Rigidity with Flexibility: Porous Triptycene Networks for Enhancing Methane Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis of PTN-70 and PTN-71
2.2.1. Synthesis of PTN-70
2.2.2. Synthesis of PTN-71
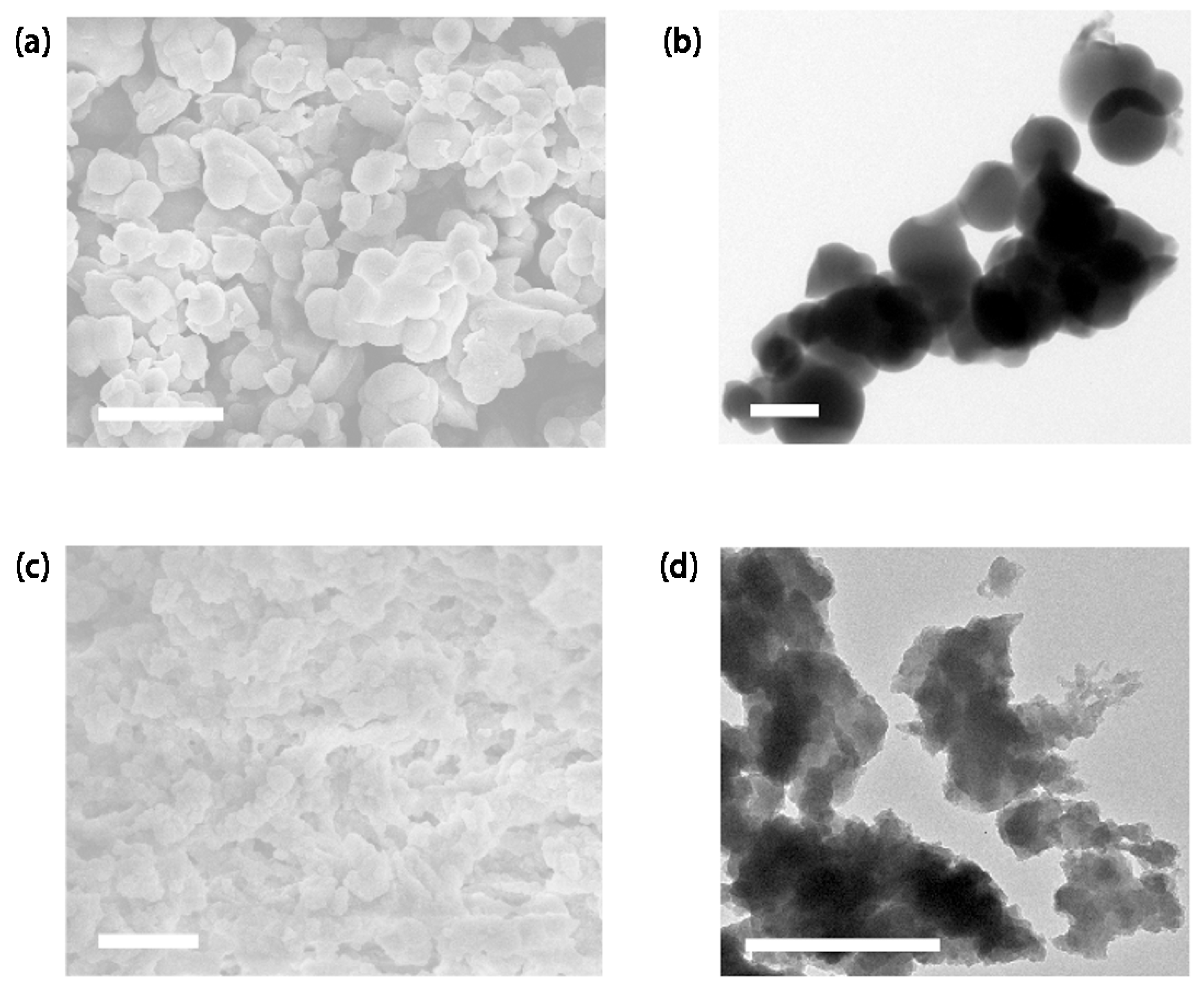
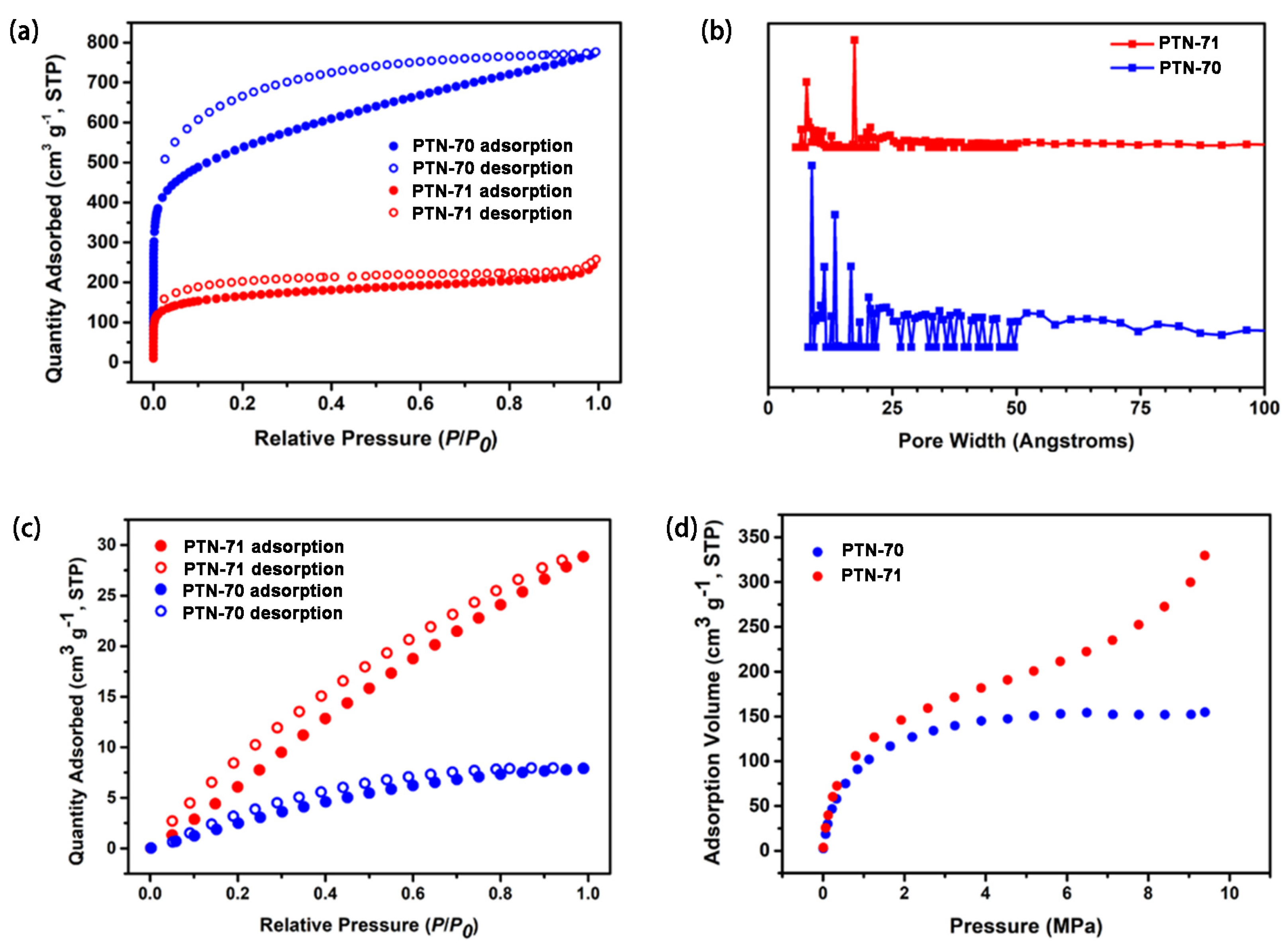
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, V.C.; Komarneni, S. Porous Adsorbents for Vehicular Natural Gas Storage: A Review. J. Porous Mater. 1998, 5, 43–58. [Google Scholar] [CrossRef]
- Zou, X.Y.; Xue, R.; An, Z.W.; Li, H.W.; Zhang, J.L.; Jiang, Y.; Huang, L.J.; Wu, W.; Wang, S.F.; Hu, G.-H.; et al. Recent Advances in Flexible CNC-Based Chiral Nematic Film Materials. Small 2023, 2023, 2303778. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Zhao, H.; An, Z.W.; Wu, W.; Jiang, Y.; Li, P.; Huang, C.-X.; Shi, D.; Li, R.K.Y.; Hu, G.-H.; et al. Self-healable, solvent response cellulose nanocrystal/ waterborne polyurethane nanocomposites with encryption capability. ACS Nano 2023, 17, 5653–5662. [Google Scholar] [CrossRef]
- Connolly, B.M.; Madden, D.; Wheatley, A.; Fairen-Jimenez, D. Shaping the Future of Fuel: Monolithic Metal-Organic Frameworks for High-Density Gas Storage. J. Am. Chem. Soc. 2020, 142, 8541–8549. [Google Scholar] [CrossRef] [PubMed]
- Rowland, C.A.; Lorzing, G.R.; Gosselin, E.J.; Trump, B.A.; Yap, G.P.A.; Brown, C.M.; Bloch, E.D. Methane Storage in Paddlewheel-Based Porous Coordination Cages. J. Am. Chem. Soc. 2018, 140, 11153–11157. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, K.; Mukherjee, P.S. Organic Imine Cages: Molecular Marriage and Applications. Angew. Chem. Int. Ed. 2019, 58, 8640–8653. [Google Scholar] [CrossRef]
- Bracco, S.; Piga, D.; Bassanetti, I.; Perego, J.; Comotti, A.; Sozzani, P. Porous 3D polymers for high pressure methane storage and carbon dioxide capture. J. Mater. Chem. A 2017, 5, 10328–10337. [Google Scholar] [CrossRef]
- Che, S.; Pang, J.D.; Kalin, A.J.; Wang, C.X.; Ji, X.Z.; Lee, J.; Cole, D.; Li, J.L.; Tu, X.M.; Zhang, Q.; et al. Rigid Ladder-Type Porous Polymer Networks for Entropically Favorable Gas Adsorption. ACS Mater. Lett. 2020, 2, 49–54. [Google Scholar] [CrossRef]
