Molecular Weight Segregation and Thermal Conductivity of Polydisperse Wax–Graphene Nanocomposites
Abstract
1. Introduction
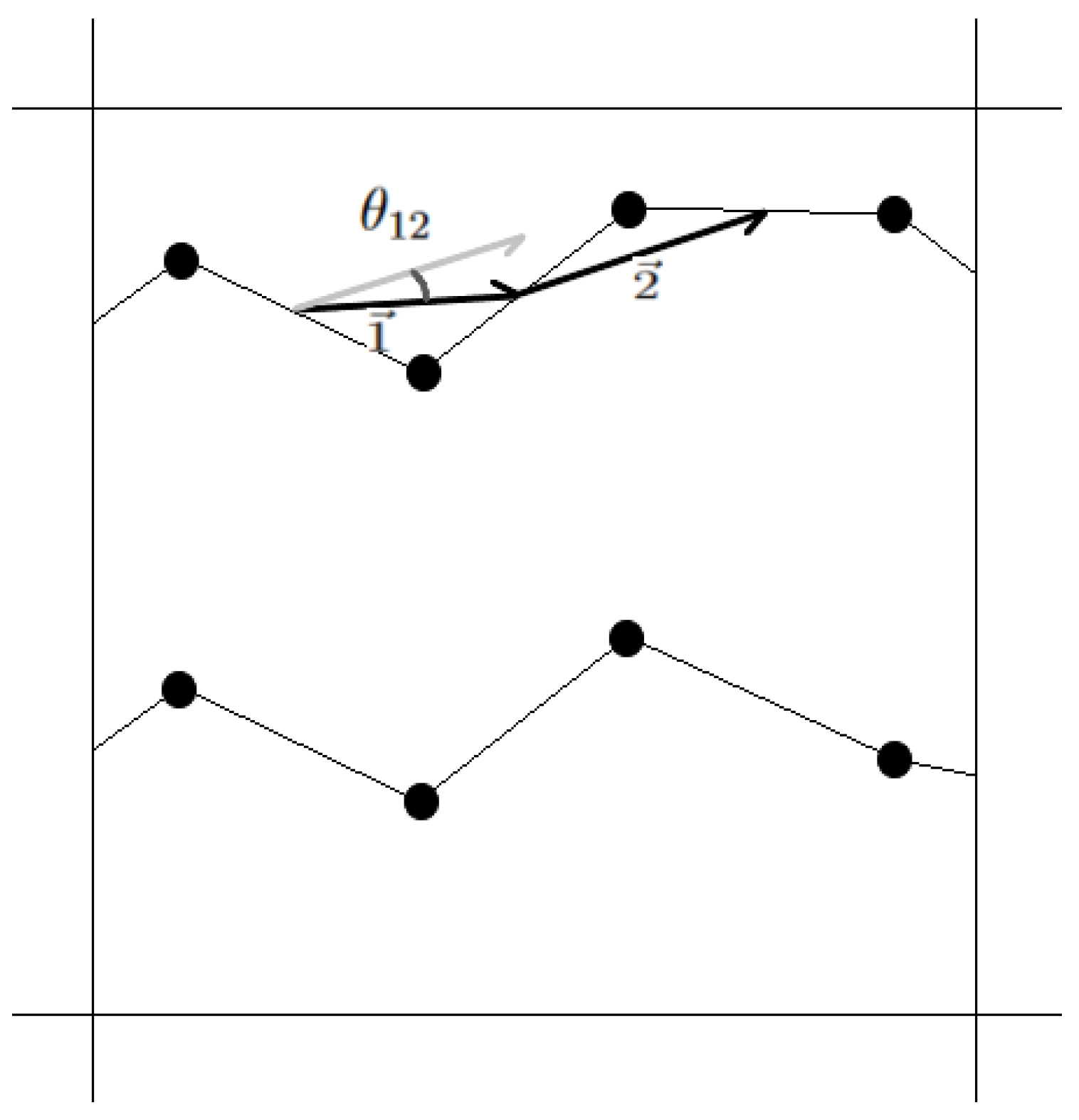
2. Models and Methods
3. Results and Discussion
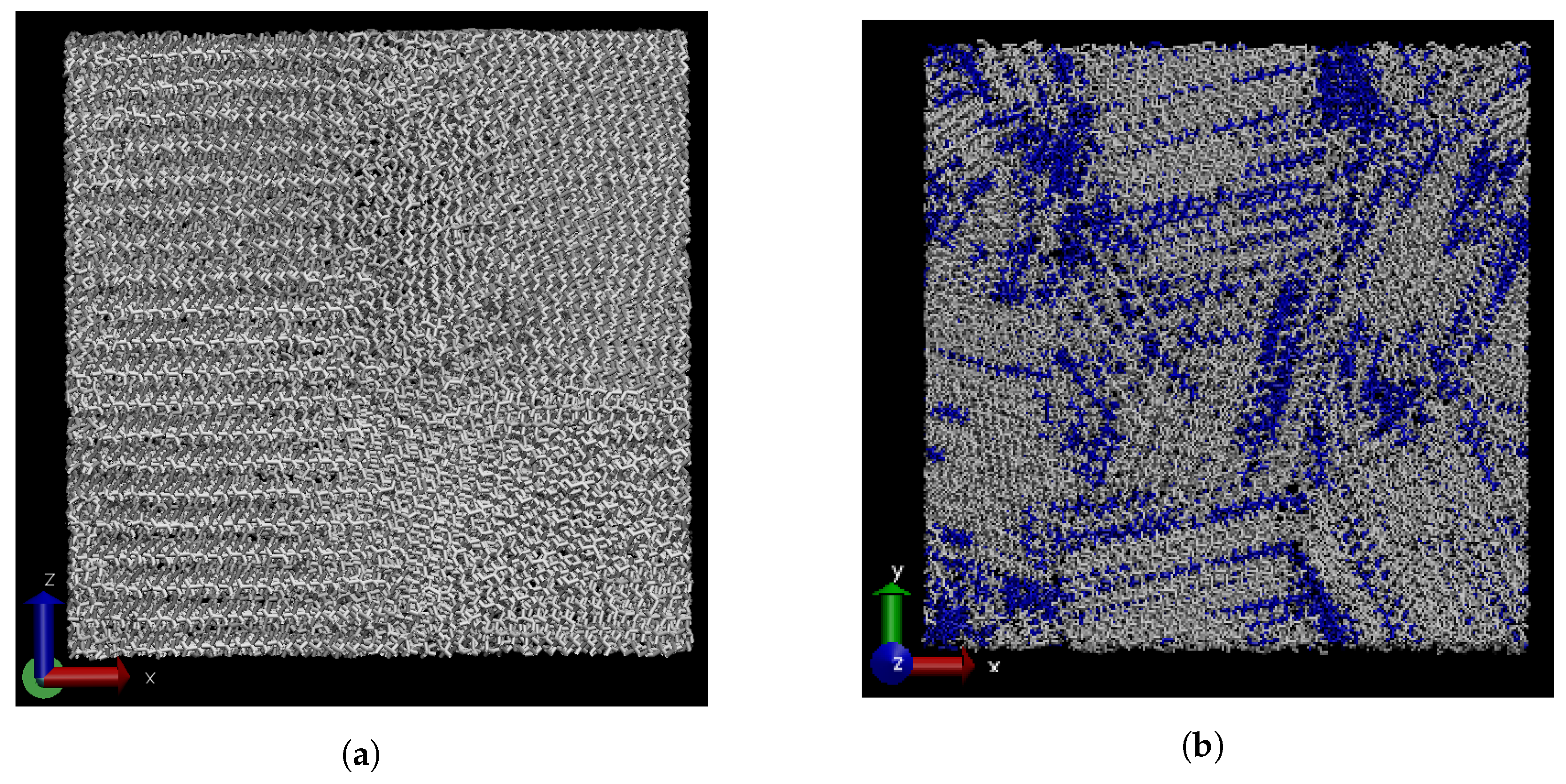
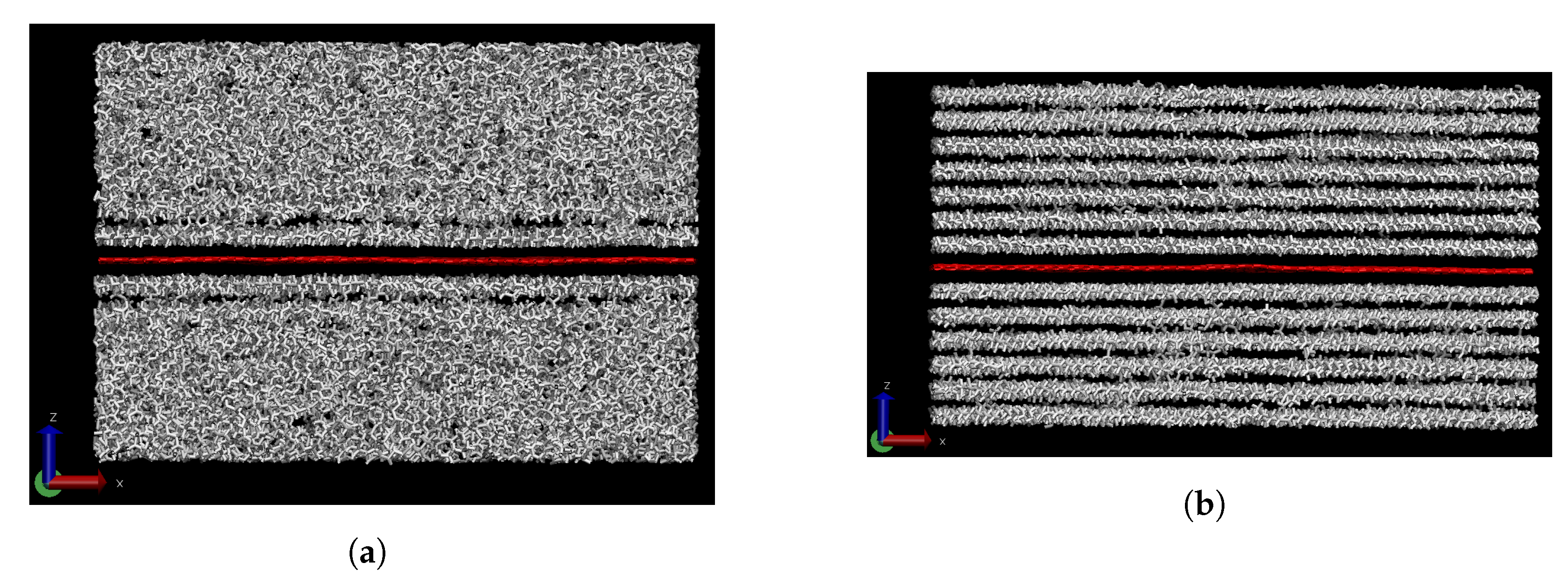
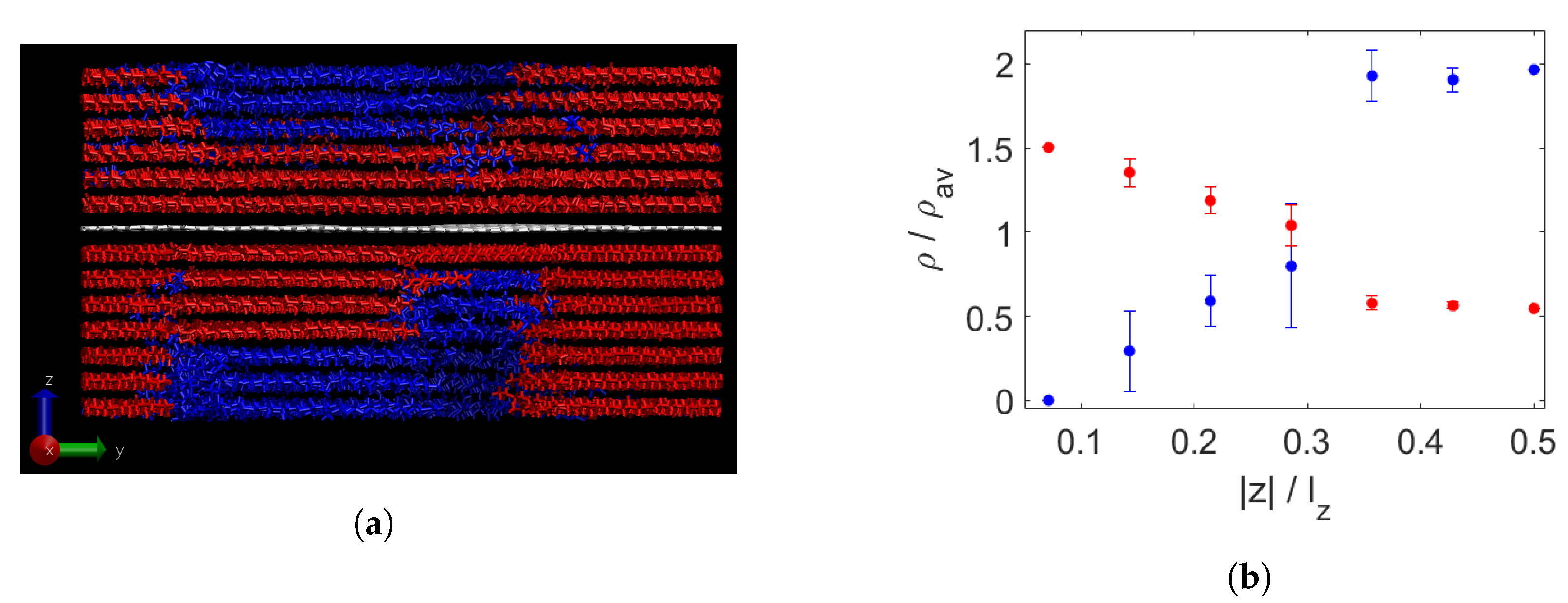
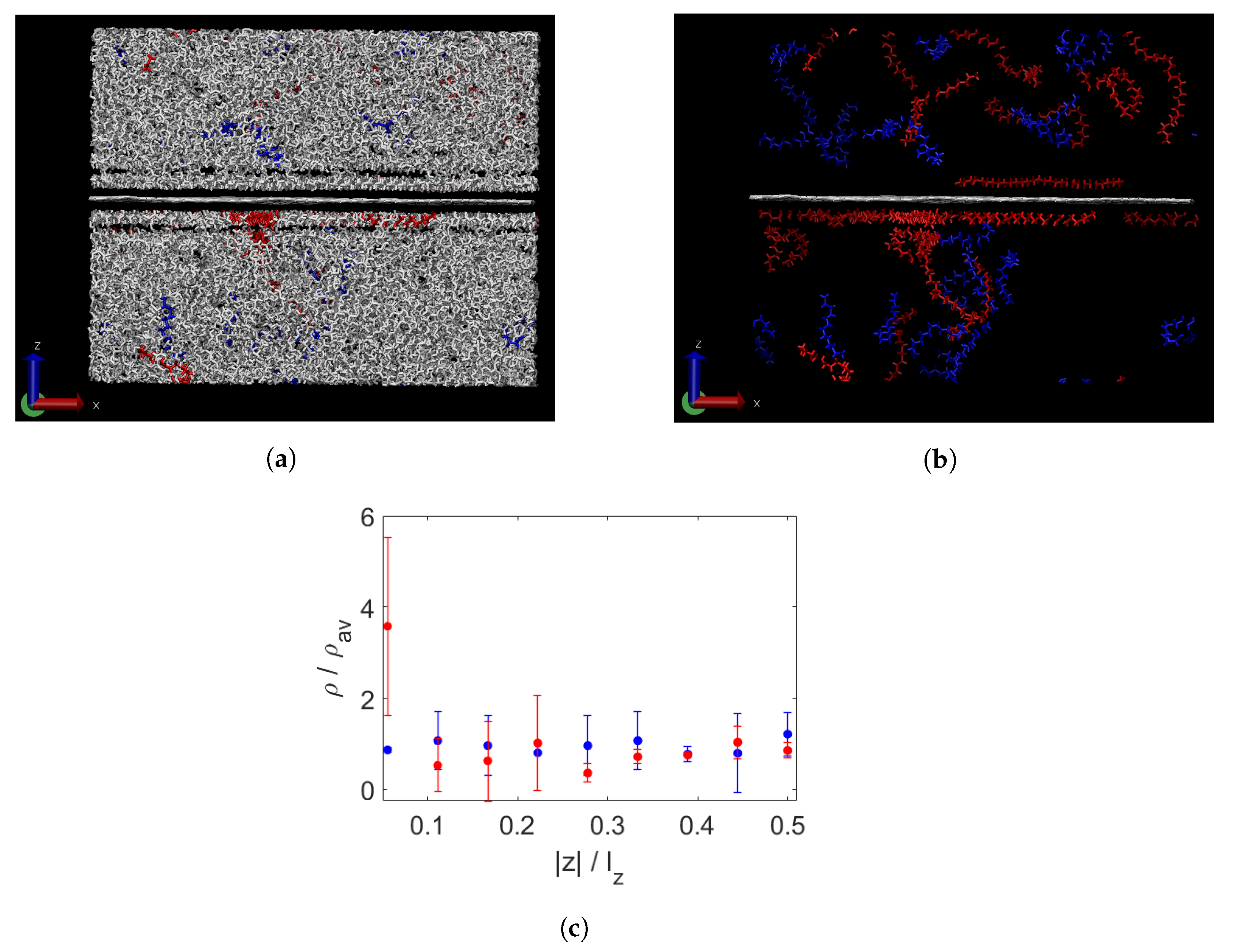
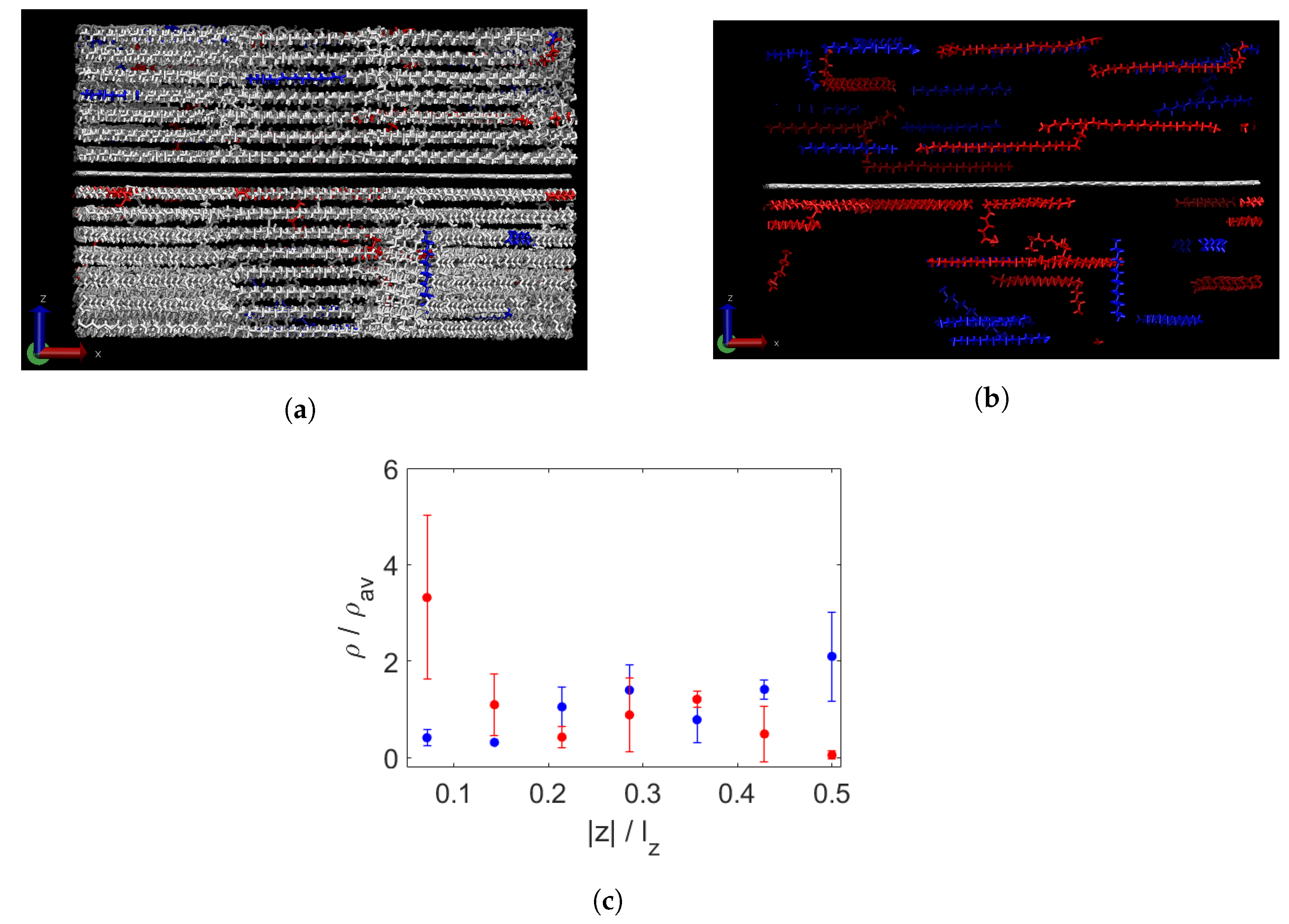
3.1. Effect of Graphene Sheets on Molecular Weight Segregation in Paraffin
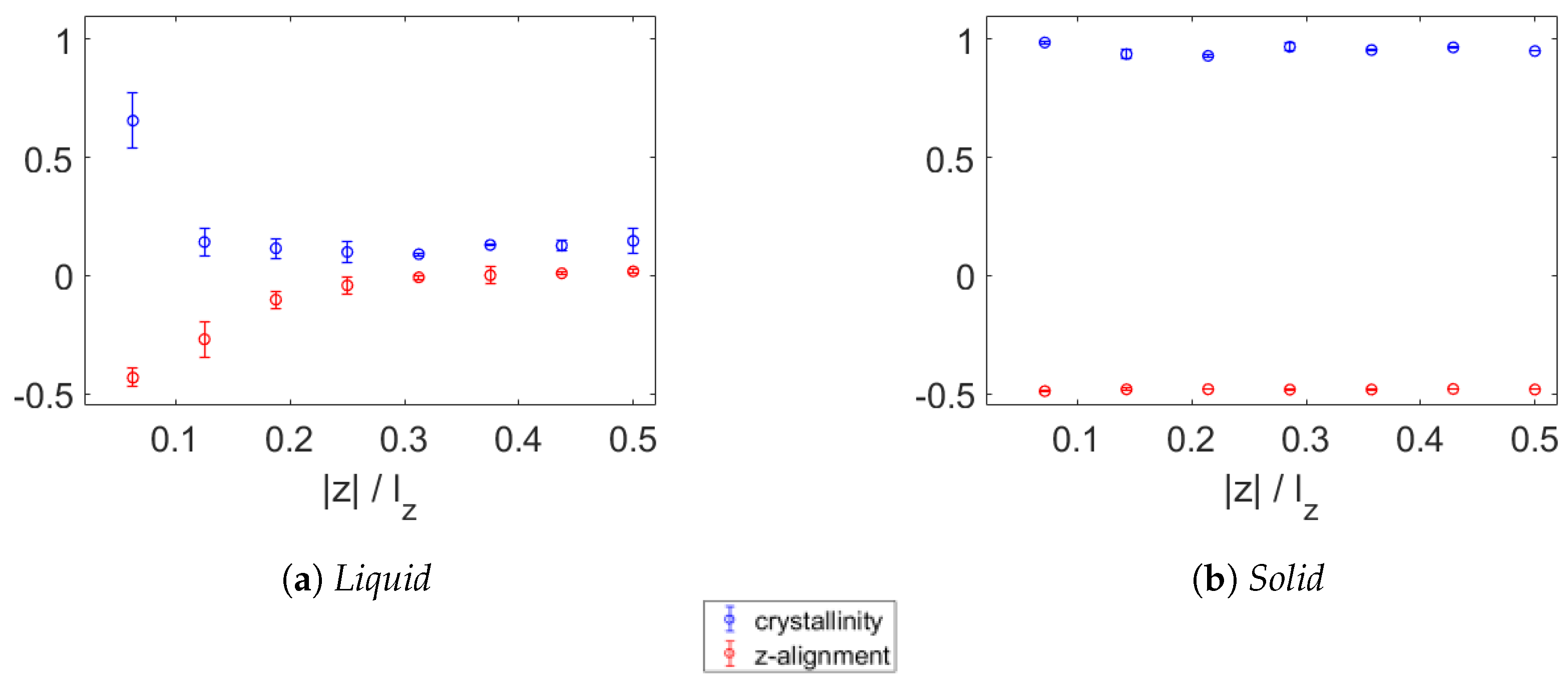
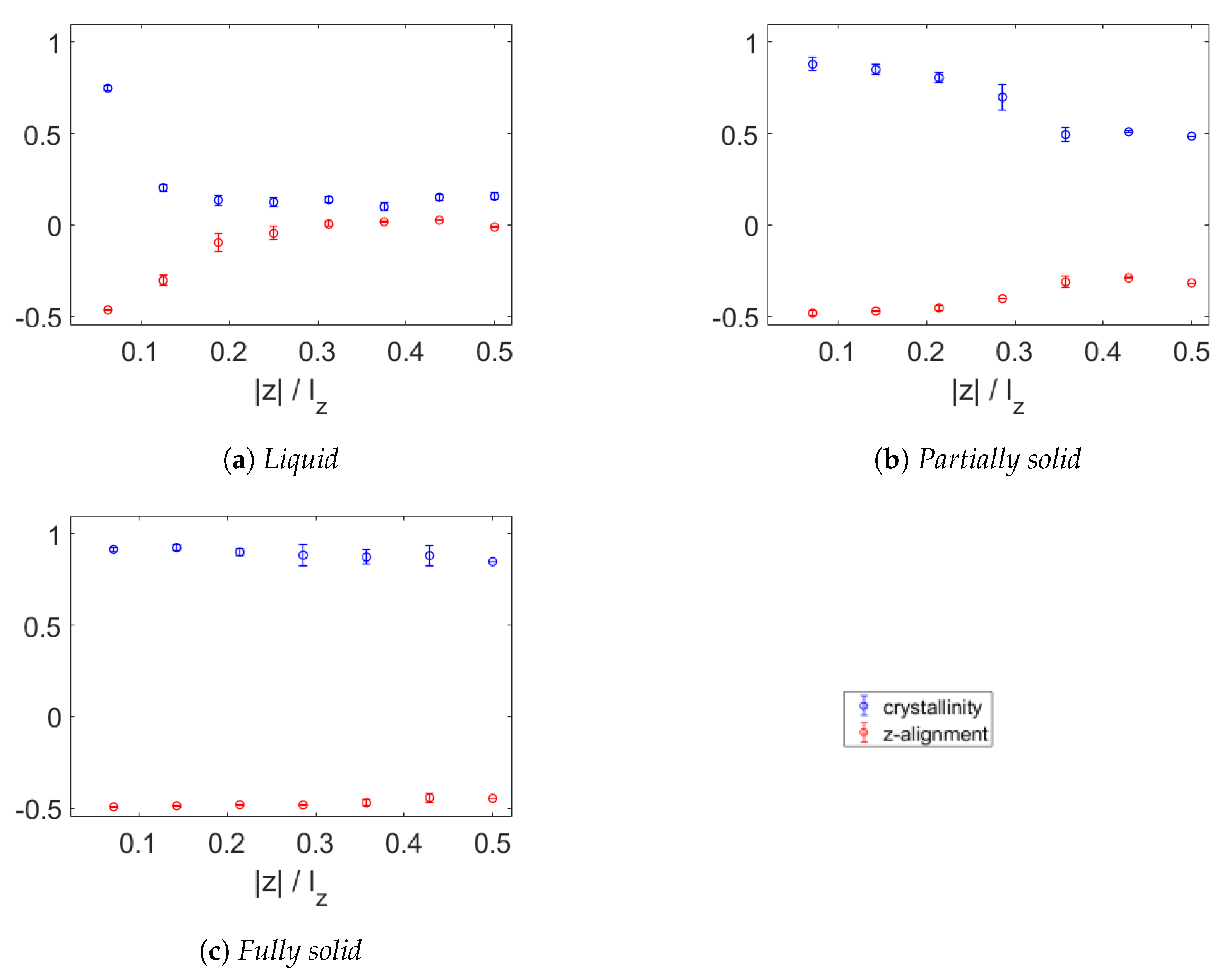
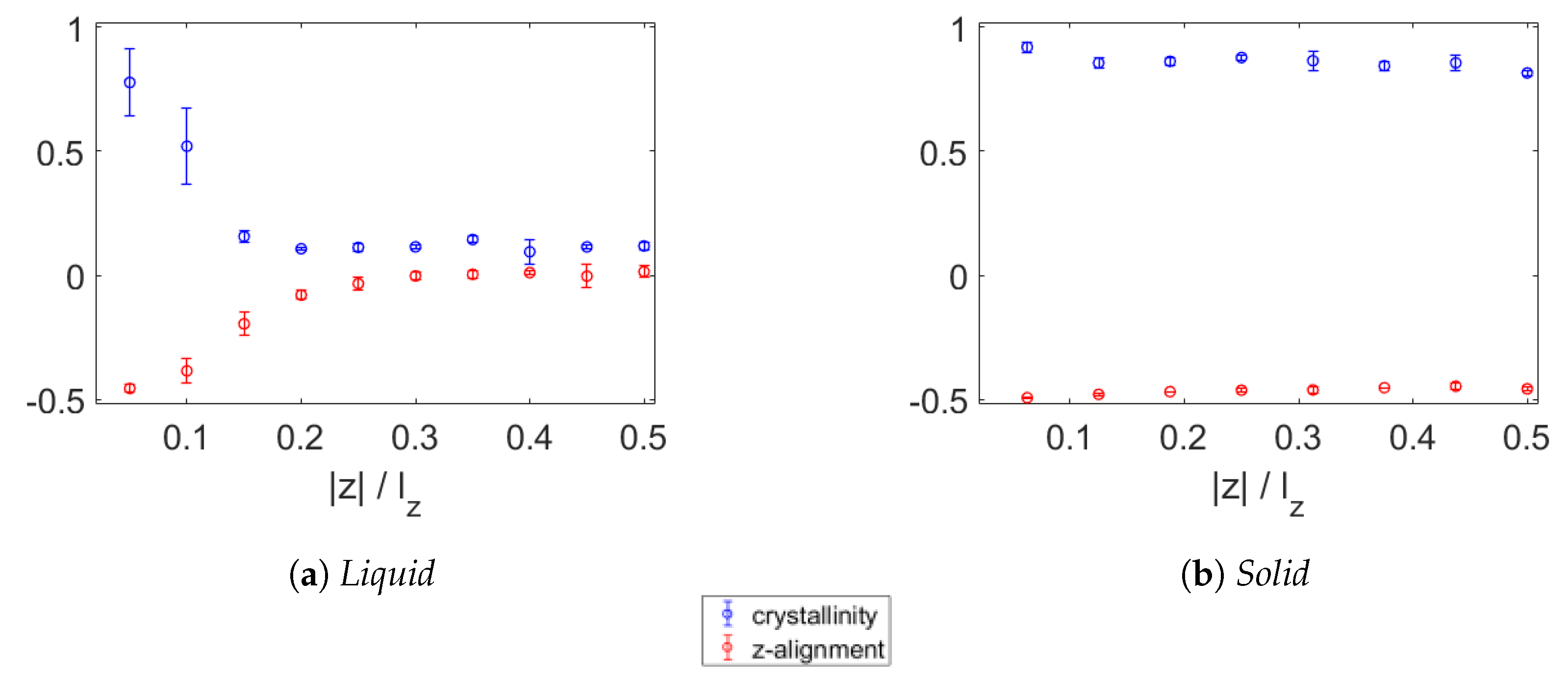
3.2. Crystallinity
3.3. Thermal Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bland, A.; Khzouz, M.; Statheros, T.; Gkanas, E.I. PCMs for residential building applications: A short review focused on disadvantages and proposals for future development. Buildings 2017, 7, 78. