UV-Accelerated Synthesis of Gold Nanoparticle–Pluronic Nanocomposites for X-ray Computed Tomography Contrast Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV-Assisted Synthesis of AuNP-PLU Colloidal Nanocomposites
2.3. Structural and Morphological Characterizations
2.4. Cell Viability Test
2.4.1. Cell Culture
2.4.2. In Vitro Cytotoxicity Assay
2.5. In Vivo Maternal and Fetal Toxicity of AuNP-PLU:2.0UV in Rats
2.6. X-ray Computed Tomography Scan
3. Results and Discussion
3.1. Effect of the UV Light on the Features of Synthesized AuNP-PLU
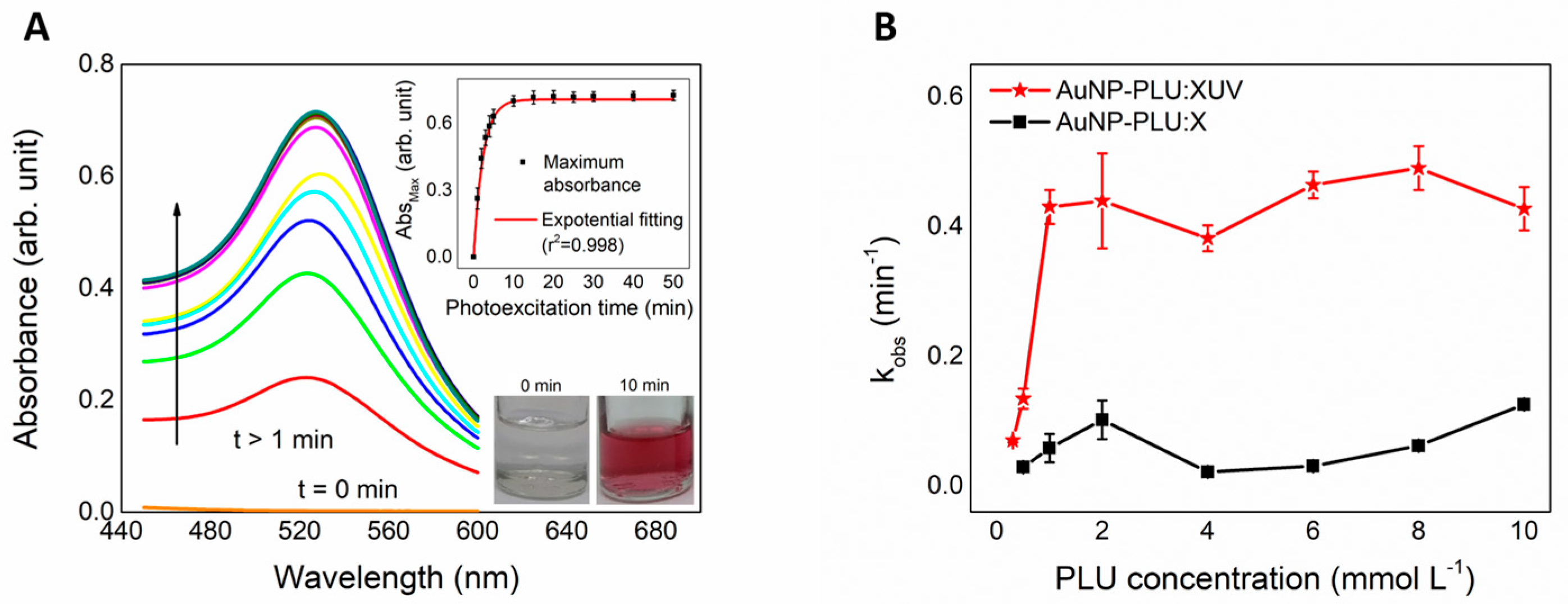
3.1.1. Kinetics of UV-Assisted Synthesis of AuNP-PLU
3.1.2. Evaluation of the Yield of AuNP-PLU-UV Production
3.1.3. Morphology of AuNP-PLU
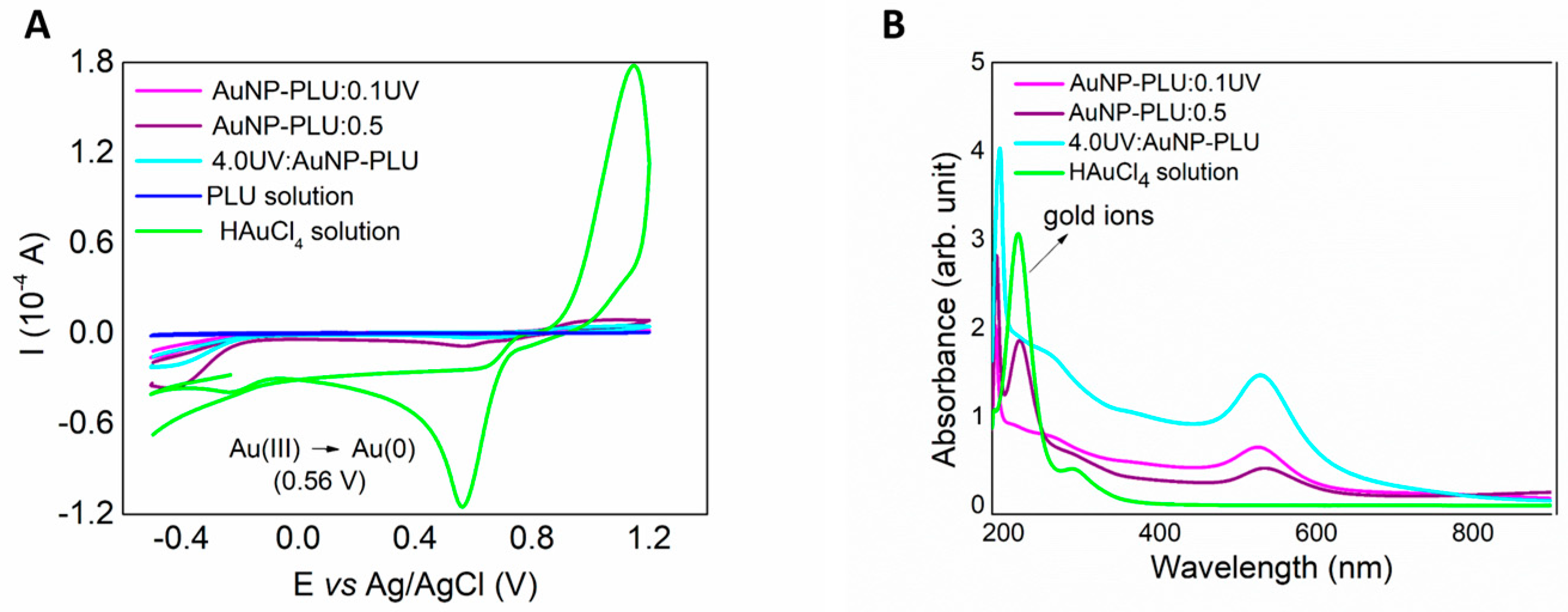
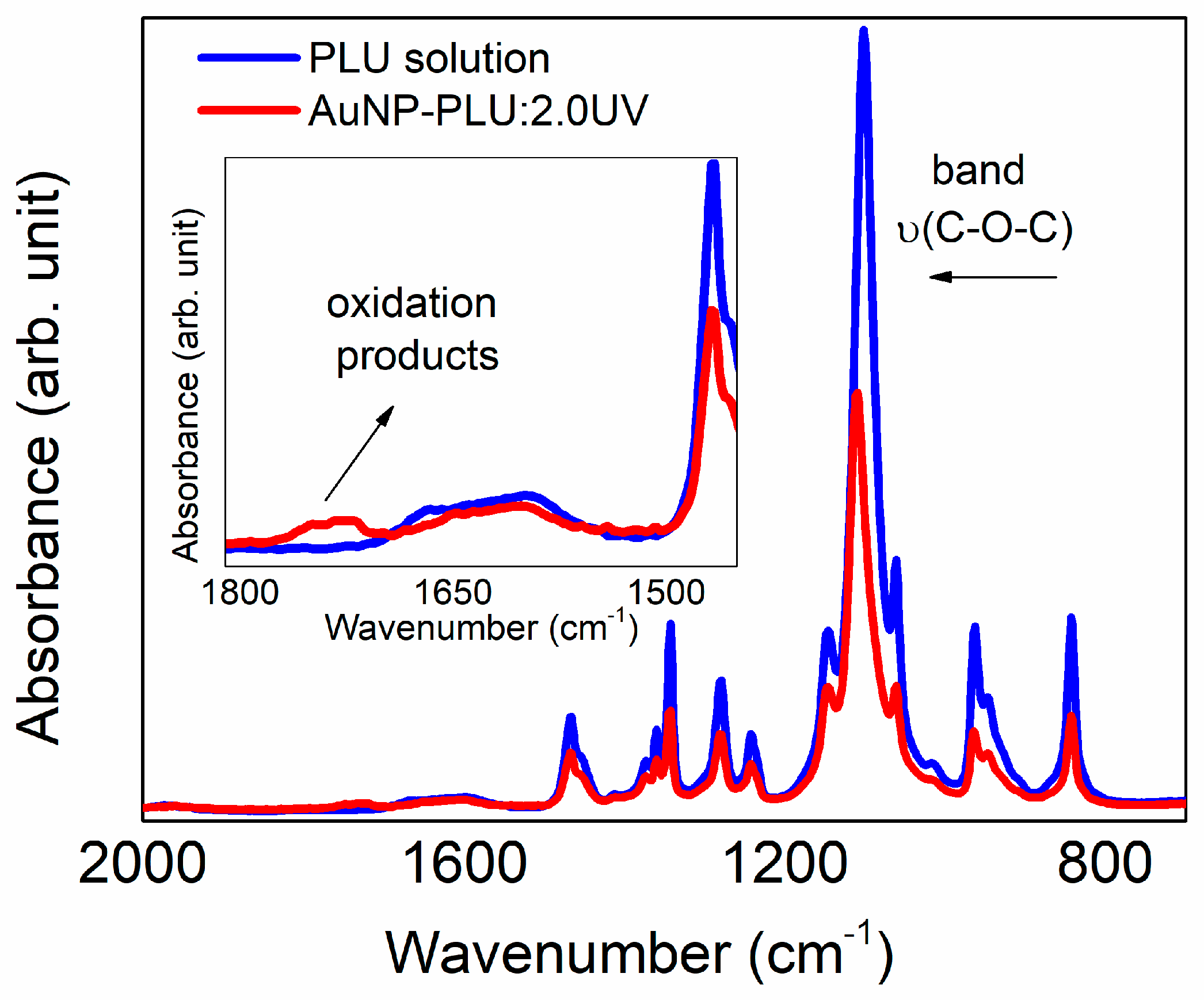
3.1.4. The Role of UV Light and PLU on the Formation of AuNP-PLU
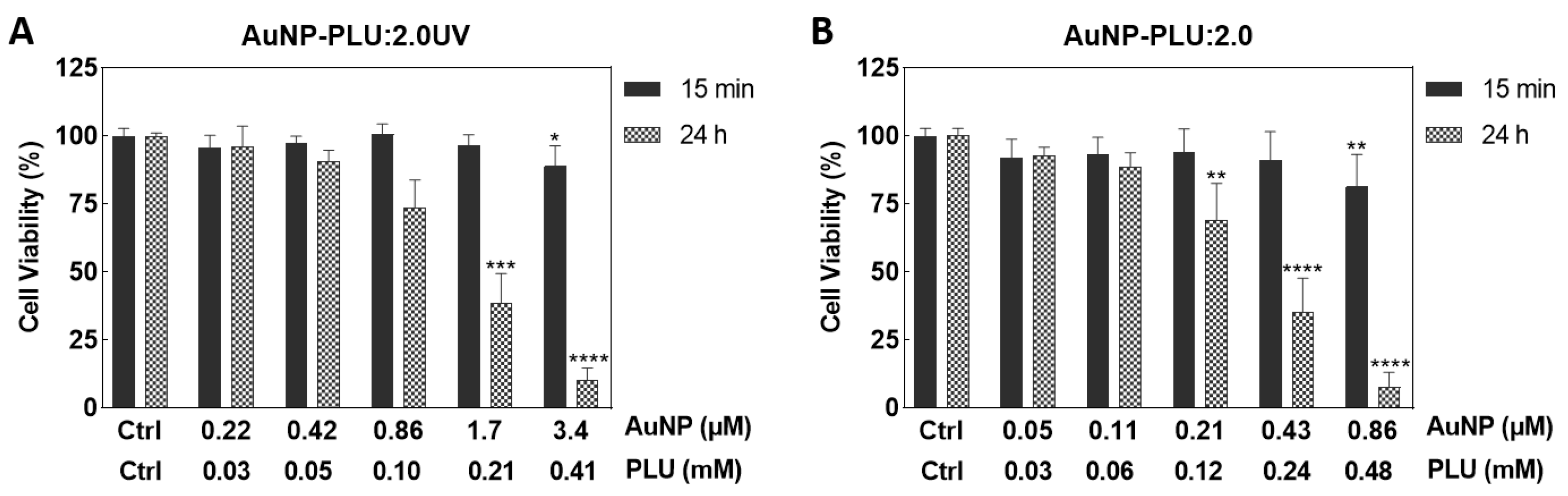
3.2. In Vitro Cytotoxicity Assay
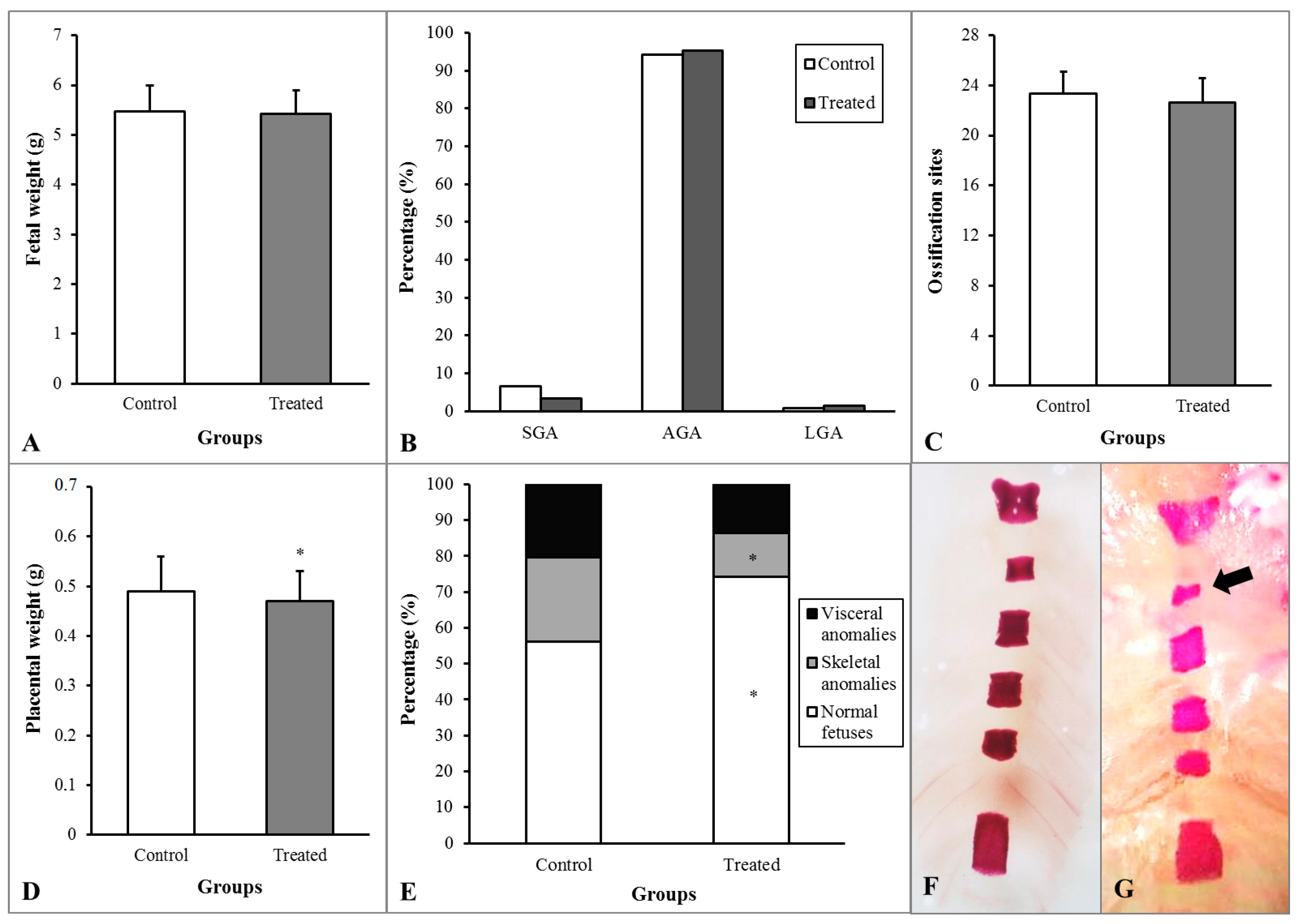
3.3. In Vivo Maternal and Fetal Toxicity in Rats
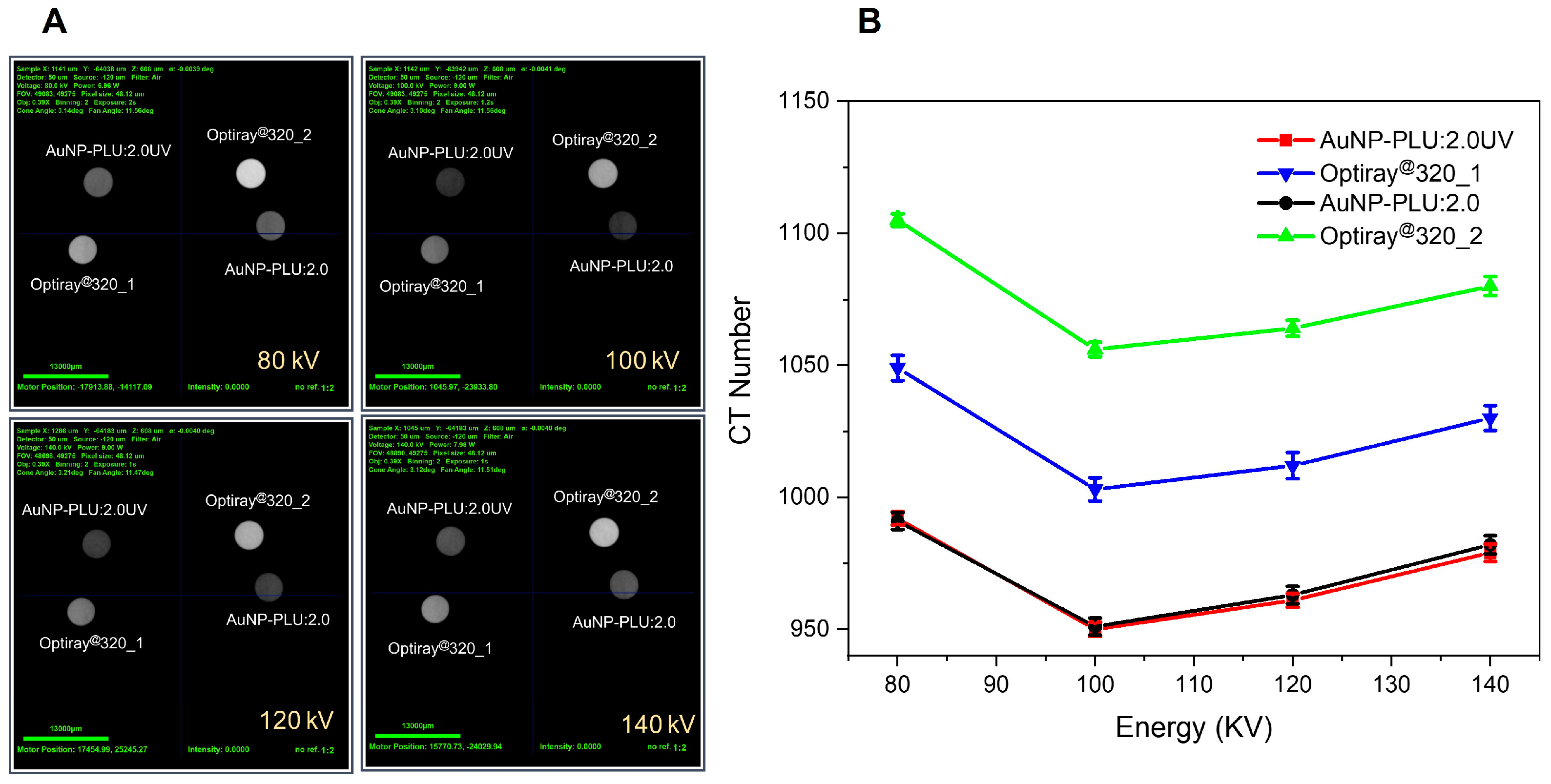
3.4. X-ray CT Scanner
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, X.Z.; Mulvaney, P. Chapter 3—Optical Properties of Strongly Coupled Plasmonic Nanoparticle Clusters. In Modern Plasmonics; Maradudin, A., Sambles, J.R., William, L.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 75–108. [Google Scholar]
- Csaki, A.; Thiele, M.; Jacqueline Jatschka, J.; Dathe, A.; Zopf, D.; Stranik, O.; Fritzsche, W. Plasmonic nanoparticle synthesis and bioconjugation for bioanalytical sensing. Eng. Life Sci. 2015, 15, 266–275. [Google Scholar] [CrossRef]
- Pallotta, A.; Boudier, A.; Creusot, B.; Brun, E.; Sicard-Roselli, C.; Bazzi, R.; Roux, S.; Clarot, I. Quality control of gold nanoparticles as pharmaceutical ingredients. Int. J. Pharm. 2019, 569, 118583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Wu, H.-Y.; Wu, D.; Wang, Y.-W.; Chang, J.H.; Zhai, Z.-B.; Meng, A.-M.; Liu, P.-X.; Zhang, L.-A.; Fan, F.-Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Shimoni, O.; Wallach, M. Novel screening test for celiac disease using peptide functionalised gold nanoparticles. World J. Gastroenterol. 2018, 24, 5379–5390. [Google Scholar] [CrossRef]
