Abstract
Failures of wound healing have been a focus of research worldwide. With the continuous development of materials science, electrospun nanofiber scaffolds loaded with metal-based nanoparticles provide new ideas and methods for research into new tissue engineering materials due to their excellent antibacterial, anti-inflammatory, and wound healing abilities. In this review, the stages of extracellular matrix and wound healing, electrospun nanofiber scaffolds, metal-based nanoparticles, and metal-based nanoparticles supported by electrospun nanofiber scaffolds are reviewed, and their characteristics and applications are introduced. We discuss in detail the current research on wound healing of metal-based nanoparticles and electrospun nanofiber scaffolds loaded with metal-based nanoparticles, and we highlight the potential mechanisms and promising applications of these scaffolds for promoting wound healing.
1. Introduction
The skin is composed of three primary layers: the epidermis, the dermis, and the subcutaneous tissue. The epidermis, the outermost layer of the skin, is a keratinized stratified squamous epithelium [1]. It is relatively thin, avascular, and does not contain blood vessels [2]. The dermal layer is intimately connected to the epidermis, and predominantly consists of fibroblasts and the extracellular matrix (ECM) they produce [3]. The ECM is a dynamic three-dimensional network surrounding the cells, composed of a variety of macromolecules such as collagen, proteoglycans, and adhesion proteins [4,5]. It provides mechanical support to the cells, and plays a role in their physiological and biochemical activities [6].
Within a day after skin injury, the body’s coagulation cascade is initially activated, leading to the formation of blood clots, while platelets continuously release signaling molecules that attract inflammatory cells to the wound site [7]. During the inflammatory phase (1–2 days), neutrophils arrive at the wound to phagocytose foreign material and release cytokines that promote the proliferation and migration of monocytes and fibroblasts [8,9]. Macrophages discharge a plethora of enzymes and cytokines to clear necrotic tissue, remodel the ECM, and foster collagen and angiogenesis [10,11]. In the proliferative phase (3–7 days), fibroblasts appear around the wound, proliferate, and differentiate to produce collagen, proteoglycans, and build a new ECM, which creates a conducive environment for the regeneration of tissue and, in conjunction with signaling molecules, regulates the growth, migration, and differentiation of cells. Therefore, the formation of the ECM is crucial for wound healing, which will be elaborated on in detail later in this review [11,12]. Finally, the remodeling phase of the wound may persist for an extended period (1 week to several weeks), during which granulation tissue re-epithelializes and the ECM is continuously synthesized and remodeled, allowing the new tissue to gradually regain a state close to that of normal skin [13,14]. Wound healing is an intricate and overlapping process, and rapid and effective treatment methods for wounds are currently a significant clinical need.
Metal-based nanoparticles (MBNPs), as emerging nanobiomaterials, have good biocompatibility, and their nanoscale size enables them to be easily taken up by cells. The metal ions produced by MBNPs can participate in normal cellular activities as biologically active molecules. These properties make MBNPs widely used in drug delivery, biological imaging, and cancer treatment [15,16]. Studies have shown that during different stages of wound healing, MBNPs and their released ions exhibit unique biological properties. For example, in the early stages of wound healing, AgNPs show excellent antibacterial and anti-inflammatory properties, preventing deterioration of the wound [17]. During the inflammatory and proliferative phases, AuNPs can exert anti-inflammatory and antioxidant effects, and promote tissue regeneration [18,19]; CuNPs mainly participate in the regulation of signaling molecules related to angiogenesis and ECM reconstruction [20,21]. However, considering the potential toxic side effects of MBNPs, the application of these nanoparticles as functional components necessitates their integration with appropriate carriers to mitigate risks [22,23]. Therefore, in recent years, researchers have often combined MBNPs with electrospinning technology to prepare regenerative scaffolds. The biological functions of MBNPs are leveraged to repair wounds. The scaffolds can wrap and fix MBNPs, enabling them to be released in situ and in a controllable manner at the wound site, further enhancing the wound healing effect.
Many regenerative scaffolds for wound healing have been developed, among which the use of nanofibers to prepare tissue engineering scaffolds is a very promising solution [24]. Electrospinning is a technique for the one-step preparation of polymer nanofibers based on electrostatic forces. Nanofibers produced by this method can uniquely assist in wound healing on the basis of the following: 1. having dimensions similar to fibrin fibers, which facilitate cell adhesion and migration [25]; 2. possessing high porosity and surface area, enabling a nanoscale simulation of the fibrous network structure of the ECM, beneficial for cell proliferation and signal transduction [26,27]; 3. exhibiting good biocompatibility and mechanical strength, which do not cause significant immune rejection reactions and can support the extensive proliferation of new cells in the early stages of wound healing, promoting the formation of new tissue [28]. Additionally, by changing the spinning parameters or combining electrospinning with mixed spinning, coaxial spinning, surface modification, and other methods, scaffolds can be generated with specific structures and specific physicochemical properties [29]. When used as carriers, functional components can be encapsulated by the nanofibers through direct mixing, enabling the controlled release of these components during application [30,31]. Therefore, electrospun fibers, with their ability to mimic the ECM and serve as carriers for various functional components that promote wound healing, have become a focal point of numerous studies.
To the best of our knowledge, a review article focused on advances in the development of metal-based nanoparticles supported by electrospun nanofiber scaffolds in wound healing has not been published to date. Therefore, the aim of this review is to summary the developments about electrospun nanofiber scaffolds loaded with metal-based nanoparticles and their application in wound healing over the last five years. The present review introduces the main functions of the ECM in various stages of wound healing. Next, the key characteristics of electrospun nanofiber scaffolds are highlighted for biomedical applications. Subsequently, the study discusses the emerging nanomaterials, MBNPs, and how they can be combined with electrospinning to synergistically promote wound healing. The focus is on the existing research regarding electrospun nanofiber scaffolds loaded with MBNPs for wound healing. Finally, conclusions and future developments and challenges are presented for the field of electrospun nanofiber scaffolds loaded with MBNPs for wound treatment. An exhaustive bibliographic search was conducted using Web of Science databases. The selected studies are from 2018 and after.
2. ECM Dynamics during Wound Healing
The ECM is composed of a tightly organized network of nanoscale fibers, including collagen fibers, elastic fibers, fibronectin, and other components; it plays a crucial role in various stages of wound healing [3]. The process of wound healing generally consists of four stages: hemostasis, inflammation, proliferation, and remodeling [32]. When the skin is injured, platelets aggregate around the wound and, together with fibrinogen in the ECM, form a blood clot. This clot releases a large quantity of cytokines and chemokines, attracting inflammatory cells and fibroblasts (Figure 1A) [33]. During the inflammation stage, inflammatory cells such as neutrophils, monocytes, and macrophages, as well as fibroblasts, migrate to the wound area through blood vessels and the ECM. Inflammatory cells clear pathogens and necrotic tissue at the wound site and release cytokines to promote fibroblasts to produce large quantities of ECM components, including collagen, hyaluronic acid, and proteoglycans. These ECM components provide support and protection for the damaged tissue (Figure 1B) [34,35]. In the proliferation phase, the newly formed ECM provides mechanical support and a survival environment for cells. Cells proliferate and differentiate extensively around the wound, filling the gap in the wound (Figure 1C) [36]. Finally, granulation tissue forms around the wound and new blood vessels are formed. Wound healing enters the remodeling phase, during which the granulation tissue undergoes epithelialization, and the ECM continuously remodels, gradually restoring the newly formed tissue to a state closer to normal tissue (Figure 1D) [3,37].
