In Situ Polymerization of Antibacterial Modification Polyamide 66 with Au@Cu2O-ZnO Ternary Heterojunction
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
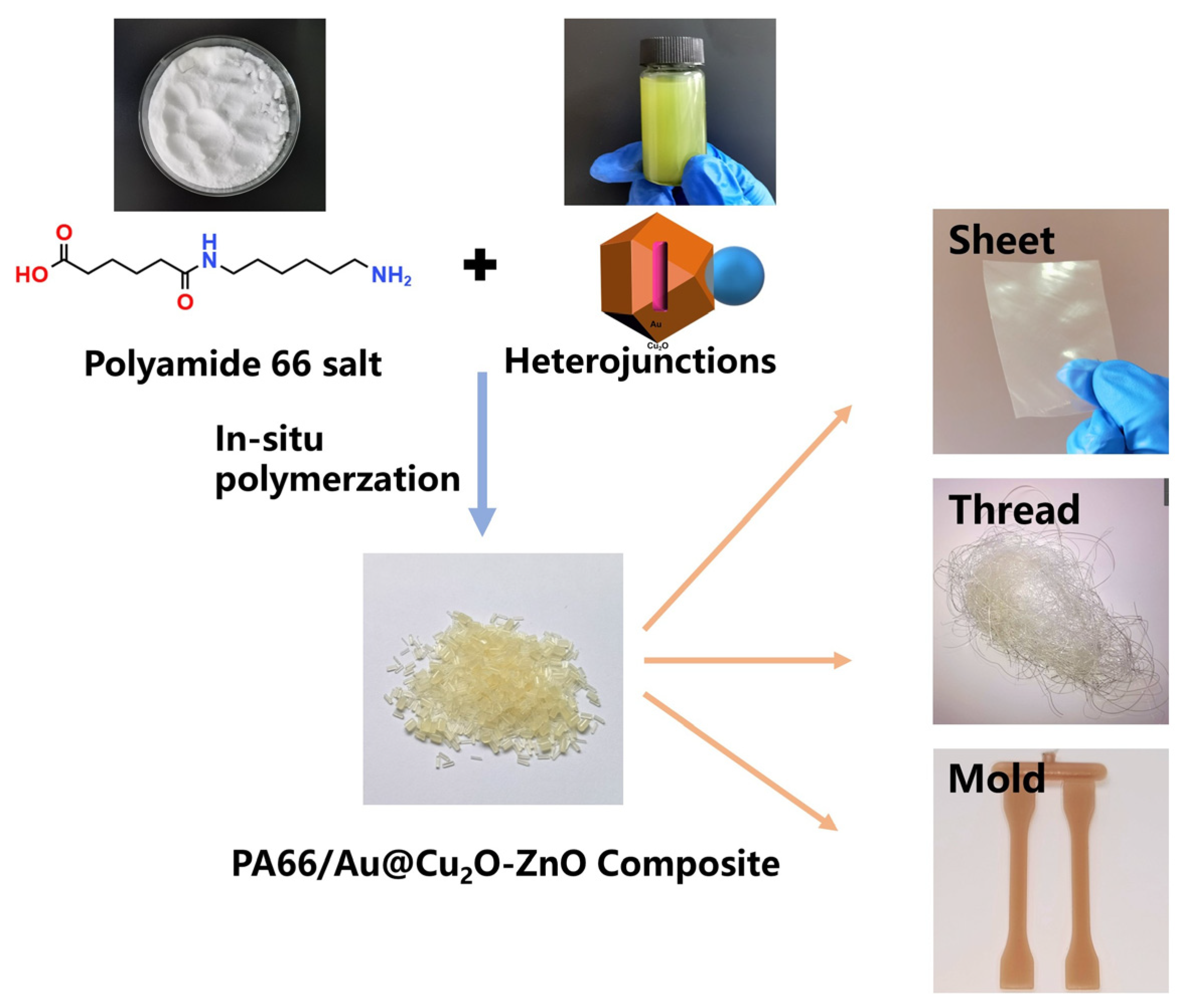
2.2. Preparation of Au@Cu2O-ZnO
2.3. Preparation of PA66/Au@Cu2O-ZnO Samples
2.4. Characterization
2.5. Antibacterial Activity of PA66/Au@Cu2O-ZnO Samples
3. Results and Discussions
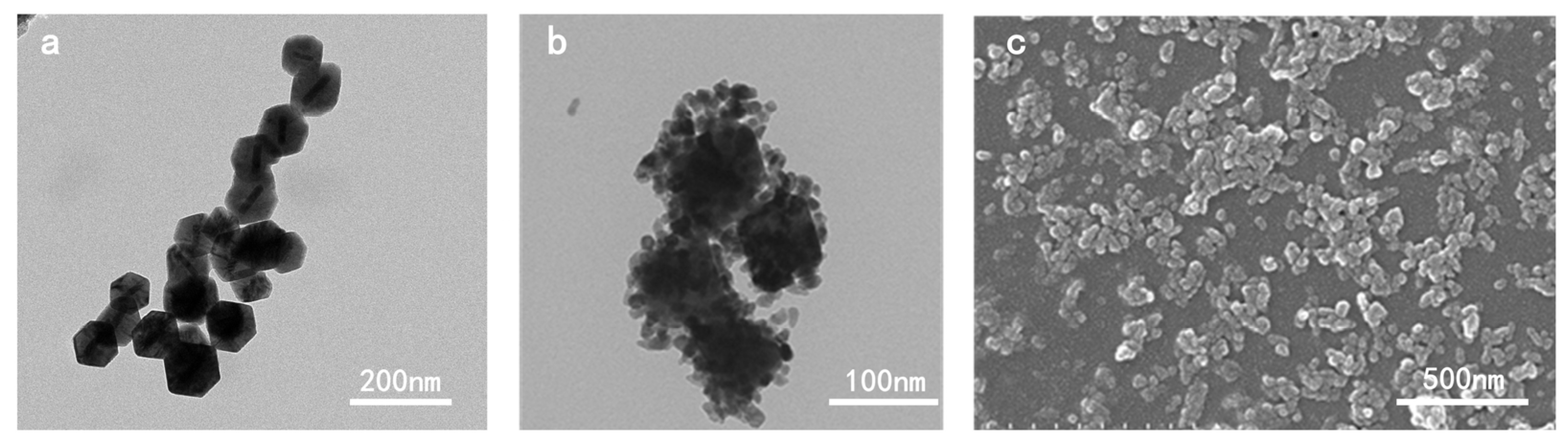
3.1. The Morphology of Au@Cu2O-ZnO
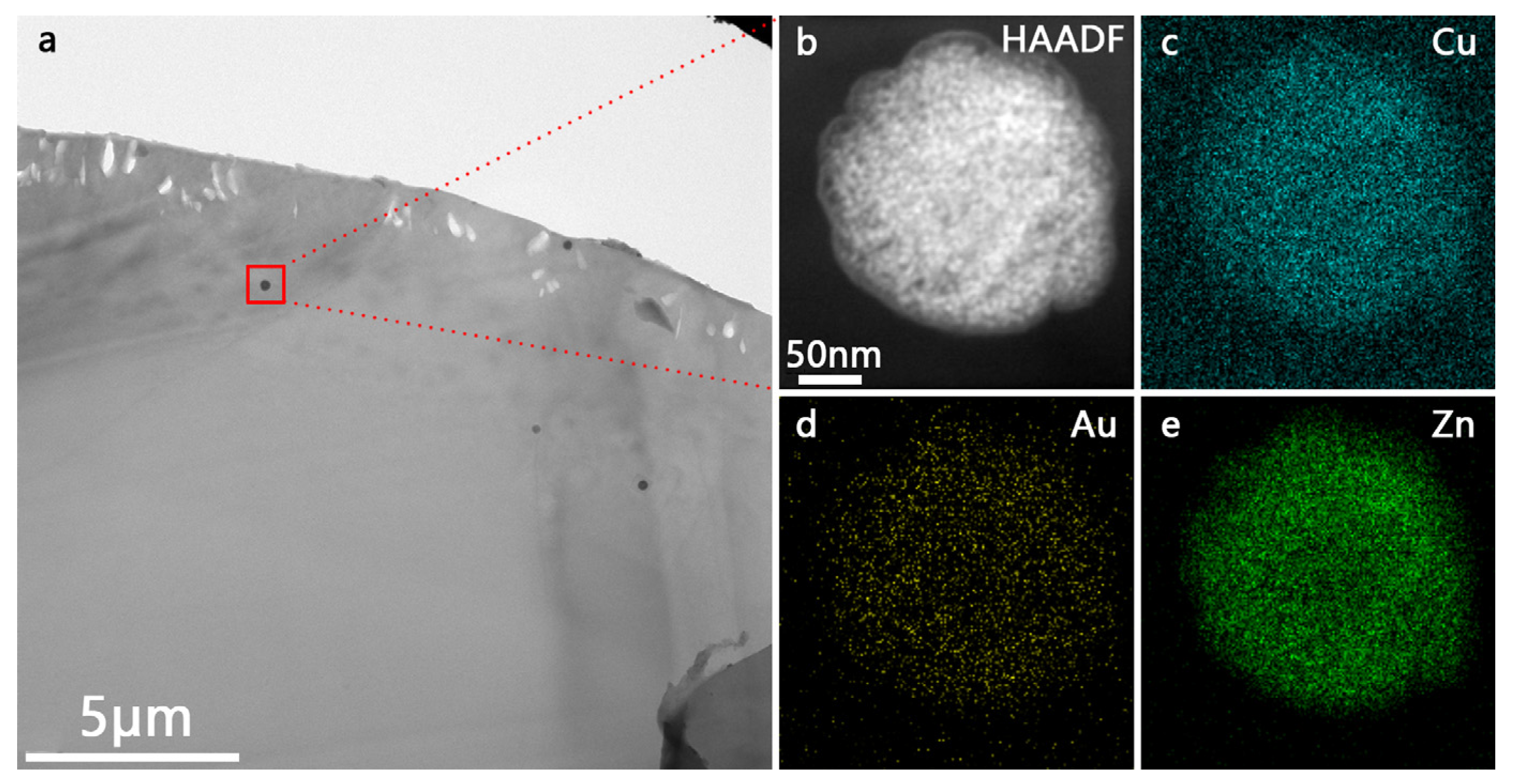
3.2. Characterization of PA66/Au@Cu2O-ZnO Composites
3.3. Crystallization and Melting Behaviors of PA66/Au@Cu2O-ZnO Composites
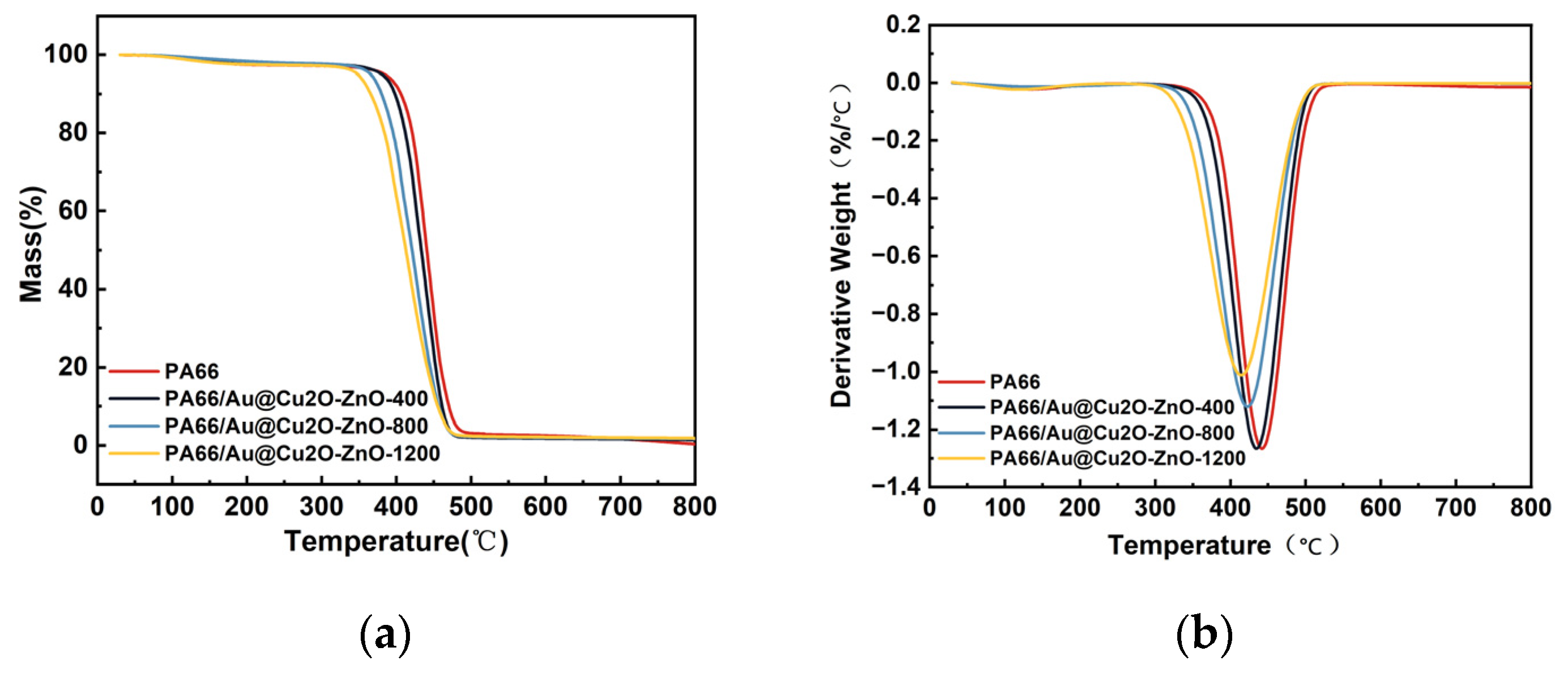
3.4. Thermal Stability of PA66/Au@Cu2O-ZnO Composites
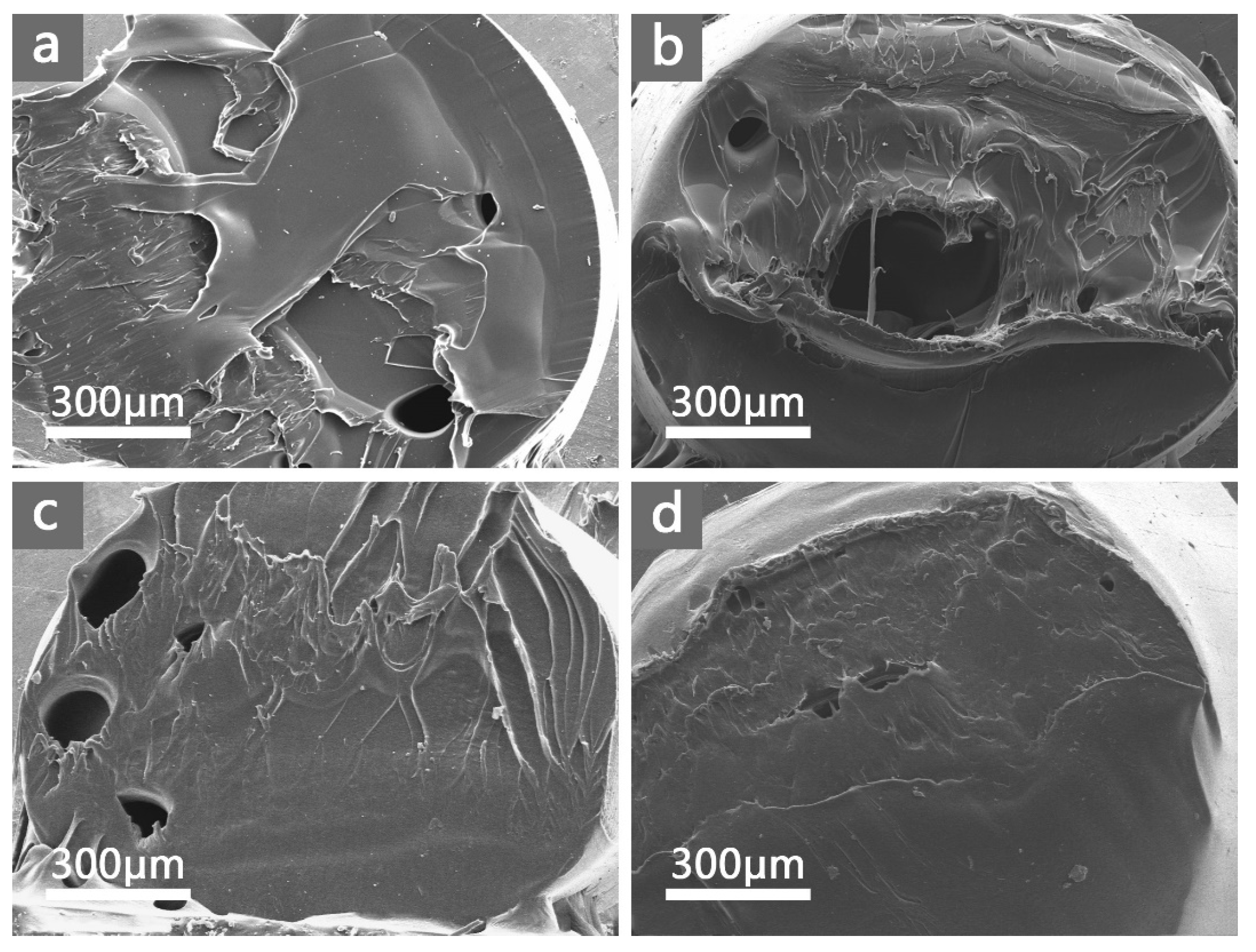
3.5. Mechanical Properties of PA66/Au@Cu2O-ZnO Composites
3.6. Evaluation of Antibacterial Performance for PA66/Au@Cu2O-ZnO Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Yang, T.; Cheng, Y.; Huang, T.; Yu, B.; Wu, Q.; Zhu, M.; Yu, H. Synthesis Phosphorus–Sulfur Reactive Flame Retardant for Polyamide 66 with High Flame Retardant Efficiency and Low Smoke. Polym. Degrad. Stab. 2023, 214, 110378. [Google Scholar] [CrossRef]
- Tamura, K.; Ohyama, S.; Umeyama, K.; Kitazawa, T.; Yamagishi, A. Preparation and Properties of Halogen-Free Flame-Retardant Layered Silicate-Polyamide 66 Nanocomposites. Appl. Clay Sci. 2016, 126, 107–112. [Google Scholar] [CrossRef]
- Chen, X.; Xu, D.; Zhang, H.; Feng, X.; Deng, J.; Pan, K. In Situ Polymerization of Flame Retardant Modification Polyamide 6,6 with 2-carboxy Ethyl (Phenyl) Phosphinic Acid. J. Appl. Polym. Sci. 2020, 137, 48687. [Google Scholar] [CrossRef]
