Flame Retardancy of Nylon 6 Fibers: A Review
Abstract
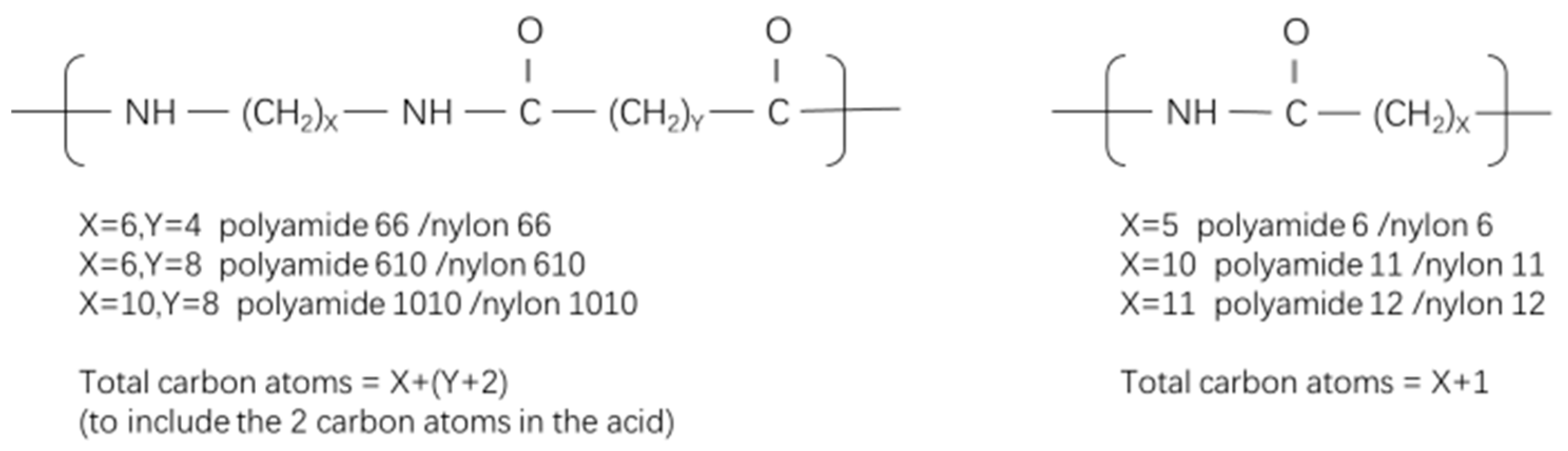
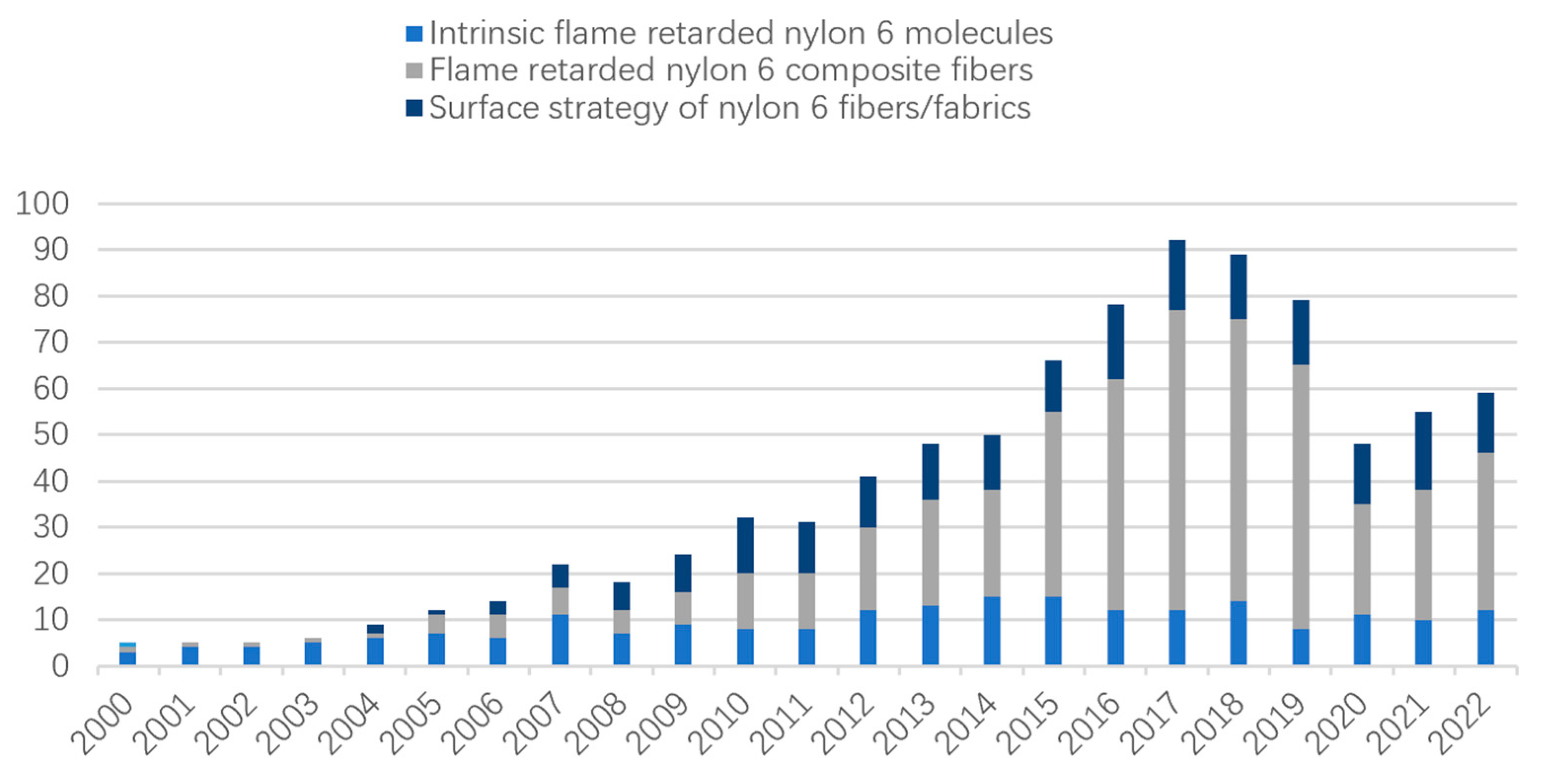
1. Introduction
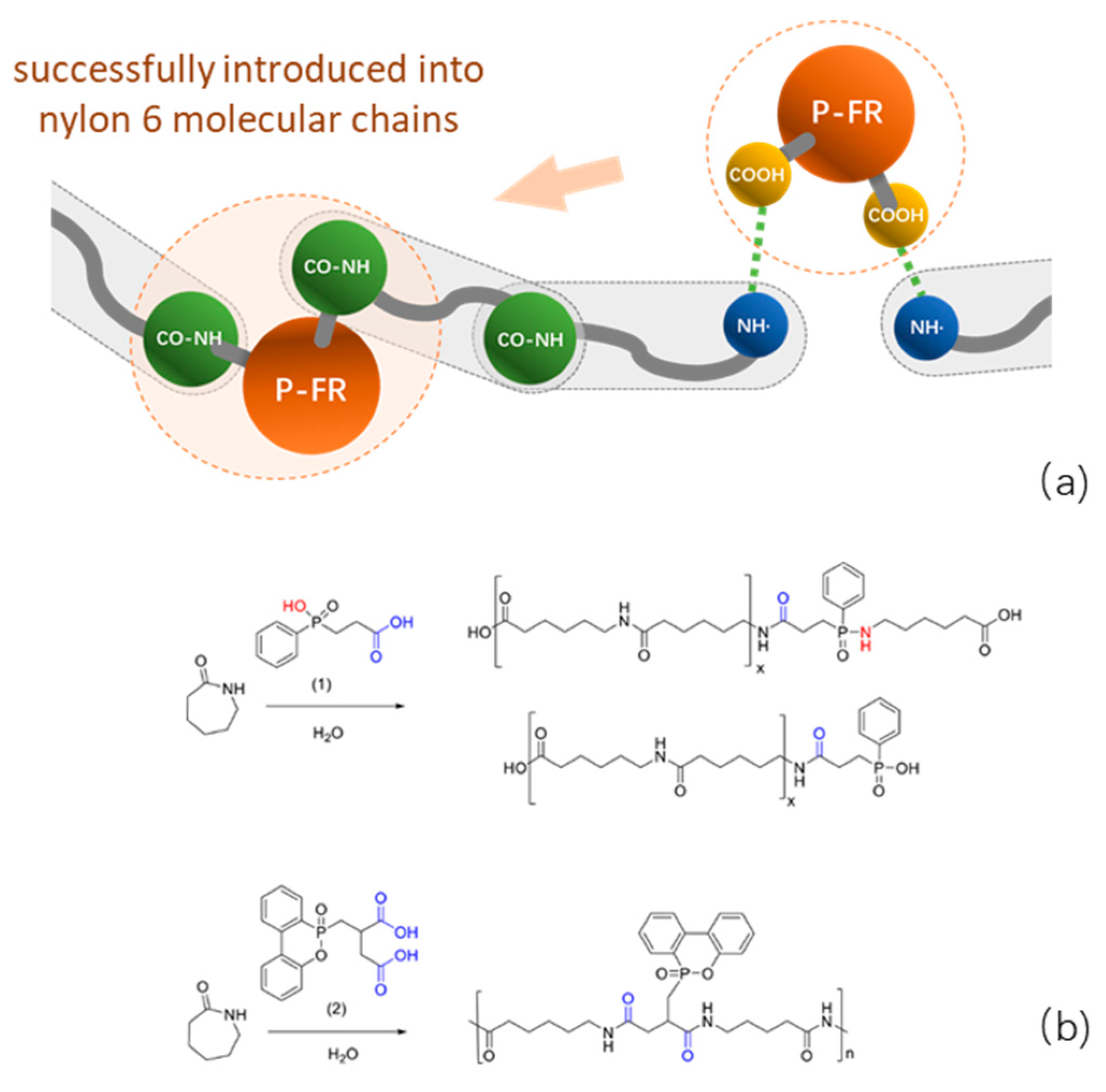
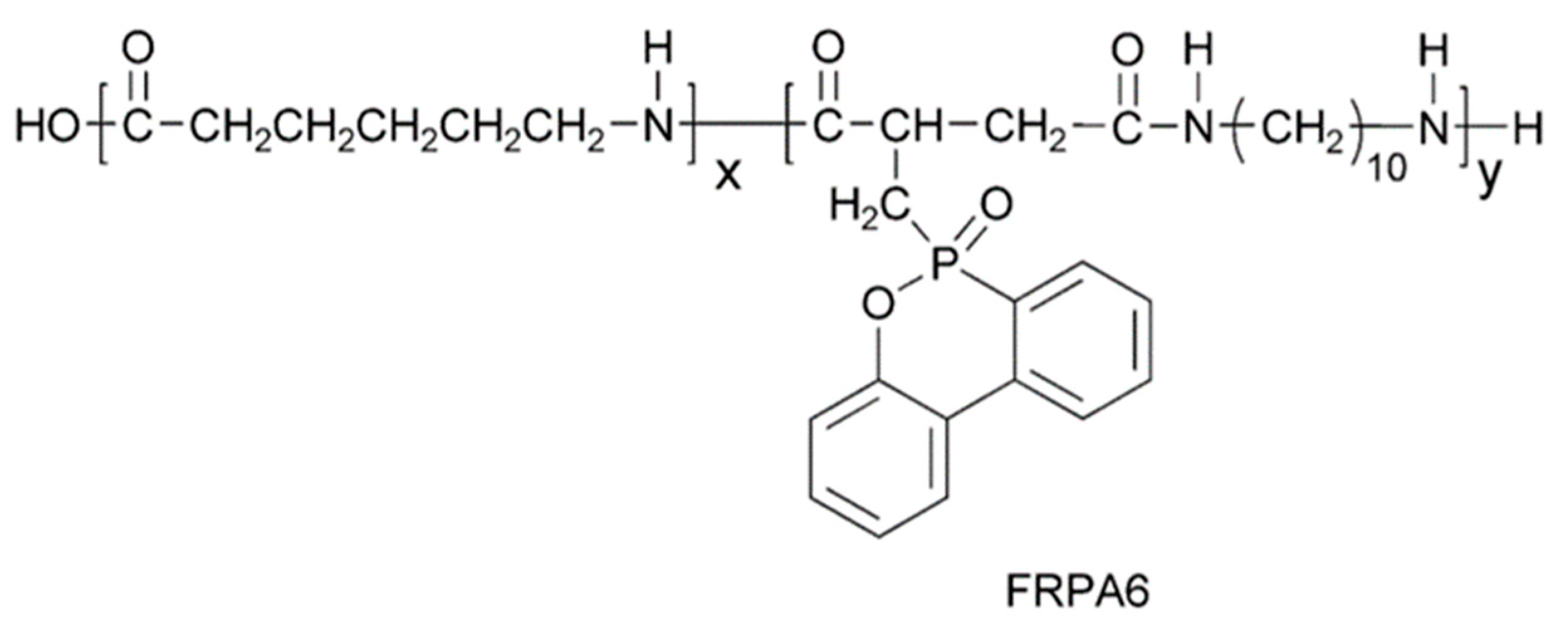
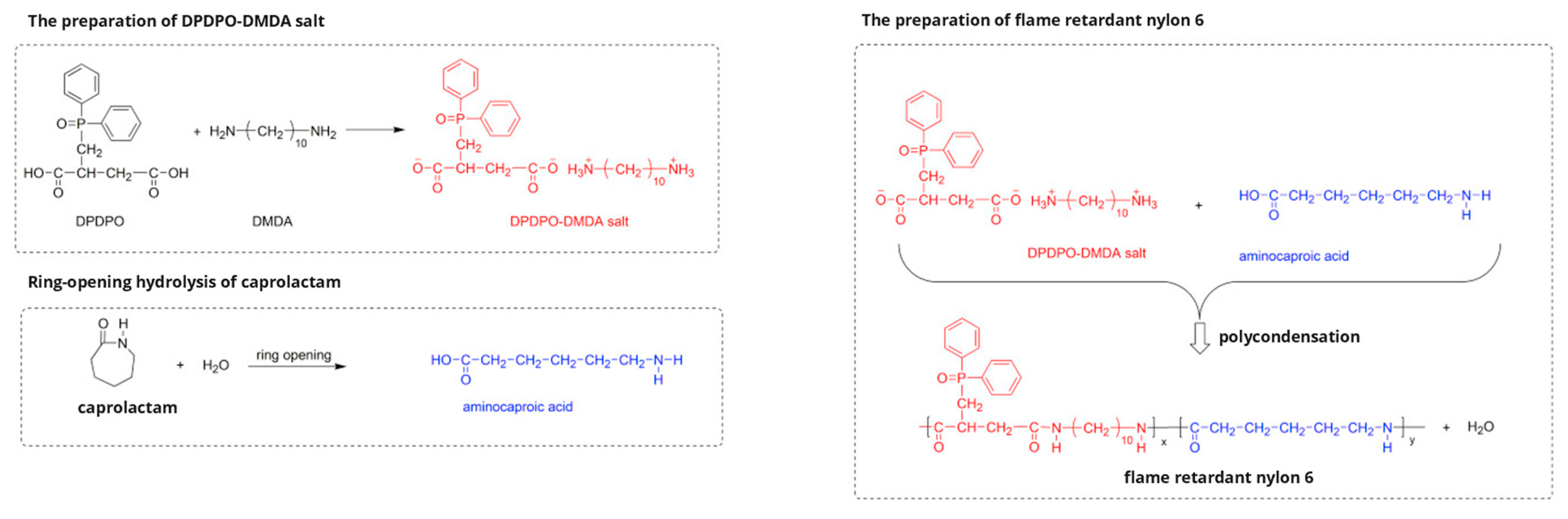
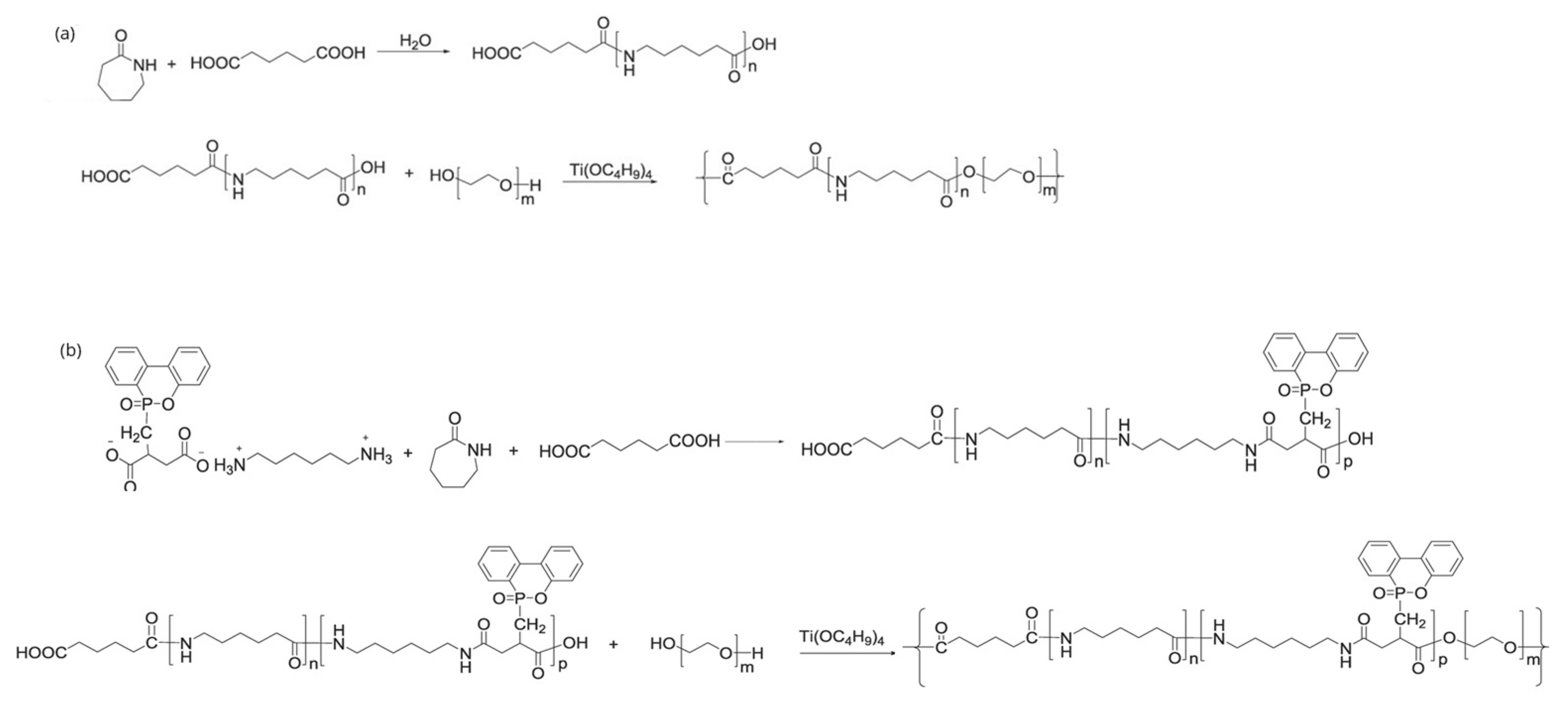
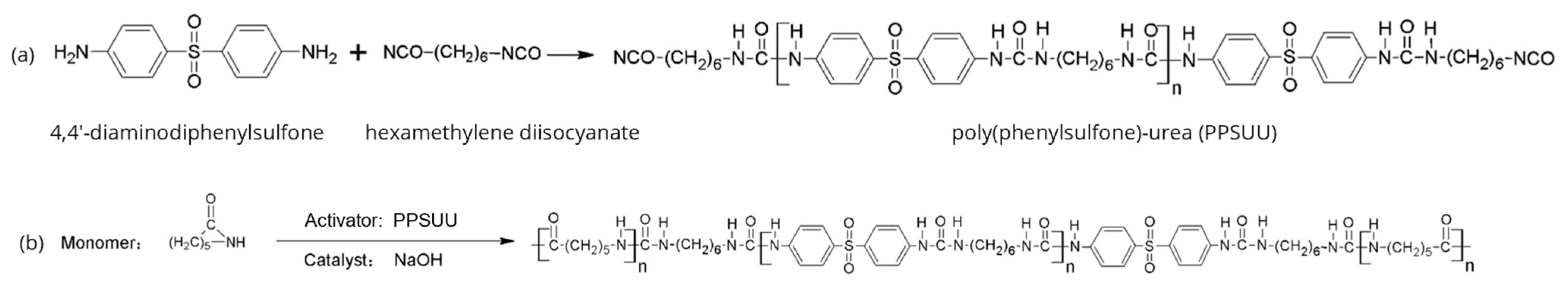
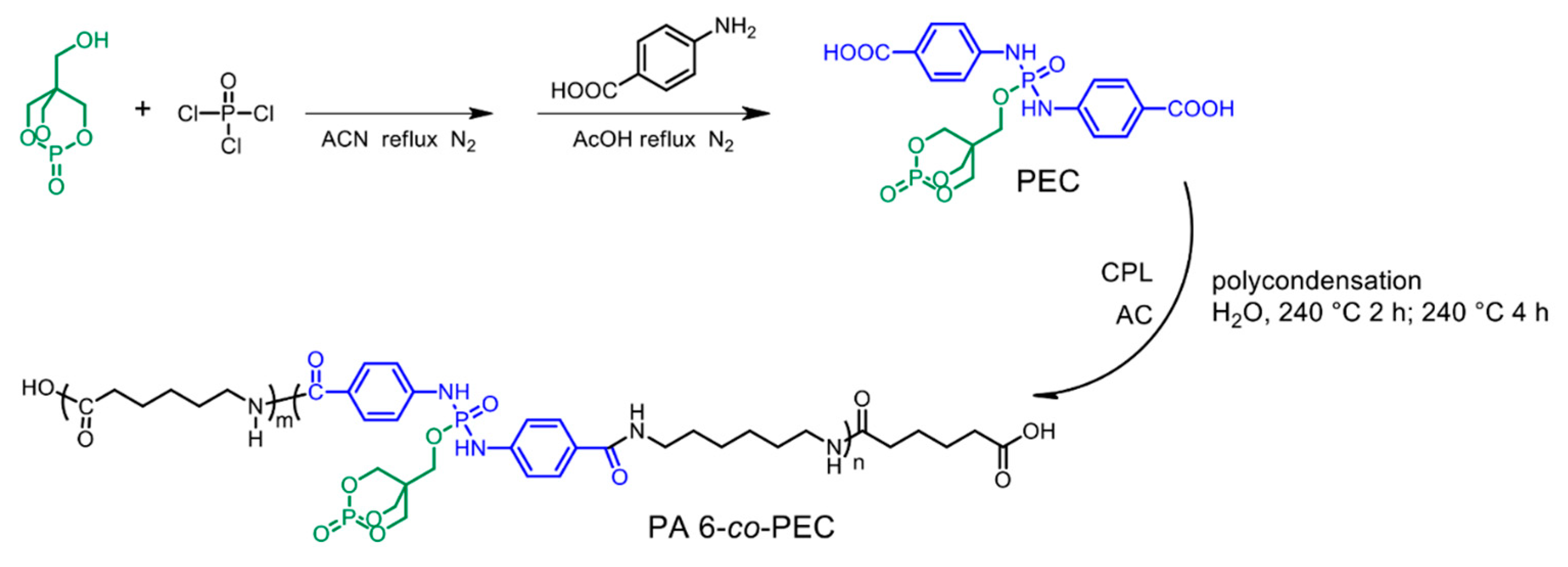
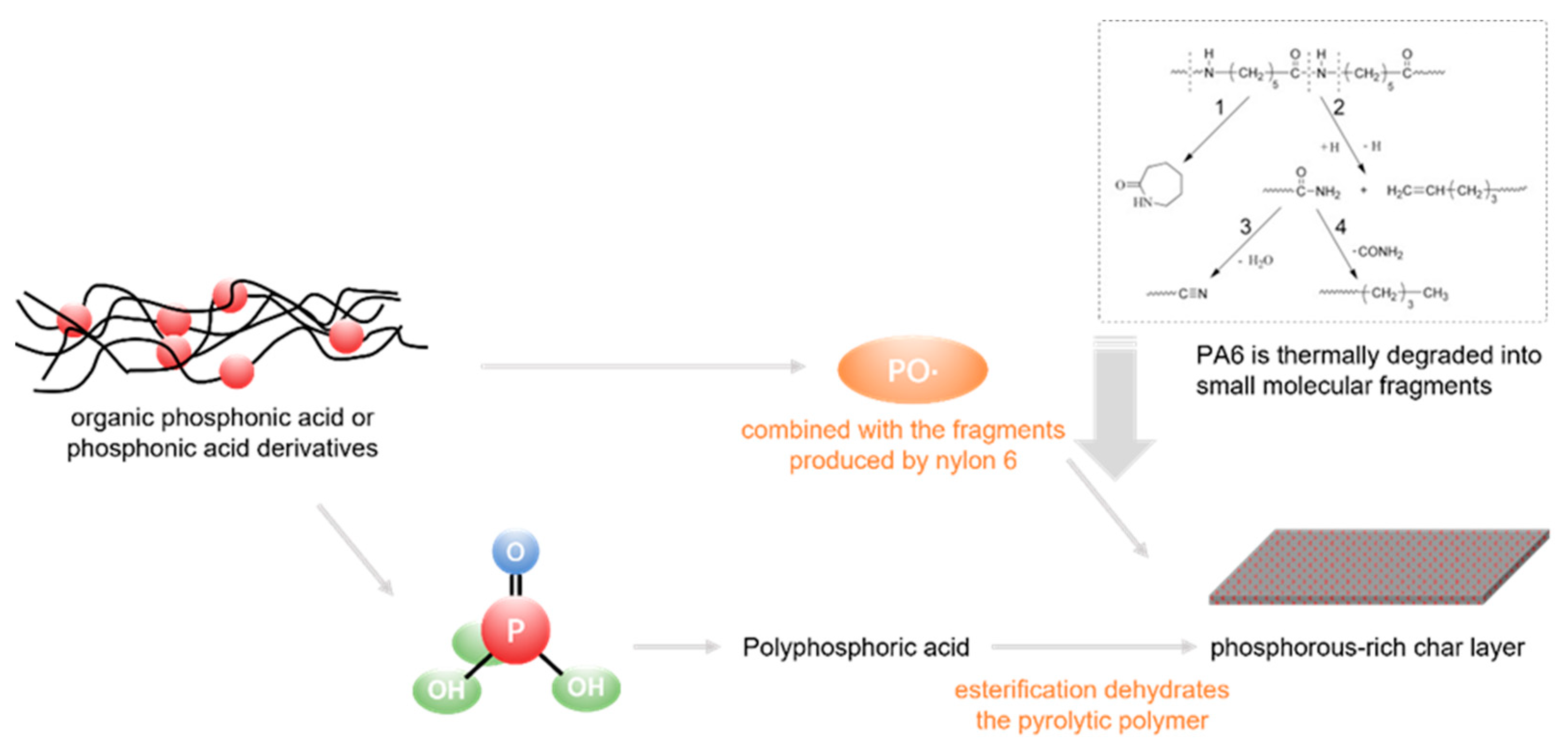
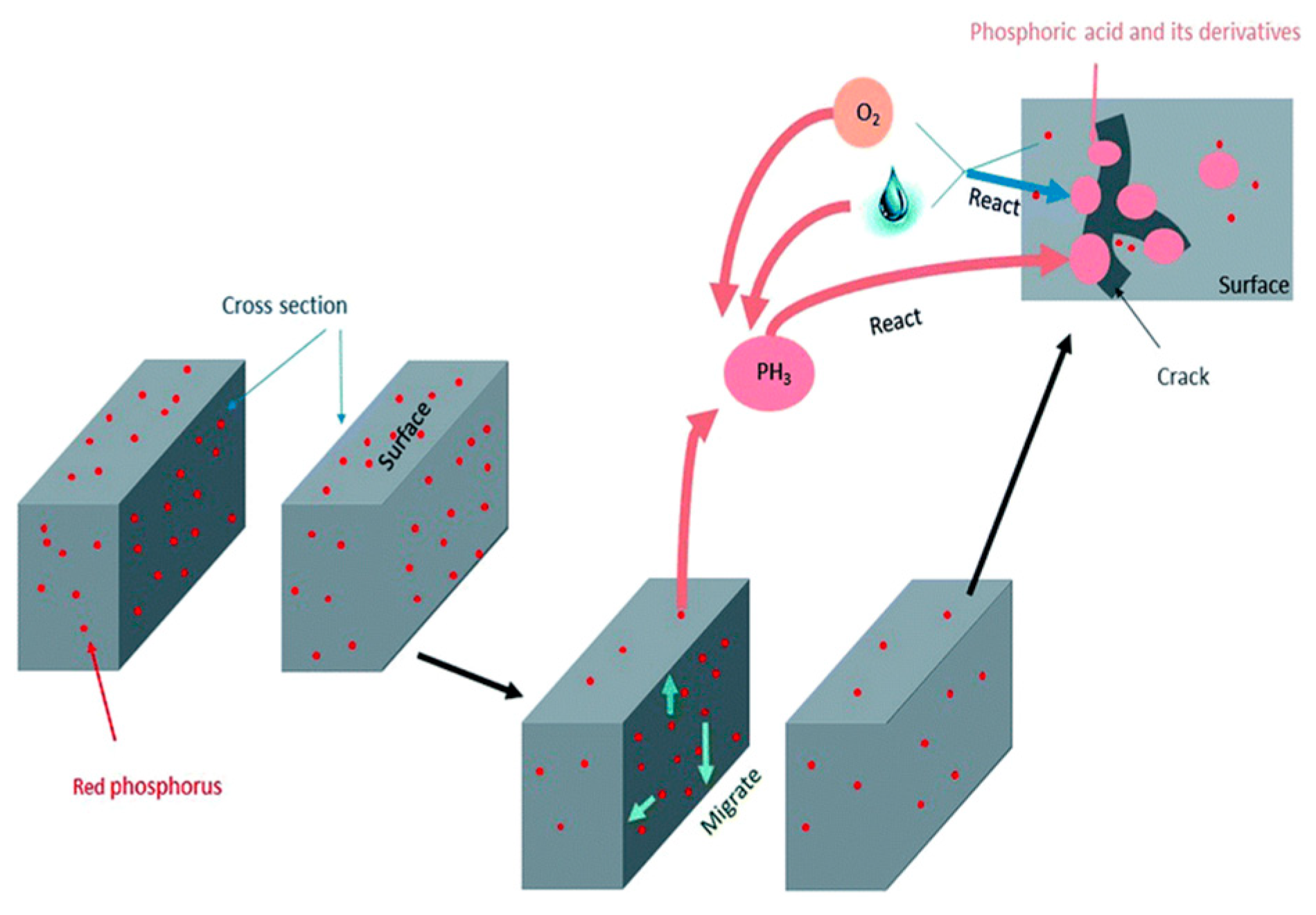
2. Intrinsic Flame-Retarded Nylon 6 Molecules
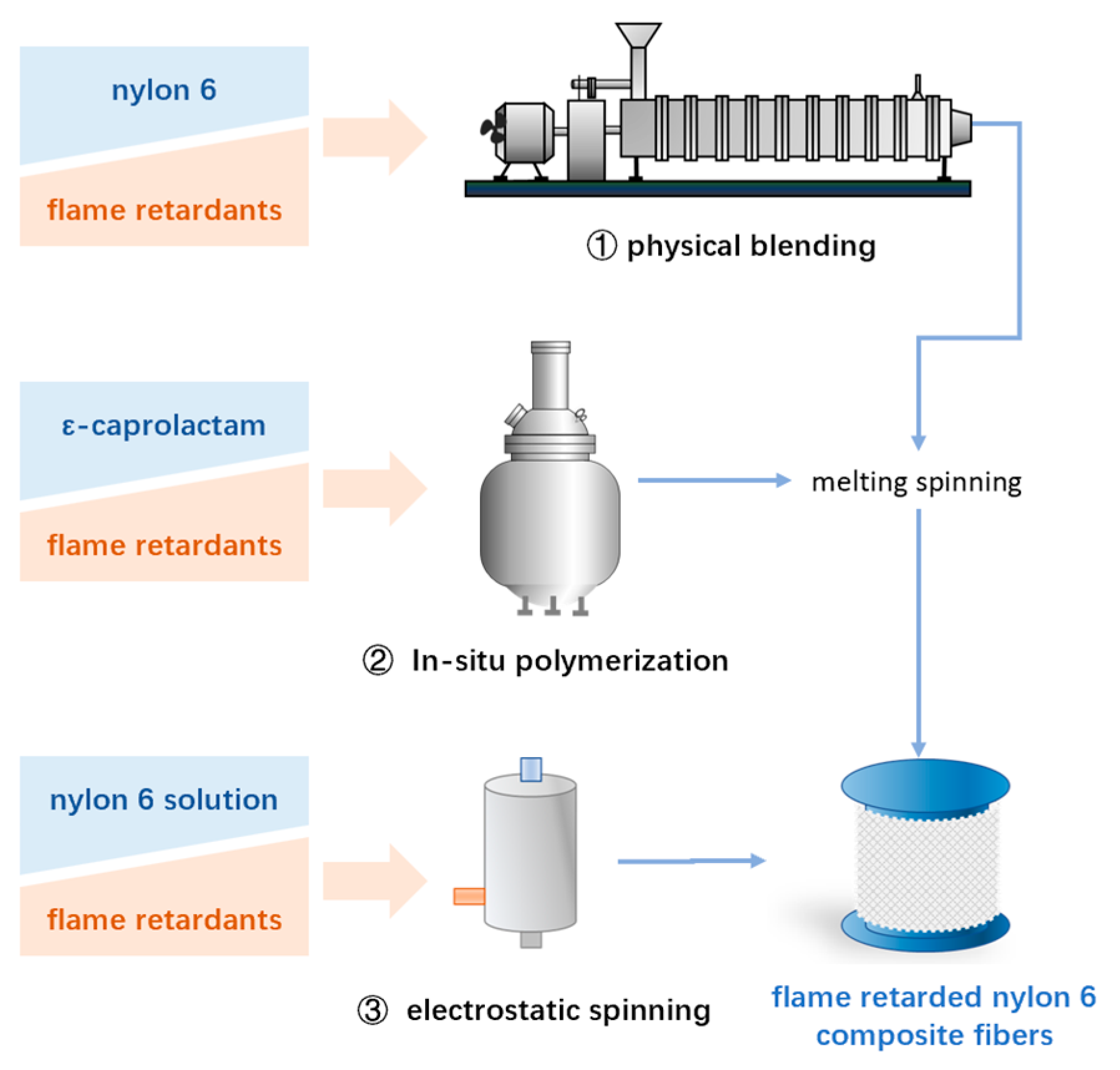
3. Flame-Retarded Composites
3.1. Physical Blending
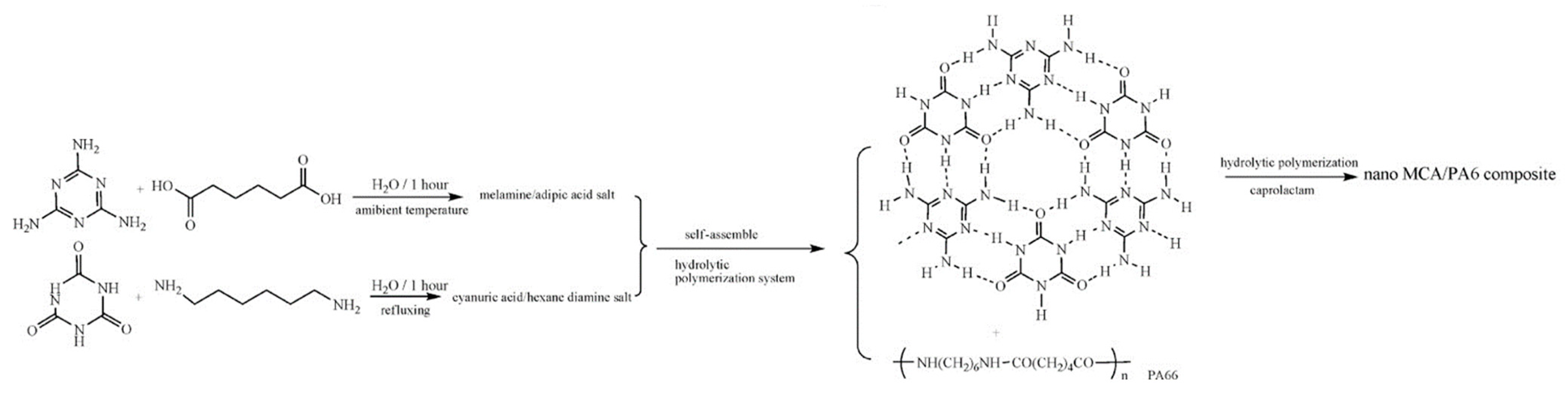
3.2. In Situ Polymerization
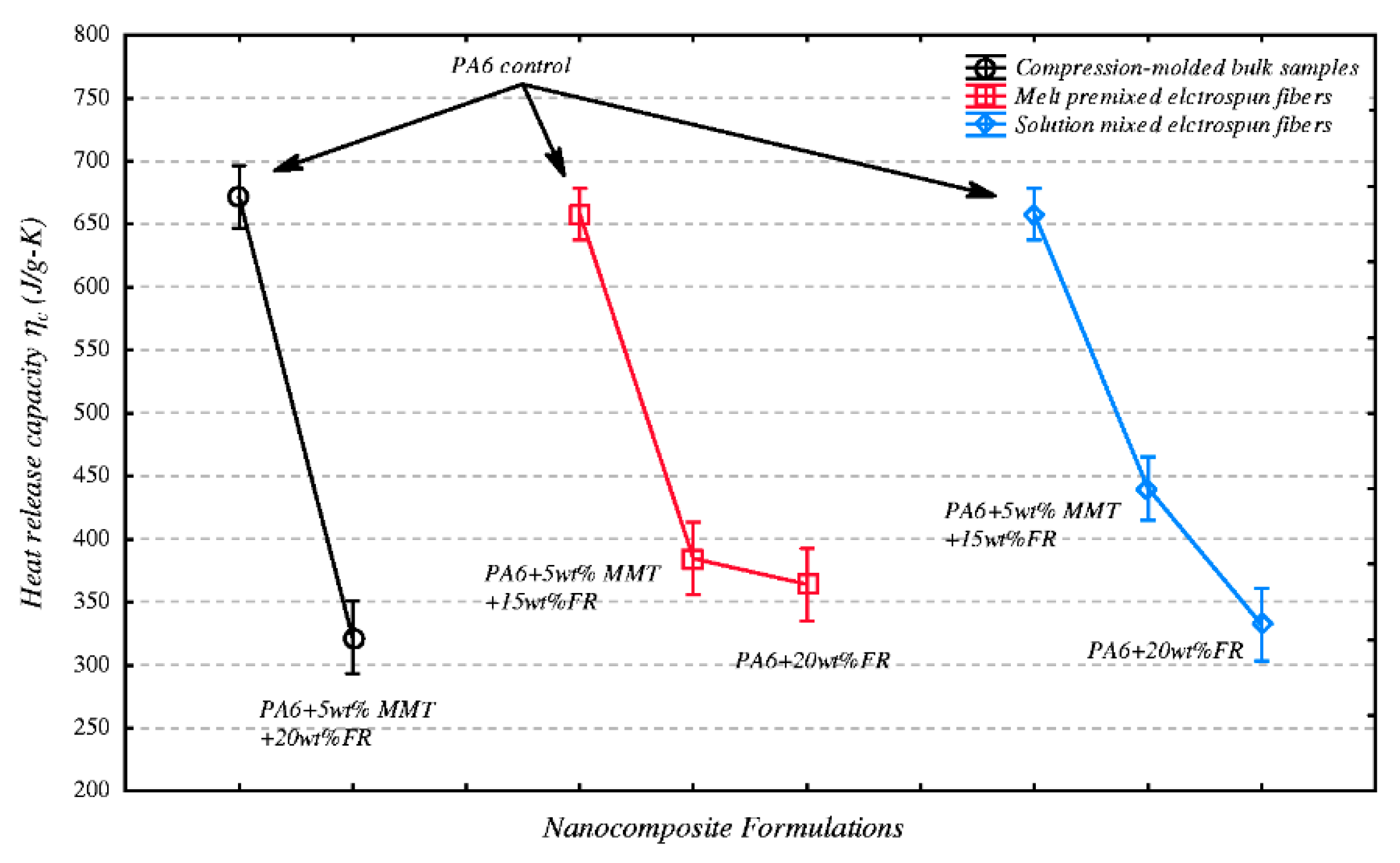
3.3. Electrostatic Spinning
4. Surface Strategy
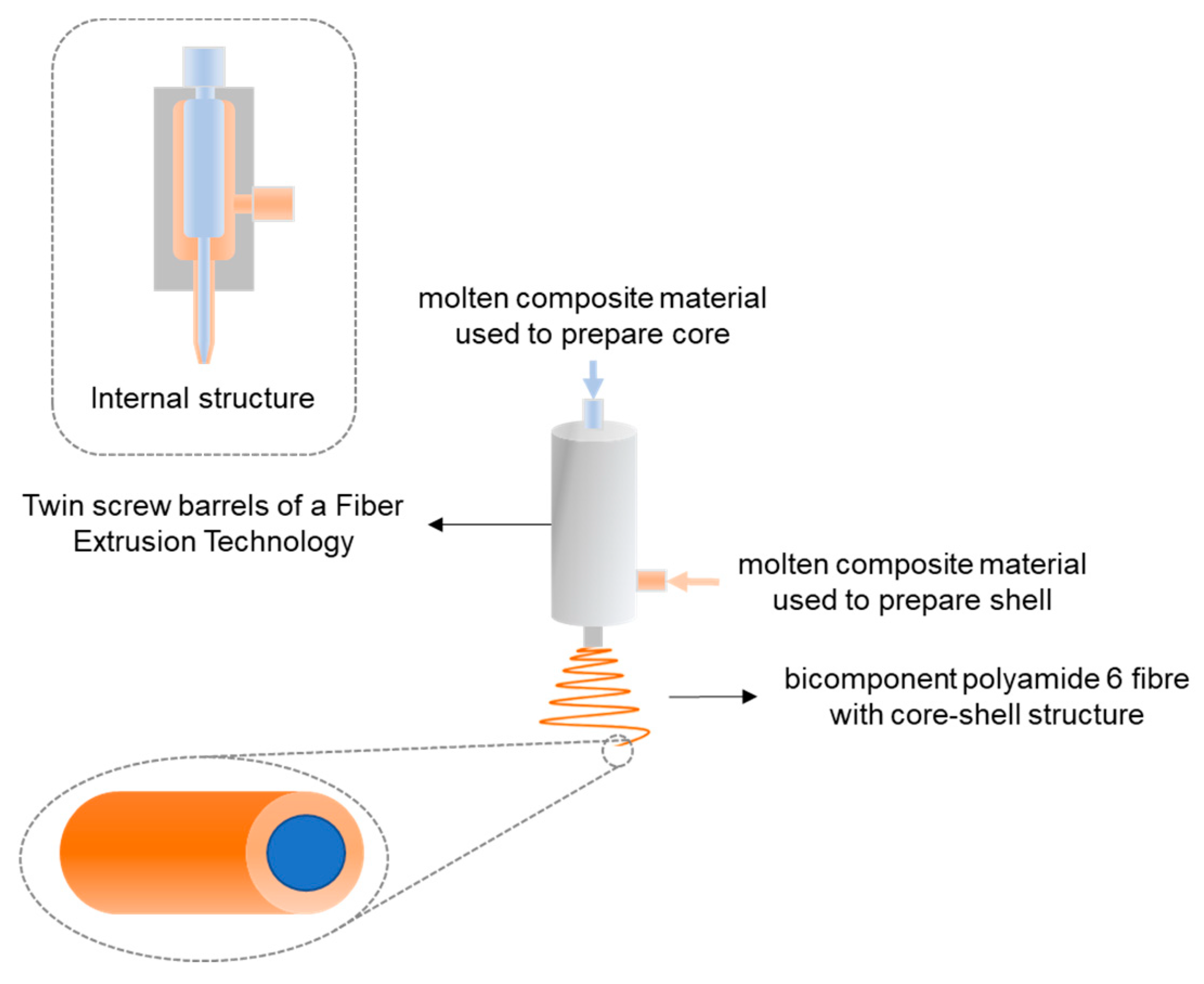
4.1. Core–Shell Structure
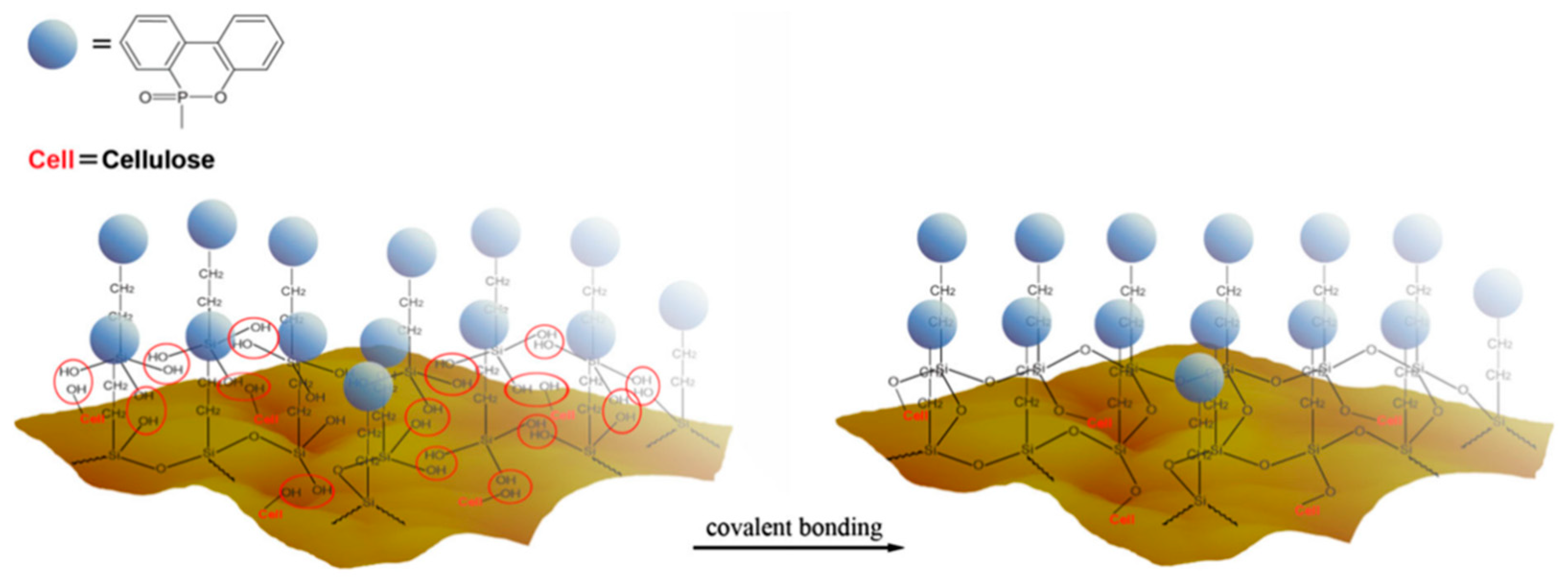
4.2. Surface Coating or Infiltration
4.3. Blended Fibers/Fabrics
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, X.-Y.; Liu, C.; Chen, Y.-X.; Yin, F.-L.; Bao, D.-M.; Zhou, G.-Y. Waterborne polyurethane composites flame retardancy based on the bi-DOPO derivatives and dangling poly(dimethylsiloxane) chains. Prog. Org. Coat. 2023, 177, 107388. [Google Scholar]
- Di, Y.; Wu, X.; Zhao, Z.; Wei, W. Experimental investigation of mechanical, thermal, and flame-retardant property of polyamide 6/phenoxyphosphazene fibers. J. Appl. Polym. Sci. 2020, 137, 48458. [Google Scholar]
- Georgios, M.; Elisabeth, G.; Tanja, S.; Michael, R.-B. Syntheses of intrinsically flame-retardant polyamide 6 fibers and fabrics. J. Appl. Polym. Sci. 2019, 136, 47829. [Google Scholar]
- Levchik, S.-V.; Weil, E.-D. Combustion and fire retardancy of aliphatic nylons. Polym. Int. 2020, 49, 1033–1073. [Google Scholar]
