Adsorption of Copper and Arsenic from Water Using a Semi-Interpenetrating Polymer Network Based on Alginate and Chitosan
Abstract
1. Introduction
Impact of Metal Ions on Human Health
2. Materials, Methods, and Characterization
2.1. Reagents
2.2. Adsorbent Synthesis
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM)
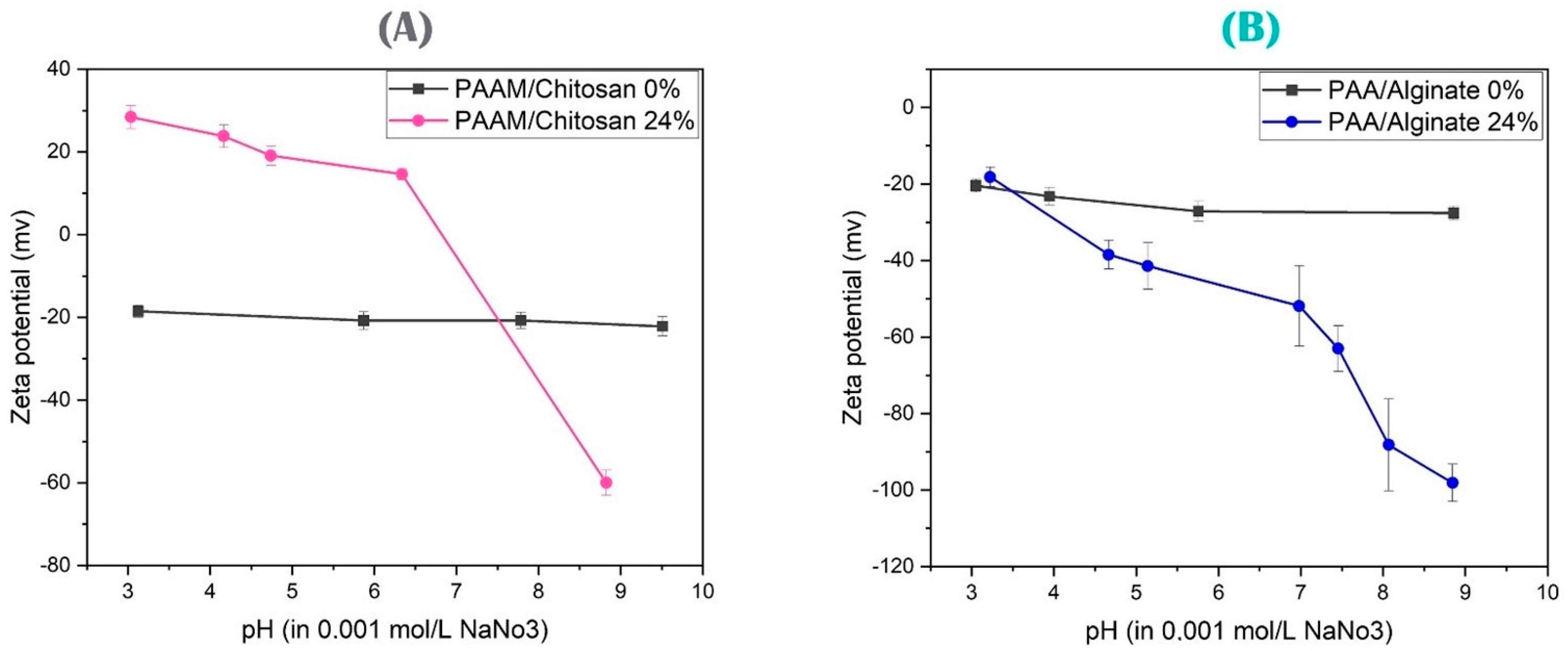
3.2. Zeta Potential Characterization
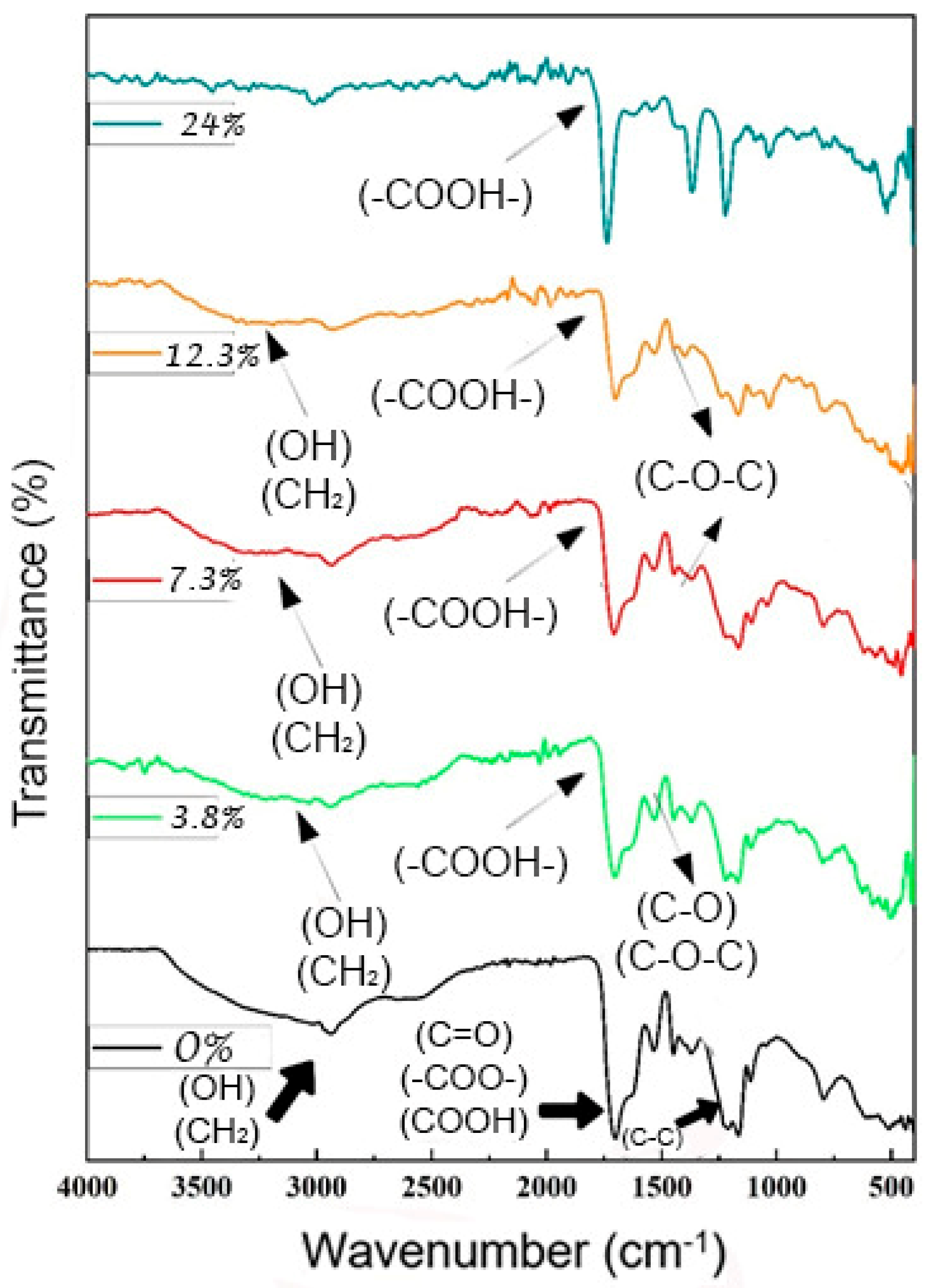
3.3. FTIR Characterization
3.4. Removal of Arsenic and Copper
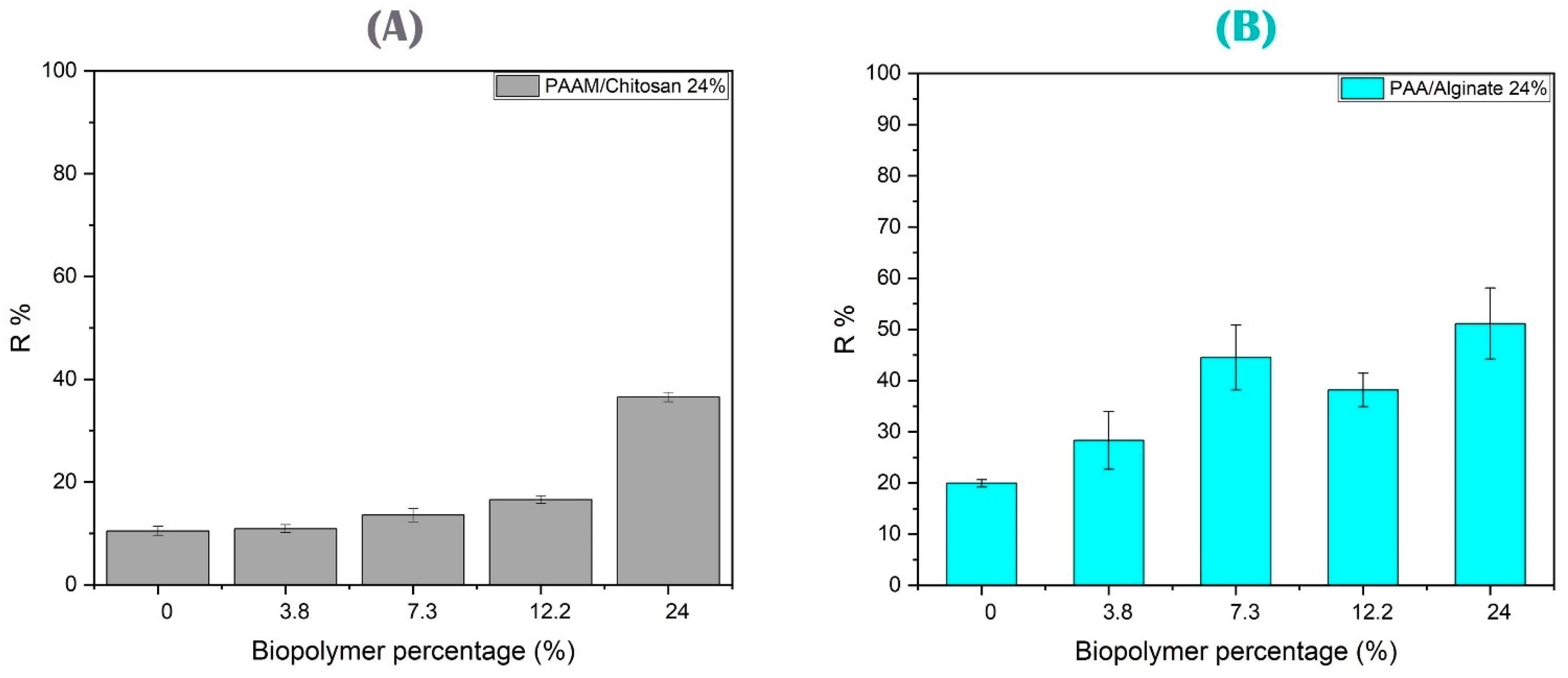
3.4.1. Effect of Biopolymer Amount on Metal Removal
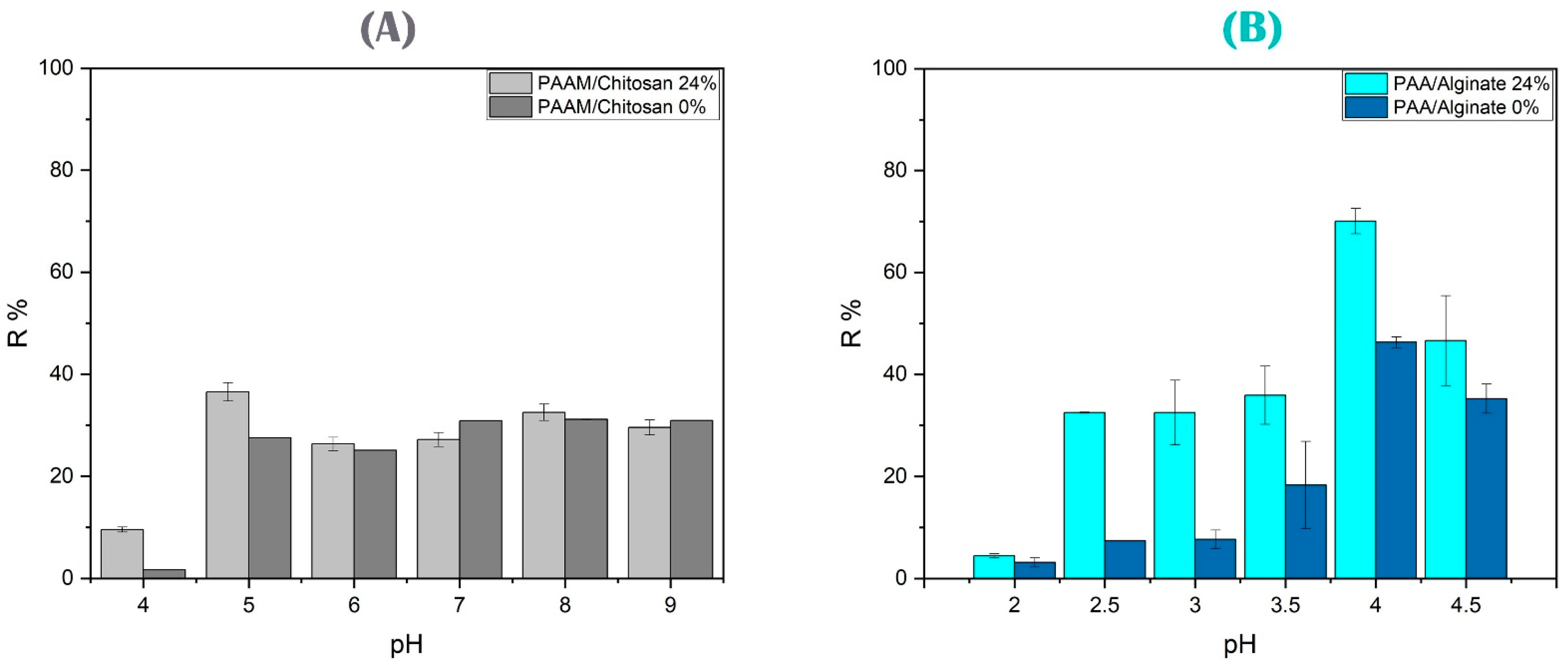
3.4.2. Effect of pH on Arsenic and Copper Ion Removal
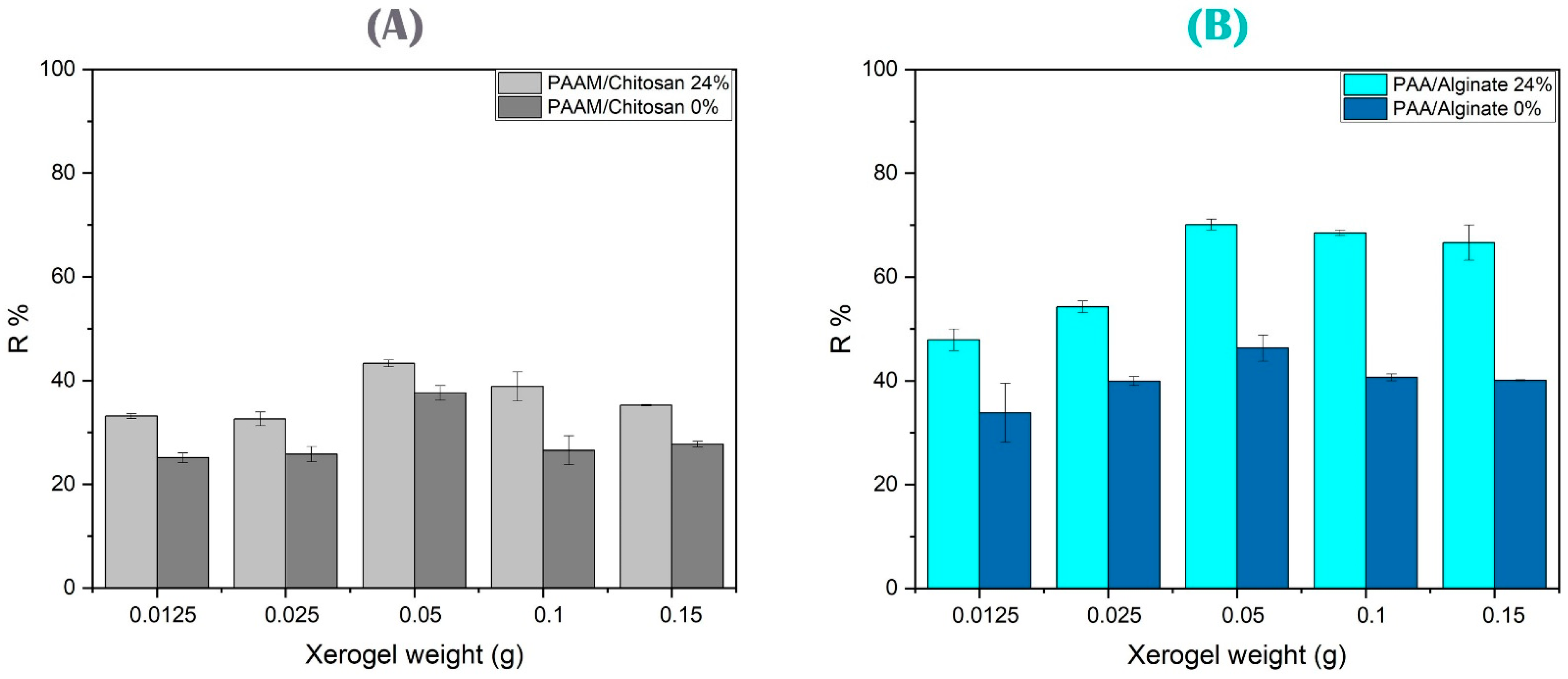
3.4.3. Effect of the Amount of Adsorbent on Arsenic and Copper Ion Removal
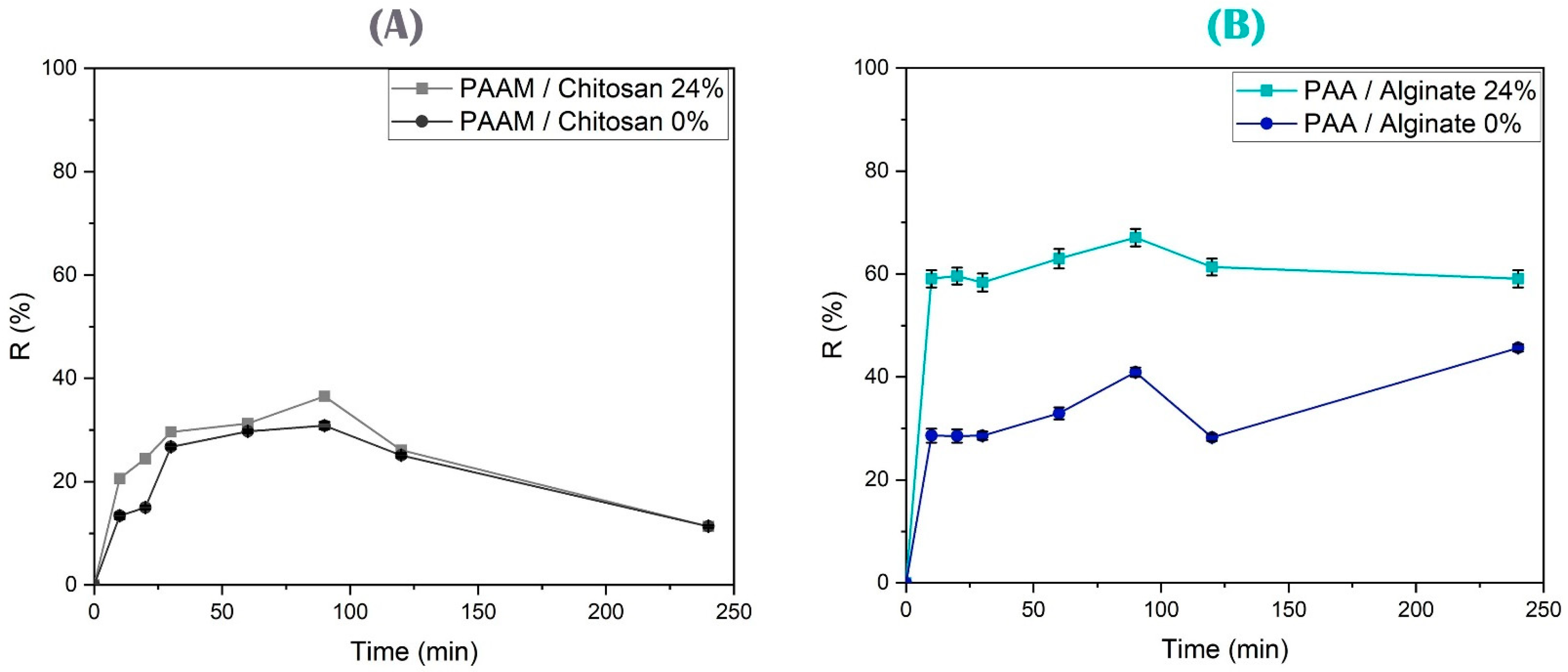
3.4.4. Effect of Contact Time on Arsenic and Copper Removal
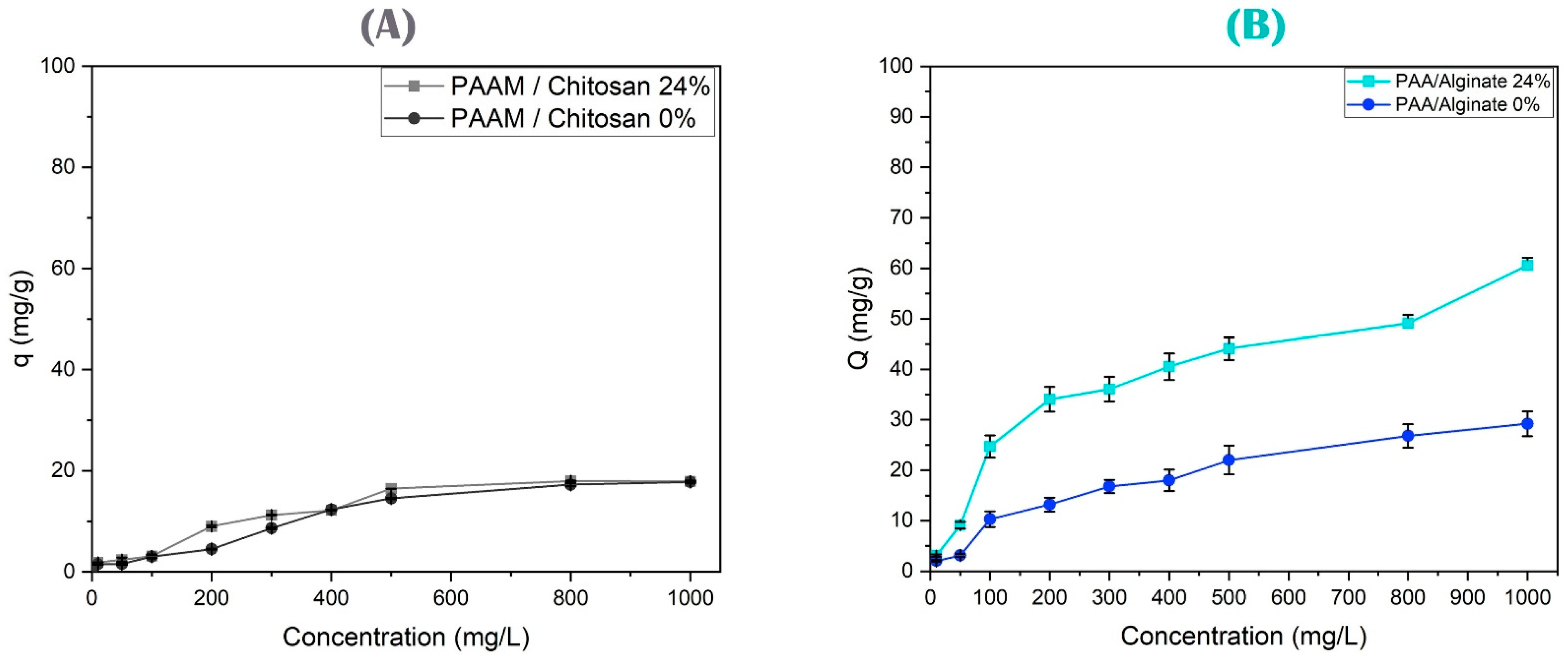
3.4.5. Effect of the Initial Arsenic or Copper Ion Concentration on Their Removal
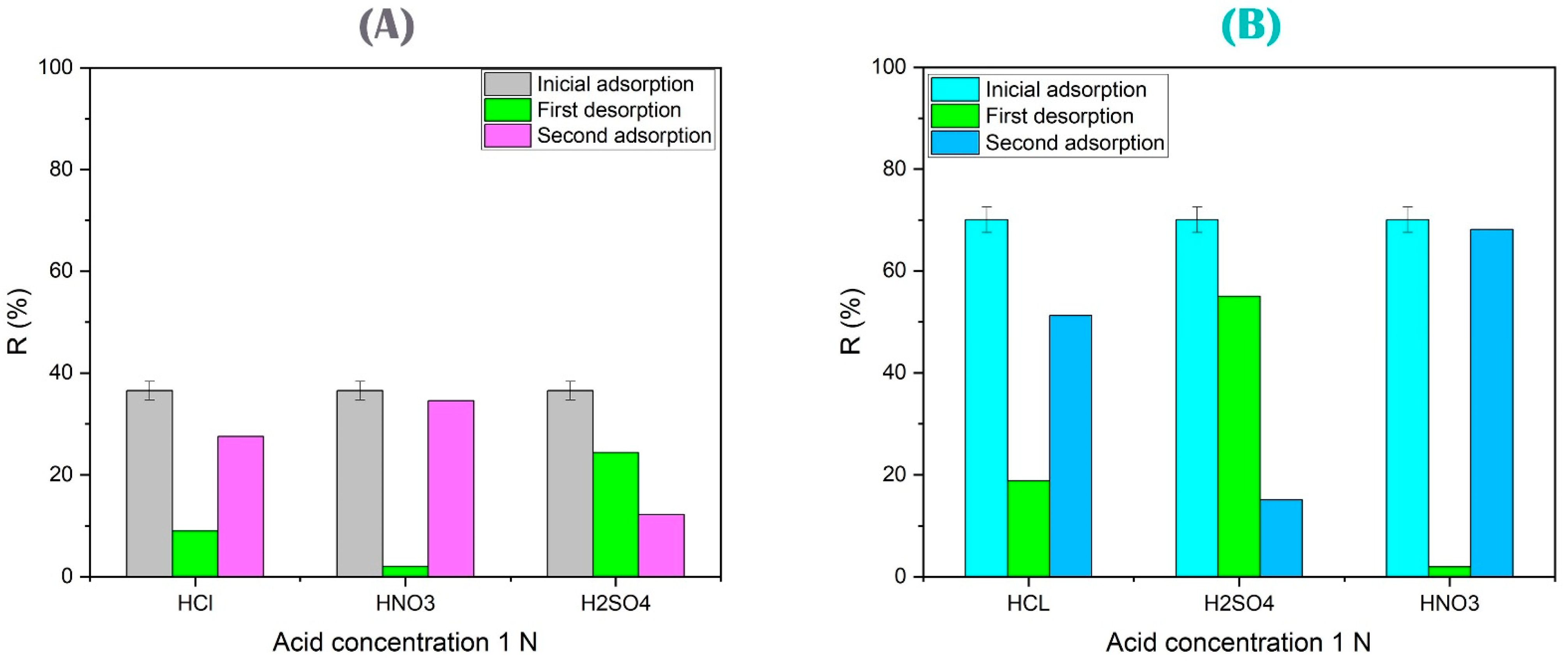
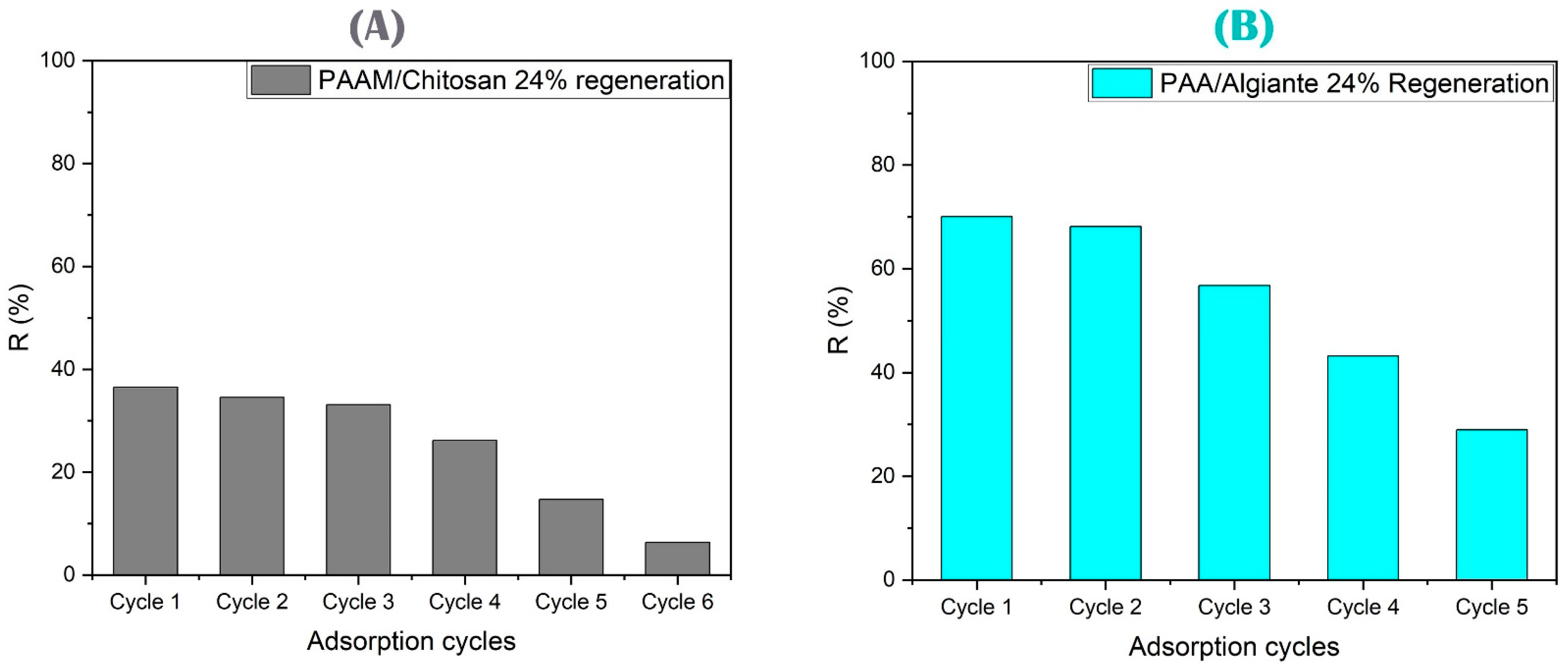
3.4.6. Regeneration and Elution Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shankar, S.; Shanker, U.; Shikha. Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Costa, F.G.E.; de Lourdes Pereira, M. Heavy Metals and Human Health. In Environmental Health—Emerging Issues and Practice; InTech: Vienna, Austria, 2012. [Google Scholar]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Current Trends of Arsenic Adsorption in Continuous Mode: Literature Review and Future Perspectives. Sustainability 2021, 13, 1186. [Google Scholar] [CrossRef]
- Bassir, R.; Prasherso, S.; Simpsonbk, B. Removal of Selected Metal Ions from Aqueous Solutions Using Chitosan Flakes. Sep. Sci. Technol. 2000, 35, 547–560. [Google Scholar] [CrossRef]
- Blandez, J.F.; Primo, A.; Asiri, A.M.; Álvaro, M.; García, H. Copper Nanoparticles Supported on Doped Graphenes as Catalyst for the Dehydrogenative Coupling of Silanes and Alcohols. Angew. Chem. 2014, 126, 12789–12794. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Biswas, S.; Meikap, B.C.; Sen, T.K. Adsorptive Removal of Aqueous Phase Copper (Cu2+) and Nickel (Ni2+) Metal Ions by Synthesized Biochar–Biopolymeric Hybrid Adsorbents and Process Optimization by Response Surface Methodology (RSM). Water Air Soil Pollut. 2019, 230, 197. [Google Scholar] [CrossRef]
- Deze, E.G.; Papageorgiou, S.K.; Favvas, E.P.; Katsaros, F.K. Porous alginate aerogel beads for effective and rapid heavy metal sorption from aqueous solutions: Effect of porosity in Cu2+ and Cd2+ ion sorption. Chem. Eng. J. 2012, 209, 537–546. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic(III) and arsenic(V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Pontoni, L.; Fabbricino, M. Use of chitosan and chitosan-derivatives to remove arsenic from aqueous solutions—A mini review. Carbohydr. Res. 2012, 356, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Terkula Iber, B.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Elchinger, P.H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Kloster, G.A.; Valiente, M.; Marcovich, N.E.; Mosiewicki, M.A. Adsorption of arsenic onto films based on chitosan and chitosan/nano-iron oxide. Int. J. Biol. Macromol. 2020, 165, 1286–1295. [Google Scholar] [CrossRef]
- Ziabari, P.D.; Dehghani Ghanateghestani, M. Experimental Study of Removal of Heavy Metal of Arsenic from Water Using Nano Absorbent Iron Oxide/N-Isopropyl Acrylamide/Chitosan. J. Water Wastewater 2019, 30, 78–89. [Google Scholar]
- Pawar, S.N. Chemical Modification of Alginate. In Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; pp. 111–155. [Google Scholar]
- Singh, L.; Pavankumar, A.R.; Lakshmanan, R.; Rajarao, G.K. Effective removal of Cu2+ ions from aqueous medium using alginate as biosorbent. Ecol. Eng. 2012, 38, 119–124. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, X.; Zhang, X.; Huang, L.; Liu, Z.; Sun, G. Highly efficient removal of trace metal ions by using poly(acrylic acid) hydrogel adsorbent. Mater. Des. 2019, 181, 107934. [Google Scholar] [CrossRef]
- Zhang, M.K.; Zhang, X.H.; Han, G.Z. Magnetic alginate/PVA hydrogel microspheres with selective adsorption performance for aromatic compounds. Sep. Purif. Technol. 2021, 278, 119547. [Google Scholar] [CrossRef]