- Lyu, W.; Chen, X.; Li, Y.; Cao, S.; Han, Y. Thermal Stability and Heat Release Effect of Flame Retarded PA66 Prepared by End-Pieces Capping Technology. Compos. Part B Eng. 2019, 167, 34–43. [Google Scholar] [CrossRef]
- Guo, S.; Xu, J.; Ni, X. Synthesis, Structures, and Properties of a New Pentaerythritol-Derived Flame Retardant Used in Polyamide 66. ACS Omega 2021, 6, 12887–12897. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Hagar, M.; Al-Mutabagani, L.A.; Abozaid, G.M.; Abdallah, S.M.; Ahmed, H.; Hassanin, A.H.; Shehata, N. Biodegradable Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19 and Anti-Multidrug Resistant Bacteria Evaluation. Materials 2021, 14, 3862. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, Z.; Zabihi, F.; Chen, Z.; Zhu, M. Progress and Perspective of Antiviral Protective Material. Adv. Fiber Mater. 2020, 2, 123–139. [Google Scholar] [CrossRef]
- Kumaran, S.; Oh, E.; Han, S.; Choi, H.-J. Photopolymerizable, Universal Antimicrobial Coating to Produce High-Performing, Multifunctional Face Masks. Nano Lett. 2021, 21, 5422–5429. [Google Scholar] [CrossRef]
- Lian, J.; Chen, J.; Luan, S.; Liu, W.; Zong, B.; Tao, Y.; Wang, X. Organocatalytic Copolymerization of Cyclic Lysine Derivative and ε-Caprolactam toward Antibacterial Nylon-6 Polymers. ACS Macro Lett. 2022, 11, 46–52. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.-M.; Su, Q.-W.; Xu, J.-L. Visible Light Response ZnO–C3N4 Thin Film Photocatalyst. Rare Met. 2021, 40, 96–104. [Google Scholar] [CrossRef]