- Georgios, M.; Elisabeth, G.; Volker, B.; Tanja, S.; Joerg, U.; Michael, R.-B. Synthesis of intrinsically flame-retardant copolyamides and their employment in PA6-fibers. Polym. Adv. Technol. 2019, 30, 2872–2882. [Google Scholar]
- Liu, K.; Li, Y.; Tao, L.; Chang, L.; Ru, X. Preparation and characterization of polyamide 6 fibre based on a phosphorus-containing flame retardant. RSC Adv. 2018, 8, 9261–9271. [Google Scholar]
- Vyverberg, F.-X.; Chapman, R.-W. Process for the Production of Pentaerytheitol Phosphate Alcohol. U.S. Patent 6,455,722, 24 September 2022. [Google Scholar]
- Liu, W.; Cui, Y.; Zhang, X.; Yuan, Y.; Zhang, W. Fire and thermal properties of PA 66 resin treated with poly-N-aniline-phenyl phosphamide as a flame retardant. Fire Mater. 2017, 41, 349–361. [Google Scholar]
- Liu, K.; Li, Y.; Tao, L.; Chang, L.; Ru, X. Synthesis and characterization of inherently flame retardant polyamide 6 based on a phosphine oxide derivative. Polym. Degrad. Stab. 2019, 163, 151–160. [Google Scholar]
- Lu, P.; Zhao, Z.-Y.; Xu, B.-R.; Li, Y.-M.; Deng, C.; Wang, Z.-Y. A novel inherently flame-retardant thermoplastic polyamide elastomer. Chem. Eng. J. 2019, 379, 122278. [Google Scholar]
- Yuan, R.-C.; Fan, S.; Wu, D.; Wang, X.; Yu, J.; Chen, L.; Li, F. Facile synthesis of polyamide 6 (PA6)-based thermoplastic elastomers with a well-defined microphase separation structure by melt polymerization. Polym. Chem. 2018, 9, 1327–1336. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Lang, F.; Song, L.; Lin, Y.; You, L.; Li, D.; Jiang, Q. Preparation and properties of wear-resistant and flame-retardant polyphenylsulfoneurea/monomer casting nylon copolymers. J. Appl. Polym. Sci. 2021, 138, 50750. [Google Scholar] [CrossRef]