- Zhang, A.J.; Zhang, Q.K.; Bai, H.; Li, L.; Li, J. Polymeric nanoporous materials fabricated with supercritical CO2 and CO2-expanded liquids. Chem. Soc. Rev. 2014, 43, 6938–6953. [Google Scholar] [CrossRef]
- Zhu, T.T.; Pei, B.Y.; Di, T.; Xia, Y.X.; Li, T.S.; Li, L. Thirty-minute preparation of microporous polyimides with large surface areas for ammonia adsorption. Green Chem. 2020, 22, 7003–7009. [Google Scholar] [CrossRef]
- Makal, T.A.; Li, J.R.; Lu, W.G.; Zhou, H.C. Methane storage in advanced porous materials. Chem. Soc. Rev. 2012, 41, 7761–7779. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Li, S.; Dai, L.; Li, J.N.; Lv, J.N.; Zhu, Z.J.J.; Yin, A.X.; Li, P.F.; Wang, B. The Synthesis of Hexaazatrinaphthylene-Based 2D Conjugated Copper Metal-Organic Framework for Highly Selective and Stable Electroreduction of CO2 to Methane. Angew. Chem. Int. Ed. 2021, 60, 16409–16415. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Preuss, K.; Titirici, M.M.; Rodriguez-Reinoso, F. Nanoporous Materials for the Onboard Storage of Natural Gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zheng, B.; Zhang, Z.H.; Li, H.X.; Xue, D.X.; Bai, J.F. Ligand-Conformer-Induced Formation of Zirconium-Organic Framework for Methane Storage and MTO Product Separation. Angew. Chem. Int. Ed. 2021, 60, 16521–16528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, T.; Dong, Y.B.; Fang, Z.B.; Xu, Y.X. Electron-donating group induced rapid synthesis of hyper-crosslinked polymers. Sci. Bull. 2022, 67, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.X.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.M.; Briggs, M.E.; Hu, C.C.; Cooper, A.I. Controlling electric double-layer capacitance and pseudocapacitance in heteroatom-doped carbons derived from hypercrosslinked microporous polymers. Nano Energy 2018, 46, 277–289. [Google Scholar] [CrossRef]