- Sajjad, S.; Arshad, F.; Uzair, B.; Leghari, S.A.K.; Noor, S.; Maaza, M. GO/Ag2O Composite Nanostructure as an Effective Antibacterial Agent. ChemistrySelect 2019, 4, 10365–10371. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, B.; Liu, X.; Li, Z.; Zhu, S.; Liang, Y.; Cui, Z.; Wu, S. Recent Progress in Photocatalytic Antibacterial. ACS Appl. Bio Mater. 2021, 4, 3909–3936. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, B.; Zhang, H.; Ding, P.; Wang, Q. Sandwiched ZnO@Au@Cu2O Nanorod Films as Efficient Visible-Light-Driven Plasmonic Photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 4066–4074. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhong, J.; Zhu, H.; Yang, Y.; Ding, M.; Luo, L.; Huo, Y.; Li, H. Hybrid Cu2O/TiO2 Nanocomposites with Enhanced Photocatalytic Antibacterial Activity toward Acinetobacter Baumannii. ACS Appl. Bio Mater. 2019, 2, 4892–4903. [Google Scholar] [CrossRef] [PubMed]
- Nithya, P.M.; Devi, L.G. Effect of Surface Ag Metallization on the Photocatalytic Properties of BaTiO3: Surface Plasmon Effect and Variation in the Schottky Barrier Height. Surf. Interfaces 2019, 15, 205–215. [Google Scholar] [CrossRef]
- Žerjav, G.; Roškarič, M.; Zavašnik, J.; Kovač, J.; Pintar, A. Effect of Au Loading on Schottky Barrier Height in TiO2 + Au Plasmonic Photocatalysts. Appl. Surf. Sci. 2022, 579, 152196. [Google Scholar] [CrossRef]
- Sun, Z.-X.; Sun, K.; Gao, M.-L.; Metin, Ö.; Jiang, H.-L. Optimizing Pt Electronic States through Formation of a Schottky Junction on Non-Reducible Metal–Organic Frameworks for Enhanced Photocatalysis. Angew. Chem. Int. Ed. 2022, 61, e202206108. [Google Scholar] [CrossRef]