- Maskawat Marjub, M.; Rahman, N.; Dafader, N.C.; Sultana Tuhen, F.; Sultana, S.; Tasneem Ahmed, F. Acrylic acid-chitosan blend hydrogel: A novel polymer adsorbent for adsorption of lead(II) and copper(II) ions from wastewater. J. Polym. Eng. 2019, 39, 883–891. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate–graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ho, J.; McCandlish, E.; Buckley, B.; Patel, R.; Li, Z.; Shapley, N.C. Copper ion adsorption by chitosan nanoparticles and alginate microparticles for water purification applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 31–41. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Ravichandar, S.; Lim, Y.C. Combined effects of adsorption and photocatalysis by hybrid TiO2/ZnO-calcium alginate beads for the removal of copper. J. Environ. Sci. 2017, 55, 214–223. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Seyedi, S.R. Kinetic and Equilibrium Studies on the Removal of Pb(II) from Aqueous Solution Using Nettle Ash. J. Chil. Chem. Soc. 2010, 55, 307–311. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Swelling, diffusion, network parameters and adsorption properties of IPN hydrogel of chitosan and acrylic copolymer. Mater. Sci. Eng. C 2014, 44, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, R.; Ray, S.K. Enhanced adsorption of synthetic dyes from aqueous solution by a semi-interpenetrating network hydrogel based on starch. J. Ind. Eng. Chem. 2014, 20, 3714–3725. [Google Scholar] [CrossRef]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan—Investigated by spectrophotometry and SEM-EDX analysis. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 275–282. [Google Scholar] [CrossRef]
- Rawat, S.; Maiti, A. Facile preparation of iron oxyhydroxide–biopolymer (Chitosan/Alginate) beads and their comparative insights into arsenic removal. Sep. Purif. Technol. 2021, 272, 118983. [Google Scholar] [CrossRef]
- Jorge Gonçalves, F.; Alves Gurgel, L.V.; Catone Soares, L.; Simões Teodoro, F.; Dias Ferreira, G.M.; Coelho, Y.L.; da Silva, L.H.M.; Prim, D.; Gil, L.F. Application of pyridine-modified chitosan derivative for simultaneous adsorption of Cu(II) and oxyanions of Cr(VI) from aqueous solution. J. Environ. Manag. 2021, 282, 111939. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Liu, C.D.; Hsieh, C.H.; Hsieh, K.H.; Lee, S.N. High dielectric constant polyaniline/poly(acrylic acid) composites prepared by in situ polymerization. Synth. Met. 2008, 158, 630–637. [Google Scholar] [CrossRef]
- Sajeesh, S.; Sharma, C.P. Poly Methacrylic Acid-Alginate Semi-IPN Microparticles for Oral Delivery of Insulin: A Preliminary Investigation. J. Biomater. Appl. 2004, 19, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly(sodium acrylate) and polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Preparation and characterization of alginate-PVA-based semi-IPN: Controlled release pH-responsive composites. Polym. Bull. 2018, 75, 1075–1099. [Google Scholar] [CrossRef]
- Mohamed, S.F.; Mahmoud, G.A.; Taleb, M.F.A. Synthesis and characterization of poly(acrylic acid)-g-sodium alginate hydrogel initiated by gamma irradiation for controlled release of chlortetracycline HCl. Monatsh. Chem. 2013, 144, 129–137. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Usman Minhas, M.; Barkat, K.; Sohail, M. Cross-Linked Sodium Alginate-g-poly(Acrylic Acid) Structure: A Potential Hydrogel Network for Controlled Delivery of Loxoprofen Sodium. Adv. Polym. Technol. 2018, 37, 985–995. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Mahmood, Z.; Nasir, S.; Jamil, N.; Sheikh, A.; Akram, A. Adsorption studies of phosphate ions on alginate-calcium carbonate composite beads. Afr. J. Environ. Sci. Technol. 2015, 9, 274–281. [Google Scholar]
- Lim, L.S.; Rosli, N.A.; Ahmad, I.; Mat Lazim, A.; Mohd Amin, M.C.I. Synthesis and Swelling Behavior of pH-Sensitive Semi-IPN Superabsorbent Hydrogels Based on Poly(acrylic acid) Reinforced with Cellulose Nanocrystals. Nanomaterials 2017, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.S.; Rao, K.M.; Rao, K.S.V.K.; Shchipunov, Y.; Ha, C.S. Synthesis of alginate based silver nanocomposite hydrogels for biomedical applications. Macromol. Res. 2014, 22, 832–842. [Google Scholar] [CrossRef]