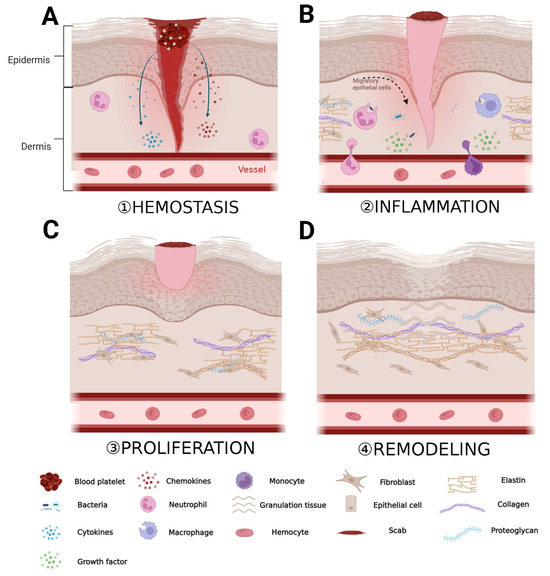
Figure 1.
Role of ECM in the various stages of wound healing. (A) Period of hemostasis; (B) period of inflammation; (C) period of proliferation; and (D) period of remodeling. “Created with BioRender.com.” Agreement number: HW262OMTG1.
The ECM plays a crucial role in the normal healing process of wounds. Electrospun nanofiber scaffolds mimic the intricate structure of the skin’s ECM at the microscale and provide robust mechanical support to promote cell proliferation, adhesion, and migration at the wound site. Additionally, the high surface area-to-volume ratio of nanofibers can aid in rapid hemostasis of the wound [38]. Incorporating functional materials into nanofibers, such as antimicrobial agents, growth factors, and metallic materials, provides additional biological functionalities that help further promote wound healing (Figure 2).
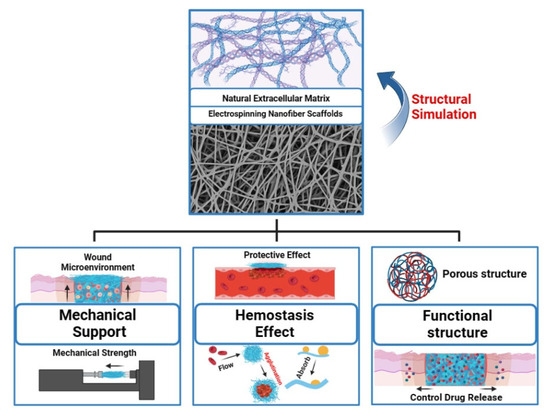
Figure 2.
Multifunctional electrospun nanofiber scaffolds for wound healing applications. “Created with BioRender.com.” Agreement number: TI2684VL3Q.
3. Electrospun Nanofiber Scaffolds in Tissue Engineering
Electrospinning is a technique that uses a polymer solution to prepare nanofibers [39]. In general, the basic equipment for electrospinning mainly includes a high-voltage power supply, a spinneret, an injection pump, and a collector plate [40]. During operation of the device, the injection pump pushes the polymer solution in the syringe to the spinneret connected to the high-voltage power supply. Under the action of surface tension, the polymer solution forms charged droplets at the nozzle of the high-voltage power supply. The electrostatic repulsion generated by the surface charge on the droplet surface is opposite to the direction of the surface tension. As the voltage intensity gradually increases, the polymer droplets are elongated under the action of the electrostatic repulsion produced by the surface charge, forming a Taylor cone [29,41]. When the electrostatic repulsion on the surface of the polymer droplet exceeds the surface tension, the droplet is elongated in the electric field, forming a charged jet. Finally, the solvent in the polymer solution evaporates, forming polymer nanofibers. The nanofibers accumulate on the collector plate in a random direction, forming a nanofiber scaffold [42,43].
Additionally, different structures and functions of scaffolds can be obtained by adjusting the spinning solution, spinning parameters, spinning environment, and other conditions [44]. When functional materials are incorporated, polymer nanofiber scaffolds can be prepared by various electrospinning techniques based on the type of functional material and solvent requirements, such as blend electrospinning, coaxial electrospinning, emulsion electrospinning, and side-by-side electrospinning, along with surface modifications, producing special structures such as the core-shell, multilayer, and Janus structures. This enables the controlled release of functional materials, such as burst release, sustained release, and multiple release, depending on the specific application (Figure 3) [45,46]. Electrospun nanofiber scaffolds possess high interconnectivity and a high specific surface area, and their porous three-dimensional structure exhibits excellent mechanical strength and contains multiple cell binding sites, which can promote the interaction between cells and the ECM when applied at an injury site [47]. During tissue remodeling, degradable nanofiber scaffolds are gradually decomposed and absorbed, without adverse effects on the structure and function of the tissue. Based on the above description, electrospun nanofiber scaffolds meet the ideal conditions for constructing tissue engineering scaffolds, as summarized in Table 1, and are ideal candidates for preparing artificial skin, wound dressings, and skin tissue scaffolds. Currently, increasingly more research is focused on using metal-based nanoparticles and electrospinning technology to prepare composite scaffolds for treating drug-resistant bacteria and difficult-to-heal wounds. The inherent biological functions of metal-based nanoparticles can endow scaffolds with unique biological activity and material properties. This type of bionanomaterial provides new ideas and unique solutions for preventing bacterial infections and treating difficult-to-heal wounds, and is expected to become an important therapeutic method and material selection in the biomedical field in the future [48].
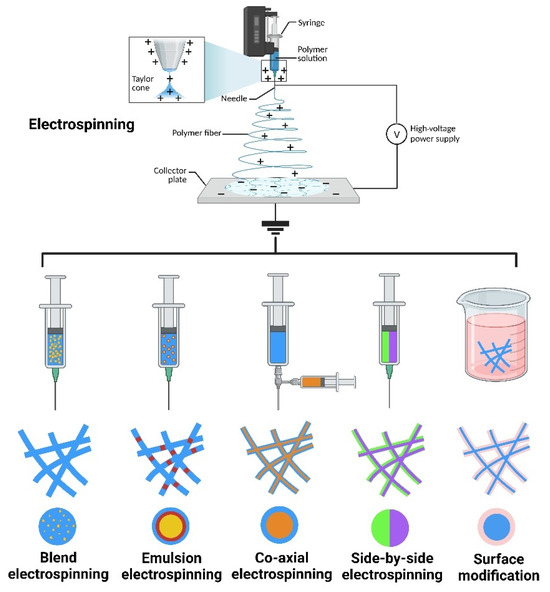
Figure 3.
The method and production of nanofiber. “Created with BioRender.com.” Agreement number: LZ2684T4E0.

Table 1.
Design requirements for tissue engineering scaffolds.
4. Metal-Based Nanoparticles in Wound Healing
MBNPs refer to small metal particles with at least one dimension in three-dimensional space in the range of 1–100 nm [58]. MBNPs are characterized by their small size, large specific surface area, and quantum size effects. These properties result in completely different physical and chemical characteristics from macroscopic metals [59]. In the past decade, many metal-based nanoparticles (such as AgNPs, AgNPs, and CuNPs) have been extensively studied and were shown to have the advantages of simple preparation, strong stability, good biocompatibility, and good biodegradation, which are very suitable characteristics for biomedical applications [60].
MBNPs can inhibit or kill microorganisms through various mechanisms. Their antibacterial mechanism differs from conventional antibiotics, which makes it difficult for bacteria to develop resistance [48,61] (Table 2). For example, 1. MBNPs with a high surface area can effectively bind to the cell wall and membrane of microorganisms, interact with the proteins on the surface, and disrupt their cellular structure [62]. 2. MBNPs can kill microorganisms by directly contacting them, inducing the production of reactive oxygen species (ROS) that can damage DNA, RNA, proteins, and other substances inside the cell (Figure 4A) [63,64]. 3. Once inside the cell, MBNPs are free to interact with cellular structures (e.g., membranes, ribosomes, proteins, DNA, RNA), disrupting cell functions [65,66]. Moreover, some metal-based nanoparticles can directly promote angiogenesis, ECM accumulation, or wound re-epithelialization through their own functions (such as anti-inflammatory, antioxidant, cell proliferation-promoting, and cytokine-regulating effects), or through combining MBNPs with photothermal therapy (PTT) and photodynamic therapy (PDT). This can improve wound healing (Figure 4B) [15,67].