- Martínez Porcel, J.E.; Churio, M.S.; Moya, S.E.; Arce, V.B.; Martire, D.O. Plasmonic silica-gold core-shell nanoparticles: Interaction with organic dyes for light-induced applications. J. Photochem. Photobiol. A Chem. 2022, 431, 114016. [Google Scholar] [CrossRef]
- Liu, J.; Shen, Y.; Guangxian, W.; Xiang, Y.; Guo, Y.; Sun, X.; Liu, Y. Highly-sensitive electrochemiluminescence biosensor for detection of inosine monophosphate in meat based on graphdiyne/AuNPs/luminol nanocomposites. Food Sci. Hum. Wellness 2023, 12, 1149–1156. [Google Scholar] [CrossRef]
- Vieira, F.M.; Gabriela Calisto, C.M.; Izumi, C. Construction of SERS substrates by gold nanoparticles assembly on polymeric matrices. Appl. Surf. Sci. 2023, 612, 155818. [Google Scholar] [CrossRef]
- Tao, Z.; Feng, J.; Yang, F.; Zhang, L.; Shen, H.; Cheng, Q.; Liu, L. Plasmon-enhanced photocatalysis using gold nanoparticles encapsulated in nanoscale molybdenum oxide shell. Nanotechnology 2023, 34, 155604. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Silvestri, A.; Zambelli, V.; Ferretti, A.M.; Salerno, D.; Bellani, G.; Polito, L. Design of functionalized gold nanoparticle probes for computed tomography imaging. Contrast Media Mol. Imaging 2016, 11, 405–414. [Google Scholar] [CrossRef]
- Tu, N.V.; Anh, N.N.; Hau, T.V.; Hao, N.V.; Huyen, N.T.; Thang, B.H.; Minh, P.N.; Chuc, N.V.; Fukata, N.; Trinh, P.V. Improving the efficiency of n-Si/PEDOT:PSS hybrid solar cells by incorporating AuNP-decorated graphene oxide as a nanoadditive for conductive polymers. RSC Adv. 2022, 12, 27625–27632. [Google Scholar] [PubMed]
- Rizalputri, L.N.; Anshori, I.; Handayani, M.; Gumilar, G.; Septiani, N.L.W.; Hartati, Y.W.; Annas, M.S.; Purwidyantri, A.; Prabowo, B.A.; Brian Yuliarto, B. Facile and controllable synthesis of monodisperse gold nanoparticle bipyramid for electrochemical dopamine sensor. Nanotechnology 2023, 35, 55502. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Xia, N.; Zhao, L.; Liu, K.; Hou, W.; Liu, L. Aptasensors for the selective detection of alpha-synuclein oligomer by colorimetry, surface plasmon resonance and electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2017, 245, 87–94. [Google Scholar] [CrossRef]