- Das, D.; Pham, H.T.T.; Lee, S.; Noh, I. Fabrication of alginate-based stimuli-responsive, non-cytotoxic, terpolymric semi-IPN hydrogel as a carrier for controlled release of bovine albumin serum and 5-amino salicylic acid. Mater. Sci. Eng. C 2019, 98, 42–53. [Google Scholar] [CrossRef]
- AL-Sabagh, A.M.; Abdeen, Z. Preparation and Characterization of Hydrogel Based on Poly(vinyl alcohol) Cross-Linked by Different Cross-Linkers Used to Dry Organic Solvents. J. Polym. Environ. 2010, 18, 576–583. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Zhao, X.; Sun, H.; Shao, M.; Xu, H. In vitro adsorption mechanism of acrylamide by lactic acid bacteria. LWT 2019, 100, 119–125. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, P.; Lan, G.; Liu, Y.; Cai, Q.; Xi, J. High crosslinked sodium carboxyl methylstarch-g-poly (acrylic acid-co-acrylamide) resin for heavy metal adsorption: Its characteristics and mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 38617–38630. [Google Scholar] [CrossRef] [PubMed]
- Faleh, Y.A.; Radhy, N.D. Removal of Metformin hydrochloride from Aqueous Solutions by using Carboxymethyl cellulose-g-poly(acrylic acid-co-acrylamide) Hydrogel: Adsorption and Thermodynamic Studies. IOP Conf. Ser. Earth Environ. Sci. 2021, 790, 012062. [Google Scholar] [CrossRef]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of sodium acrylate and acrylamide copolymer/GO hydrogels and their effective adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959. [Google Scholar] [CrossRef]
- Ma, Q.; Shuler, P.J.; Aften, C.W.; Tang, Y. Theoretical studies of hydrolysis and stability of polyacrylamide polymers. Polym. Degrad. Stab. 2015, 121, 69–77. [Google Scholar] [CrossRef]
- Guan, Q.; Zheng, H.; Zhai, J.; Zhao, C.; Zheng, X.; Tang, X.; Chen, W.; Sun, Y. Effect of Template on Structure and Properties of Cationic Polyacrylamide: Characterization and Mechanism. Ind. Eng. Chem. Res. 2014, 53, 5624–5635. [Google Scholar] [CrossRef]
- Chen, H.; Wang, A. Adsorption characteristics of Cu(II) from aqueous solution onto poly(acrylamide)/attapulgite composite. J. Hazard. Mater. 2009, 165, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.L.A.; Alleoni, L.R.F.; Camargo, O.A.; Casagrande, J.C. Copper Adsorption in Oxidic Soils after Removal of Organic Matter and Iron Oxides. Commun. Soil Sci. Plant Anal. 2006, 33, 3581–3592. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Sinha Ray, S. The adsorption of Pb2+ and Cu2+ onto gum ghatti-grafted poly(acrylamide-co-acrylonitrile) biodegradable hydrogel: Isotherms and kinetic models. J. Phys. Chem. A 2015, 119, 2026–2039. [Google Scholar] [CrossRef]
- Pinho De Aguiar, K.L.N.; Frias De Oliveira, P.; Elias Mansur, C.R. A comprehensive review of in situ polymer hydrogels for conformance control of oil reservoirs. Oil Gas Sci. Technol. 2020, 75, 8. [Google Scholar] [CrossRef]
- Husar, B.; Hatzenbichler, M.; Mironov, V.; Liska, R.; Stampfl, J.; Ovsianikov, A. Photopolymerization-based additive manufacturing for the development of 3D porous scaffolds. In Biomaterials for Bone Regeneration: Novel Techniques and Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 149–201. [Google Scholar]
- Ketova, Y. Relevant directions in development of polymer compositions for conditions of operated in Perm region reservoirs. Bull. Perm Natl. Res. Polytech. Univ. Geol. Oil Gas Min. 2017, 16, 342–349. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Dogan, N.O.; Kizilel, S. Deep Insight into PEGylation of Bioadhesive Chitosan Nanoparticles: Sensitivity Study for the Key Parameters Through Artificial Neural Network Model. ACS Appl. Mater. Interfaces 2018, 10, 33945–33955. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Islam, S.; Arnold, L.; Padhye, R. Comparison and characterisation of regenerated chitosan from 1-butyl-3-methylimidazolium chloride and chitosan from crab shells. BioMed Res. Int. 2015, 2015, 874316. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.K.; Saha, B.; Qadir, M.D.; Gafur, M.D.; Masum, S. Chromium(VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- ALSamman, M.M.; Sánchez, J. Chitosan- and Alginate-Based Hydrogels for the Adsorption of Anionic and Cationic Dyes from Water. Polymers 2022, 14, 1498. [Google Scholar] [CrossRef]