- Liang, Y.; Li, W.; Wang, X.; Zhou, R.; Ding, H. TiO2–ZnO/Au Ternary Heterojunction Nanocomposite: Excellent Antibacterial Property and Visible-Light Photocatalytic Hydrogen Production Efficiency. Ceram. Int. 2022, 48, 2826–2832. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, M.; Zhang, X.; Zhuo, M.; Zhou, Q.; Su, Y.; Zheng, M.; Yuan, G.; Wang, Z. Flowerlike CuO/Au Nanoparticle Heterostructures for Nonenzymatic Glucose Detection. ACS Appl. Nano Mater. 2021, 4, 5808–5815. [Google Scholar] [CrossRef]
- Su, Y.; Guo, H.; Wang, Z.; Long, Y.; Li, W.; Tu, Y. Au@Cu2O Core-Shell Structure for High Sensitive Non-Enzymatic Glucose Sensor. Sens. Actuators B Chem. 2018, 255, 2510–2519. [Google Scholar] [CrossRef]
- Lu, W.-C.; Kumar, S.S.; Chen, Y.-C.; Hsu, C.-M.; Lin, H.-N. Au/Cu2O/ZnO Ternary Nanocomposite for Low Concentration NO2 Gas Sensing at Room Temperature. Mater. Lett. 2019, 256, 126657. [Google Scholar] [CrossRef]
- Li, J.-M.; Tsao, C.-W.; Fang, M.-J.; Chen, C.-C.; Liu, C.-W.; Hsu, Y.-J. TiO2-Au-Cu2O Photocathodes: Au-Mediated Z-Scheme Charge Transfer for Efficient Solar-Driven Photoelectrochemical Reduction. ACS Appl. Nano Mater. 2018, 1, 6843–6853. [Google Scholar] [CrossRef]
- Sangeetha, P.; Chen, Y.-W. Preferential Oxidation of CO in H2 Stream on Au/CeO2–TiO2 Catalysts. Int. J. Hydrogen Energy 2009, 34, 7342–7347. [Google Scholar] [CrossRef]