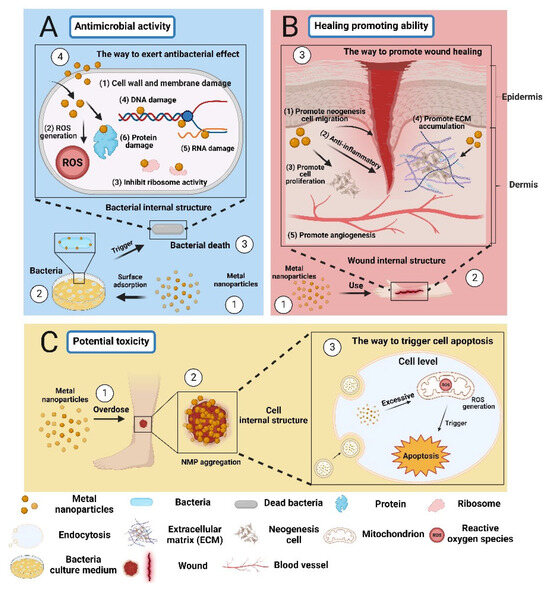
Figure 4.
The effects of metal-based nanoparticles: beneficial and harmful aspects in wound healing. (A) The antimicrobial activity of MBNPs (B) The healing promoting ability of MBNPs (C) The potential toxicity of MBNPs. “Created with BioRender.com.” Agreement number: CN2684TD8X.
However, the safety and efficacy of metal nanoparticles as functional components in medical products need to be evaluated by regulatory agencies due to their different physicochemical properties and biological effects [68]. For instance, the US FDA guidance document “Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology” emphasizes the deliberate manipulation and control of dimensions to produce specific properties. The emergence of new properties or phenomena may raise questions about safety, effectiveness, performance, quality, or public health impact, warranting further evaluation [69]. Moreover, there have been studies showing that direct application of MBNPs to the wound surface can lead to the aggregation of NPs around the wound, and high local concentrations can potentially cause toxicity and affect cell regeneration (Figure 4C) [23,62]. Therefore, in recent years, researchers have combined MBNPs with electrospinning technology, using polymer nanofibers to wrap MBNPs, which can not only use metal-based nanoparticles for in situ wound treatment but also control the release rate of nanoparticles, which has great potential in biomedical applications [70,71,72] (Table 3). In this review, research on the use of electrospun nanofibers loaded with metal nanoparticles for wound healing is clarified. The following is a brief summary of the characteristics and applications of various MBNPs.


Table 2.
Comparative analysis of metal-based nanoparticles’ antibacterial effects.
Table 2.
Comparative analysis of metal-based nanoparticles’ antibacterial effects.
| MBNPs Type | Nanomaterial Properties | Bacterial Species | Exposure Time | Biological Activity | References |
|---|---|---|---|---|---|
| AgNPs | Spherical; 40 nm | E. coli MTCC 062 | 18 h | MIC = 3.6 µg/mL | [73] |
| P. aeruginosa MTCC 424 | MIC = 2.7 µg/mL | ||||
| Spherical; 18.936 ± 7.789 nm | E. coli (ATCC25922) | 24 h | MIC = 50 μg/mL | [74] | |
| P. aeruginosa (ATCC27853) | MIC = 6.25 μg/mL | ||||
| AuNPs | Spherical; 40 nm | E. coli (ATCC No. 25922) | 24 h | MIC = 3.9 μg/mL | [75] |
| P. aeruginosa (PTCC No. 1707) | MIC = 1.95 μg/mL | ||||
| S. aureus (ATCC No. 25923) | MIC = 3.9 μg/mL | ||||
| B. subtilis (ATCC No. 21332) | MIC = 15.62 μg/mL | ||||
| Spherical; 3.5 nm | P. aeruginosa | 24 h | MIC = 100 μg/mL | [76] | |
| S. aureus | MIC = 100 μg/mL | ||||
| E. coli | MIC = 100 μg/mL | ||||
| Star; 26.0 ± 2.6 nm | S. aureus (ATCC 12600) | 24 h | MIC = 250 μg/mL | [77] | |
| CuNPs | Spherical; 38 nm | E. coli | 24 h | MIC = 350 μg/mL | [78] |
| S. aureus | MIC = 150 μg/mL | ||||
| C. albicans | MIC = 300 μg/mL | ||||
| Spherical; 17.85 nm | P. aeruginosa | 24 h | Z = 16.00 ± 1.63 mm | [79] | |
| S. aureus | Z = 9.67 ± 0.47 mm | ||||
| Spherical; 11–33 nm | S. aureus | 24 h | MIC = 31.25 μg/mL | [80] | |
| B. cereus | MIC = 62.5 μg/mL | ||||
| E. coli | MIC = 125 μg/mL | ||||
| K. pneumoniae | MIC = 125 μg/mL |
MIC, minimal inhibitory concentration; Z, zone of inhibition.

Table 3.
Compilation of patents on electrospun nanofibers loaded with metal-based nanoparticles.
4.1. Silver-Based Nanoparticles in Wound Healing
Silver-based materials have been used since ancient times to control wound infections. With the development of nanomedicine, silver nanoparticles (AgNPs) have attracted considerable research attention due to their simple and diverse synthesis methods, nontraditional and effective antibacterial mechanisms, and low toxicity [81]. Currently, the antibacterial mechanism of silver nanoparticles is summarized as follows: 1. AgNPs absorb on and penetrate the cell wall and membrane of bacteria to destroy their structure [82]; 2. AgNPs destroy intracellular structures within bacteria, and release Ag+ into the cytoplasm that specifically binds to proteins, resulting in enzyme inactivation [83]; 3. AgNPs generate a large amount of ROS within the bacteria, leading to oxidative stress in the bacteria [84]; and 4. AgNPs induce structural and permeability changes in bacteria, leading to the dissipation of proton power and the destruction of cell membranes [85,86].
Additionally, during the inflammatory phase, AgNPs can reduce the inflammatory response and exert anti-inflammatory effects by diminishing the production of pro-inflammatory cytokines such as interleukin-8 (IL-8), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [87,88,89,90]. Concurrently, AgNPs also promote cellular proliferation at the wound site and induce the differentiation of fibroblasts into myofibroblasts, accelerating wound closure [91,92]. Furthermore, during the remodeling phase of the wound, AgNPs facilitate the accumulation of the ECM and stimulate angiogenesis by modulating the release of signaling molecules such as transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF), thereby hastening the restoration of skin structure [93,94,95] (Table 4).

Table 4.
Applications of silver-based nanoparticles in wound healing.
Regarding the toxic effects of AgNPs, extensive research indicates that exposure to environments with high concentrations or particle sizes smaller than 10 nm can cause varying degrees of damage to most human cell lines (such as macrophages, erythrocytes, hepatocytes, etc.) [103,104,105,106,107]. These nanoparticles may also be transported via the bloodstream to organs such as the liver, spleen, kidneys, and lungs, inducing inflammation, damage, and even death in animals [108,109,110,111,112,113]. Therefore, the use of AgNPs in wound treatment necessitates finding reasonable methods to deliver AgNPs that ensure their efficacy while minimizing toxicity as much as possible.
4.2. Gold-Based Nanoparticles in Wound Healing
Since the 20th century, gold salt drugs have been used to treat diseases such as arthritis [114]. In the 21st century, AuNPs have become widely used in biomedical imaging, targeted therapy, antibacterial treatment, and cancer treatment due to their superior photoelectric and physical properties and biocompatibility [115,116,117]. Regarding the antibacterial activity and wound healing ability of AuNPs, Zhan et al. summarized that AuNPs have broad-spectrum antibacterial properties, and can be combined with photothermal effects to perform targeted ablation and sterilization at the affected area [118]. Furthermore, multiple studies have indicated that the large surface area and electron-accepting tendency of AuNPs enable them to interact with ROS and participate in the regulation of cytokines (such as the IL family, TNF-α) and growth factors (such as VEGF, FGF, TGF-β), exerting antioxidant and anti-inflammatory effects [119,120,121]. Additionally, they can synergistically promote wound healing through the combination of photothermal therapy (PTT) and photobiomodulation therapy (PBMT) (Table 5).