- Doherty, B.; Csáki, A.; Thiele, M.; Zeisberger, M.; Schwuchow, A.; Kobelke, J.; Fritzsche, W.; Schmidt, M.A. Nanoparticle functionalised smallcore suspended-core fibre—A novel platform for efficient sensing. Biomed. Opt. Express 2017, 8, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Jing, C.; Long, Y.-T. Single plasmonic nanoparticles as ultrasensitive sensors. Analyst 2017, 142, 409–420. [Google Scholar] [CrossRef]
- Zhenga, K.; Zhoua, D.; Wud, L.; Lia, J.; Zhaoa, B.; Zhange, S.; Heb, R.; Xiaof, L.; Zoyae, I.; Yub, L.; et al. Gold-nanoparticle-based multistage drug delivery system for antitumor therapy. Drug Deliv. 2022, 29, 3186–3196. [Google Scholar] [CrossRef]
- Irvin-Choy, N.S.; Nelson, K.M.; Dang, M.N.; Gleghorn, J.P.; Day, E.S. Gold nanoparticle biodistribution in pregnant mice following intravenous administration varies with gestational age. Nanomedicine 2021, 36, 102412. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Du, L.; Tian, X.; Fan, Z.; Sun, C.; Liu, Y.; Keelan, J.A.; Nie, G. Effects of nanoparticle size and gestational age on maternal biodistribution and toxicity of gold nanoparticles in pregnant mice. Toxicol Lett. 2014, 230, 10–18. [Google Scholar] [CrossRef]
- Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schaffler, M.; Tian, F.; Schmid, G.; Oberdörster, G.; Kreyling, W.G. Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat. Part Fibre Toxicol. 2014, 11, 33. [Google Scholar] [CrossRef]
- Alexandridis, P. Gold Nanoparticle Synthesis, Morphology Control, and Stabilization Facilitated by Functional Polymers. Chem. Eng. Technol. 2011, 34, 15–28. [Google Scholar] [CrossRef]
- Alexandridis, P.; Tsianou, M. Block copolymer-directed metal nanoparticle morphogenesis and organization. Eur. Polym. J. 2011, 47, 569–583. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Nguyen, H.V.; Phan, V.M.; Park, B.J.; Seo, T.S. Serially diluting centrifugal microfluidics for high-throughput gold nanoparticle synthesis using an automated and portable workstation. Chem. Eng. J. 2023, 452, 139044. [Google Scholar] [CrossRef]
- Cabrera, G.F.S.; Balbin, M.M.; Eugenio, P.J.G.; Zapanta, C.S.; Monserate, J.J.; Salazar, J.R.; Mingala, C.N. Green synthesis of gold nanoparticles reduced and stabilized by sodium glutamate and sodium dodecyl sulfate. Biochem. Biophys. Res. Commun. 2017, 484, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Shajkumar, A.; Nandan, B.; Sanwaria, S.; Albrecht, V.; Libera, M.; Lee, M.-H.; Auffermann, G.; Stamm, M.; Horechyy, A. Silica-supported Au@hollow-SiO2 particles with outstanding catalytic activity prepared via block copolymer template approach. J. Colloid Interface Sci. 2017, 491, 246–254. [Google Scholar] [CrossRef]