- Song, L.; Yang, H.-Y.; Li, D.-X.; Jiang, Q.-B.; Zeng, Z. Polydimethylsiloxane/monomer casting nylon copolymers: Preparation, flame-retardant properties, and wear-resistant properties. J. Appl. Polym. Sci. 2019, 137, 48753. [Google Scholar] [CrossRef]
- Zhuang, Y.-F.; Cao, X.-Y.; Zhang, J.-N.; Ma, X.-Y.; Shang, X.-X.; Lu, J.-X.; Yang, S.-L.; Zheng, K.; Ma, Y.-M. Monomer casting nylon/graphene nanocomposite with both improved thermal conductivity and mechanical performance. Compos. Part A-Appl. Sci. Manuf. 2019, 120, 49. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Ranganathan, P.; Lee, Y.-H.; Rwei, S.-P. New strategy and polymer design to synthesize polyamide 66 (PA66) copolymers with aromatic moieties from recycled PET. ACS Sustain. Chem. Eng. 2021, 9, 3518–3528. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, B.-W.; He, F.-M.; Zhang, Q.; Wang, A.; Guo, D.-M.; Zhao, H.-B.; Chen, L.; Wang, Y.-Z. High-performance flame-retardant aliphatic polyamide via enhanced chain entanglement. Chem. Eng. J. 2023, 455, 140637. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakane, T.; Uchida, M.; Kaneko, Y. Mechanical modeling and testing of different polyamides considering molecular chain structure, crystallinity, and large strains. Int. J. Solids Struct. 2022, 111419, 239–240. [Google Scholar] [CrossRef]
- Gao, J.-C.; Wu, Y.-D.; Li, J.; Peng, X.-Q.; Yin, D.-W.; Jin, H.-L.; Wang, S.; Wang, J.-C.; Wang, X.-H.; Jin, M.-J.; et al. A review of the recent developments in flame-retardant nylon composites. Compos. Part C 2022, 9, 100297. [Google Scholar]
- Tang, L.; Li, Y.; Chen, Y.; Ji, P.; Wang, C.; Wang, H.; Huang, Q. Preparation and characterization of graphene reinforced PA6 fiber. J. Appl. Polym. Sci. 2017, 135, 45834. [Google Scholar] [CrossRef]