Table 5.
Applications of gold-based nanoparticles in wound healing.
Currently, there is still much controversy surrounding the toxic effects of AuNPs. The main findings suggest that the toxicity of AuNPs is related to their size, shape, and dose (Woźniak et al. [128]; Isoda et al. [129]). However, there is a significant variation in the doses used in the different studies, and even contradictory results have been reported in different toxicity studies (Tao et al. [130]; Rambanapasi et al. [131]; Lopez-Chaves et al., [132]). Therefore, a systematic investigation is still needed to fully understand the toxic effects of AuNPs.
4.3. Copper-Based Nanoparticles in Wound Healing
Copper, a chemical element with atomic number 29 and symbol Cu, is a bioactive metal that has been extensively studied. In biological systems, copper is often present in the form of Cu2+, and plays a crucial role in the formation of various connective tissues in bones, blood vessels, as well as in lipid metabolism, carbohydrate metabolism, iron metabolism, and antioxidant defense mechanisms [133,134,135]. Despite its prominent physiological roles, an excess of copper ions in the body may lead to cellular oxidative stress, resulting in DNA and protein damage. Therefore, careful management of copper intake is necessary [136,137].
In recent years, copper-based nanoparticles have been shown to exist in the body not as ions, significantly reducing their toxicity compared to conventional copper-based materials [138,139,140,141]. Moreover, several studies have indicated that copper-based nanoparticles are more inclined to bind to bacterial cell membranes, disrupting their external structure and inducing the generation of ROS, further inhibiting bacterial growth [142,143,144]. In terms of wound healing, CuNPs can participate in the regulation of various stages of behavior, including hemostasis and the inflammatory phase, by regulating the release of platelet-derived growth factor (PDGF) and hypoxia-inducible factor-1-alpha (HIF-1), and catalyzing superoxide dismutase (SOD) activity [145,146,147,148]. During the proliferation and remodeling phase, they stimulate the production of signaling molecules such as VEGF, FGF, and TGF-β, accelerating ECM deposition and angiogenesis to promote wound healing [149,150,151,152] (Table 6).

Table 6.
Applications of copper-based nanoparticles in wound healing.
5. Electrospun Nanofiber Scaffolds Loaded with Metal-Based Nanoparticles for Skin Regeneration
Currently, various metals (such as silver, gold, and copper) have been widely used in the study of MBNPs. Silver nanoparticles are the most widely used metal-based nanoparticles, and have been widely used in commercialization and wound research due to their superior antibacterial properties and green preparation methods [89]. Gold has been used in cosmetic and anti-inflammatory applications since ancient times, and because of its superior ductility and ability to combine with photothermal and targeted therapies, gold nanoparticles are often used in medical research [115]. Copper is an essential trace element in all human tissues, and is vital in multiple metabolic pathways [138]. The following section summarizes the recent research on electrospun nanofibers loaded with MBNPs for wound healing, classified by metal type.
5.1. Electrospun Nanofiber Scaffolds Loaded with Silver-Based Nanoparticles
Allizond et al. reported a one-step method for preparing electrospun nanofibers containing AgNPs from polylactic acid/polyethylene oxide (PLA/PEO). The experimental results showed that AgNP–PLA/PEO nanofibers effectively killed S. epidermidis and E. coli by rapidly releasing silver ions [156]. Spagnol et al. modified cellulose whiskers (CWs) to produce carboxylated CWs (CWSAc) with carboxyl groups (-COO-), and then immersed them in a silver nitrate solution (AgNO3) to synthesize AgNPs in situ and anchor AgNPs on the surface. They prepared CWSAc/AgNPs and combined them with electrospinning to prepare polyvinyl alcohol (PVA) antibacterial fibers loaded with CWSAc/AgNPs. The antibacterial fibers exhibited inhibition zones up to 11 mm in diameter against E. coli, P. aeruginosa, and S. aureus in antibacterial tests [157]. However, recent studies have found that using AgNPs alone as antibacterial agents had weaker antibacterial effects against Gram-positive bacteria. Therefore, researchers wrapped AgNPs and ciprofloxacin separately with polyvinylpyrrolidone and ethyl cellulose, respectively, and prepared Janus-structured nanofibers using side-by-side electrospinning technology. The experimental results showed that ciprofloxacin and AgNPs were wrapped on both sides of the nanofiber and were released separately in a controlled manner. Antibacterial tests showed that the synergistic effect of AgNPs and ciprofloxacin had strong antibacterial activity against Gram-positive and Gram-negative bacteria, providing a new idea for the research of novel electrospun nanofiber membranes [158].
To minimize the potential toxicity of AgNPs, researchers have recently found that plant extracts and natural polymers can be used for the in situ synthesis of AgNPs in electrospinning solutions. Kohsari et al. used sorghum extract to reduce a silver nitrate solution (AgNO3) to prepare “green” AgNPs, and combined them with chitosan/polyethylene oxide nanofibers through an electrospinning technique. The experimental results showed that AgNPs, when combined with electrospinning, exhibited sustained release and superior antibacterial properties, indicating their potential for practical applications [159]. El-Aassar et al. prepared biologically active AgNP spheres (Ag–PGA/HA) using polygalacturonic acid (PGA) and hyaluronic acid (HA), and loaded them onto PVA nanofibers. The antibacterial results showed that Ag–PGA/HA–PVA nanofibers exhibited significant antibacterial effects. On the eighth day of use in rat wounds, Ag–PGA/HA–PVA nanofibers significantly promoted wound healing. Subsequent histopathological results showed that collagen deposition at the wound site was tightly packed and the wound was completely epithelialized [160]. Therefore, electrospun nanofibers loaded with green AgNPs also exhibited positive effects on wound repair.
5.2. Electrospun Nanofiber Scaffolds Loaded with Gold-Based Nanoparticles
Gold nanoparticles have good surface modifiability and are excellent antibiotic carriers. Yang et al. developed a polycaprolactone (PCL)/gelatin nanofiber loaded with gold nanoparticles (Au–APA) modified with antibiotic drug intermediates (APA). The nanofiber had good biocompatibility, and an analysis of its healing effects on rat wound infection and antibiotic-resistant bacterial infection models showed better therapeutic effects on infected wounds than did the controls [73]. Based on the photothermal effect of gold nanoparticles, Tian et al. designed a composite nanoparticle (Au@CD) composed of AuNPs and carbon dots (N,S-CDs), which was combined with electrospinning and used for PTT in animal models. The results showed that Au@CD nanofibers had good antibacterial activity and effectively promoted collagen accumulation and blood vessel generation at the wound site, providing a new approach to promoting wound healing [161]. By leveraging the similar crystal structures of AgNPs and AuNPs, a characteristic that also makes them easily co-prepared, Bai et al. developed a sea urchin-like Au–Ag bimetallic nanoparticle-modified polyacrylonitrile (PAN) nanofiber mat. Experimental results showed that the nanofiber mat enhanced osteoblast activity, had good antibacterial activity, was nonirritating to the skin, and had a significant promoting effect on the healing of infected wounds [70]. Considering the potential toxicity of AuNPs prepared by reducing agents such as NaBH4, researchers have recently turned to natural polymers such as chitosan and xanthan gum to synthesize biogenic AuNPs. Then, these biogenic AuNPs were combined with drugs such as moxifloxacin hydrochloride, peppermint oil, and a nanoemulsion (SNE) to produce antibacterial and anti-inflammatory nanofibers. This approach provides a new strategy and method for the development of efficient and environmentally friendly wound healing materials [162,163].