- Makhado, E.; Hato, M.J. Preparation and Characterization of Sodium Alginate-Based Oxidized Multi-Walled Carbon Nanotubes Hydrogel Nanocomposite and its Adsorption Behaviour for Methylene Blue Dye. Front. Chem. 2021, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Deepa, G.; Thulasidasan, A.K.T.; Anto, R.J.; Jisha Pillai, J.; Vinod Kumar, G.S. Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy. Int. J. Nanomed. 2012, 7, 4077–4088. [Google Scholar]
- Pooley, S.A.; Rivas, B.L.; Lillo, F.E.; Del, C.; Pizarro, G. Hydrogels from Acrylic Acid with N,N-dimethylacrylamide: Synthesis, Characterization, and Water Absorption Properties. J. Chil. Chem. Soc. 2010, 55, 19–24. [Google Scholar] [CrossRef]
- Hirashima, Y.; Sato, H.; Suzuki, A. ATR-FTIR Spectroscopic Study on Hydrogen Bonding of Poly(N-isopropylacrylamide-co-sodium acrylate) Gel. Macromolecules 2005, 38, 9280–9286. [Google Scholar] [CrossRef]
- Choi, J.S.; Lingamdinne, L.P.; Yang, J.K.; Chang, Y.Y.; Koduru, J.R. Fabrication of chitosan/graphene oxide-gadolinium nanorods as a novel nanocomposite for arsenic removal from aqueous solutions. J. Mol. Liq. 2020, 320, 114410. [Google Scholar] [CrossRef]
- Ayub, A.; Raza, Z.A.; Majeed, M.I.; Tariq, M.R.; Irfan, A. Development of sustainable magnetic chitosan biosorbent beads for kinetic remediation of arsenic contaminated water. Int. J. Biol. Macromol. 2020, 163, 603–617. [Google Scholar] [CrossRef]
- Das, T.K.; Sakthivel, T.S.; Jeyaranjan, A.; Seal, S.; Bezbaruah, A.N. Ultra-high arsenic adsorption by graphene oxide iron nanohybrid: Removal mechanisms and potential applications. Chemosphere 2020, 253, 126702. [Google Scholar] [CrossRef]
- El Kaim Billah, R.; Aminul Islam, M.; Lgaz, H.; Lima, E.C.; Abdellaoui, Y.; Rakhila, Y.; Goudali, O.; Majdoubi, H.; Alrashdi, A.A.; Agunaou, M.; et al. Shellfish waste-derived mesoporous chitosan for impressive removal of arsenic(V) from aqueous solutions: A combined experimental and computational approach. Arab. J. Chem. 2022, 15, 104123. [Google Scholar] [CrossRef]
- Ayub, A.; Raza, Z.A. Arsenic removal approaches: A focus on chitosan biosorption to conserve the water sources. Int. J. Biol. Macromol. 2021, 192, 1196–1216. [Google Scholar] [CrossRef] [PubMed]
- Pincus, L.N.; Petrović, P.V.; Gonzalez, I.S.; Stavitski, E.; Fishman, Z.S.; Rudel, H.E.; Anastas, P.T.; Zimmerman, J.B. Selective adsorption of arsenic over phosphate by transition metal cross-linked chitosan. Chem. Eng. J. 2021, 412, 128582. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2022, 422, 126863. [Google Scholar] [CrossRef]
- Lim, S.F.; Zheng, Y.M.; Chen, J.P. Organic arsenic adsorption onto a magnetic sorbent. Langmuir 2009, 25, 4973–4978. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; He, Y.; Luo, X. Phenolic hydroxyl derived copper alginate microspheres as superior adsorbent for effective adsorption of tetracycline. Int. J. Biol. Macromol. 2019, 136, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, A. Removal of heavy metals using polyvinyl alcohol semi-IPN poly(acrylic acid)/tourmaline composite optimized with response surface methodology. Chem. Eng. J. 2010, 162, 186–193. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Y.; Song, X.; Zhu, J.L.; Li, J. A smart thermoresponsive adsorption system for efficient copper ion removal based on alginate-g-poly(N-isopropylacrylamide) graft copolymer. Carbohydr. Polym. 2019, 219, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Lenoble, V.; Bouras, O.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenic Adsorption onto Pillared Clays and Iron Oxides. J. Colloid Interface Sci. 2002, 255, 52–58. [Google Scholar] [CrossRef]
- Lobo, C.; Castellari, J.; Colman Lerner, J.; Bertola, N.; Zaritzky, N. Functional iron chitosan microspheres synthesized by ionotropic gelation for the removal of arsenic (V) from water. Int. J. Biol. Macromol. 2020, 164, 1575–1583. [Google Scholar] [CrossRef]
- Bajpai, A.K. Facile preparation of ionotropically crosslinked chitosan-alginate nanosorbents by water-in-oil (W/O) microemulsion technique: Optimization and study of arsenic (V) removal. J. Water Process Eng. 2019, 32, 100920. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Weerasundara, L.; Ok, Y.S.; Kumarathilaka, P.; Marchuk, A.; Bundschuh, J. Assessment and optimization of As(V) adsorption on hydrogel composite integrating chitosan-polyvinyl alcohol and Fe3O4 nanoparticles and evaluation of their regeneration and reusable capabilities in aqueous media. Sci. Total Environ. 2023, 855, 158877. [Google Scholar] [CrossRef]
