Polyurethane Adhesives with Chemically Debondable Properties via Diels–Alder Bonds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
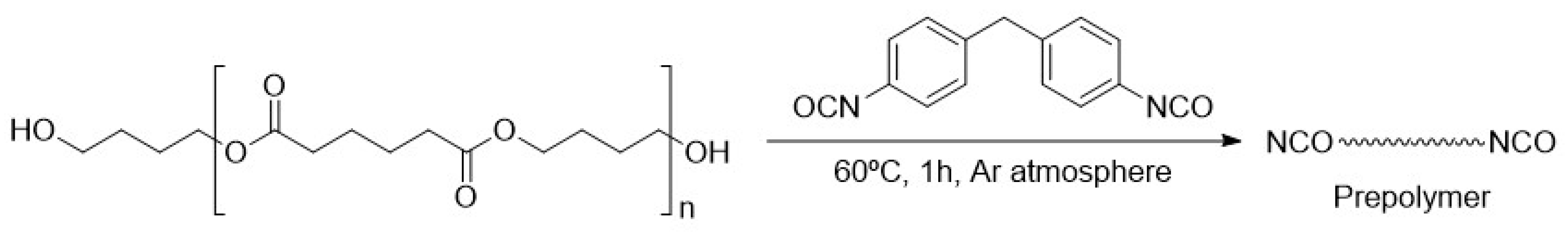
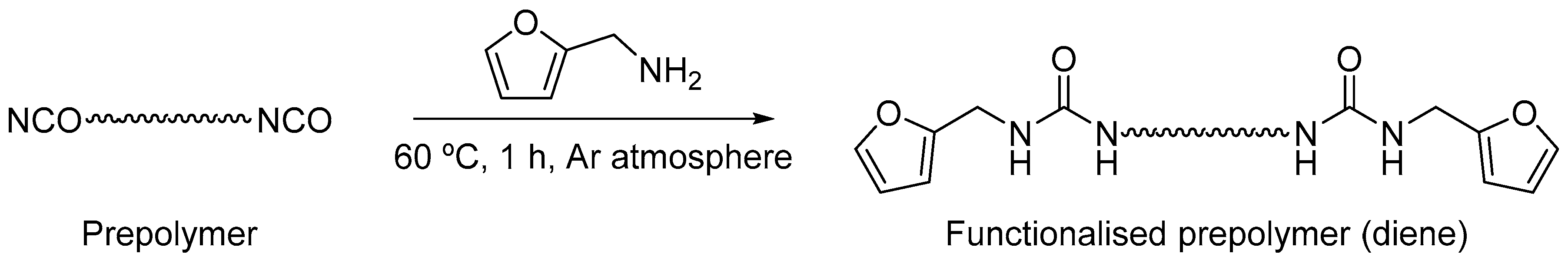
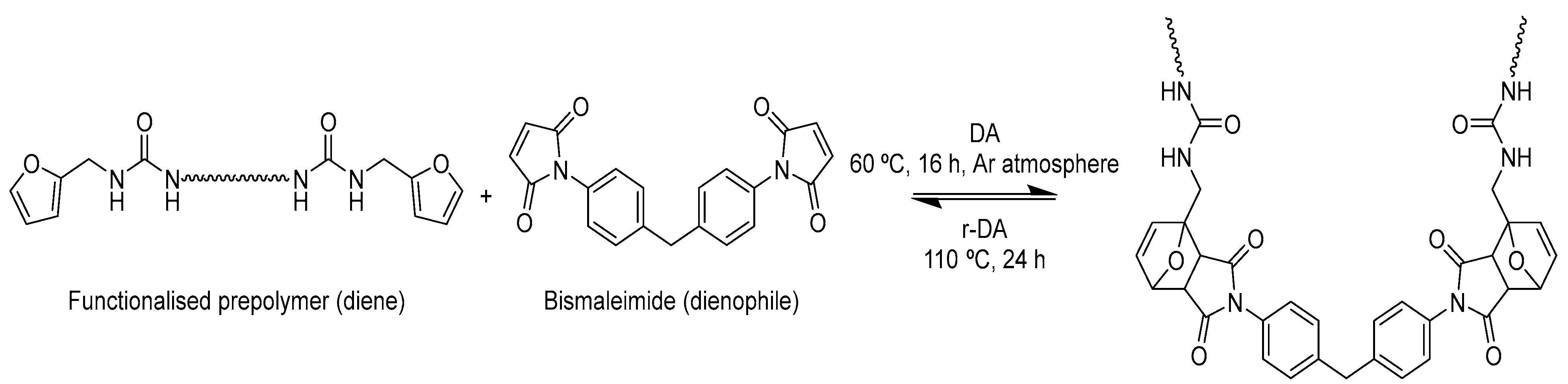
2.2. General Procedure for the Functionalisation of Polyurethane by DA/r-DA Reaction
2.3. Characterisation Techniques
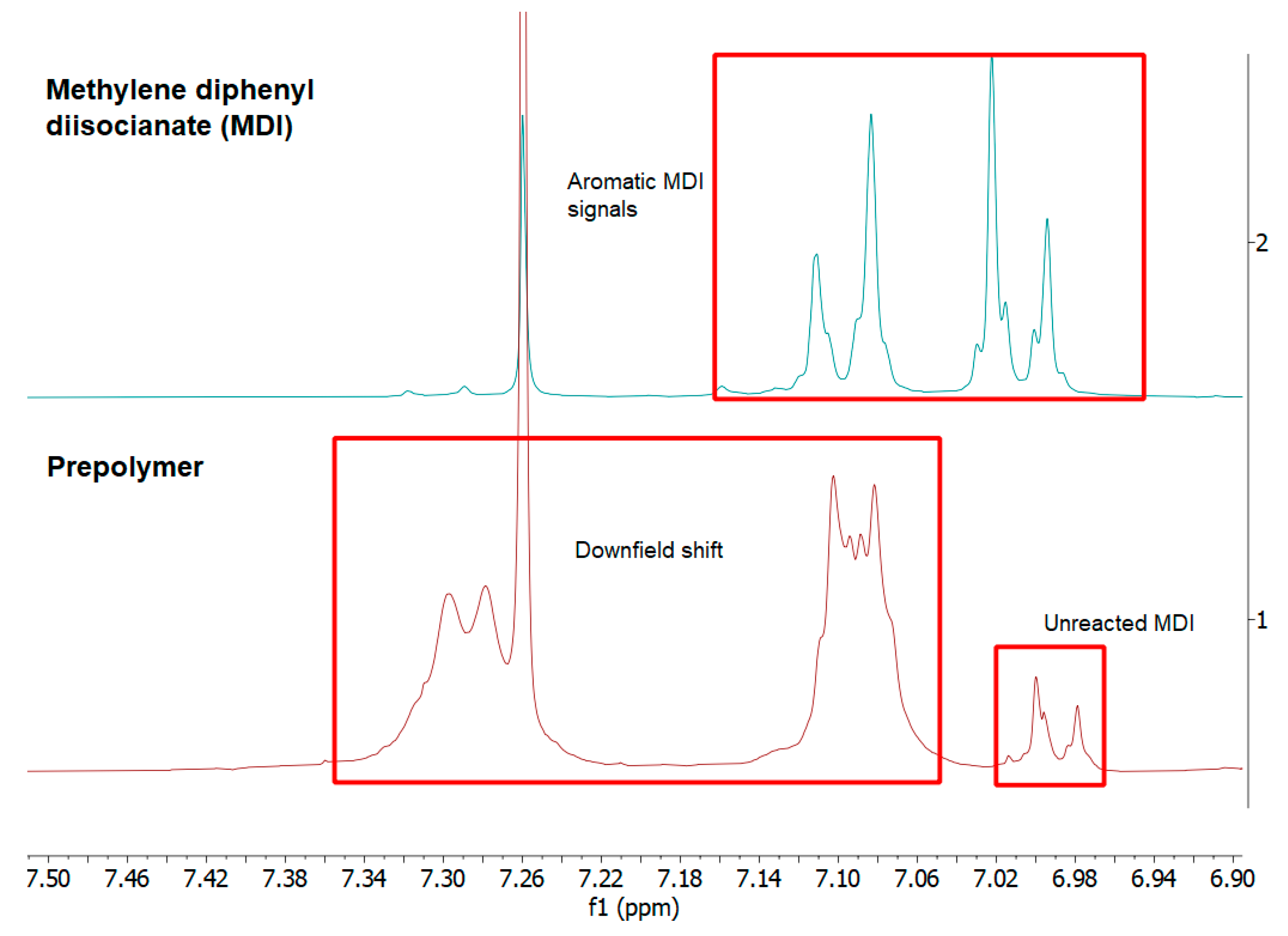
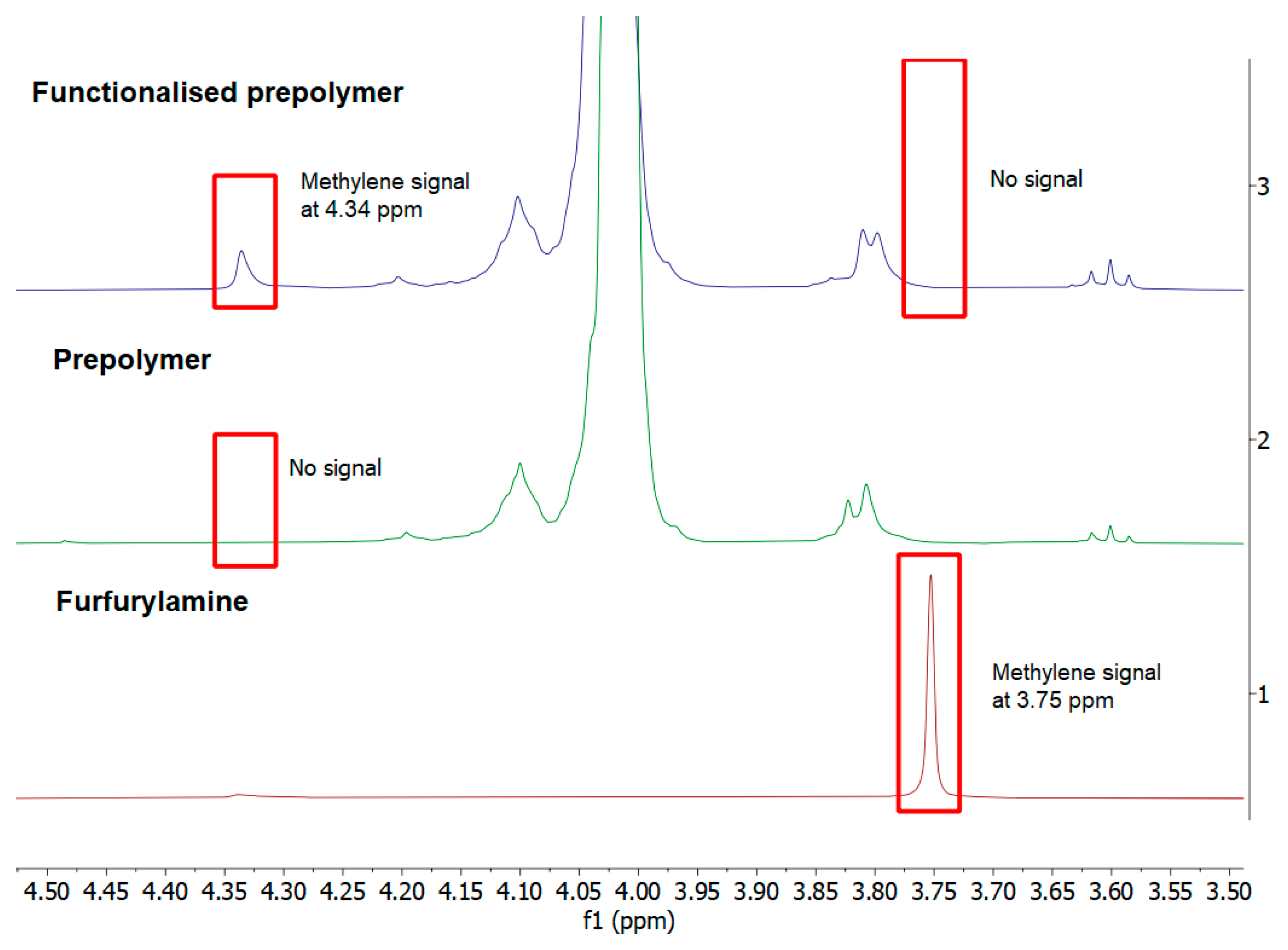
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Griffini, G.; Rigatelli, B.; Turri, S. Diels-Alder Macromolecular Networks in Recyclable, Repairable and Reprocessable Polymer Composites for the Circular Economy—A Review. Macromol. Mater. Eng. 2023, 308, 2300133. [Google Scholar] [CrossRef]
- Miravalle, E.; Bracco, P.; Brunella, V.; Barolo, C.; Zanetti, M. Improving Sustainability through Covalent Adaptable Networks in the Recycling of Polyurethane Plastics. Polymers 2023, 15, 3780. [Google Scholar] [CrossRef] [PubMed]
- Banea, M.D. Debonding on Demand of Adhesively Bonded Joints: A Critical Review. Rev. Adhes. Adhes. 2019, 7, 33–50. [Google Scholar] [CrossRef]
- Pradeep, S.V.; Kandasubramanian, B.; Sidharth, S. A Review on Recent Trends in Bio-Based Pressure Sensitive Adhesives. J. Adhes. 2023, 99, 2145–2166. [Google Scholar] [CrossRef]
- Bibi, I.; Ahmad, H.; Farid, A.; Iqbal, H.; Habib, N.; Atif, M. A Comprehensive Study of Electrically Switchable Adhesives: Bonding and Debonding on Demand. Mater. Today Commun. 2023, 35, 106293. [Google Scholar] [CrossRef]
- Moon, H.H.; Cha, I.; Hegazy, H.A.; Kim, K.; Song, C. Covalent Adaptive Polymer Networks for Switchable Adhesives. Bull. Korean Chem. Soc. 2023, 44, 750–767. [Google Scholar] [CrossRef]
- Hohl, D.K.; Weder, C. (De)Bonding on Demand with Optically Switchable Adhesives. Adv. Opt. Mater. 2019, 7, 1900230. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, N.; Rabnawaz, M. Covalent Adaptable Network and Self-Healing Materials: Current Trends and Future Prospects in Sustainability. Polymers 2020, 12, 2027. [Google Scholar] [CrossRef]