- Maguire, P.; Rutherford, D.; Macias-Montero, M.; Mahony, C.; Kelsey, C.; Tweedie, M.; Pérez-Martin, F.; McQuaid, H.; Diver, D.; Mariotti, D. Continuous In-Flight Synthesis for On-Demand Delivery of Ligand-Free Colloidal Gold Nanoparticles. Nano Lett. 2017, 17, 1336–1343. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef]
- Sakai, T.; Alexandridis, P. Single-Step Synthesis and Stabilization of Metal Nanoparticles in Aqueous Pluronic Block Copolymer Solutions at Ambient Temperature. Langmuir 2004, 20, 8426–8430. [Google Scholar] [CrossRef]
- Grzelczak, M.; Liz-Marzan, L.M. The relevance of light in the formation of colloidal metal nanoparticles. Chem. Soc. Rev. 2014, 43, 2089–2097. [Google Scholar] [CrossRef]
- Gomes, D.S.; Paterno, L.G.; Santos, A.B.; Garay, A.V.; Mertz, D.; Freitas, S.M.; Soler, M.A. New insights on the formation of gold nanoparticles and Pluronic nanocomposites: Kinetics and thermodynamics parameters. J. Mol. Liq. 2018, 268, 181–189. [Google Scholar] [CrossRef]
- Santos, D.C.; de Souza, V.C.; Vasconcelos, D.A.; Andrade, G.R.S.; Gimenez, I.F.; Teixeira, Z. Triblock copolymer-mediated synthesis of catalytically active gold nanostructures. J. Nanopart. Res. 2018, 20, 105. [Google Scholar] [CrossRef]
- Sokolsky-Papkov, M.; Kabanov, A. Synthesis of Well-Defined Gold Nanoparticles Using Pluronic: The Role of Radicals and Surfactants in Nanoparticles Formation. Polymers 2019, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Pal, A. Photoinitiated gold sol generation in aqueous Triton X-100 and its analytical application for spectrophotometric determination of gold. Talanta 1998, 46, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Ghosh, S.K.; Kundu, S.; Esumi, K.; Pal, T. UV Photoactivation for Size and Shape Controlled Synthesis and Coalescence of Gold Nanoparticles in Micelles. Langmuir 2002, 18, 7792–7797. [Google Scholar] [CrossRef]
- Shang, Y.; Min, C.; Hu, J.; Wang, T.; Liu, H.; Hu, Y. Synthesis of gold nanoparticles by reduction of HAuCl4 under UV irradiation. Solid State Sci. 2013, 15, 17–23. [Google Scholar] [CrossRef]