5.3. Electrospun Nanofiber Scaffolds Loaded with Copper-Based Nanoparticles
Jahangirian et al. prepared PVA electrospun membranes loaded with CuNPs by blending CuNPs with PVA and CS. These membranes were used as the inner layer and combined with an outer layer of PVP electrospun membranes to form wound dressings for a full-thickness skin defect model in rats. The experimental results showed that CuNPs could be uniformly dispersed in the polymer fibers and exhibited excellent antibacterial effects against Bacillus cereus, S. aureus, E. coli, and P. aeruginosa. Furthermore, the use of PVA/CS/CuNPs composites in treating rat wounds led to rapid healing within a short period of time [164]. The “green” synthesis of CuNPs as implantable materials has been a focus of research. Fahimirad et al. designed electrospun membranes with PCL as the inner support layer and PVA as the outer covering layer. They incorporated CuNPs synthesized using Quercus infectoria galls (QLG) extract into the PVA spinning solution, resulting in PCL/PVA nanofibers loaded with QLG and CuNPs (PCL/PVA/QLG/CuNPs). Analysis using FTIR spectroscopy, XRD, and other techniques confirmed the successful synthesis of CuNPs combined with the functional components of QLG, which were uniformly dispersed in the PVA nanofibers. Cell viability, antimicrobial, and animal experiment results demonstrated that the PCL/PVA/QLG/CuNPs nanofiber membrane was completely non-toxic and exhibited significant antimicrobial activity against S. aureus. The wound healing effects on non-infected and infected S. aureus wounds were improved by 77.6% and 73.8%, respectively [165].
When used as an antimicrobial material, it is important to avoid potential toxic side effects of CuNPs by achieving controlled release during application. J. Ahire et al. incorporated CuNPs into a solution of PDLLA and PEO at a concentration of 150 mg/mL−1 to prepare CuNP-loaded nanofibers using a one-step method. They evaluated the release capacity of CuNPs in the nanofibers, their cytotoxicity, and antimicrobial activity against P. aeruginosa and S. aureus. The experimental results showed that CuNPs in the CuNPs/PDLLA/PEO nanofibers existed in an irregular form, and the release rate could be adjusted by varying the ratio of PDLLA and PEO. The released CuNPs exhibited significant inhibitory effects against P. aeruginosa and S. aureus. Additionally, cytotoxicity experiments demonstrated that CuNPs had toxic side effects on a small number of cell lines [166]. Therefore, achieving controlled release of NPs remains an important topic for further research and application.
6. Conclusions and Future Directions
Since trauma is a major health problem that all countries across the globe face, current research on new functional materials with properties that promote wound healing is making new progress every day. Based on current research, MBNPs involving gold, silver, and copper have functions that promote wound healing, such as exerting antibacterial and anti-inflammatory activity, and promoting cell proliferation, angiogenesis, and tissue remodeling [121,167,168]. Additionally, some MBNPs have expanded their biological applications through their unique properties. For example, gold nanoparticles have photothermal effects and can be used in photodynamic therapy [169,170]. Although there is considerable research on MBNPs in biomedical applications, we should also pay attention to the nanotoxicity of nanomaterials. Nanotoxicity is related to factors such as the size, shape, surface area, and structure of nanomaterials. Studies have revealed that metal nanoparticles may have certain toxicity to cells after entering the human body, and nanoparticles easily accumulate in organs such as the liver and kidneys through the bloodstream [171,172,173,174]. Therefore, before being used in clinical research in the future, MBNPs must be stabilized in a carrier through embedding, solidification, and other methods to reduce their potential toxicity [175]. Additionally, there has been controversy regarding the synthesis of MBNPs for biomedical applications in recent years, and green synthesis is considered to be a safer and friendlier synthesis for human health than traditional physical and chemical synthesis, which deserves further research [176].
Electrospinning technology can rapidly prepare large quantities of polymer nanofiber scaffolds, which can be used to cover or fill wounds for in situ treatment [177]. For example, biomimetic nanofiber scaffolds can be prepared according to the structure and materials of damaged tissue, which can provide a survival environment for new cells in the early stage of wound healing, promoting cell proliferation and migration [178]. In the future, research on nanofiber scaffolds for wound healing can focus more on the dynamic changes in the ECM in different healing stages and strive to simulate the structure of the ECM in a more detailed way. Furthermore, adding functional materials such as growth factors and antibacterial materials to nanofibers, or preparing composite nanofiber scaffolds can help regulate cell signaling pathways and achieve intelligent treatment of wounds in different healing stages.
In summary, electrospun nanofiber scaffolds loaded with metal-based nanoparticles, as a stable carrier for MBNPs, have the superior properties of nanofibers and can better treat wounds by combining the properties of MBNPs. Moreover, for the needs of different healing stages, composite and multi-level nanofibers could be prepared with different MBNPs to achieve diverse and efficient trauma treatment. These research results could provide new ideas and methods for trauma treatment and make valuable contributions to the development of nanomedicine.
Author Contributions
Conceptualization, Z.D. and P.Z.; methodology, Z.Y.; software, X.W.; validation, Z.D.; formal analysis, Z.D.; investigation, Z.D.; resources, Z.Y.; data curation, Z.D.; writing—original draft preparation, Z.D. and P.Z.; writing—review and editing, X.W.; visualization, X.M.; supervision, X.M.; project administration, X.M.; funding acquisition, P.Z. and X.M. All authors have read and agreed to the published version of the manuscript.
Funding
Military Logistics Key Open Research Projects (China BHJ17L018); National Natural Science Foundation of China (81602408); sponsored by Beijing Nova Program (20220484218).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the references listed below.
Acknowledgments
We thank Biorender (www.biorender.com) for their drawing assistance (Graphical Abstract Agreement number: TT2684V71G) during the writing of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venus, M.; Waterman, J.; McNab, I. Basic physiology of the skin. Surgery 2011, 29, 471–474. [Google Scholar] [CrossRef]
- Tobin, D.J. Biochemistry of human skin—Our brain on the outside. Chem. Soc. Rev. 2006, 35, 52–67. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef]
- Nystrom, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, X.; Jin, L.; Shan, S.; Yang, J.; Zhou, J. Recent advances in strategies to target the behavior of macrophages in wound healing. Biomed. Pharmacother. 2023, 165, 115199. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Kimura, S.; Tsuji, T. Mechanical and Immunological Regulation in Wound Healing and Skin Reconstruction. Int. J. Mol. Sci. 2021, 22, 5474. [Google Scholar] [CrossRef]
- Bando, T.; Yokoyama, H.; Nakamura, H. Wound repair, remodeling, and regeneration. Dev. Growth Differ. 2018, 60, 303–305. [Google Scholar] [CrossRef]
- Spielman, A.F.; Griffin, M.F.; Parker, J.; Cotterell, A.C.; Wan, D.C.; Longaker, M.T. Beyond the Scar: A Basic Science Review of Wound Remodeling. Adv. Wound Care 2023, 12, 57–67. [Google Scholar] [CrossRef]
- Qin, W.; Wu, Y.; Liu, J.; Yuan, X.; Gao, J. A Comprehensive Review of the Application of Nanoparticles in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives. Int. J. Nanomed. 2022, 17, 6007–6029. [Google Scholar] [CrossRef]