- Yue, T.; Liang, X.; Zhang, J.; Lei, I.M.; Liu, J. Polyurethane Vitrimers: Chemistry, Properties and Applications. J. Polym. Sci. 2022, 61, 2233–2253. [Google Scholar] [CrossRef]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.K.; Martinez, A.M.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward Stimuli-Responsive Dynamic Thermosets through Continuous Development and Improvements in Covalent Adaptable Networks (CANs). Adv. Mater. 2020, 32, e1906876. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Yang, G.; Yao, Y.; Wei, X.; Pan, T.; Wu, J.; Tian, M.; Yin, P. Recent Advances in Recyclable Thermosets and Thermoset Composites Based on Covalent Adaptable Networks. J. Mater. Sci. Technol. 2021, 92, 75–87. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, T.; Zhang, Y.; Guo, B.; Xu, J. Reprocessable Cross-Linked Polyurethane with Dynamic and Tunable Phenol–Carbamate Network. ACS Sustain. Chem. Eng. 2020, 8, 1207–1218. [Google Scholar] [CrossRef]
- Elling, B.R.; Dichtel, W.R. Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Liu, G.; Dong, X.; Liu, X.; Xu, J.; Wang, D. Facile Fabrication of Fast Recyclable and Multiple Self-healing Epoxy Materials through Diels-alder Adduct Cross-linker. J. Polym. Sci. Part Polym. Chem. 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Jia, P.; Zhang, M.; Bo, C.; Feng, G.; Hu, L.; Zhou, Y. Tunable “Soft and Stiff”, Self-Healing, Recyclable, Thermadapt Shape Memory Biomass Polymers Based on Multiple Hydrogen Bonds and Dynamic Imine Bonds. J. Mater. Chem. A 2019, 7, 13400–13410. [Google Scholar] [CrossRef]
- Ozawa, M.; Shibata, M. Reprocessable Bismaleimide-Diamine Thermosets Based on Disulfide Bonds. React. Funct. Polym. 2020, 146, 104404. [Google Scholar] [CrossRef]