- Liu, N.; Ma, H.; Sun, R.; Zhang, Q.-P.; Tan, B.; Zhang, C. Porous Triptycene Network Based on Tröger’s Base for CO2 Capture and Iodine Enrichment. ACS Appl. Mater. Interfaces 2023, 15, 30402–30408. [Google Scholar] [CrossRef]
- Eckstein, B.J.; Brown, L.C.; Noll, B.C.; Moghadasnia, M.P.; Balaich, G.J.; McGuirk, C.M. A Porous Chalcogen-Bonded Organic Framework. J. Am. Chem. Soc. 2021, 143, 20207–20215. [Google Scholar] [CrossRef]
- Soto, C.; Torres-Cuevas, E.S.; Gonzalez-Ortega, A.; Palacio, L.; Pradanos, P.; Freeman, B.D.; Lozano, A.E.; Hernandez, A. Hydrogen Recovery by Mixed Matrix Membranes Made from 6FCl-APAF HPA with Different Contents of a Porous Polymer Network and Their Thermal Rearrangement. Polymers 2021, 13, 4343. [Google Scholar] [CrossRef]
- Zotkin, M.A.; Alentiev, D.A.; Shorunov, S.V.; Sokolov, S.E.; Gavrilova, N.N.; Bermeshev, M.V. Microporous polynorbornenes bearing carbocyclic substituents: Structure-property study. Polymer 2023, 269, 125732. [Google Scholar] [CrossRef]
- Mason, J.A.; Oktawiec, J.; Taylor, M.K.; Hudson, M.R.; Rodriguez, J.; Bachman, J.E.; Gonzalez, M.I.; Cervellino, A.; Guagliardi, A.; Brown, C.M.; et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management. Nature 2015, 527, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Rozyyev, V.; Thirion, D.; Ullah, R.; Lee, J.; Jung, M.; Oh, H.; Atilhan, M.; Yavuz, C.T. High-capacity methane storage in flexible alkane-linked porous aromatic network polymers. Nat. Energy 2019, 4, 604–611. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Filinchuk, Y.; Férey, G. How Hydration Drastically Improves Adsorption Selectivity for CO2 over CH4 in the Flexible Chromium Terephthalate MIL-53. Angew. Chem. Int. Ed. 2006, 45, 7751–7754. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Zhan, Z.; Yu, J.C.; Li, S.Q.; Yi, X.X.; Wang, J.Y.; Wang, S.L.; Tan, B. Ultrathin Hollow Co/N/C Spheres from Hyper-Crosslinked Polymers by a New Universal Strategy with Boosted ORR Efficiency. Small 2023, 19, 2207646. [Google Scholar] [CrossRef]