- Hao, W.; Mourad, K.; Koo, J.-H. Inherently flame retardant nylon 6 nanocomposite fibers. Fibers Polym. 2018, 19, 1500–1512. [Google Scholar]
- Zhang, W.; Xu, B.; Gong, C.; Yi, C.; Zhang, S. Antibacterial and anti-flaming PA6 composite with metathetically prepared nano AgCl@BaSO4 co-precipitates. Front. Chem. Sci. Eng. 2021, 15, 340–350. [Google Scholar] [CrossRef]
- Xiang, H.-X.; Li, L.; Wei, C.; Yu, S.; Sun, B.; Zhu, M. Flame retardancy of polyamide 6 hybrid fibers:combined effects ofα-zirconium phosphate and ammonium sulfamate. Prog. Nat. Sci. 2017, 27, 369–373. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Zhou, D.-X.; Zhang, J.-X.; Zheng, Y.-D. Fabrication of Polyamide 6 Nanocomposite with Improved Thermal Conductivity and Mechanical Properties via Incorporation of Low Graphene Content. Ind. Eng. Chem. Res. 2018, 57, 10967–10976. [Google Scholar] [CrossRef]
- Liu, T.; Wang, R.-T. Preparation and performance of pentaerythritol phosphate/zinc diethyl phosphate synergistic flame retardant polyamide 6. Text. Res. J. 2018, 39, 8–14. [Google Scholar]
- Zhe, H.; Bo, R.; Jin, W. High-efficiency ammonium polyphosphate intumescent encapsulated polypropylene flame retardant. J. Appl. Polym. Sci. 2021, 138, 50413. [Google Scholar]
- Zheng, T.; Xia, W.; Guo, J.; Wang, K.; Liu, Y. Preparation of flame-retardant polyamide 6 by incorporating MgO combined with g-C3N4. Polym. Adv. Technol. 2020, 31, 1963–1971. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, W.; Liu, Y. A novel phosphorus-nitrogen flame retardant for improving the flame retardancy of polyamide 6: Preparation, properties, and flame retardancy mechanism. Polym. Adv. Technol. 2021, 32, 2508–2516. [Google Scholar] [CrossRef]