- Hammer, L.; Van Zee, N.J.; Nicolaÿ, R. Dually Crosslinked Polymer Networks Incorporating Dynamic Covalent Bonds. Polymers 2021, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. The Furan/Maleimide Diels–Alder Reaction: A Versatile Click–Unclick Tool in Macromolecular Synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Du, P.; Wu, M.; Liu, X.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Diels-Alder-based Crosslinked Self-healing Polyurethane/Urea from Polymeric Methylene Diphenyl Diisocyanate. J. Appl. Polym. Sci. 2014, 131, app.40234. [Google Scholar] [CrossRef]
- Wu, M.; Liu, Y.; Du, P.; Wang, X.; Yang, B. Polyurethane Hot Melt Adhesive Based on Diels-Alder Reaction. Int. J. Adhes. Adhes. 2020, 100, 102597. [Google Scholar] [CrossRef]
- Soavi, G.; Portone, F.; Battegazzore, D.; Paravidino, C.; Arrigo, R.; Pedrini, A.; Pinalli, R.; Fina, A.; Dalcanale, E. Phenoxy Resin-Based Vinylogous Urethane Covalent Adaptable Networks. React. Funct. Polym. 2023, 191, 105681. [Google Scholar] [CrossRef]
- Sridhar, L.M.; Slark, A.T.; Wilson, J.A. Furan Functionalized Polyesters and Polyurethanes for Thermally Reversible Reactive Hotmelt Adhesives. In Furan Derivatives—Recent Advances and Applications; Khan, A., Muzibur Rahman, M., Ramesh, M., Ahmad Khan, S., Mohammed Ahmed Asiri, A., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-707-4. [Google Scholar]
- Liu, T.; Hao, C.; Zhang, S.; Yang, X.; Wang, L.; Han, J.; Li, Y.; Xin, J.; Zhang, J. A Self-Healable High Glass Transition Temperature Bioepoxy Material Based on Vitrimer Chemistry. Macromolecules 2018, 51, 5577–5585. [Google Scholar] [CrossRef]
- Ruiz De Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy Resin with Exchangeable Disulfide Crosslinks to Obtain Reprocessable, Repairable and Recyclable Fiber-Reinforced Thermoset Composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Fairbanks, B.D.; Liang, H.; Ke, J.; Zhu, C. Fast Self-Healing Engineered by UV-Curable Polyurethane Contained Diels-Alder Structure. Prog. Org. Coat. 2019, 131, 131–136. [Google Scholar] [CrossRef]
- Budd, M.E.; Stephens, R.; Afsar, A.; Salimi, S.; Hayes, W. Exploiting Thermally-Reversible Covalent Bonds for the Controlled Release of Microencapsulated Isocyanate Crosslinkers. React. Funct. Polym. 2019, 135, 23–31. [Google Scholar] [CrossRef]