- Teixeira, P.R.; Santos, M.S.; Silva, A.L.G.; Báo, S.N.; Azevedo, R.B.; Sales, M.J.A.; Paterno, L.G. Photochemically-assisted synthesis of non-toxic and biocompatible gold nanoparticles. Colloid Surf. B-Biointerfaces 2016, 148, 317–323. [Google Scholar] [CrossRef]
- Mallick, K.; Wang, Z.; Pal, T. Seed-mediated successive growth of gold particles accomplished by UV irradiation: A photochemical approach for size-controlled synthesis. J. Photochem. Photobiol. A-Chem. 2001, 140, 75–80. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Soares, T.S.; Moraes-Souza, R.Q.; Carneiro, T.B.; Araujo-Silva, V.C.; Schavinski, A.Z.; Gratão, T.B.; Damasceno, D.C.; Volpato, G.T. Maternal-fetal outcomes of exercise applied in rats with mild hyperglycemia after embryonic implantation. Birth Defects Res. 2021, 113, 287–298. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Gallego, F.Q.; Sinzato, Y.K.; Miranda, C.A.; Iessi, I.L.; Dallaqua, B.; Volpato, G.T.; Scarano, W.R.; SanMartín, S.; Damasceno, D.C. Pancreatic islet response to diabetes during pregnancy in rats. Life Sci. 2018, 214, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paula, V.G.; Cruz, L.L.; Sene, L.B.; Gratão, T.B.; Soares, T.S.; Moraes-Souza, R.Q.; Damasceno, D.C.; Volpato, G.T. Maternal-fetal repercussions of Phyllanthus niruri L. treatment during rat pregnancy. J. Ethnopharmacol. 2020, 254, 112728. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G. Methods for administering agents and detecting malformations in experimental animal. In Teratology: Principles and Techniques; Wilson, J.C., Warkany, J., Eds.; University of Chicago Press: Chicago, IL, USA, 1965; pp. 47–74. [Google Scholar]
- Staples, R.E.; Schnell, V.L. Refinements in rapid clearing technique in the KOH-alizarin red S method for fetal bone. Stain Technol. 1964, 39, 61–63. [Google Scholar]
- Aliverti, V.; Bonanomi, L.; Giavini, E.; Leone, V.G.; Mariani, L. The extent of fetal ossification as an index of delayed development in teratogenic studies on the rat. Teratology 1979, 20, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Souza, R.Q.; Reinaque, A.P.; Soares, T.S.; Silva, A.L.T.; Giunchetti, R.C.; Takano, M.A.S.; Akamatsu, M.A.; Kubrusly, F.S.; Lúcio-Macarini, F.; Raw, I.; et al. Safety evaluation of a vaccine: Effect in maternal reproductive outcome and fetal anomaly frequency in rats using a leishmanial vaccine as a model. PLoS ONE 2017, 12, e0172525. [Google Scholar] [CrossRef] [PubMed]