- Borbón, S.; Lugo, S.; Pourjafari, D.; Pineda Aguilar, N.; Oskam, G.; López, I. Open-Circuit Voltage (VOC) Enhancement in TiO2-Based DSSCs: Incorporation of ZnO Nanoflowers and Au Nanoparticles. ACS Omega 2020, 5, 10977–10986. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Pei, F.; Luo, X.; Hu, H.; Qian, H.; Wen, P.; Miao, K.; Guo, S.; Wang, W.; Feng, G. Fabrication of ZnO/Au@Cu2O Heterojunction towards Deeply Oxidative Photodegradation of Organic Dyes. Sep. Purif. Technol. 2021, 262, 118301. [Google Scholar] [CrossRef]
- ISO 307: 2019; Plastics—Polyamides—Determination of Viscosity Number. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 527-1:2012; Plastics—Determination of tensile properties—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2012.
- GB/T 23763-2009; Photo-Catalytic Antimicrobial Materials and Products—Assessment for Antimicrobial Activity and Efficacy. Standardization Administration of China: Beijing, China, 2009.
- Sagdinc, S.; Bayarı, S. Spectroscopic Studies on the Interaction of Ofloxacin with Metals. J. Mol. Struct. 2004, 691, 107–113. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, S.; He, Y.; Cao, X.; Shang, J.; Liu, Q.; Ye, G.; Zheng, K.; Ma, Y. Intrinsically Flame-Retardant Polyamide 66 with High Flame Retardancy and Mechanical Properties. RSC Adv. 2021, 11, 433–441. [Google Scholar] [CrossRef]
- Gong, B.; Peng, Q.; Jur, J.S.; Devine, C.K.; Lee, K.; Parsons, G.N. Sequential Vapor Infiltration of Metal Oxides into Sacrificial Polyester Fibers: Shape Replication and Controlled Porosity of Microporous/Mesoporous Oxide Monoliths. Chem. Mater. 2011, 23, 3476–3485. [Google Scholar] [CrossRef]