- Nune, K.C.; Misra, R.D.K. Biological activity of nanostructured metallic materials for biomedical applications. Mater. Technol. 2016, 31, 772–781. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Hernández-Escobar, S.; Wagley, Y.; Acevedo, P.; Cramer, M.; Badylak, S.; Hammer, N.D.; Hardy, J.; Hankenson, K.; Chatzistavrou, X. Silver-releasing bioactive glass nanoparticles for infected tissue regeneration. Biomater. Adv. 2023, 154, 213656. [Google Scholar] [CrossRef]
- Rezaei Kolarijani, N.; Cheraghali, D.; Khastar, H.; Ehterami, A.; Alizade, M.; Vaez, A.; Amini, S.M.; Salehi, M. Nanofibrous polycaprolactone/gelatin scaffold containing gold nanoparticles: Physicochemical and biological characterization for wound healing. Wound Repair. Regen. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Yadid, M.; Feiner, R.; Dvir, T. Gold Nanoparticle-Integrated Scaffolds for Tissue Engineering and Regenerative Medicine. Nano Lett. 2019, 19, 2198–2206. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Jia, Q.; He, L.; Duan, S.; Fu, Y.; Zhang, Z.; Du, M. Copper-based polymer-metal–organic framework embedded with Ag nanoparticles: Long-acting and intelligent antibacterial activity and accelerated wound healing. Chem. Eng. J. 2022, 435, 134915. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, 2106049. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. WIREs Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Fibrin mechanical properties and their structural origins. Matrix Biol. 2017, 60–61, 110–123. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Xia, Y. Multiple facets for extracellular matrix mimicking in regenerative medicine. Nanomedicine 2015, 10, 689–692. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Zong, H.; Xia, X.; Liang, Y.; Dai, S.; Alsaedi, A.; Hayat, T.; Kong, F.; Pan, J.H. Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater. Sci. Eng. C 2018, 92, 1075–1091. [Google Scholar] [CrossRef]
- CeCe, R.; Jining, L.; Islam, M.; Korvink, J.G.; Sharma, B. An Overview of the Electrospinning of Polymeric Nanofibers for Biomedical Applications Related to Drug Delivery. Adv. Eng. Mater. 2023, 35, 2301297. [Google Scholar] [CrossRef]
- Bian, D.; Wu, Y.; Song, G. Basic fibroblast growth factor combined with extracellular matrix-inspired mimetic systems for effective skin regeneration and wound healing. Mater. Today Commun. 2023, 35, 105876. [Google Scholar] [CrossRef]
- Briquez, P.S.; Hubbell, J.A.; Martino, M.M. Extracellular Matrix-Inspired Growth Factor Delivery Systems for Skin Wound Healing. Adv. Wound Care 2015, 4, 479–489. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair. Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Cárdenas-León, C.G.; Mäemets-Allas, K.; Klaas, M.; Lagus, H.; Kankuri, E.; Jaks, V. Matricellular proteins in cutaneous wound healing. Front. Cell Dev. Biol. 2022, 10, 1073320. [Google Scholar] [CrossRef]
- Chester, D.; Brown, A.C. The role of biophysical properties of provisional matrix proteins in wound repair. Matrix Biol. 2017, 60–61, 124–140. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G.; Fukunishi, T.; Best, C.A.; Ong, C.S.; Groehl, T.; Reinhardt, J.; Yi, T.; Miyachi, H.; et al. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostructure Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Medeiros, G.B.; Lima, F.A.; de Almeida, D.S.; Guerra, V.G.; Aguiar, M.L. Modification and Functionalization of Fibers Formed by Electrospinning: A Review. Membranes 2022, 12, 861. [Google Scholar] [CrossRef]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef]
- Hernandez-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. A 2021, 109, 1751–1764. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, Y.; Lim, C.T. Fabrication of Large Pores in Electrospun Nanofibrous Scaffolds for Cellular Infiltration: A Review. Tissue Eng. Part B Rev. 2011, 18, 77–87. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.y.; Zare, E.N.; Borzacchiello, A.; Niu, L.n.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Li, Z.; Xie, M.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent Progress in Tissue Engineering and Regenerative Medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Meyer, U. Fundamentals of Tissue Engineering and Regenerative Medicine; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Yang, Y.; Ritchie, A.C.; Everitt, N.M. Comparison of glutaraldehyde and procyanidin cross-linked scaffolds for soft tissue engineering. Mater. Sci. Eng. C 2017, 80, 263–273. [Google Scholar] [CrossRef]
- Abdelaal, O.A.M.; Darwish, S.M.H. Review of Rapid Prototyping Techniques for Tissue Engineering Scaffolds Fabrication. In Characterization and Development of Biosystems and Biomaterials; Öchsner, A., da Silva, L.F.M., Altenbach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–54. [Google Scholar]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Synthetic-based blended electrospun scaffolds in tissue engineering applications. J. Mater. Sci. 2022, 57, 4020–4079. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef]
- Maximilien, J.; Beyazit, S.; Rossi, C.; Haupt, K.; Tse Sum Bui, B. Nanoparticles in Biomedical Applications. In Measuring Biological Impacts of Nanomaterials; Bioanalytical Reviews; Springer: Berlin/Heidelberg, Germany, 2015; pp. 177–210. [Google Scholar]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Vimbela, G.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/metal oxide nanocomposites for bactericidal effect: A review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Kumar, A.; Shah, S.R.; Jayeoye, T.J.; Kumar, A.; Parihar, A.; Prajapati, B.; Singh, S.; Kapoor, D.U. Biogenic metallic nanoparticles: Biomedical, analytical, food preservation, and applications in other consumable products. Front. Nanotechnol. 2023, 5, 1175149. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Tyner, K.M.; Zou, P.; Yang, X.; Zhang, H.; Cruz, C.N.; Lee, S.L. Product quality for nanomaterials: Current U.S. experience and perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 640–654. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Guidance, Compliance, & Regulatory Information. 2015. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/ (accessed on 1 December 2023).
- Bai, M.-Y.; Ku, F.-Y.; Shyu, J.-F.; Hayashi, T.; Wu, C.-C. Evaluation of Polyacrylonitrile Nonwoven Mats and Silver–Gold Bimetallic Nanoparticle-Decorated Nonwoven Mats for Potential Promotion of Wound Healing In Vitro and In Vivo and Bone Growth In Vitro. Polymers 2021, 13, 516. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef]
- Morsy, R.; Hosny, M.; Reicha, F.; Elnimr, T. Developing a potential antibacterial long-term degradable electrospun gelatin-based composites mats for wound dressing applications. React. Funct. Polym. 2017, 114, 8–12. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Sayed, R.; Saad, H.; Hagagy, N. Silver nanoparticles: Characterization and antibacterial properties. Rend. Lincei. Sci. Fis. E Nat. 2018, 29, 81–86. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B Biol. 2018, 184, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ayyanaar, S.; Kesavan, M.P. One-pot biogenic synthesis of gold nanoparticles@saponins niosomes: Sustainable nanomedicine for antibacterial, anti-inflammatory and anticancer therapeutics. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132229. [Google Scholar] [CrossRef]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid Interface Sci. 2021, 586, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Selvarani, M. INVESTIGATION OF THE SYNERGISTIC ANTIBACTERIAL ACTION OF COPPER NANOPARTICLES ON CERTAIN ANTIBIOTICS AGAINST HUMAN PATHOGENS. Int. J. Pharm. Pharm. Sci. 2018, 10, 83–86. [Google Scholar] [CrossRef][Green Version]
- Shiravand, S.; Azarbani, F. Phytosynthesis, characterization, antibacterial and cytotoxic effects of copper nanoparticles. Green Chem. Lett. Rev. 2017, 10, 241–249. [Google Scholar] [CrossRef]