- Jelena, V.; Marija, Č.; Ivan, J.; Barbara, S.; Andrej, D.; Younes, S.; Matic, Š.; Ervin, Š.; Barbara, G.; Mirjam, L.; et al. In situ prepared polyamide 6/DOPO-derivative nanocomposite for melt-spinning of flame retardant textile filaments. Polym. Degrad. Stab. 2019, 166, 50–59. [Google Scholar]
- Jelena, V.; Marija, C.; Nataša, C.-K.; Matic, Š.; Žiga, Š.; Ivan, J. Effect of Different Flame-Retardant Bridged DOPO Derivatives on Properties of in Situ Produced Fibers-Forming Polyamide 6. Polymers 2020, 12, 657. [Google Scholar]
- Hou, W.; Fu, Y.; Zeng, C.; Liu, N.; Yin, C. Enhancement of flame retardancy and mechanical properties of polyamide 6 by incorporating melamine cyanurate combined with attapulgite. J. Appl. Polym. Sci. 2020, 137, 47298. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Lin, Y.; Sha, K.; Xiao, R. Preparation and characterizations of flame retardant melamine cyanurate/polyamide 6 composite fibers via in situ polymerization. Text. Res. J. 2016, 87, 561–569. [Google Scholar] [CrossRef]
- Sha, K.; Hu, Y.L.; Wang, Y.-H.; Xiao, R. Preparation of flame retardant Polyamide 6/melamine cyanurate via in situ polymerisation and its characterisation. Mater. Res. Innov. 2014, 18, S4-843–S4-847. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Xu, W.; Liu, Y.-C.; Xia, J.-K.; Xu, W.-J. Preparation and Characterization of Flame-Retardant Melamine Cyanurate/Polyamide 6 Nanocomposites by In Situ Polymerization. J. Appl. Polym. Sci. 2009, 113, 2109–2116. [Google Scholar] [CrossRef]
- Hao, W.; Mourad, K.; Joseph, H.-K. Flame retardant polyamide 6/nanoclay/ intumescent nanocomposite fibers through electrospinning. Text. Res. J. 2014, 84, 1106–1118. [Google Scholar]