- Akyildiz, H.I.; Diler, S.; Islam, S. Evaluation of TiO2 and ZnO Atomic Layer Deposition Coated Polyamide 66 Fabrics for Photocatalytic Activity and Antibacterial Applications. J. Vac. Sci. Technol. A Vac. Surf. Film. 2021, 39, 022405. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A Novel Approach Toward Fabrication of High Performance Thin Film Composite Polyamide Membranes. Sci. Rep. 2016, 6, 22069. [Google Scholar] [CrossRef] [PubMed]
- Mejía, M.I.; Marín, J.M.; Restrepo, G.; Pulgarín, C.; Mielczarski, E.; Mielczarski, J.; Stolitchnov, I.; Kiwi, J. Innovative UVC Light (185 Nm) and Radio-Frequency-Plasma Pretreatment of Nylon Surfaces at Atmospheric Pressure and Their Implications in Photocatalytic Processes. ACS Appl. Mater. Interfaces 2009, 1, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Wu, J.; Takawira, C.M.; Wang, J. Surface Modification of Polyamide 6,6 Fabrics with an Alkaline Protease—Subtilisin. J. Eng. Fibers Fabr. 2016, 11, 155892501601100. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.B.; Zuo, Y.; Lv, G.Y.; Mu, Y.H. Influences of the Crystallization Behavior of N-HA Reinforced PA66 Biocomposite on Its Mechanical Properties. Mater. Sci. Forum 2006, 510–511, 898–901. [Google Scholar] [CrossRef]
- Zheng, J.; Siegel, R.W.; Toney, C.G. Polymer Crystalline Structure and Morphology Changes in Nylon-6/ZnO Nanocomposites. J. Polym. Sci. B Polym. Phys. 2003, 41, 1033–1050. [Google Scholar] [CrossRef]
- Cui, L.; Yeh, J.-T. Nylon 6 Crystal-Phase Transition in Nylon 6/Clay/Poly(Vinyl Alcohol) Nanocomposites. J. Appl. Polym. Sci. 2010, 118, 1683–1690. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Wang, M.-Z. Structure and Thermal Behaviour of Nylon 6 Nanocomposites. J. Appl. Polym. Sci. 2006, 100, 3116–3122. [Google Scholar] [CrossRef]