- Huang, H.; Yang, Q.; Zhang, L.; Huang, C.; Liang, Y. Polyacrylamide modified kaolin enhances adsorption of sodium alginate/carboxymethyl chitosan hydrogel beads for copper ions. Chem. Eng. Res. Des. 2022, 180, 296–305. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jin, H.J.; Park, S.S.; Kim, S.J.; Cho, S.Y. Adsorption equilibrium of copper ion and phenol by powdered activated carbon, alginate bead and alginate-activated carbon bead. J. Ind. Eng. Chem. 2008, 14, 714–719. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Fatinathan, S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Ahmadpoor, F.; Shojaosadati, S.A.; Mousavi, S.Z. Magnetic silica coated iron carbide/alginate beads: Synthesis and application for adsorption of Cu(II) from aqueous solutions. Int. J. Biol. Macromol. 2019, 128, 941–947. [Google Scholar] [CrossRef]
- Zheng, Y.; Hua, S.; Wang, A. Adsorption behavior of Cu2+ from aqueous solutions onto starch-g-poly(acrylic acid)/sodium humate hydrogels. Desalination 2010, 263, 170–175. [Google Scholar] [CrossRef]
- Bayramoǧlu, G.; Yakup Arica, M. Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for biosorption of Cu(II), Zn(II) and Ni(II): Kinetics and equilibrium studies. Bioresour. Technol. 2009, 100, 186–193. [Google Scholar] [CrossRef]
- Zhan, W.; Xu, C.; Qian, G.; Huang, G.; Tang, X.; Lin, B. Adsorption of Cu(II), Zn(II), and Pb(II) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Adv. 2018, 8, 18723–18733. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Roméo, M. Arsenic adsorption onto hematite and goethite. Comptes Rendus Chim. 2009, 12, 876–881. [Google Scholar] [CrossRef]
- Pena, M.; Meng, X.; Korfiatis, G.P.; Jing, C. Adsorption mechanism of arsenic on nanocrystalline titanium dioxide. Environ. Sci. Technol. 2006, 40, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, S.; Xu, K.; Zhao, W.; Hao, R.; Zhang, J.; Li, D. Adsorption of As(V) by magnetic alginate-chitosan porous beads based on iron sludge. J. Clean. Prod. 2022, 359, 132117. [Google Scholar] [CrossRef]
- Poon, L.; Younus, S.; Wilson, L.D. Adsorption study of an organo-arsenical with chitosan-based sorbents. J. Colloid Interface Sci. 2014, 420, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Veglio’, F.; Esposito, A.; Reverberi, A.P. Copper adsorption on calcium alginate beads: Equilibrium pH-related models. Hydrometallurgy 2002, 65, 43–57. [Google Scholar] [CrossRef]
- Dil, N.N.; Sadeghi, M. Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu(II) metal ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar] [CrossRef]
- Gabris, M.A.; Rezania, S.; Rafieizonooz, M.; Khankhaje, E.; Devanesan, S.; AlSalhi, M.S.; Aljaafreh, M.J.; Shadravan, A. Chitosan magnetic graphene grafted polyaniline doped with cobalt oxide for removal of Arsenic(V) from water. Environ. Res. 2022, 207, 112209. [Google Scholar] [CrossRef]
- Chutia, P.; Kato, S.; Kojima, T.; Satokawa, S. Arsenic adsorption from aqueous solution on synthetic zeolites. J. Hazard. Mater. 2009, 162, 440–447. [Google Scholar] [CrossRef]
- Stollenwerk, K.G. Geochemical Processes Controlling Transport of Arsenic in Groundwater: A Review of Adsorption. In Arsenic in Ground Water; Springer: Berlin/Heidelberg, Germany, 2003; pp. 67–100. [Google Scholar]
- Bisaria, K.; Sinha, S.; Iqbal, H.M.N.; Singh, R. Ultrasonication expedited As(III) adsorption onto chitosan impregnated Ni–Fe layered double hydroxide biosorbent: Optimization studies and artificial intelligence modelling. Environ. Res. 2022, 212, 113184. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Al-Ashkar, E.A.; El-Ghaffar, M.A.A. Preparation and characterization of eco-friendly poly(p-phenylenediamine) and its composite with chitosan for removal of copper ions from aqueous solutions. Trans. Nonferrous Met. Soc. China 2015, 25, 3808–3819. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Wang, A. Fast removal of copper ions from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite composites. J. Hazard. Mater. 2009, 168, 970–977. [Google Scholar] [CrossRef]