- Spiesschaert, Y.; Guerre, M.; De Baere, I.; Van Paepegem, W.; Winne, J.M.; Du Prez, F.E. Dynamic Curing Agents for Amine-Hardened Epoxy Vitrimers with Short (Re)Processing Times. Macromolecules 2020, 53, 2485–2495. [Google Scholar] [CrossRef]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous Urethane Vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Tellers, J.; Pinalli, R.; Soliman, M.; Vachon, J.; Dalcanale, E. Reprocessable Vinylogous Urethane Cross-Linked Polyethylene via Reactive Extrusion. Polym. Chem. 2019, 10, 5534–5542. [Google Scholar] [CrossRef]
- Heo, Y.; Sodano, H.A. Self-Healing Polyurethanes with Shape Recovery. Adv. Funct. Mater. 2014, 24, 5261–5268. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Torkelson, J.M. Recyclable Polymer Networks Containing Hydroxyurethane Dynamic Cross-Links: Tuning Morphology, Cross-Link Density, and Associated Properties with Chain Extenders. Polymer 2019, 178, 121604. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Zhang, L.; Guo, Y.; Song, J.; Lou, J.; Guan, Q.; He, C.; You, Z. Strong, Detachable, and Self-Healing Dynamic Crosslinked Hot Melt Polyurethane Adhesive. Mater. Chem. Front. 2019, 3, 1833–1839. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, H.; Wang, Y.; Ye, J.; Guo, L.; He, L.; Li, X.; Qiu, T.; Tuo, X. Dual Dynamic Network System Constructed by Waterborne Polyurethane for Improved and Recoverable Performances. Chem. Eng. J. 2022, 442, 136204. [Google Scholar] [CrossRef]
- Kaiser, K.M.A. Recycling of Multilayer Packaging Using a Reversible Cross-linking Adhesive. J. Appl. Polym. Sci. 2020, 137, 49230. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Quiles-Díaz, S.; Seyler, H.; Ellis, G.J.; Shuttleworth, P.S.; Gómez-Fatou, M.A. Remotely Triggered Reversible Bonds in Adhesives for Sustainable Multi-Layered Packaging. Sustain. Mater. Technol. 2023, 36, e00632. [Google Scholar] [CrossRef]
- UNE-EN 827:2006; Adhesives—Determination of Conventional Solids Content and Constant Mass Solids Content. European Standard Committee: Brussels, Belgium, 2006.