- Duemichen, E.; Braun, U.; Sturm, H.; Kraemer, R.; Deglmann, P.; Gaan, S.; Senz, R. A New Molecular Understanding of the Thermal Degradation of PA 66 Doped with Metal Oxides: Experiment and Computation. Polym. Degrad. Stab. 2015, 120, 340–356. [Google Scholar] [CrossRef]
- Du, L.; Qu, B.; Zhang, M. Thermal Properties and Combustion Characterization of Nylon 6/MgAl-LDH Nanocomposites via Organic Modification and Melt Intercalation. Polym. Degrad. Stab. 2007, 92, 497–502. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Zhang, J.; Xiao, R. Preparation and Characterizations of Inherent Flame Retarded Polyamide 66 Containing the Phosphorus Linking Pendent Group: Preparation and Characterizations of Flame Retarded Polyamide 66. Polym Adv Technol 2018, 29, 951–960. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
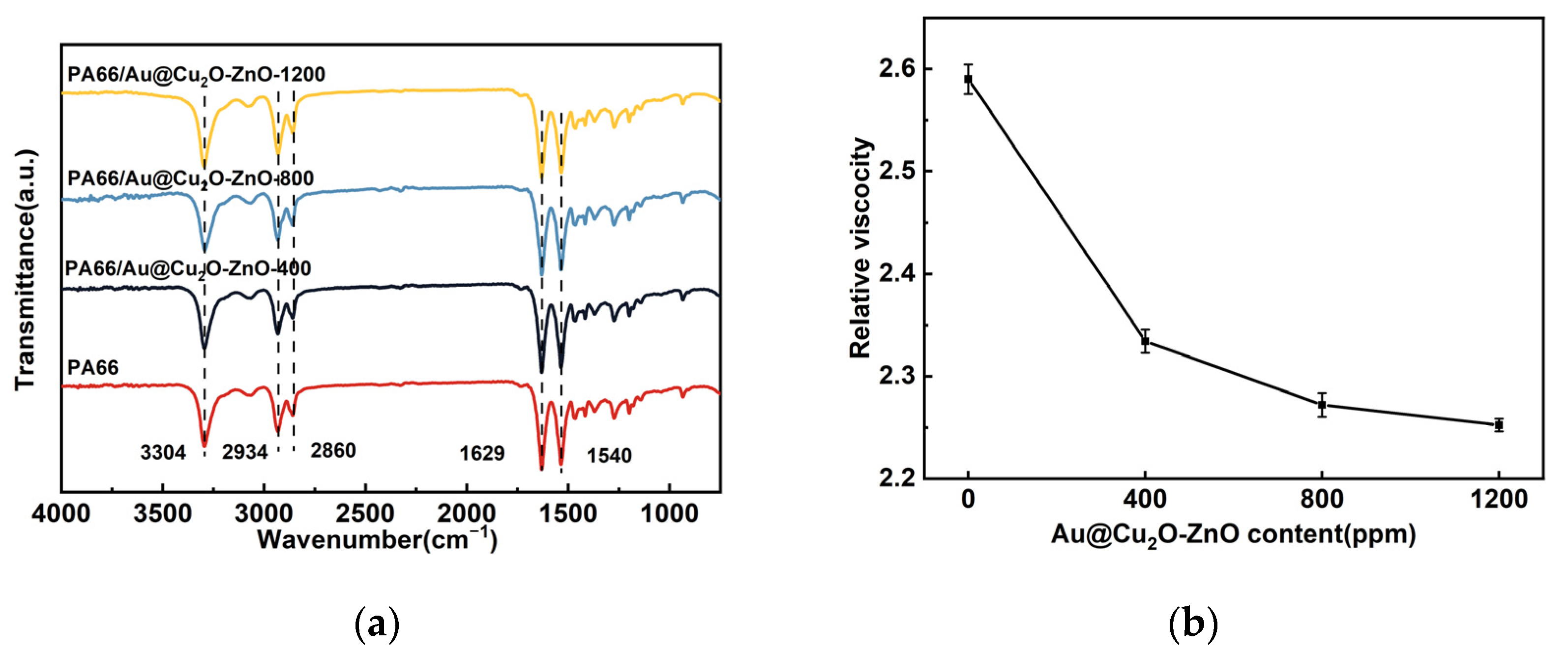
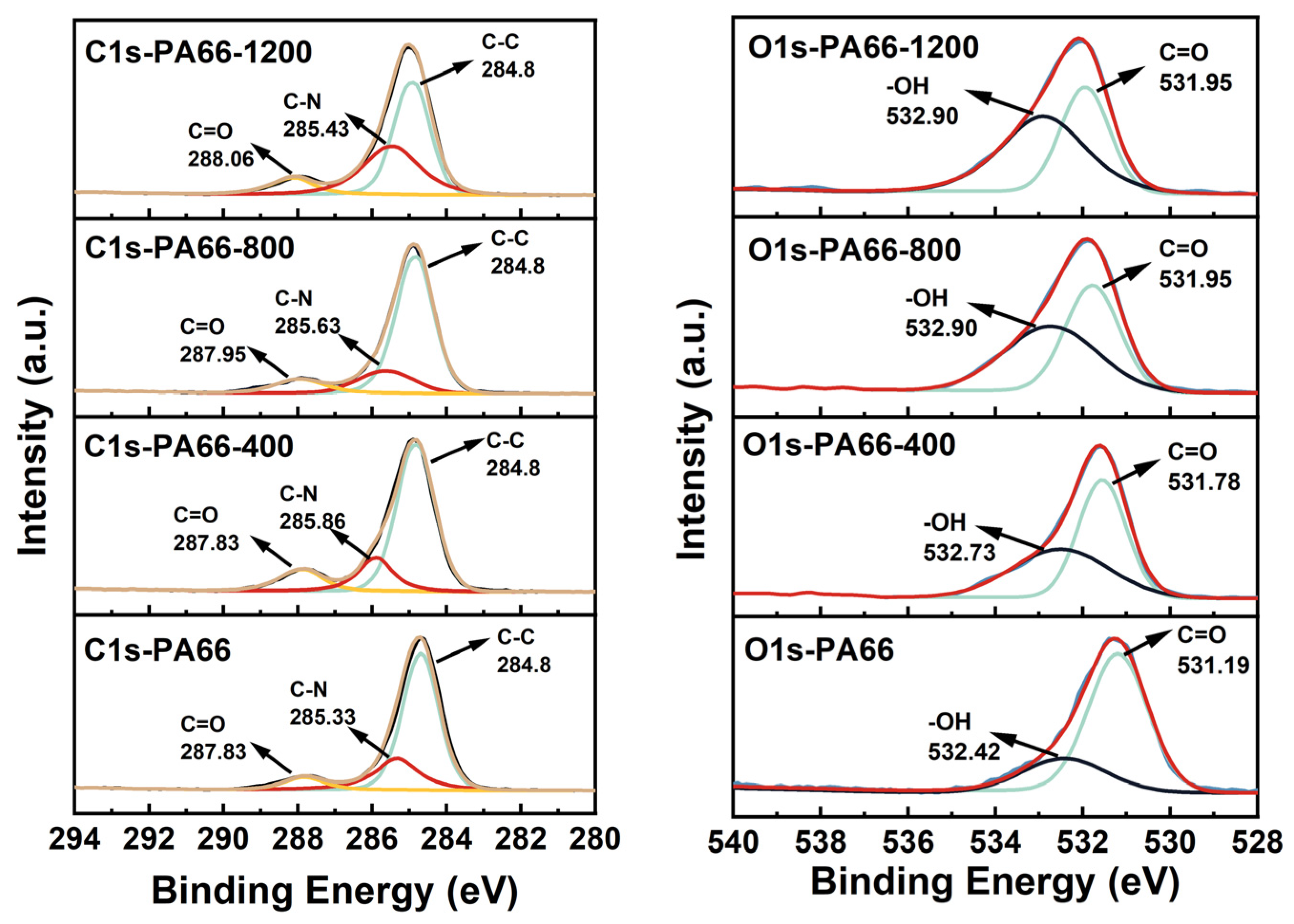
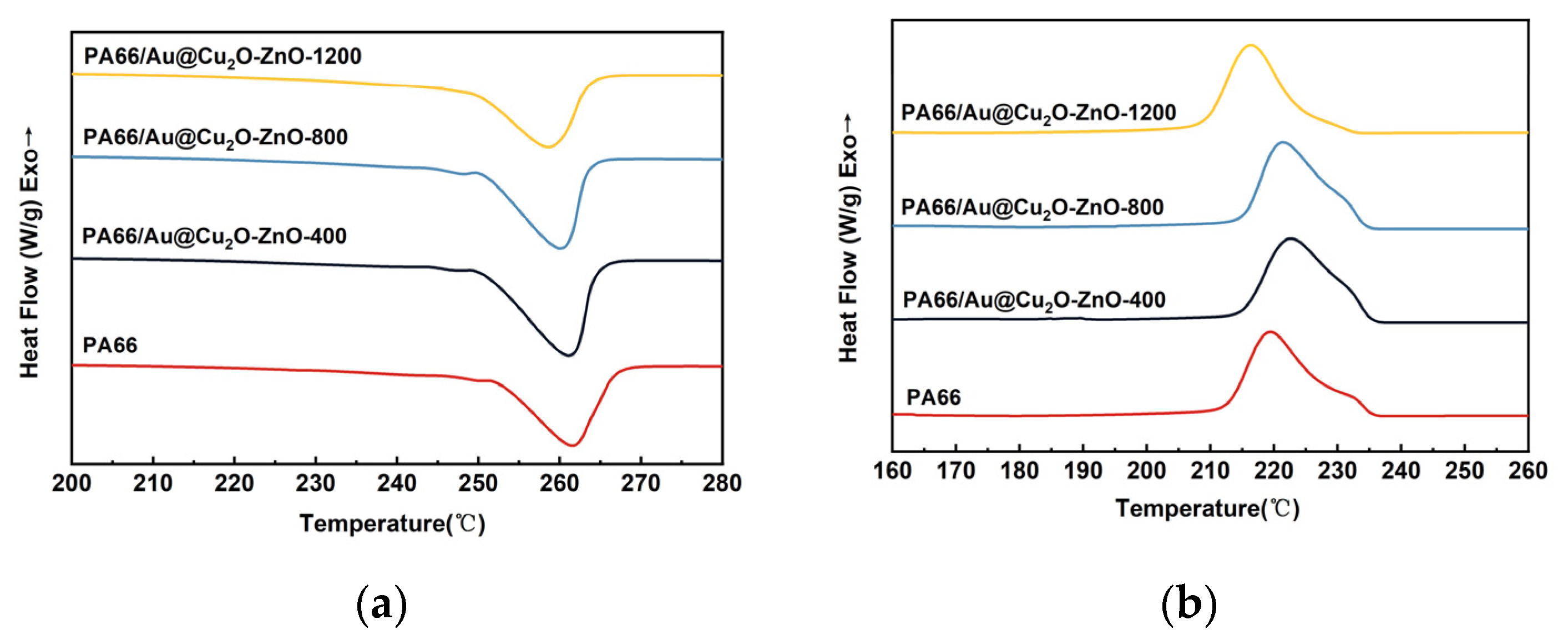
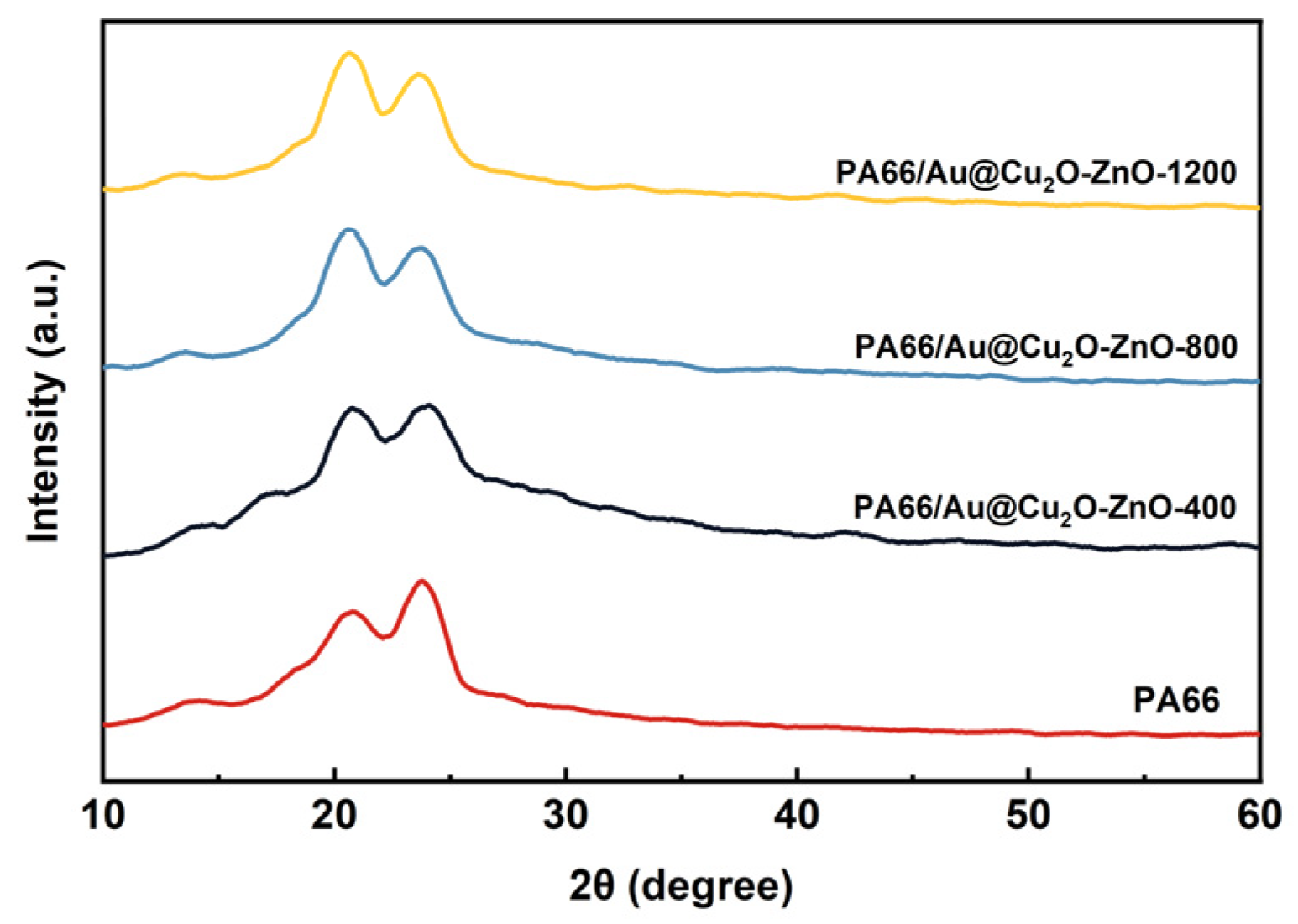

| Sample | Tm, °C | ΔHm, J g−1 | Tc, °C | Xc, % |
|---|---|---|---|---|
| PA66 | 261.62 | 49.65 | 219.60 | 26.13 |
| 400 ppm | 261.03 | 60.42 | 222.65 | 31.80 |
| 800 ppm | 260.10 | 54.19 | 221.47 | 28.52 |
| 1200 ppm | 258.70 | 43.78 | 216.41 | 23.04 |
| Sample | T5%, °C | Tmax, °C | Char Residue at 600 °C | Char Residue at 800 °C |
|---|---|---|---|---|
| PA66 | 385 | 441.6 | 2.55% | 0.37% |
| 400 ppm | 381.4 | 435 | 1.83% | 1.46% |
| 800 ppm | 365.7 | 423.3 | 2.04% | 1.77% |