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Gasia, J.; Miró, L.; de Gracia, A.; Barreneche, C.; Cabeza, L.F. Experimental evaluation of a paraffin as phase change material for thermal energy storage in laboratory equipment and in a shell-and-tube heat exchanger. Appl. Sci. 2016, 6, 112. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Mhadhbi, M. Phase Change Materials and Their Applications; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Kim, S.; Drzal, L.T. High latent heat storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2009, 93, 136–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, X.; Muthu, J.; Mabrouki, T.; Fontaine, M.; Gong, Y.; Rabczuk, T. Load transfer of graphene/carbon nanotube/polyethylene hybrid nanocomposite by molecular dynamics simulation. Compos. Part B Eng. 2014, 63, 27–33. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Boomstra, M.W.; van Asseldonk, M.W.; Geurts, B.J.; Nazarychev, V.M.; Lyulin, A.V. Effects of branching and polydispersity on thermal conductivity of paraffin waxes. Int. J. Heat Mass Transf. 2022, 195, 123192. [Google Scholar] [CrossRef]
- Velez, C.; Khayet, M.; Ortiz de Zarate, J. Temperature-dependent thermal properties of solid/liquid phase change even-numbered n-alkanes: n-Hexadecane, n-octadecane and n-eicosane. Appl. Energy 2015, 143, 383–394. [Google Scholar]
- Fan, L.W.; Fang, X.; Wang, X.; Zeng, Y.; Xiao, Y.Q.; Yu, Z.T.; Xu, X.; Hu, Y.C.; Cen, K.F. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials. Appl. Energy 2013, 110, 163–172. [Google Scholar] [CrossRef]
- Huang, Y.R.; Chuang, P.H.; Chen, C.L. Molecular-dynamics calculation of the thermal conduction in phase change materials of graphene paraffin nanocomposites. Int. J. Heat Mass Transf. 2015, 91, 45–51. [Google Scholar] [CrossRef]
- Chen, G.; Su, Y.; Jiang, D.; Pan, L.; Li, S. An experimental and numerical investigation on a paraffin wax/graphene oxide/carbon nanotubes composite material for solar thermal storage applications. Appl. Energy 2020, 264, 114786. [Google Scholar] [CrossRef]