- Carbonell-Blasco, M.P.; Pérez-Limiñana, M.A.; Ruzafa-Silvestre, C.; Arán-Ais, F.; Orgilés-Calpena, E. Influence of Biobased Polyol Type on the Properties of Polyurethane Hotmelt Adhesives for Footwear Joints. Appl. Adhes. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Carbonell-Blasco, M.P.; Ruzafa-Silvestre, C.; Mateu-Romero, B.; Orgilés-Calpena, E.; Arán-Ais, F. Cleaner Technologies to Minimise the Environmental Footprint of the Footwear Bonding Process. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2022, 095440622211400. [Google Scholar] [CrossRef]
- EN 1392:2007; Adhesives for Leather and Footwear Materials-Solvent-Based and Dispersion Adhesives-Testing of Bond Strength under Specified Conditions. European Standard Committee: Brussels, Belgium, 2007.
- Orgilés-Calpena, E.; Arán-Aís, F.; Torró-Palau, A.M.; Montiel-Parreño, E.; Orgilés-Barceló, C. Synthesis of Polyurethanes from CO2-Based Polyols: A Challenge for Sustainable Adhesives. Int. J. Adhes. Adhes. 2016, 67, 63–68. [Google Scholar] [CrossRef]
- Blasco, M.P.C.; Limiñana, M.Á.P.; Silvestre, C.R.; Calpena, E.O.; Aís, F.A. Sustainable Reactive Polyurethane Hot Melt Adhesives Based on Vegetable Polyols for Footwear Industry. Polymers 2022, 14, 284. [Google Scholar] [CrossRef]
- Arán-Ais, F.; Ruzafa-Silvestre, C.; Carbonell-Blasco, M.; Pérez-Limiñana, M.; Orgilés-Calpena, E. Sustainable Adhesives and Adhesion Processes for the Footwear Industry. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2020, 235, 585–596. [Google Scholar] [CrossRef]