- Zhao, M.; Yi, D.; Camino, G.; Frache, A.; Yang, R. Interdigitated crystalline MMT–MCA in polyamide 6. RSC Adv. 2017, 7, 861–869. [Google Scholar] [CrossRef]
- Zhao, M.; Yi, D.; Yang, R. Enhanced mechanical properties and fire retardancy of polyamide 6 nanocomposites based on interdigitated crystalline montmorillonite–melamine cyanurate. J. Appl. Polym. Sci. 2018, 135, 46039. [Google Scholar] [CrossRef]
- Chen, Z.; Du, Z.-X.; Yang, R.-J. Failure behavior of nylon products for red phosphorus flame retardant electrical connectors. RSC Adv. 2019, 9, 24935–24941. [Google Scholar] [CrossRef]
- Mohammadreza, N.; Ali, Z.; Richard, K.; Rasoul, E.N.; Saied, N.K.; Seeram, R. Recent advances in core/shell bicomponent fibers and nanofibers, A review. J. Appl. Polym. Sci. 2018, 135, 46265. [Google Scholar]
- Horrocks, A.-R.; Sitpalan, A.; Kandola, B.-K. Design and characterisation of bicomponent polyamide 6 fibres with specific locations of each flame retardant component for enhanced flame retardancy. Polym. Test. 2019, 79, 106041. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C.; Hsu, P.-C.; Xu, J.-X.; Kong, B.; Wu, T.; Zhang, R.-F.; Zhou, G.-M.; Huang, W.; Sun, J.; et al. coreshell nanofibrous materials with high particulate matter removal efficiencies and thermally triggered flame retardant properties. ACS Cent. Sci. 2018, 4, 894–898. [Google Scholar] [CrossRef]
- Zhu, J.; Shentu, X.; Xu, X.; Zheng, A.; Wei, D. Preparation of graphene oxide modified glass fibers and their application in flame retardant polyamide 6. Polym. Adv. Technol. 2020, 31, 1709–1718. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Chen, X.; Hong, W.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. A halogen-free, flame retardant, waterborne polyurethane coating based on the synergistic effect of phosphorus and silicon. Prog. Org. Coat. 2021, 158, 106359. [Google Scholar] [CrossRef]