| 1200 ppm | 348.3 | 413.6 | 2.17% | 1.88% |
| Bacterium | Sample | Antibacterial Rate (%) | |
|---|---|---|---|
| Under Light | No Light | ||
| E. coli | PA66 | No effect | No effect |
| PA66/Au@Cu2O-ZnO-400 | 15.73 | 44.18 | |
| PA66/Au@Cu2O-ZnO-800 | >99 | 98.63 | |
| PA66/Au@Cu2O-ZnO-1200 | >99 | >99 | |
| S. aureus | PA66 | No effect | No effect |
| PA66/Au@Cu2O-ZnO-400 | 96.36 | 16.67 | |
| PA66/Au@Cu2O-ZnO-800 | >99 | 70 | |
| PA66/Au@Cu2O-ZnO-1200 | >99 | 72.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zheng, M.; Zhao, S.; Cao, Z.; Pan, K.; Feng, X.; Zhang, H.; Zheng, M.; Wang, C. In Situ Polymerization of Antibacterial Modification Polyamide 66 with Au@Cu2O-ZnO Ternary Heterojunction. Polymers 2024, 16, 158. https://doi.org/10.3390/polym16010158
Li X, Zheng M, Zhao S, Cao Z, Pan K, Feng X, Zhang H, Zheng M, Wang C. In Situ Polymerization of Antibacterial Modification Polyamide 66 with Au@Cu2O-ZnO Ternary Heterojunction. Polymers. 2024; 16(1):158. https://doi.org/10.3390/polym16010158
Chicago/Turabian StyleLi, Xiang, Mi Zheng, Shikun Zhao, Zhiwen Cao, Kai Pan, Xinxing Feng, Hua Zhang, Min Zheng, and Cheng Wang. 2024. "In Situ Polymerization of Antibacterial Modification Polyamide 66 with Au@Cu2O-ZnO Ternary Heterojunction" Polymers 16, no. 1: 158. https://doi.org/10.3390/polym16010158
APA StyleLi, X., Zheng, M., Zhao, S., Cao, Z., Pan, K., Feng, X., Zhang, H., Zheng, M., & Wang, C. (2024). In Situ Polymerization of Antibacterial Modification Polyamide 66 with Au@Cu2O-ZnO Ternary Heterojunction. Polymers, 16(1), 158. https://doi.org/10.3390/polym16010158