- Nam, G.; Rangasamy, S.; Purushothaman, B.; Song, J.M. The Application of Bactericidal Silver Nanoparticles in Wound Treatment. Nanomater. Nanotechnol. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernandez, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, X.; Ahmed, T.; Shang, Q.; Zhang, S.; Ma, Z.; Yin, Y. Toxicity and action mechanisms of silver nanoparticles against the mycotoxin-producing fungus Fusarium graminearum. J. Adv. Res. 2022, 38, 1–12. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, P.; Du, J.; Zhao, X.; Wong, K.K.Y. Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. J. Pediatr. Surg. 2017, 52, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Padwad, Y.S.; Yadav, S.K. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int. J. Biol. Macromol. 2017, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; El-Rafie, M.H.; El-Sheikh, M.A.; Seleem, A.A.; El-Naggar, M.E. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.B.; Dananjaya, S.H.S.; Nikapitiya, C.; Park, B.K.; Gooneratne, R.; Kim, T.-Y.; Lee, J.; Kim, C.-H.; De Zoysa, M. Silver nanoparticles enhance wound healing in zebrafish (Danio rerio). Fish Shellfish Immunol. 2017, 68, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Frankova, J.; Pivodova, V.; Vagnerova, H.; Juranova, J.; Ulrichova, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, e137–e142. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K.; et al. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C 2020, 112, 110925. [Google Scholar] [CrossRef]
- Stefan, L.M.; Iosageanu, A.; Ilie, D.; Stanciuc, A.-M.; Matei, C.; Berger, D.; Craciunescu, O. Extracellular matrix biomimetic polymeric membranes enriched with silver nanoparticles for wound healing. Biomed. Mater. 2021, 16, 035010. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles from Azadirachta indica. Front. Microbiol. 2021, 12, 611560. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.Y.; Ho, C.-M.; Lok, C.-N.; Yu, W.-Y.; Che, C.-M.; Chiu, J.-F.; Tam, P.K.H. Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Alsareii, S.A.; Manaa Alamri, A.; AlAsmari, M.Y.; Bawahab, M.A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J.H.; Kumbar, V. Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory, and Cytotoxic Activity. Molecules 2022, 27, 6306. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Green Synthesis of Silver Nanoparticles by the Cyanobacteria Synechocystis sp.: Characterization, Antimicrobial and Diabetic Wound-Healing Actions. Mar. Drugs 2022, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Hu, Y.; Chang, L.; Xu, S.; Mei, X.; Chen, Z. Electrospinning of antibacterial and anti-inflammatory Ag@hesperidin core-shell nanoparticles into nanofibers used for promoting infected wound healing. Regen. Biomater. 2022, 9, rbac012. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yin, H.; Chen, X.; Chen, T.H.; Liu, H.M.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Liu, Y.W.; Hu, X.K.; et al. Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chen, S.; Ge, W.; Zhao, Y.; Xu, X.; Wang, S.; Zhang, J. Riclin-Capped Silver Nanoparticles as an Antibacterial and Anti-Inflammatory Wound Dressing. Int. J. Nanomed. 2022, 17, 2629–2641. [Google Scholar] [CrossRef]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef]
- Younis, N.S.; El Semary, N.A.; Mohamed, M.E. Silver nanoparticles green synthesis via cyanobacterium Phormidium sp.: Characterization, wound healing, antioxidant, antibacterial, and anti-inflammatory activities. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3083–3096. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Irving, E.S.; Hayashi, Y.; Sutherland, D.S.; Thorsen, K.; Autrup, H.; Beer, C. Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol. Sci. 2012, 130, 145–157. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Interactions of manufactured silver nanoparticles of different sizes with normal human dermal fibroblasts. Int. Wound J. 2016, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jarak, I.; Carrola, J.; Barros, A.S.; Gil, A.M.; Pereira, M.d.L.; Corvo, M.L.; Duarte, I.F. From the Cover: Metabolism Modulation in Different Organs by Silver Nanoparticles: An NMR Metabolomics Study of a Mouse Model. Toxicol. Sci. 2017, 159, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Oczkowski, M.; Krawczyńska, A.; Chwastowska, J.; Sadowska-Bratek, M.; Chajduk, E.; Wojewódzka, M.; Dušinská, M.; et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol. 2012, 32, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, B.K.; Hirn, S.; Kermanizadeh, A.; Kanase, N.; Fytianos, K.; Wenk, A.; Haberl, N.; Brunelli, A.; Kreyling, W.G.; Stone, V. Effects of Silver Nanoparticles on the Liver and Hepatocytes In Vitro. Toxicol. Sci. 2012, 131, 537–547. [Google Scholar] [CrossRef]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Liu, M.-S.; Huang, L.-J.; Lue, S.-I.; Lin, L.-C.; Kwan, A.-L.; Yang, R.-C. Bioenergetic failure correlates with autophagy and apoptosis in rat liver following silver nanoparticle intraperitoneal administration. Part. Fibre Toxicol. 2013, 10, 40. [Google Scholar] [CrossRef]
- Jessop, J.D.; Currey, H.L. Influence of gold salts on adjuvant arthritis in the rat. Ann. Rheum. Dis. 1968, 27, 577. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Lopes, T.S.; Alves, G.G.; Pereira, M.R.; Granjeiro, J.M.; Leite, P.E.C. Advances and potential application of gold nanoparticles in nanomedicine. J. Cell. Biochem. 2019, 120, 16370–16378. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, J.; Tang, H.; Xia, D.; Lin, H. Antibacterial Properties of Gold Nanoparticles in the Modification of Medical Implants: A Systematic Review. Pharmaceutics 2022, 14, 2654. [Google Scholar] [CrossRef]
- Hornos Carneiro, M.F.; Barbosa, F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Health Part B 2016, 19, 129–148. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef]
- Han, R.; Xiao, Y.; Bai, Q.; Choi, C.H.J. Self-therapeutic metal-based nanoparticles for treating inflammatory diseases. Acta Pharm. Sin. B 2023, 13, 1847–1865. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, R.; Hu, E.; Qian, P.; Lu, B.; Lan, G.; Lu, F. Protein-reduced gold nanoparticles mixed with gentamicin sulfate and loaded into konjac/gelatin sponge heal wounds and kill drug-resistant bacteria. Int. J. Biol. Macromol. 2020, 148, 921–931. [Google Scholar] [CrossRef]
- Leu, J.G.; Chen, S.A.; Chen, H.M.; Wu, W.M.; Hung, C.F.; Yao, Y.D.; Tu, C.S.; Liang, Y.J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and alpha-lipoic acid. Nanomedicine 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, J.; Jang, M.; Kwak, M.H.; Go, J.; Kho, E.K.; Song, S.H.; Sung, J.E.; Lee, J.; Hwang, D.Y. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater. Sci. 2015, 3, 509–519. [Google Scholar] [CrossRef]
- Marza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Pestean, C.; Nagy, A.; Tabaran, F.; Licarete, E.; Suarasan, S.; Dreanca, A.; et al. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed. Mater. 2019, 14, 025011. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Marimuthu Prabhu, N.; Jeyakanthan, J.; Saravanan, M. Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in vitro and in vivo Conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef]
- Wei, S.C.; Chang, L.; Huang, C.C.; Chang, H.T. Dual-functional gold nanoparticles with antimicrobial and proangiogenic activities improve the healing of multidrug-resistant bacteria-infected wounds in diabetic mice. Biomater. Sci. 2019, 7, 4482–4490. [Google Scholar] [CrossRef]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef]
- Isoda, K.; Tanaka, A.; Fuzimori, C.; Echigoya, M.; Taira, Y.; Taira, I.; Shimizu, Y.; Akimoto, Y.; Kawakami, H.; Ishida, I. Toxicity of Gold Nanoparticles in Mice due to Nanoparticle/Drug Interaction Induces Acute Kidney Damage. Nanoscale Res. Lett. 2020, 15, 141. [Google Scholar] [CrossRef]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Rambanapasi, C.; Zeevaart, J.R.; Buntting, H.; Bester, C.; Kotze, D.; Hayeshi, R.; Grobler, A. Bioaccumulation and Subchronic Toxicity of 14 nm Gold Nanoparticles in Rats. Molecules 2016, 21, 763. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Oliveri, V. Biomedical applications of copper ionophores. Coord. Chem. Rev. 2020, 422, 213474. [Google Scholar] [CrossRef]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Gubler, C.J.; Cartwright, G.E.; Wintrobe, M.M. Studies On Copper Metabolism: Xx. Enzyme Activities And Iron Metabolism In Copper And Iron Deficiencies. J. Biol. Chem. 1957, 224, 533–546. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.-L.; Qiao, N.; Hu, L.-M.; Ma, Y.-J.; Liang, X.-H.; Liu, J.-L.; Yang, Z.-M. Effects of Excess Copper Ions on Decidualization of Human Endometrial Stromal Cells. Biol. Trace Elem. Res. 2017, 177, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Arnal, N.; de Alaniz, M.J.T.; Marra, C.A. Carnosine and neocuproine as neutralizing agents for copper overload-induced damages in cultured human cells. Chem.-Biol. Interact. 2011, 192, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Budych, M.J.; Staszak, K.; Staszak, M. Copper and Copper-Based Nanoparticles in Medicine—Perspectives and Challenges. Molecules 2023, 28, 6687. [Google Scholar] [CrossRef] [PubMed]
