W.; Jia, Y.; Yuan, X.; Zhou, J.; Liu, T.; Hou, J. Field Application of Nanoscale Polymer Microspheres for In-Depth Profile Control in the Ultralow Permeability Oil Reservoir. Front. Chem. 2020, 8, 805. [Google Scholar] [CrossRef]
- Wang, H.; Lin, M.; Chen, D.; Dong, Z.; Yang, Z.; Zhang, J. Research on the rheological properties of cross-linked polymer microspheres with different microstructures. Powder Technol. 2018, 331, 310–321. [Google Scholar] [CrossRef]
- Lai, Y.Y. The Preparation and Performance Evaluation of Low Interfacial Tension Carbon Dioxide Foam System. Master’s Thesis, China University of Petroleum (Beijing), Beijing, China, 2015. [Google Scholar]
- Gong, J.; Ji, Y.; Wang, Y.; Fan, H.; Wei, Z.; Li, C. Preparation and interfacial behavior of surface-active microspheres for both emulsion stabilization and profile control. J. Pet. Sci. Eng. 2022, 208, 109414. [Google Scholar] [CrossRef]
- Wang, H.; Lin, M.; Zhao, Q.; Xu, C.; Dong, Z.; Yang, Z.; Zhang, J. Fabrication, formation mechanism, and thermal degradation process of AM/AMPS/NVP terpolymeric microspheres with different microstructures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124401. [Google Scholar] [CrossRef]






| Ion Species | K++Na+ | Ca2+ | Mg2+ | SO42− | HCO3− | CO32− | Cl− | Salinity |
|---|---|---|---|---|---|---|---|---|
| Content mg/L | 2428.01 | 14.85 | 7.48 | 54.1 | 2160.08 | 197.66 | 2266.88 | 7156.5 |
| Core | Length (cm) | Diameter (cm) | Gas Permeability (mD) | Water Permeability (mD) | Pore Volume (mL) | Porosity (%) | Saturated Oil (mL) |
|---|---|---|---|---|---|---|---|
| low permeability | 10.009 | 3.792 | 21 | 11.9 | 18.54 | 16.47 | 8.2 |
| high permeability | 9.973 | 3.792 | 95 | 34.0 | 17.44 | 15.49 | 15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Peng, B. Preparation of Preformed Submicron Crosslinked Polymer Coils for Conformance Control in Low-Permeability Reservoirs. Polymers 2024, 16, 39. https://doi.org/10.3390/polym16010039
Liu J, Peng B. Preparation of Preformed Submicron Crosslinked Polymer Coils for Conformance Control in Low-Permeability Reservoirs. Polymers. 2024; 16(1):39. https://doi.org/10.3390/polym16010039
Chicago/Turabian StyleLiu, Jianwei, and Bo Peng. 2024. "Preparation of Preformed Submicron Crosslinked Polymer Coils for Conformance Control in Low-Permeability Reservoirs" Polymers 16, no. 1: 39. https://doi.org/10.3390/polym16010039
APA StyleLiu, J., & Peng, B. (2024). Preparation of Preformed Submicron Crosslinked Polymer Coils for Conformance Control in Low-Permeability Reservoirs. Polymers, 16(1), 39. https://doi.org/10.3390/polym16010039