- Qiao, S.; Li, Z.; Zhang, B.; Li, Q.; Jin, W.; Zhang, Y.; Wang, W.; Li, Q.; Liu, X. Flexible chain & rigid skeleton complementation polycarbazole microporous system for gas storage. Micropor. Mesopor. Mater. 2019, 284, 205–211. [Google Scholar]
- An, Z.-W.; Ye, K.; Xue, R.; Zhao, H.; Liu, Y.; Li, P.; Chen, Z.-M.; Huang, C.-X.; Hu, G.-H. Recent advances in self-healing polyurethane based on dynamic covalent bonds combined with other self-healing methods. Nanosacle 2023, 15, 7591. [Google Scholar] [CrossRef]
- Li, H.-W.; Zhang, J.-L.; Xue, R.; An, Z.-W.; Wu, W.; Liu, Y.; Hu, G.-H.; Zhao, H. Construction of self-healable and recyclable waterborne polyurethane-MOF membranes for adsorption of dye wastewater. Sep. Purif. Technol. 2023, 320, 12415. [Google Scholar] [CrossRef]
- Dai, L.; Dong, A.W.; Meng, X.J.; Liu, H.Y.; Li, Y.T.; Li, P.F.; Wang, B. Enhancement of Visible-Light-Driven Hydrogen Evolution Activity of 2D pi-Conjugated Bipyridine-Based Covalent Organic Frameworks via Post-Protonation. Angew. Chem. Int. Ed. 2023, 475, 146264. [Google Scholar] [CrossRef]
- Li, M.P.; Ren, H.; Sun, F.X.; Tian, Y.Y.; Zhu, Y.L.; Li, J.L.; Mu, X.; Xu, J.; Deng, F.; Zhu, G.S. Construction of Porous Aromatic Frameworks with Exceptional Porosity via Building Unit Engineering. Adv. Mater. 2018, 30, 1804169. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.C.; Li, J.W.; Li, X.L.; Zhao, T.Y.; Ren, H.; Sun, F.X. Imine-linked porous aromatic frameworks based on spirobifluorene building blocks for CO2 separation. Micropor. Mesopor. Mater. 2022, 334, 111779. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Wang, Z.; Zhang, Z.W.; Zhai, T.L.; Chen, J.J.; Ma, H.; Tan, B.; Zhang, C. Triptycene-based Chiral Porous Polyimides for Enantioselective Membrane Separation. Angew. Chem. Int. Ed. 2021, 60, 12781–12785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, H.; Zhai, T.L.; Cheng, G.; Xu, Q.; Liu, J.M.; Yang, J.K.; Zhang, Q.M.; Zhang, Q.P.; Zheng, Y.S.; et al. Networked Cages for Enhanced CO2 Capture and Sensing. Adv. Sci. 2018, 5, 1800141. [Google Scholar] [CrossRef]