- Zhang, H.; Omer, A.M.; Hu, Z.; Yang, L.Y.; Ji, C.; Ouyang, X.K. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu(II) adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Kuczajowska-Zadrożna, M.; Filipkowska, U.; Jóźwiak, T. Adsorption of Cu(II) and Cd(II) from aqueous solutions by chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Pandey, A.; Bera, D.; Shukla, A.; Ray, L. Studies on Cr(VI), Pb(II) and Cu(II) adsorption–desorption using calcium alginate as biopolymer. Chem. Speciat. Bioavailab. 2015, 19, 17–24. [Google Scholar] [CrossRef]
- Xie, J.; Liu, X.; Liang, J. Absorbency and adsorption of poly(acrylic acid-co-acrylamide) hydrogel. J. Appl. Polym. Sci. 2007, 106, 1606–1613. [Google Scholar] [CrossRef]
- Gu, Z.; Deng, B.; Yang, J. Synthesis and evaluation of iron-containing ordered mesoporous carbon (FeOMC) for arsenic adsorption. Microporous Mesoporous Mater. 2007, 102, 265–273. [Google Scholar] [CrossRef]
- Akha, N.Z.; Salehi, S.; Anbia, M. Removal of arsenic by metal organic framework/chitosan/carbon nanocomposites: Modeling, optimization, and adsorption studies. Int. J. Biol. Macromol. 2022, 208, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Islam, S.M.; Liu, H.; Zhao, J.; Sun, G.; Li, H.; Ma, S.; Kanatzidis, M.G. Selective and Efficient Removal of Toxic Oxoanions of As(III), As(V), and Cr(VI) by Layered Double Hydroxide Intercalated with MoS42−. Chem. Mater. 2017, 29, 3274–3284. [Google Scholar] [CrossRef]
- Mcafee, B.J.; Gould, W.D.; Nadeau, J.C.; Da Costa, A.C.A. Biosorption of metal ions using chitosan, chitin, and biomass of Rhizopus oryzae. Sep. Sci. Technol. 2001, 36, 3207–3222. [Google Scholar] [CrossRef]
- Qureshi, I.; Memon, S.; Yilmaz, M. An excellent arsenic(V) sorption behavior of p-tert-butylcalix[8]areneoctamide impregnated resin. Comptes Rendus Chim. 2010, 13, 1416–1423. [Google Scholar] [CrossRef]
- Penke, Y.K.; Anantharaman, G.; Ramkumar, J.; Kar, K.K. Aluminum substituted nickel ferrite (Ni–Al–Fe): A ternary metal oxide adsorbent for arsenic adsorption in aqueous medium. RSC Adv. 2016, 6, 55608–55617. [Google Scholar] [CrossRef]
- Jiang, X.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Shi, Z. Versatile core/shell-like alginate@polyethylenimine composites for efficient removal of multiple heavy metal ions (Pb2+, Cu2+, CrO42−): Batch and fixed-bed studies. Mater. Res. Bull. 2019, 118, 110526. [Google Scholar] [CrossRef]
- Hajeeth, T.; Vijayalakshmi, K.; Gomathi, T.; Sudha, P.N. Removal of Cu(II) and Ni(II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int. J. Biol. Macromol. 2013, 62, 59–65. [Google Scholar] [CrossRef]
- Çavuş, S.; Gürdaǧ, G.; Yaşar, M.; Güçlü, K.; Gürkaynak, M.A. The competitive heavy metal removal by hydroxyethyl cellulose-g-poly(acrylic acid) copolymer and its sodium salt: The effect of copper content on the adsorption capacity. Polym. Bull. 2006, 57, 445–456. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, Q.L.; Hu, S.R.; Ni, J.C.; He, Y.S. Adsorption mechanism of Cu(II) ions from aqueous solution by glutaraldehyde crosslinked humic acid-immobilized sodium alginate porous membrane adsorbent. Chem. Eng. J. 2011, 173, 511–519. [Google Scholar] [CrossRef]
- Hu, X.; Long, L.; Gong, T.; Zhang, J.; Yan, J.; Xue, Y. Enhanced alginate-based microsphere with the pore-forming agent for efficient removal of Cu(II). Chemosphere 2020, 240, 124860. [Google Scholar] [CrossRef]
- Yi, X.; Sun, F.; Han, Z.; Han, F.; He, J.; Ou, M.; Gu, J.; Xu, X. Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu(II) and U(VI) removal. Ecotoxicol. Environ. Saf. 2018, 158, 309–318. [Google Scholar] [CrossRef]
- Ji, C.; Yang, S.; Tao, E.; Cheng, Y.; Hao, X.; Li, Y. Three-dimensional network graphene oxide/sodium alginate aerogel beads with slit-shaped structure: Synthesis, performance and selective adsorption mechanism for Cu(II). J. Environ. Chem. Eng. 2021, 9, 106819. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, L.; Lin, Z.; Jiang, H.; Zhang, A. Adsorption of Pb2+, Cu2+ and Cd2+ by sulfhydryl modified chitosan beads. Carbohydr. Polym. 2021, 274, 118622. [Google Scholar] [CrossRef]
- Tao, X.; Wang, S.; Li, Z.; Zhou, S. Green synthesis of network nanostructured calcium alginate hydrogel and its removal performance of Cd2+ and Cu2+ ions. Mater. Chem. Phys. 2021, 258, 123931. [Google Scholar] [CrossRef]