- UNE-EN ISO 17708:2019; Footwear-Test Methods for Whole Shoe-Upper Sole Adhesion. ISO: Geneva, Switzerland, 2019.
- Turkenburg, D.H.; Van Bracht, H.; Funke, B.; Schmider, M.; Janke, D.; Fischer, H.R. Polyurethane Adhesives Containing Diels–Alder-based Thermoreversible Bonds. J. Appl. Polym. Sci. 2017, 134, app.44972. [Google Scholar] [CrossRef]
- Quiles-Díaz, S.; Seyler, H.; Ellis, G.J.; Shuttleworth, P.S.; Flores, A.; Gómez-Fatou, M.A.; Salavagione, H.J. Designing New Sustainable Polyurethane Adhesives: Influence of the Nature and Content of Diels–Alder Adducts on Their Thermoreversible Behavior. Polymers 2022, 14, 3402. [Google Scholar] [CrossRef]
- Du, L.; Liu, Z.; Ye, Z.; Hao, X.; Ou, R.; Liu, T.; Wang, Q. Dynamic Cross-Linked Polyurethane Hot-Melt Adhesive with High Biomass Content and High Adhesive Strength Simultaneously. Eur. Polym. J. 2023, 182, 111732. [Google Scholar] [CrossRef]
- Li, Q.; Ma, S.; Li, P.; Wang, B.; Feng, H.; Lu, N.; Wang, S.; Liu, Y.; Xu, X.; Zhu, J. Biosourced Acetal and Diels–Alder Adduct Concurrent Polyurethane Covalent Adaptable Network. Macromolecules 2021, 54, 1742–1753. [Google Scholar] [CrossRef]
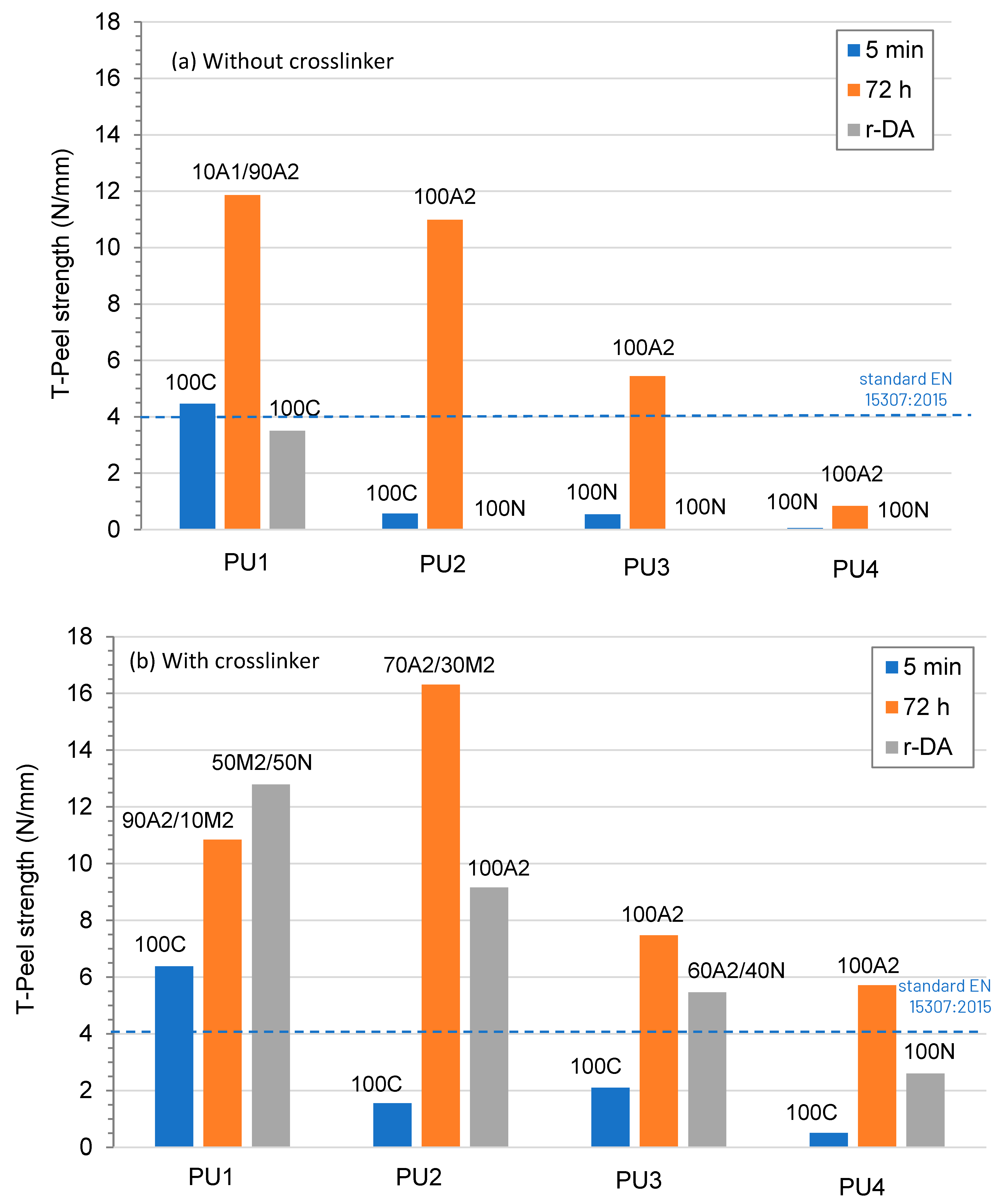
- UNE-EN 15307:2015; Adhesive for Leather and Footwear Materials-Sole-Upper Bonds. Minimum Strength Requirements. European Standard Committee: Brussels, Belgium, 2015.