- Letelier, M.E.; Sánchez-Jofré, S.; Peredo-Silva, L.; Cortés-Troncoso, J.; Aracena-Parks, P. Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects. Chem.-Biol. Interact. 2010, 188, 220–227. [Google Scholar] [CrossRef]
- Razmara, P.; Pyle, G.G. Effect of copper nanoparticles and copper ions on the architecture of rainbow trout olfactory mucosa. Ecotoxicol. Environ. Saf. 2021, 227, 112876. [Google Scholar] [CrossRef]
- Kim, J.-C.; Lee, I.-C.; Ko, J.-W.; Park, S.-H.; Lim, J.-O.; Shin, I.-S.; Moon, C.; Kim, S.-H.; Her, J.-D. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomed. 2016, 11, 2883–2900. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the Antibacterial Mechanism of CuO Nanoparticles: Revealing the Route of Induced Oxidative Stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Ashino, T.; Sudhahar, V.; Urao, N.; Oshikawa, J.; Chen, G.-F.; Wang, H.; Huo, Y.; Finney, L.; Vogt, S.; McKinney, R.D.; et al. Unexpected Role of the Copper Transporter ATP7A in PDGF-Induced Vascular Smooth Muscle Cell Migration. Circ. Res. 2010, 107, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Schuschke, D.A.; Saari, J.T.; West, C.A.; Miller, F.N. Dietary Copper Deficiency Increases the Mast Cell Population of the Rat. Proc. Soc. Exp. Biol. Med. 1994, 207, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Sudhahar, V.; Chen, G.F.; Kim, H.W.; Youn, S.W.; Finney, L.; Vogt, S.; Yang, J.; Kweon, J.; Surenkhuu, B.; et al. Endothelial Antioxidant-1: A Key Mediator of Copper-dependent Wound Healing in vivo. Sci. Rep. 2016, 6, 33783. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef] [PubMed]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef] [PubMed]
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The neglected role of copper ions in wound healing. J. Inorg. Biochem. 2016, 161, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Narayanan, K.; Thakar, M.B.; Jagani, H.V.; Venkata Rao, J. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.; Ríos, G.; Sepúlveda, N.; Salvo, J.; Souza-Mello, V.; Farías, J. Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review. Pharmaceutics 2022, 14, 1838. [Google Scholar] [CrossRef]
- Gopal, A.; Kant, V.; Gopalakrishnan, A.; Tandan, S.K.; Kumar, D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharmacol. 2014, 731, 8–19. [Google Scholar] [CrossRef]
- Geng, X.; Liu, K.; Wang, J.; Su, X.; Shi, Y.; Zhao, L. Preparation of Ultra-Small Copper Nanoparticles-Loaded Self-Healing Hydrogels with Antibacterial, Inflammation-Suppressing and Angiogenesis-Enhancing Properties for Promoting Diabetic Wound Healing. Int. J. Nanomed. 2023, 18, 3339–3358. [Google Scholar] [CrossRef]
- Volodina, L.A.; Bayder, L.M.; Rakhmetova, A.A.; Bogoslovskaja, O.A.; Olkhovskaya, I.P.; Gluschenko, N.N. Copper-induced change in the ESR signal of hemoglobin nitrosyl complexes in wound by the action of copper nanoparticles. Biophysics 2013, 58, 693–698. [Google Scholar] [CrossRef]
- Allizond, V.; Banche, G.; Salvoni, M.; Malandrino, M.; Cecone, C.; Cuffini, A.M.; Bracco, P. Facile One-Step Electrospinning Process to Prepare AgNPs-Loaded PLA and PLA/PEO Mats with Antibacterial Activity. Polymers 2023, 15, 1470. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.; Fragal, E.H.; Pereira, A.G.B.; Nakamura, C.V.; Muniz, E.C.; Follmann, H.D.M.; Silva, R.; Rubira, A.F. Cellulose nanowhiskers decorated with silver nanoparticles as an additive to antibacterial polymers membranes fabricated by electrospinning. J. Colloid Interface Sci. 2018, 531, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Yu, D.-G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Kohsari, I.; Shariatinia, Z.; Pourmortazavi, S.M. Antibacterial electrospun chitosan-polyethylene oxide nanocomposite mats containing bioactive silver nanoparticles. Carbohydr. Polym. 2016, 140, 287–298. [Google Scholar] [CrossRef] [PubMed]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Hong, J.; Li, C.; Qiu, Y.; Li, M.; Qin, Z.; Ghiladi, R.A.; Yin, X. Electrospinning membranes with Au@carbon dots: Low toxicity and efficient antibacterial photothermal therapy. Biomater. Adv. 2022, 142, 213155. [Google Scholar] [CrossRef]
- Teaima, M.H.; Abdelnaby, F.A.; Fadel, M.; El-Nabarawi, M.A.; Shoueir, K.R. Synthesis of Biocompatible and Environmentally Nanofibrous Mats Loaded with Moxifloxacin as a Model Drug for Biomedical Applications. Pharmaceutics 2020, 12, 1029. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shalaby, E.S.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. Technol. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, M.S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Fahimirad, S.; Satei, P.; Ganji, A.; Abtahi, H. Wound healing performance of PVA/PCL based electrospun nanofiber incorporated green synthetized CuNPs and Quercus infectoria extracts. J. Biomater. Sci. Polym. Ed. 2023, 34, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Hattingh, M.; Neveling, D.P.; Dicks, L.M.T. Copper-Containing Anti-Biofilm Nanofiber Scaffolds as a Wound Dressing Material. PLoS ONE 2016, 11, e0152755. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Buyana, B.; Feketshane, Z.; Aderibigbe, B.A. Electrospun Nanofibers/Nanofibrous Scaffolds Loaded with Silver Nanoparticles as Effective Antibacterial Wound Dressing Materials. Pharmaceutics 2021, 13, 964. [Google Scholar] [CrossRef] [PubMed]
- Zafar, N.; Madni, A.; Khalid, A.; Khan, T.; Kousar, R.; Naz, S.S.; Wahid, F. Pharmaceutical and Biomedical Applications of Green Synthesized Metal and Metal Oxide Nanoparticles. Curr. Pharm. Des. 2020, 26, 5844–5865. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Bidin, N.; Islam, S.; Shukri, W.N.B.W.M.; Zakaria, N.; Musa, N.; Krishnan, G. Influence of gold nanoparticles on wound healing treatment in rat model: Photobiomodulation therapy. Lasers Surg. Med. 2017, 49, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Xiao, Z.; Zhang, G.; Wei, L.; Cao, J.; Zhang, Z.; Li, X.; Song, Z.; Wang, W.; Liu, J.; et al. An injectable multifunctional hydrogel for eradication of bacterial biofilms and wound healing. Acta Biomater. 2023, 161, 112–133. [Google Scholar] [CrossRef] [PubMed]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 102316. [Google Scholar] [CrossRef]
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Yao, C.; Li, C.; Xing, X.; Huang, Y.; Gu, T.; Wu, M. Toxic effects of metal oxide nanoparticles and their underlying mechanisms. Sci. China Mater. 2017, 60, 93–108. [Google Scholar] [CrossRef]
- Bakshi, M.S. Nanotoxicity in Systemic Circulation and Wound Healing. Chem. Res. Toxicol. 2017, 30, 1253–1274. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Joshi, P.B.; Chavali, M.S. Updates on Biogenic Metallic and Metal Oxide Nanoparticles: Therapy, Drug Delivery and Cytotoxicity. Pharmaceutics 2023, 15, 1650. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.M.; Seabra, A.B. Bactericidal and Virucidal Activities of Biogenic Metal-Based Nanoparticles: Advances and Perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Haldar, S.; Gupta, S.; Chauhan, S.; Mago, V.; Roy, P.; Lahiri, D. Single unit functionally graded bioresorbable electrospun scaffold for scar-free full-thickness skin wound healing. Biomater. Adv. 2022, 139, 212980. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun nanofibers promote wound healing: Theories, techniques, and perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).