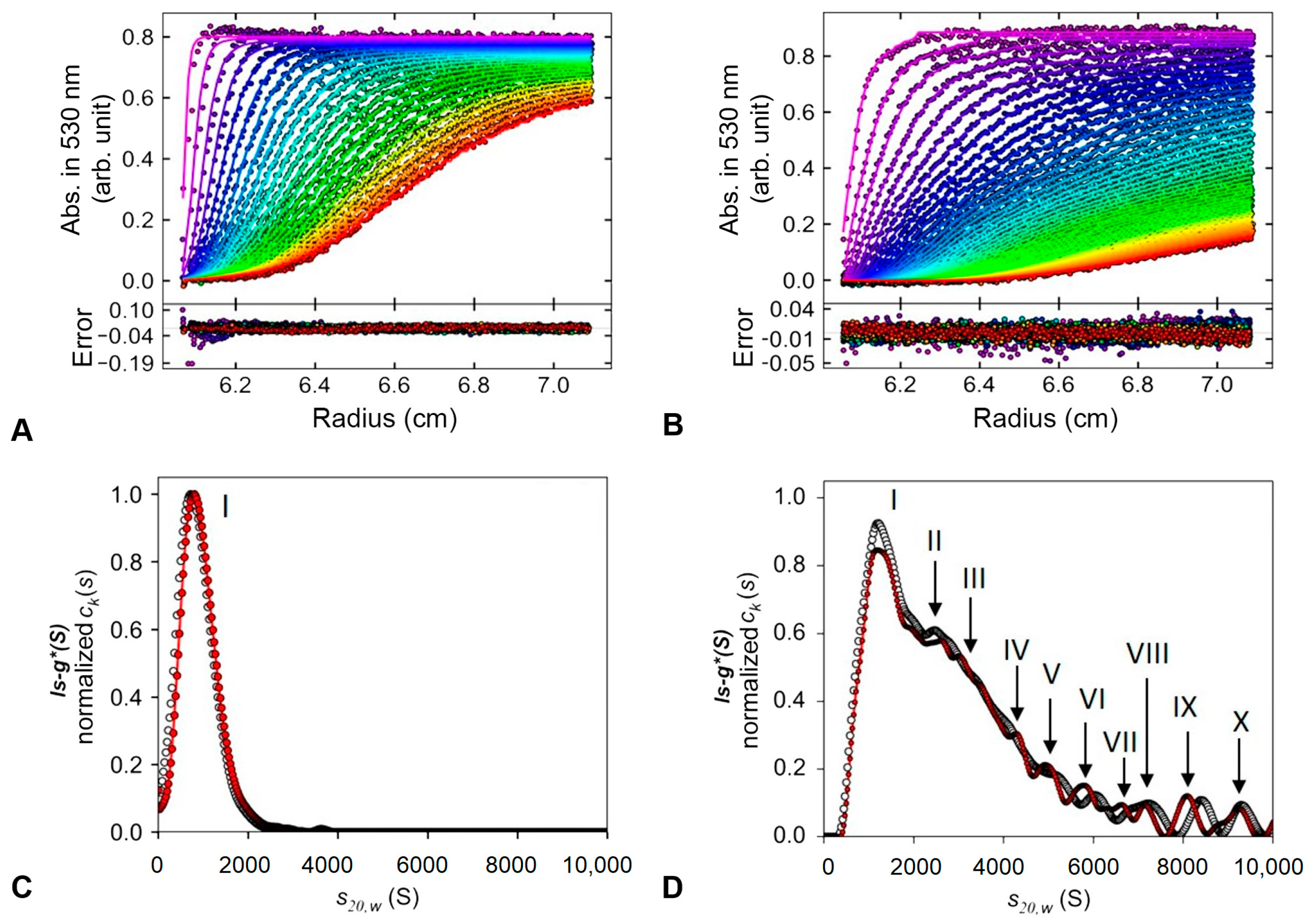
- Correia, J.J.; Stafford, W.F. Sedimentation Velocity: A Classical Perspective. In Methods in Enzymology; Cole, J.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 562, pp. 49–80. [Google Scholar]
- Shiraishi, Y.; Tanaka, H.; Sakamoto, H.; Ichikawa, S.; Takayuki, H. Photoreductive synthesis of monodispersed Au nanoparticles with citric acid as reductant and surface stabilizing reagent. RSC Adv. 2017, 7, 6187–6192. [Google Scholar] [CrossRef]
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.H.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Aengenheister, L.; Dietrich, D.; Sadeghpour, A.; Manser, P.; Diener, L.; Wichser, A.; Karst, U.; Peter Wick, P.; Buerki-Thurnherr, T. Gold nanoparticle distribution in advanced in vitro and ex vivo human placental barrier models. J Nanobiotechnol. 2018, 16, 79. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, Y.; Zhao, X.; Sheng, L.; Wang, L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomed. 2017, 12, 6197–6204. [Google Scholar] [CrossRef]
- Mohamadzadeh, N.; Zirak Javanmard, M.; Karimipour, M.; Farjah, G. Developmental toxicity of the neural tube induced by titanium dioxide nanoparticles in mouse embryos. Avicenna J. Med. Biotechnol. 2021, 13, 74–80. [Google Scholar] [CrossRef]
- Leal-Silva, T.; Souza, M.R.; Cruz, L.L.; Moraes-Souza, R.Q.; Paula, V.G.; Soares, T.S.; Dela Justina, V.; Giachini, F.R.; Daasceno, D.C.; Américo, M.F.; et al. Toxicological effects of the Morinda citrifolia L. fruit extract on maternal reproduction and fetal development in rats. Drug Chem. Toxicol. 2023, 46, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Souza, R.Q.; Soares, T.S.; Carmo, N.O.; Damasceno, D.C.; Campos, K.E.; Volpato, G.T. Adverse effects of Croton urucurana B. exposure during rat pregnancy. J. Ethnopharmacol. 2017, 199, 328–333. [Google Scholar] [CrossRef]