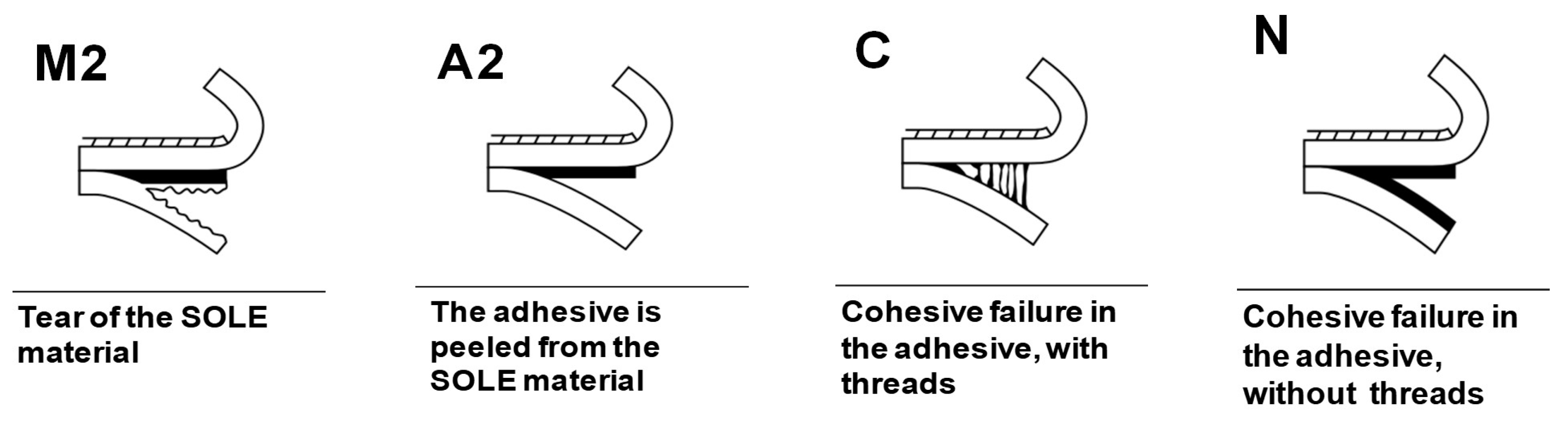
| Adhesive | NCO/OH | DA Conversion (%) | r-DA Conversion (%) |
|---|---|---|---|
| PU1 | 1.4 | - | - |
| PU2 | 1.4 | 69% | 37% |
| PU3 | 1.4 | 68% | 57% |
| PU4 | 1.1 | 63% | 62% |
| Adhesive | Solids Content (wt%) |
|---|---|
| PU1 | 16.6 ± 0.1 |
| PU2 | 16.3 ± 0.1 |
| PU3 | 17.7 ± 0.04 |
| PU4 | 17.6 ± 0.1 |
| Speed (rpm) | PU1 | PU2 | PU3 | PU4 |
|---|---|---|---|---|
| 10 | 250 | 10 | 100 | 30 |
| 20 | 245 | 15 | 100 | 30 |
| 30 | 247 | 20 | 103 | 30 |
| 50 | 246 | 26 | 108 | 36 |
| 60 | 248 | 28 | 110 | 40 |
| 100 | 249 | 36 | 120 | 49 |
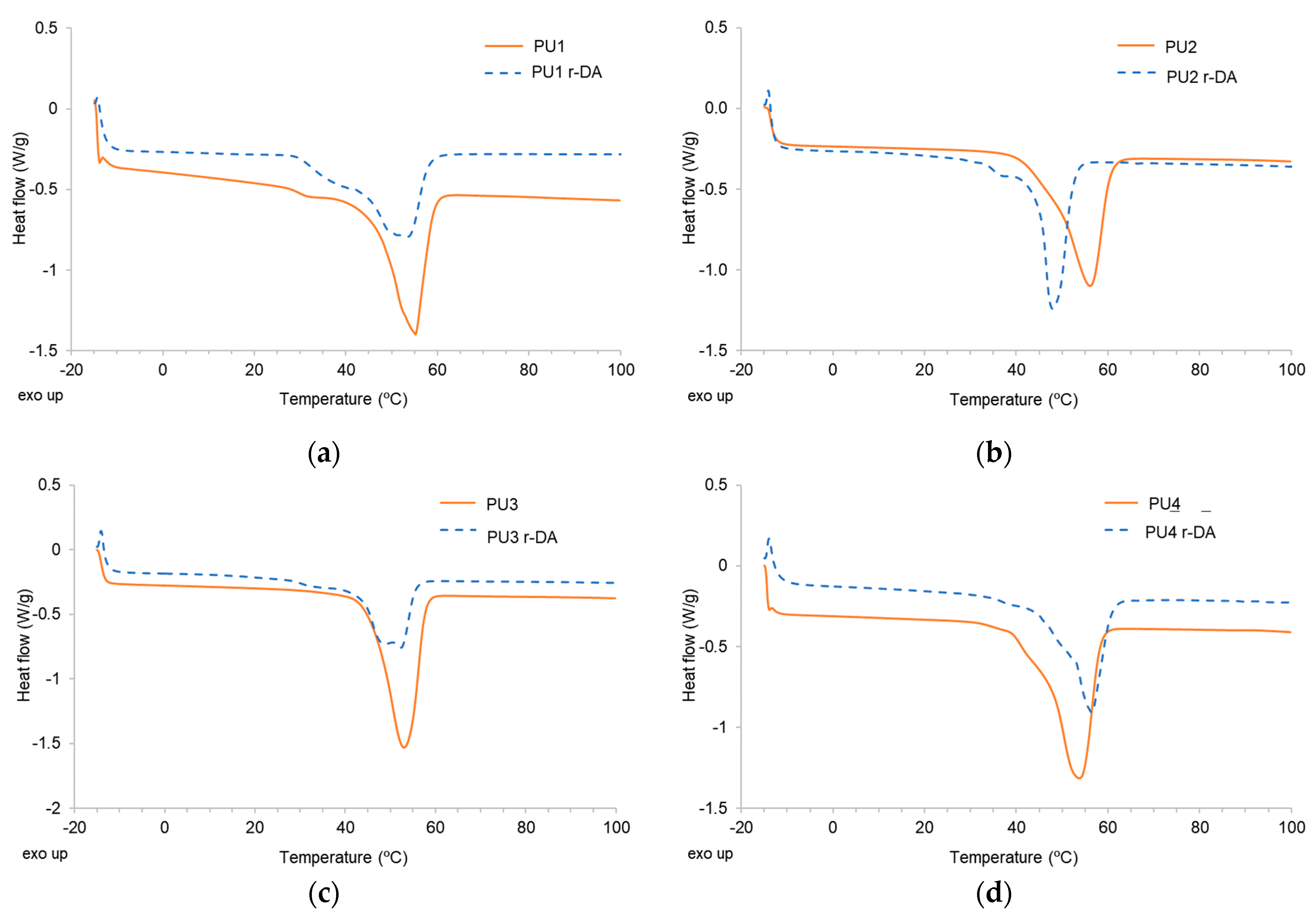
| Adhesive | Tm (°C) | ΔHm (J/g) | Adhesive | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|
| PU1 | 55 | −54.48 | PU1 r-DA | 53 | −33.97 |
| PU2 | 56 | −47.70 | PU2 r-DA | 48 | −38.27 |
| PU3 | 53 | −57.84 | PU3 r-DA | 52 | −36.13 |
| PU4 | 54 | −55.60 | PU4 r-DA | 56 | −39.13 |
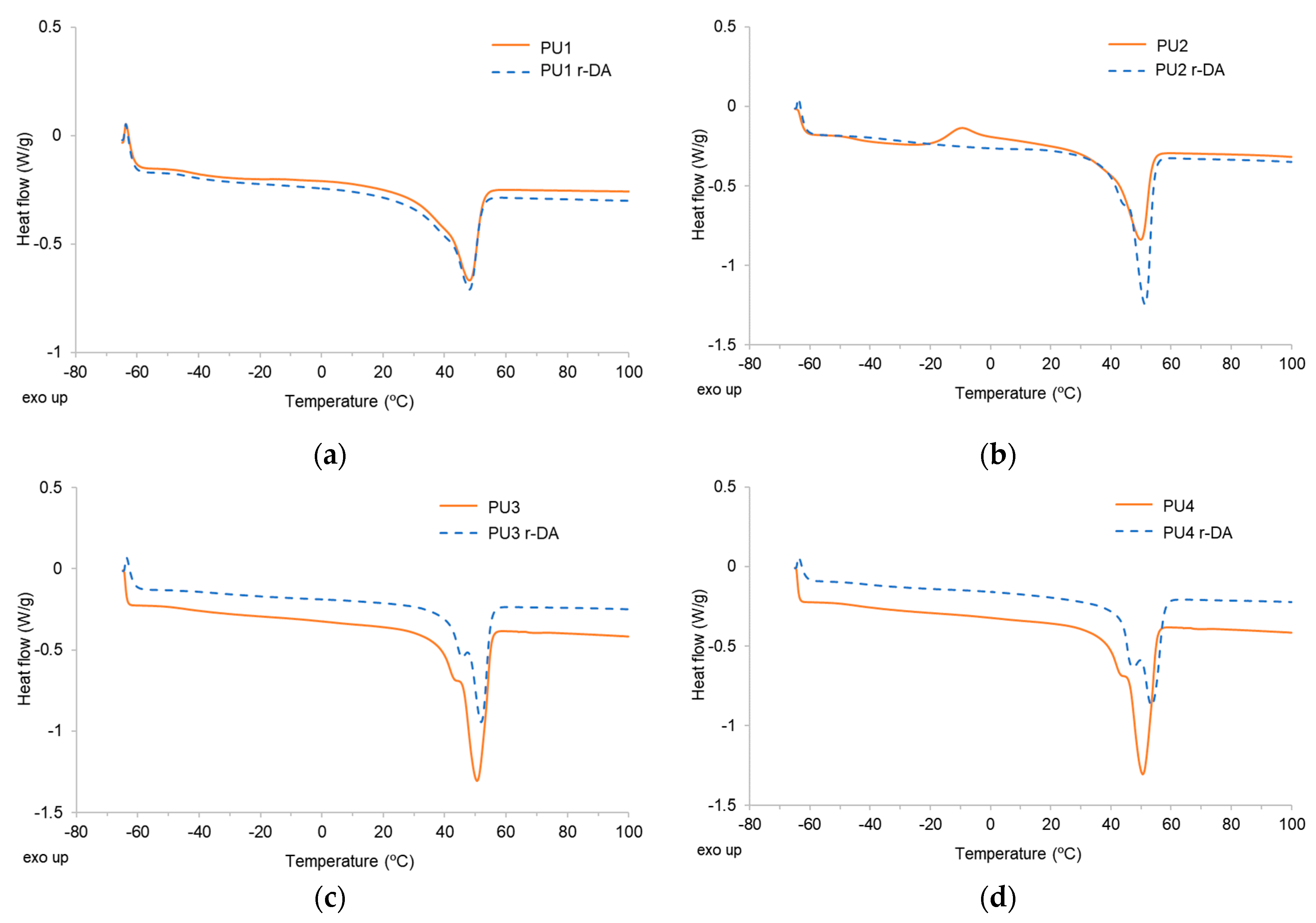
| Adhesive | Tm (°C) | ΔHm (J/g) | Adhesive | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|
| PU1 | 48 | −33.51 | PU1 r-DA | 48 | −32.54 |
| PU2 | 50 | −34.65 | PU2 r-DA | 51 | −45.57 |
| PU3 | 50 | −43.50 | PU3 r-DA | 52 | −35.40 |
| PU4 | 50 | −52.50 | PU4 r-DA | 53 | −43.48 |
| Adhesive | Td (֯C) | Δmd (%) | Residue (%) | Adhesive | Td (֯C) | Δmd (%) |
|---|---|---|---|---|---|---|
| PU1 | 415 | 95 | 5 | PU1 r-DA | 410 | 95 |