- Saito, K.; Nakamura, J.; Natori, A. Ballistic thermal conductance of a graphene sheet. Phys. Rev. B-Condens. Matter Mater. Phys. 2007, 76, 1–4. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef]
- Babaei, H.; Keblinski, P.; Khodadadi, J.M. Improvement in thermal conductivity of paraffin by adding high aspect-ratio carbon-based nano-fillers. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2013, 377, 1358–1361. [Google Scholar] [CrossRef]
- Borhani Zarandi, M.; Amrollahi Bioki, H.; Mirbagheri, Z.; Tabbakh, F.; Mirjalili, G. Effect of crystallinity and irradiation on thermal properties and specific heat capacity of LDPE & LDPE/EVA. Appl. Radiat. Isot. 2012, 70, 1–5. [Google Scholar] [CrossRef]
- Luo, C.; Sommer, J.U.; Schreiner, E.; Castro, I.G.; Tinsley, J.; Weiss, H. Length-dependent segregation in crystallization of n-alkanes: MD simulations. J. Non. Cryst. Solids 2015, 407, 206–212. [Google Scholar] [CrossRef]
- Zeng, Y.; Khodadadi, J.M. Molecular Dynamics Simulations of the Crystallization Process of n -Alkane Mixtures and the Resulting Thermal Conductivity. Energy Fuels 2018, 32, 11253–11260. [Google Scholar] [CrossRef]
- Wu, D.T.; Fredrickson, G.H.; Carton, J.; Ajdari, A.; Leibler, L. Distribution of chain ends at the surface of a polymer melt: Compensation effects and surface tension. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 2373–2389. [Google Scholar] [CrossRef]
- Hariharan, A.; Kumar, S.K.; Russel, T.P. A Lattice Model for the Surface Segregation of Polymer Chains Due to Molecular Weight Effects. Macromolecules 1990, 23, 3584–3592. [Google Scholar] [CrossRef]
- Chaudhry, A.U.; Mabrouk, A.; Abdala, A. Thermally enhanced pristine polyolefins: Fundamentals, progress and prospective. J. Mater. Res. Technol. 2020, 9, 10796–10806. [Google Scholar] [CrossRef]
- Ramos, J.; Vega, J.F.; Martínez-Salazar, J. Predicting experimental results for polyethylene by computer simulation. Eur. Polym. J. 2018, 99, 298–331. [Google Scholar] [CrossRef]