- Šehić, A.; Tomšič, B.; Jerman, I.; Vasiljević, J.; Medved, J.; Simončič, B. Synergistic inhibitory action of P- and Si-containing precursors in sol-gel coatings on the thermal degradation of polyamide 6. Polym. Degrad. Stab. 2016, 128, 245–252. [Google Scholar] [CrossRef]
- Jelena, V.; Ivan, J.; Gregor, J.; Jenny, A.; Giulio, M.; Milena, Z.; Brigita, T.; Barbara, S. Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose 2015, 22, 1893–1910. [Google Scholar]
- Aleksandra, B.; Timea, S.; Lea, B.; Daniel, R.; Maciej, H.; Sabyasachi, G. Bridged DOPO derivatives as flame retardants for PA6. Polym. Degrad. Stab. 2014, 107, 158–165. [Google Scholar]
- Liu, L.-J.; Yang, R.-J.; Xia, Z.-H.; Li, D.-H. Interfacial bonding flame retarded coating of nylon 6: Anti-dropping, self-extinguishment and water-proof. Prog. Org. Coat. 2022, 170, 106913. [Google Scholar] [CrossRef]
- Andrzej, G.; Janusz, F.; Czesław, Ś.; Anna, B.; Tadeusz, G.; Jarosław, J. Modification of polyamide 6 fibers with water-glass in the bath method. Polimery 2017, 62, 861–867. [Google Scholar]
- Pan, Y.; Song, L.; Wang, W.-M.; Zhao, H.-T. Polydimethylsiloxane wrapped aluminum diethylphosphinate for enhancing the flame retardancy of polyamide 6. J. Appl. Polym. Sci. 2020, 137, 49027. [Google Scholar] [CrossRef]
- Sabet, M.; Soleimani, H. The influence of graphene on the mechanical, thermal, and flame retardant characteristics of polyamide 6. Mater. Technol. 2022, 37, 1251–1262. [Google Scholar] [CrossRef]
- Kolibaba, T.-J.; Nigam, A.; Tai, B.-L. Environmentally benign flame retardant Polyamide-6 filament for additive manufacturing. Macromol. Mater. Eng. 2021, 306, 2100245. [Google Scholar] [CrossRef]