| PU2 | 411 | 94 | 6 | PU2 r-DA | 409 | 93 |
| PU3 | 412 | 90 | 10 | PU3 r-DA | 412 | 94 |
| PU4 | 413 | 95 | 5 | PU4 r-DA | 411 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonell-Blasco, M.P.; Moyano, M.A.; Hernández-Fernández, C.; Sierra-Molero, F.J.; Pastor, I.M.; Alonso, D.A.; Arán-Aís, F.; Orgilés-Calpena, E. Polyurethane Adhesives with Chemically Debondable Properties via Diels–Alder Bonds. Polymers 2024, 16, 21. https://doi.org/10.3390/polym16010021
Carbonell-Blasco MP, Moyano MA, Hernández-Fernández C, Sierra-Molero FJ, Pastor IM, Alonso DA, Arán-Aís F, Orgilés-Calpena E. Polyurethane Adhesives with Chemically Debondable Properties via Diels–Alder Bonds. Polymers. 2024; 16(1):21. https://doi.org/10.3390/polym16010021
Chicago/Turabian StyleCarbonell-Blasco, María Pilar, María Alejandra Moyano, Carlota Hernández-Fernández, Francisco J. Sierra-Molero, Isidro M. Pastor, Diego A. Alonso, Francisca Arán-Aís, and Elena Orgilés-Calpena. 2024. "Polyurethane Adhesives with Chemically Debondable Properties via Diels–Alder Bonds" Polymers 16, no. 1: 21. https://doi.org/10.3390/polym16010021
APA StyleCarbonell-Blasco, M. P., Moyano, M. A., Hernández-Fernández, C., Sierra-Molero, F. J., Pastor, I. M., Alonso, D. A., Arán-Aís, F., & Orgilés-Calpena, E. (2024). Polyurethane Adhesives with Chemically Debondable Properties via Diels–Alder Bonds. Polymers, 16(1), 21. https://doi.org/10.3390/polym16010021