- Tong, X.; Yang, P.; Zeng, M.; Wang, Q. Confinement Effect of Graphene Interface on Phase Transition of n-Eicosane: Molecular Dynamics Simulations. Langmuir 2020, 36, 8422–8434. [Google Scholar] [CrossRef]
- Deng, S.; Wang, D.; Wang, X.; Wei, Y.; Waterhouse, G.I.; Lan, X.Z. Effect of nanopore confinement on the thermal and structural properties of heneicosan. Thermochim. Acta 2018, 664, 57–63. [Google Scholar] [CrossRef]
- Fu, D.; Liu, Y.; Liu, G.; Su, Y.; Wang, D. Confined crystallization of binary n-alkane mixtures: Stabilization of a new rotator phase by enhanced surface freezing and weakened intermolecular interactions. Phys. Chem. Chem. Phys. 2011, 13, 15031–15036. [Google Scholar] [CrossRef]
- Ocko, B.M.; Wu, X.Z.; Sirota, E.B.; Sinha, S.K.; Gang, O.; Deutsch, M. Surface freezing in chain molecules: Normal alkanes. Phys. Rev. E 1997, 55, 3164. [Google Scholar] [CrossRef]
- Green, M.S. Markoff random processes and the statistical mechanics of time-dependent phenomena. II. Irreversible processes in fluids. J. Chem. Phys. 1954, 22, 398–413. [Google Scholar] [CrossRef]
- Kubo, R. Statistical’Mechanical Theory of Irreversible Processes. I. General Theory and Simple Applications to Magnetic and Conduction Problems. J. Phys. Soc. Jpn. 1957, 12, 570–586. [Google Scholar] [CrossRef]
- Müller-Plathe, F. A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1997, 106, 6082–6085. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Nazarychev, V.M.; Glova, A.D.; Volgin, I.V.; Larin, S.V.; Lyulin, A.V.; Lyulin, S.V.; Gurtovenko, A.A. Evaluation of thermal conductivity of organic phase-change materials from equilibrium and non-equilibrium computer simulations: Paraffin as a test case. Int. J. Heat Mass Transf. 2021, 165, 120639. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giambasu, G.; Gilson, M.K.; et al. AmberTools20. 2020. Available online: https://ambermd.org/doc12/Amber20.pdf (accessed on 27 February 2023).