- Godiya, C.B.; Liang, M.; Sayed, S.M.; Li, D.; Lu, X. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions. J. Environ. Manag. 2019, 232, 829–841. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 2020, 744, 140653. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Hao, J.A.; Tong, Y. Alginate modified graphitic carbon nitride composite hydrogels for efficient removal of Pb(II), Ni(II) and Cu(II) from water. Int. J. Biol. Macromol. 2020, 148, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, Y.; Ju, B.; Tian, Y. Preparation of thermoresponsive alginate/starch ether composite hydrogel and its application to the removal of Cu(II) from aqueous solution. Bioresour. Technol. 2019, 294, 122192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, F.; Xia, B.; Du, Q.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. J. Hazard. Mater. 2010, 177, 876–880. [Google Scholar] [CrossRef]
- Li, Y.; Xia, B.; Zhao, Q.; Liu, F.; Zhang, P.; Du, Q.; Wang, D.; Li, D.; Wang, Z.; Xia, Y. Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin. J. Environ. Sci. 2011, 23, 404–411. [Google Scholar] [CrossRef]
- Dong, Y.; Sang, D.; He, C.; Sheng, X.; Lei, L. Mxene/alginate composites for lead and copper ion removal from aqueous solutions. RSC Adv. 2019, 9, 29015–29022. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, Y.; Zhang, X.F.; Yao, J. In-situ gelation of sodium alginate supported on melamine sponge for efficient removal of copper ions. J. Colloid Interface Sci. 2018, 512, 7–13. [Google Scholar] [CrossRef]
- Tan, W.S.; Ting, A.S.Y. Alginate-immobilized bentonite clay: Adsorption efficacy and reusability for Cu(II) removal from aqueous solution. Bioresour. Technol. 2014, 160, 115–118. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, J.; Wang, B.; Wang, H.; He, Q.; Song, J.; Zhang, H.; Tang, M.; Zhou, L.; Gao, Y.; et al. Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight. J. Hazard. Mater. 2021, 418, 126358. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, Z.; Zhao, X.; He, H. Efficient removal of lead and copper ions from water by enhanced strength-toughness alginate composite fibers. Int. J. Biol. Macromol. 2019, 134, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Q.; Zhao, X.; Wang, Z. Highly efficient removal of copper ions from water by using a novel alginate-polyethyleneimine hybrid aerogel. Int. J. Biol. Macromol. 2019, 138, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chauhan, K.; Priya, V.; Singhal, R.K. A greener approach for impressive removal of As(III)/As(V) from an ultra-low concentration using a highly efficient chitosan thiomer as a new adsorbent. RSC Adv. 2016, 6, 64946–64961. [Google Scholar] [CrossRef]
- Kwok, K.C.M.; Koong, L.F.; Chen, G.; McKay, G. Mechanism of arsenic removal using chitosan and nanochitosan. J. Colloid Interface Sci. 2014, 416, 1–10. [Google Scholar] [CrossRef]
- Min, L.L.; Zhong, L.B.; Zheng, Y.M.; Liu, Q.; Yuan, Z.H.; Yang, L.M. Functionalized chitosan electrospun nanofiber for effective removal of trace arsenate from water. Sci. Rep. 2016, 6, 32480. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Ouyang, X.K.; Liang, X.X.; Yu, D.; Yang, L.Y.; Huang, F. Fabrication of chitosan-based MCS/ZnO@Alg gel microspheres for efficient adsorption of As(V). Int. J. Biol. Macromol. 2019, 139, 886–895. [Google Scholar] [CrossRef]
- Song, Y.; Wang, S.; Yang, L.Y.; Yu, D.; Wang, Y.G.; Ouyang, X.K. Facile fabrication of core–shell/bead-like ethylenediamine-functionalized Al-pillared montmorillonite/calcium alginate for As(V) ion adsorption. Int. J. Biol. Macromol. 2019, 131, 971–979. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Chen, L.; Huang, X.; Liu, J. Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem. Eng. J. 2014, 251, 25–34. [Google Scholar] [CrossRef]
- Awual, M.R.; El-Safty, S.A.; Jyo, A. Removal of trace arsenic(V) and phosphate from water by a highly selective ligand exchange adsorbent. J. Environ. Sci. 2011, 23, 1947–1954. [Google Scholar] [CrossRef]
- Santra, D.; Sarkar, M. Optimization of process variables and mechanism of arsenic (V) adsorption onto cellulose nanocomposite. J. Mol. Liq. 2016, 224, 290–302. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, F.; Xu, K.; Zhang, J.; Li, D. Optimization and regeneration of chitosan-alginate hybrid adsorbent embedding iron-manganese sludge for arsenic removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125500. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Studies on sorption, desorption, regeneration and reuse of sugar-beet pectin gels for heavy metal removal. J. Hazard. Mater. 2010, 178, 243–248. [Google Scholar] [CrossRef] [PubMed]