- Withers, P.J.; Bouman, C.; Carmignato, S.; Cnudde, V.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Du Plessis, A.; Stock, S.R. X-ray computed tomography. Nat. Rev. Methods Prim. 2021, 1, 18. [Google Scholar] [CrossRef]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. BioMed Res Int. 2014, 2014, 741018. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.N.; Schabel, C.; Krauss, B.; Claussen, C.D.; Nikolaou, K.; Thomas, C. Potential of gadolinium as contrast material in second generation dual energy computed tomography—An ex vivo phantom study. Clin. Imaging 2017, 43, 74–79. [Google Scholar] [CrossRef]
- Mesbahi, A.; Famouri, F.; Ahar, M.; Ghaffari, M.; Ghavami, S. A study on the imaging characteristics of Gold nanoparticles as a contrast agent in X-ray computed tomography. Pol. J. Med. Phys. Eng. 2017, 23, 9–14. [Google Scholar] [CrossRef]
- Santos-Martinez, M.J.; Rahme, K.; Corbalan, J.J.; Faulkner, C.; Holmes, J.D.; Tajber, L.; Medina, C.; Radomski, M.W. Pegylation Increases Platelet Biocompatibility of Gold Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1004–1015. [Google Scholar] [CrossRef]


| Samples | Concentration (mmol L−1) | |
|---|---|---|
| PLU (X) | HAuCl4 (Y) | |
| AuNP-PLU:0.1UV | 0.1 | 2.0 |
| AuNP-PLU:0.3UV | 0.3 | |
| AuNP-PLU:0.5UV | 0.5 | |
| AuNP-PLU:1.0UV | 1.0 | |
| AuNP-PLU:2.0UV | 2.0 | |
| AuNP-PLU:4.0UV | 4.0 | |
| AuNP-PLU:6.0UV | 6.0 | |
| AuNP-PLU:8.0UV | 8.0 | |
| AuNP-PLU:10.0UV | 10.0 | |
| 1.0UV:AuNP-PLU | 2.0 | 1.0 |
| 3.0UV:AuNP-PLU | 3.0 | |
| 4.0UV:AuNP-PLU | 4.0 | |
| Synthesis Methods | Distribution at 530 nm | Distribution at 260 nm | ||||
|---|---|---|---|---|---|---|
| ls-g* (S) Peaks | S20,w | Percentage Ratio (%) | ls-g* (S) Peaks | S20,w | Percentage Ratio (%) | |
| Environment light (Control) | I | 1451.17 | 43.21 | I | 1418.534 | 44.17 |
| II | 2613.09 | 13.30 | II | 3555.02 | 43.80 | |
| III | 3477.32 | 20.04 | III | 6118.22 | 2.71 | |
| IV | 4403.81 | 5.24 | IV | 7145.20 | 3.26 | |
| V | 5030.07 | 4.98 | V | 8441.53 | 2.02 | |
| VI | 5811.97 | 3.67 | VI | 9364.58 | 1.69 | |
| VII | 6343.14 | 0.85 | ||||
| VIII | 6681.68 | 1.44 | ||||
| IX | 7198.76 | 1.44 | ||||
| X | 8125.44 | 2.16 | ||||
| photoexcited synthesis (UV) | I | 947.10 | 99.36 | I | 857.78 | 99.15 |
| Groups | ||
|---|---|---|
| Control (n = 12) | Treated (n = 12) | |
| Weight gain in pregnancy (g) | ||
| 1st week | 19.75 ± 5.96 | 20.42 ± 7.95 |
| 2nd week | 22.75 ± 10.60 | 20.75 ± 4.90 |
| 3rd week | 79.67 ± 15.76 | 79.67 ± 15.76 |