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Lindahl; Abraham; Hess; van der Spoel. GROMACS 2020.4 Source code. 2020. [Google Scholar] [CrossRef]
- Sousa Da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; C Berendsen, H.J.; E M Fraaije, J.G. LINCS: A linear constraint solver for molecular simulations. J. Comp. Chem. 1997, 18, 1463–1472. [Google Scholar]
- Glova, A.D.; Volgin, I.V.; Nazarychev, V.M.; Larin, S.V.; Lyulin Ab, S.V.; Gurtovenko, A.A.; Lyulin, S.V.; Gurtovenko, A.A. Toward realistic computer modeling of paraffin-based composite materials: Critical assessment of atomic-scale models of paraffins. RSC Adv. 2019, 9, 38834–38847. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Shirts, M.R.; Klein, C.; Swails, J.M.; Yin, J.; Gilson, M.K.; Mobley, D.L.; Case, D.A.; Zhong, E.D. Lessons learned from comparing molecular dynamics engines on the SAMPL5 dataset. J. Comput. Aided. Mol. Des. 2017, 31, 147–161. [Google Scholar] [CrossRef]
- Yamamoto, T. Molecular Dynamics Simulation of Stretch-Induced Crystallization in Polyethylene: Emergence of Fiber Structure and Molecular Network. Macromolecules 2019, 52, 1695–1706. [Google Scholar] [CrossRef]
- Boone, P.; Babaei, H.; Wilmer, C.E. Heat Flux for Many-Body Interactions: Corrections to LAMMPS. J. Chem. Theory Comput. 2019, 15, 5579–5587. [Google Scholar] [CrossRef]
- Babaei, H.; Keblinski, P.; Khodadadi, J.M. Thermal conductivity enhancement of paraffins by increasing the alignment of molecules through adding CNT/graphene. Int. J. Heat Mass Transf. 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Wang, X.; Kaviany, M.; Huang, B. Phonon coupling and transport in individual polyethylene chains: A comparison study with the bulk crystal. Nanoscale 2017, 9, 18022–18031. [Google Scholar] [CrossRef]
- Ramsey, J.C.; Fricke, A.L.; Caskey, J.A. Thermal conductivity of polymer melts. J. Appl. Polym. Sci. 1973, 17, 1597–1605. [Google Scholar] [CrossRef]
- Wang, Q.; Keffer, D.J.; Petrovan, S.; Thomas, J.B. Molecular dynamics simulation of poly(ethylene terephthalate) oligomers. J. Phys. Chem. B 2010, 114, 786–795. [Google Scholar] [CrossRef]


| Wax Type | Phase | Distance from Graphene | TC [W/(m K)] |
|---|---|---|---|
| eicosane | liquid | first two layers (2 above, 2 below) | 0.21 ± 0.06 |
| remaining paraffin | 0.16 ± 0.01 | ||
| solid | first two layers (2 above, 2 below) | 0.35 ± 0.07 | |
| remaining layers | 0.23 ± 0.04 | ||
| bidisperse | liquid | first two layers (2 above, 2 below) | 0.25 ± 0.06 |
| remaining paraffin | 0.18 ± 0.01 | ||
| semi-solid | first four layers (4 above, 4 below) | 0.30 ± 0.02 | |
| remaining paraffin | 0.17 ± 0.01 | ||
| solid | first four layers (4 above, 4 below) | 0.28 ± 0.02 | |
| remaining layers | 0.13 ± 0.02 | ||
| polydisperse | liquid | first two layers (2 above, 2 below) | 0.19 ± 0.03 |
| remaining paraffin | 0.17 ± 0.01 | ||
| solid | first two layers (2 above, 2 below) | 0.21 ±0.04 | |
| remaining layers | 0.18 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boomstra, M.; Geurts, B.; Lyulin, A. Molecular Weight Segregation and Thermal Conductivity of Polydisperse Wax–Graphene Nanocomposites. Polymers 2023, 15, 2175. https://doi.org/10.3390/polym15092175
Boomstra M, Geurts B, Lyulin A. Molecular Weight Segregation and Thermal Conductivity of Polydisperse Wax–Graphene Nanocomposites. Polymers. 2023; 15(9):2175. https://doi.org/10.3390/polym15092175
Chicago/Turabian StyleBoomstra, Maarten, Bernard Geurts, and Alexey Lyulin. 2023. "Molecular Weight Segregation and Thermal Conductivity of Polydisperse Wax–Graphene Nanocomposites" Polymers 15, no. 9: 2175. https://doi.org/10.3390/polym15092175
APA StyleBoomstra, M., Geurts, B., & Lyulin, A. (2023). Molecular Weight Segregation and Thermal Conductivity of Polydisperse Wax–Graphene Nanocomposites. Polymers, 15(9), 2175. https://doi.org/10.3390/polym15092175