- Wood, C.D.; Tan, B.; Trewin, A.; Su, F.; Rosseinsky, M.J.; Bradshaw, D.; Sun, Y.; Zhou, L.; Cooper, A.I. Microporous Organic Polymers for Methane Storage. Adv. Mater. 2008, 20, 1916–1921. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Burress, J.; Yildirim, T. Graphene oxide derived carbons (GODCs): Synthesis and gas adsorption properties. Energy Environ. Sci. 2012, 5, 6453–6459. [Google Scholar] [CrossRef]
- Mahmoudian, L.; Rashidi, A.; Dehghani, H.; Rahighi, R. Single-step scalable synthesis of three-dimensional highly porous graphene with favorable methane adsorption. Chem. Eng. J. 2016, 304, 784–792. [Google Scholar] [CrossRef]
- Liu, B.S.; Wang, W.S.; Wang, N.; Au, C.T. Preparation of activated carbon with high surface area for high-capacity methane storage. J. Energy Chem. 2014, 23, 662–668. [Google Scholar] [CrossRef]
- Li, H.X.; Zhang, Z.H.; Fang, H.; Xue, D.X.; Bai, J.F. Synthesis, structure and high methane storage of pure D6R Yb(Y) nonanuclear cluster-based zeolite-like metal-organic frameworks. J. Mater. Chem. A 2022, 10, 14795–14798. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Ma, H.; Yang, B.-B.; Wang, Z.; Meng, X.-G.; Bu, J.-H.; Zhang, C. Rigidity with Flexibility: Porous Triptycene Networks for Enhancing Methane Storage. Polymers 2024, 16, 156. https://doi.org/10.3390/polym16010156
Guo F, Ma H, Yang B-B, Wang Z, Meng X-G, Bu J-H, Zhang C. Rigidity with Flexibility: Porous Triptycene Networks for Enhancing Methane Storage. Polymers. 2024; 16(1):156. https://doi.org/10.3390/polym16010156
Chicago/Turabian StyleGuo, Fei, Hui Ma, Bin-Bin Yang, Zhen Wang, Xiang-Gao Meng, Jian-Hua Bu, and Chun Zhang. 2024. "Rigidity with Flexibility: Porous Triptycene Networks for Enhancing Methane Storage" Polymers 16, no. 1: 156. https://doi.org/10.3390/polym16010156
APA StyleGuo, F., Ma, H., Yang, B.-B., Wang, Z., Meng, X.-G., Bu, J.-H., & Zhang, C. (2024). Rigidity with Flexibility: Porous Triptycene Networks for Enhancing Methane Storage. Polymers, 16(1), 156. https://doi.org/10.3390/polym16010156