| Total body weight gain—WG | 121.1 ± 12.2 | 115.3 ± 19.0 |
| Gravid uterus weight—GUW (g) | 72.5 ± 19.3 | 79.4 ± 12.1 |
| BWG minus GUW (g) | 44.27 ± 14.96 | 38.34 ± 10.65 |
| Daily food consumption (g) | 20.32 ± 2.11 | 21.39 ± 2.60 |
| Daily water intake (mL) | 55.55 ± 6.96 | 56.48 ± 12.64 |
| Relative organ weight (g/100g) | ||
| Heart | 0.31 ± 0.03 | 0.31 ± 0.03 |
| Liver | 3.94 ± 0.41 | 3.98 ± 0.31 |
| Spleen | 0.22 ± 0.06 | 0.20 ± 0.02 |
| Kidneys | 0.55 ± 0.04 | 0.55 ± 0.03 |
| Biochemical serum parameters | ||
| ALT (U/L) | 41.41 ± 10.61 | 39.50 ± 8.79 |
| AST (U/L) | 145.82 ± 32.42 | 127.98 ± 42.13 |
| Urea (mg/dL) | 45.43 ± 8.69 | 49.80 ± 7.70 |
| Creatinine (mg/dL) | 0.62 ± 0.16 | 0.58 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, D.S.B.; Paterno, L.G.; Santos, A.B.S.; Barbosa, D.P.P.; Holtz, B.M.; Souza, M.R.; Moraes-Souza, R.Q.; Garay, A.V.; de Andrade, L.R.; Sartoratto, P.P.C.; et al. UV-Accelerated Synthesis of Gold Nanoparticle–Pluronic Nanocomposites for X-ray Computed Tomography Contrast Enhancement. Polymers 2023, 15, 2163. https://doi.org/10.3390/polym15092163
Gomes DSB, Paterno LG, Santos ABS, Barbosa DPP, Holtz BM, Souza MR, Moraes-Souza RQ, Garay AV, de Andrade LR, Sartoratto PPC, et al. UV-Accelerated Synthesis of Gold Nanoparticle–Pluronic Nanocomposites for X-ray Computed Tomography Contrast Enhancement. Polymers. 2023; 15(9):2163. https://doi.org/10.3390/polym15092163
Chicago/Turabian StyleGomes, Deizilene S. B., Leonardo G. Paterno, Aline B. S. Santos, Debora P. P. Barbosa, Beatriz M. Holtz, Maysa R. Souza, Rafaianne Q. Moraes-Souza, Aisel V. Garay, Laise R. de Andrade, Patricia P. C. Sartoratto, and et al. 2023. "UV-Accelerated Synthesis of Gold Nanoparticle–Pluronic Nanocomposites for X-ray Computed Tomography Contrast Enhancement" Polymers 15, no. 9: 2163. https://doi.org/10.3390/polym15092163
APA StyleGomes, D. S. B., Paterno, L. G., Santos, A. B. S., Barbosa, D. P. P., Holtz, B. M., Souza, M. R., Moraes-Souza, R. Q., Garay, A. V., de Andrade, L. R., Sartoratto, P. P. C., Mertz, D., Volpato, G. T., Freitas, S. M., & Soler, M. A. G. (2023). UV-Accelerated Synthesis of Gold Nanoparticle–Pluronic Nanocomposites for X-ray Computed Tomography Contrast Enhancement. Polymers, 15(9), 2163. https://doi.org/10.3390/polym15092163