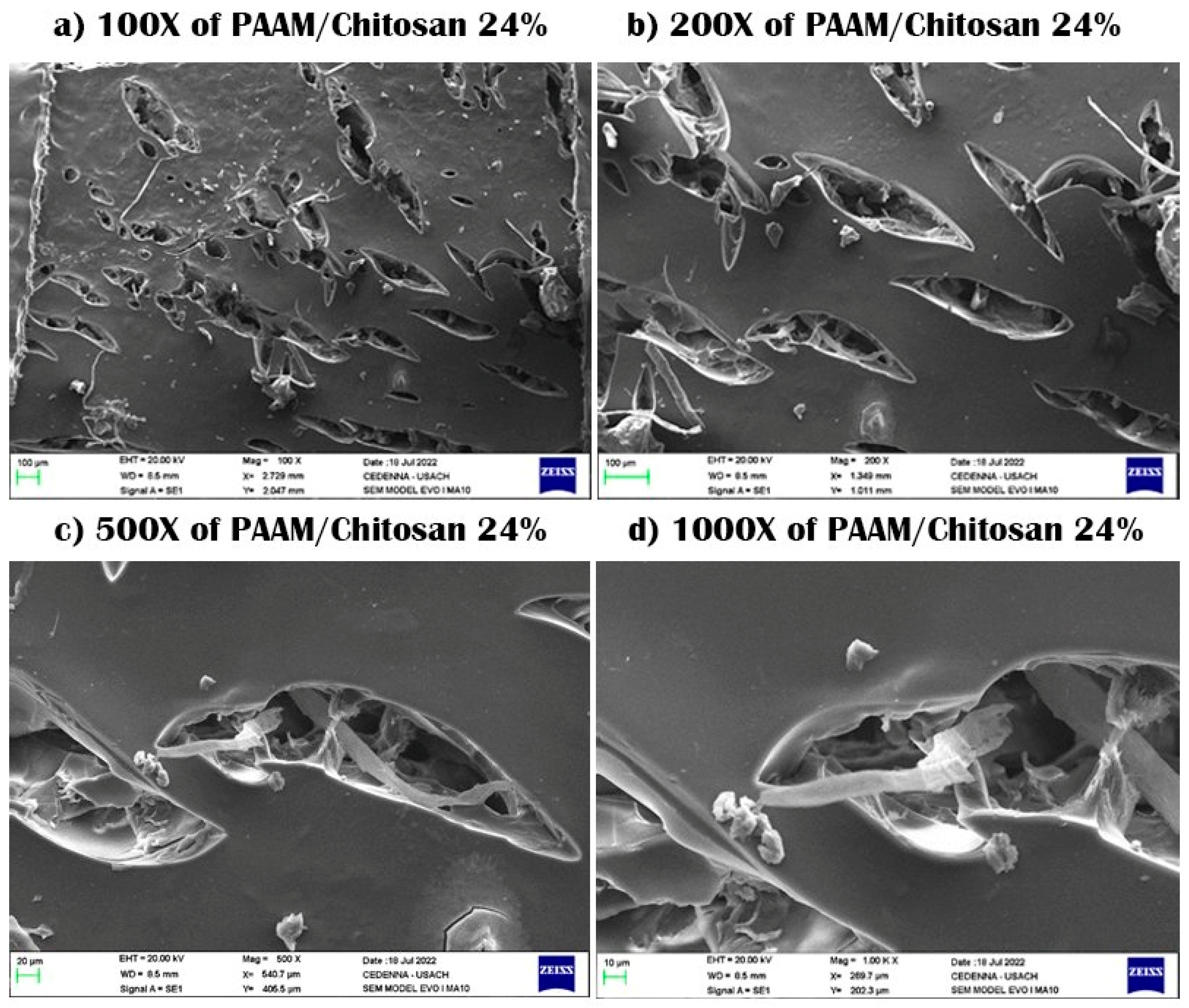
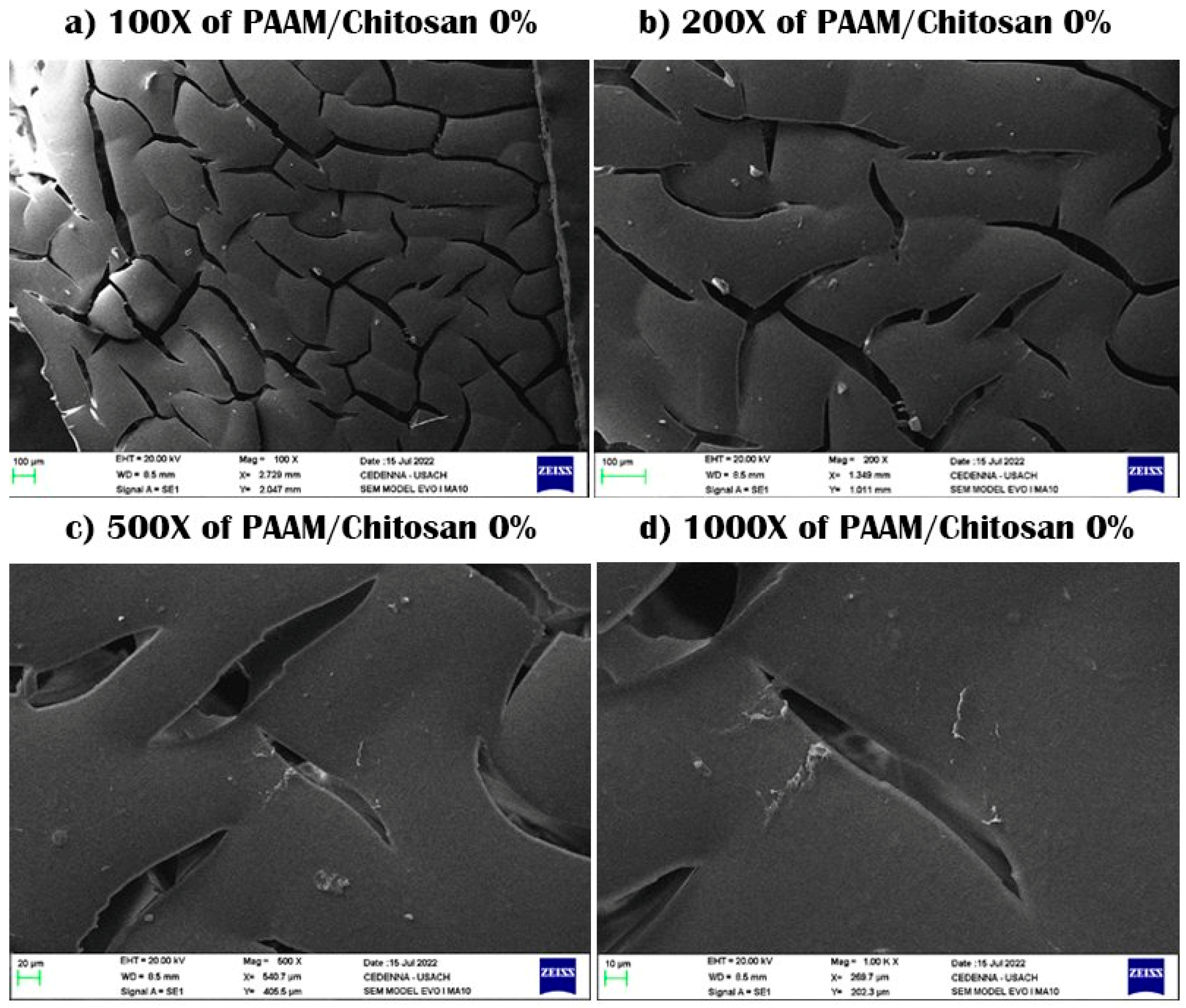
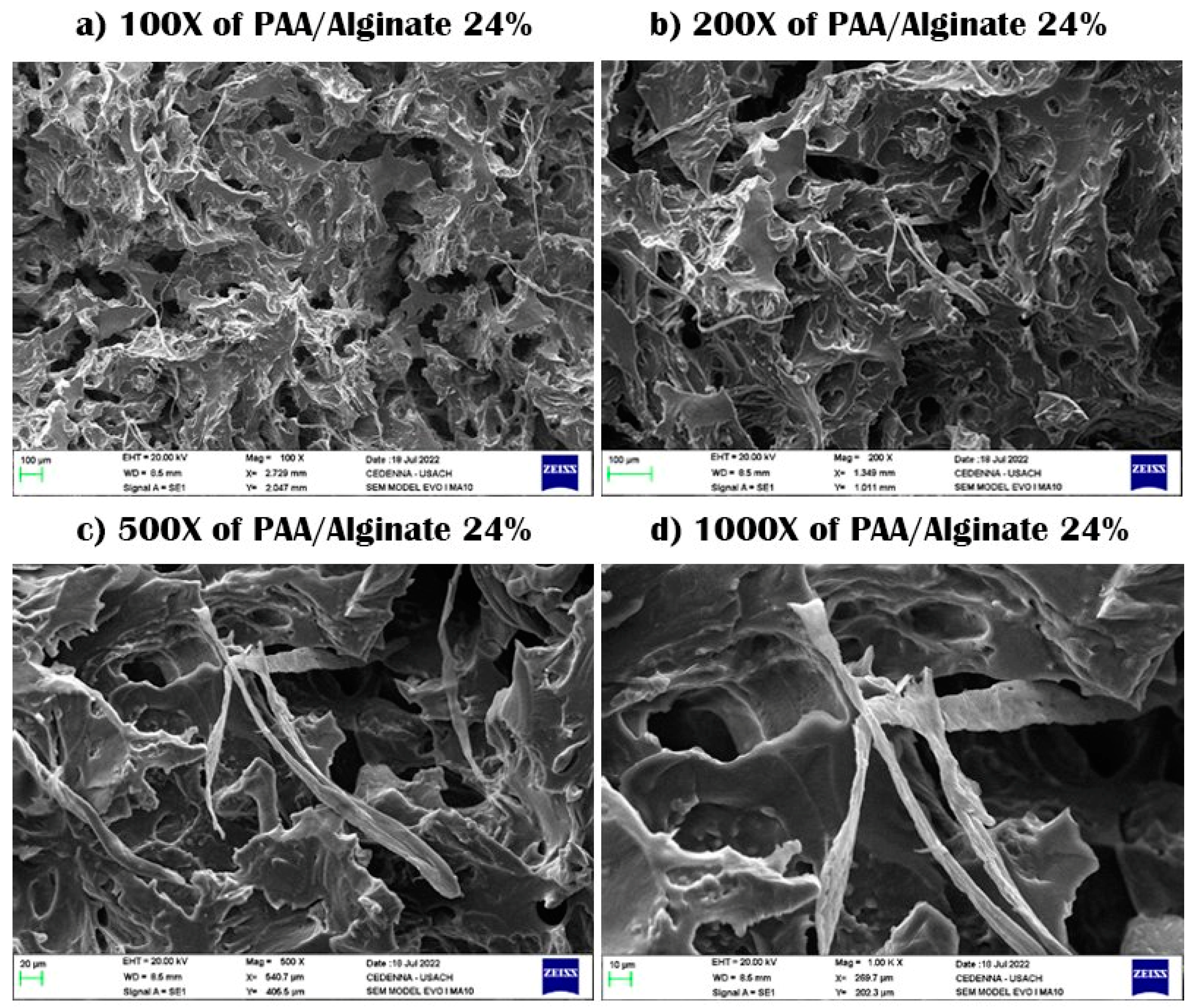


| Sample No. | Percentage (%) | PAA (mL) ± 0.005 mL | MBA (g) ± 0.005 g | PSA (g) ± 0.0005 g | Alginate (g) ± 0.005 g | Yields (%) |
| 1 | 0 | 1.37 (1.489 g) | 0.3114 | 0.0116 | 0 | 94.3 |
| 2 | 3.8 | 1.37 (1.489 g) | 0.3114 | 0.0116 | 0.07107 | 96.63 |
| 3 | 7.3 | 1.37 (1.489 g) | 0.3114 | 0.0116 | 0.14214 | 97.12 |
| 4 | 12.3 | 1.37 (1.489 g) | 0.3114 | 0.0116 | 0.28428 | 98.6 |
| 5 | 24 | 1.37 (1.489 g) | 0.3114 | 0.0116 | 0.56856 | 94.46 |
| 6 | 40 | 1.37 (1.489 g) | 0.3114 | 0.0116 | 1.208 | 91.33 |
| 7 | 55.66 | 1.37 (1.489 g) | 0.3114 | 0.0116 | 2.27424 | 95.46 |
| Sample No. | Percentage (%) | PAM (g) ± 0.005 g | MBA (g) ± 0.005 g | PSA (g) ± 0.0005 g | Chitosan (g) ± 0.005 g | Yields (%) |
| 8 | 0 | 1.4412 | 0.3114 | 0.0116 | 0 | 94.6 |
| 9 | 3.9 | 1.4412 | 0.3114 | 0.0116 | 0.0720 | 95.7 |
| 10 | 7.6 | 1.4412 | 0.3114 | 0.0116 | 0.14412 | 99.1 |
| 11 | 14 | 1.4412 | 0.3114 | 0.0116 | 0.28824 | 99.75 |
| 12 | 24 | 1.4412 | 0.3114 | 0.0116 | 0.57648 | 96.02 |
| 13 | 40 | 1.4412 | 0.3114 | 0.0116 | 1.1761 | 96.4 |
| 14 | 60 | 1.4412 | 0.3114 | 0.0116 | 2.6463 | 94.7 |
| Adsorbent | Adsorbate | Adsorption Capacity (mg/g) | pH | Concentration (mg/L) | R% | Ref. |
| 3D network nanostructured sodium alginate | Cu(II) | 13.38 | 7 | 1000 | 82.73 | [122] |
| Alginate/polyethyleneimine | Cu(II) | 322.6 | 5.5 | 200 | 45.7 | [125] |
| Chitosan/sodium alginate/calcium ion | Cu(II) | 70.83 | 3 | 20–100 | [126] | |
| Sodium alginate-grafted polyacrylamide/graphene oxide | Cu(II) | 68.76 | 5 | 25–250 | 80 | [127] |
| Alginate-modified graphitic carbon nitride | Cu(II) | 168.2 | 6 | 500 | 95 | [128] |
| Alginate/starch ether composite | Cu(II) | 247.16 | 4 | 100–1000 | 78.03 | [129] |
| Carbon nanotube/calcium alginate composites | Cu(II) | 25.81 | 5.5 | 25–250 | 92.21 | [130] |
| Calcium alginate-immobilized kaolin | Cu(II) | 47.46 | 5 | 15 | 79.43 | [131] |
| Mxene/alginate | Cu(II) | 87.6 | 5 | 10 | 63.5 | [132] |
| Sodium alginate supported on melamine sponge | Cu(II) | 83.0 | 50–250 | 66.6 | [133] | |
| Alginate-immobilized bentonite | Cu(II) | 114.70 | 4 | 400 | [134] | |
| Alginate-based porous nanocomposite hydrogels | Cu(II) | 87.2 | 3 | 20–500 | [135] | |
| Strength-toughness alginate composite fibers | Cu(II) | 102.4 | 10 | 78.6 | [136] | |
| alginate-polyethyleneimine hybrid aerogel | Cu(II) | 214.4 | 4 | -- | 95.1 | [137] |
| PAA/alginate 24% | Cu(II) | 63.59 | 4 | 1000 | This study | |
| Adsorbent | Adsorbate | Adsorption Capacity (mg/g) | pH | Concentration (mg/L) | R% | Ref. |
| Chitosan thiomer | As(V) | 17.66 | 6–8 | 0.050 | 87% | [138] |
| Chitosan and nanochitosan | As(V) | 39.68 | 4 | 0.250–11 | [139] | |
| Chitosan-related electrospun nanofiber | As(V) | 0.5 | 7.2 | 0.1–0.75 | 3% | [140] |
| Iron-functionalized chitosan-based electrospun nanofiber | As(V) | 11.2 | 7.2 | 0.1–0.75 | 98% | [140] |
| Chitosan-based MCS/ZnO@Alg gel microspheres | As(V) | 63.69 | 4 | 10 | 99% | [141] |
| Core–shell/bead-like ethylenediamine-functionalized Al-pillared montmorillonite/calcium alginate | As(V) | 61.94 | 4 | 50 | 94.85% | [142] |
| Magnetic nanoparticle-impregnated chitosan beads | As(V) | 35.7 | 6.8 | 2000 | 88.2% | [143] |
| PAAM/chitosan 24% | As(V) | 17.8 | 5 | 1000 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ALSamman, M.T.; Sánchez, J. Adsorption of Copper and Arsenic from Water Using a Semi-Interpenetrating Polymer Network Based on Alginate and Chitosan. Polymers 2023, 15, 2192. https://doi.org/10.3390/polym15092192
ALSamman MT, Sánchez J. Adsorption of Copper and Arsenic from Water Using a Semi-Interpenetrating Polymer Network Based on Alginate and Chitosan. Polymers. 2023; 15(9):2192. https://doi.org/10.3390/polym15092192
Chicago/Turabian StyleALSamman, Mohammad T., and Julio Sánchez. 2023. "Adsorption of Copper and Arsenic from Water Using a Semi-Interpenetrating Polymer Network Based on Alginate and Chitosan" Polymers 15, no. 9: 2192. https://doi.org/10.3390/polym15092192
APA StyleALSamman, M. T., & Sánchez, J. (2023). Adsorption of Copper and Arsenic from Water Using a Semi-Interpenetrating Polymer Network Based on Alginate and Chitosan. Polymers, 15(9), 2192. https://doi.org/10.3390/polym15092192