- Cui, M.-J.; Li, J.; Gao, Q.; Xiang, J.; Chen, Y.; Yan, J.; Fan, H.-J. A novel strategy to fabricate nylon 6 based flame retardant microfiber nonwoven fabric with durability. Colloids Surf. 2022, 641, 128482. [Google Scholar] [CrossRef]
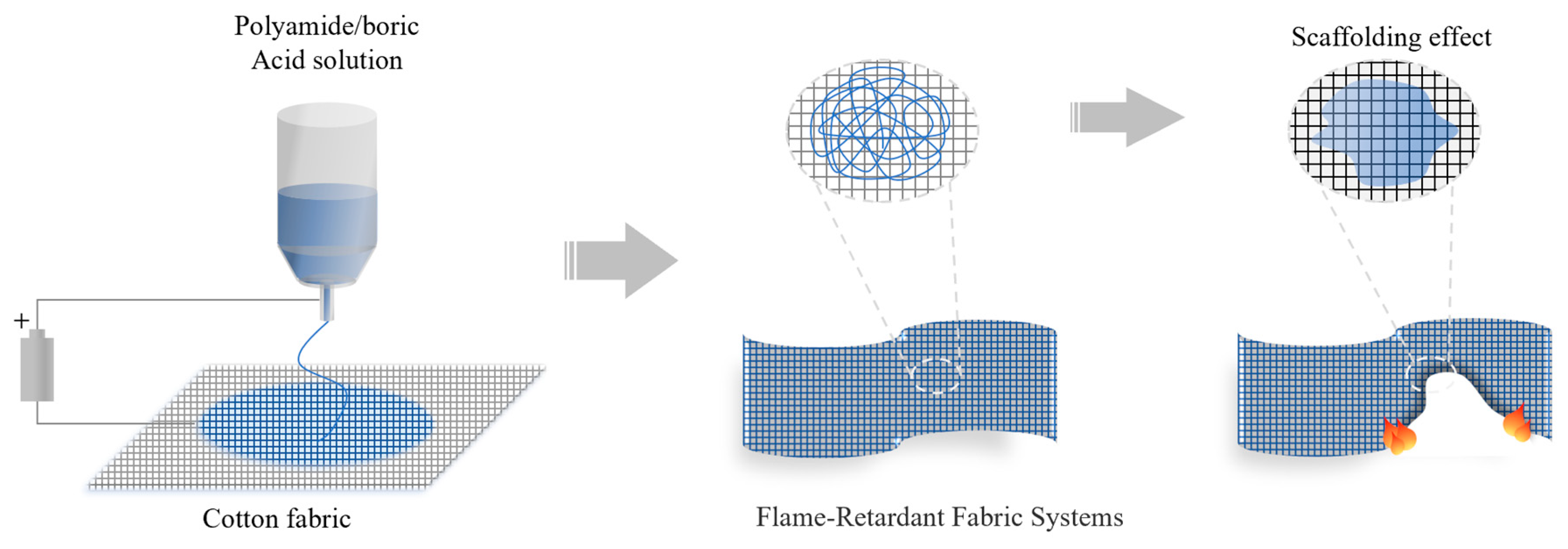
- Selvakumar, N.; Azhagurajan, A.; Natarajan, T.S.; Mohideen, A.K. Flame-retardant fabric systems based on electrospun polyamide/boric acid nanocomposite fibers. J. Appl. Polym. Sci. 2012, 126, 614–619. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, C.-Q.; Zhao, T. Heat release properties and flammability of the nylon/cotton blend fabric treated with a crosslinkable organophosphorus flame retardant system. J. Anal. Appl. Pyrolysis 2014, 110, 205–212. [Google Scholar] [CrossRef]


| Flame Retardants | Molecular Structures | References |
|---|---|---|
| 3-hydroxyphenylphosphinylpro-pionic acid (3-HPP) | 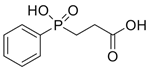 | [5] |
| 9,10-dihydro-10-[2,3-di(hydroxylcarbonyl) propyl]-10-phosphaphenanthrene-10-oxide (DDP) | 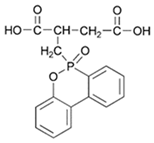 | [6] |
| 1-oxyphosa-4-hydroxymethyl-2,6,7-trioxabicyclic [2,2.2] octane (PEPA) | 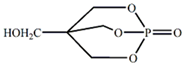 | [7] |
| 2,3-dicarboxy propyl diphenyl phosphine oxide (DPDPO) | 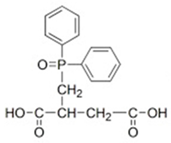 | [8] |
| Sample | LOI% | Ignition of Cotton (p/p) | UL 94 Rating |
|---|---|---|---|
| Pure PA6 | 25.5 ± 0.2 | Yes (5/5) | V-2 |
| 0.5 wt.% | 26.4 ± 0.1 | Yes (5/5) | V-2 |
| 1.5 wt.% | 30.0 ± 0.2 | No (3/5) | V-1 |
| 2.5 wt.% | 30.6 ± 0.3 | No (0/5) | V-0 |
| Formulation | TM (°C) | pHRR (W·g−1) | THR (kJ·g−1) | P content (wt.%) |
|---|---|---|---|---|
| PA6 | 480 ± 3 | 528 ± 8 | 26.1 ± 0.3 | – |
| PA(16P + 8Si) | 430 ± 1 | 459 ± 2 | 23.5 ± 0.4 | 1.22 |
| DiDopoMeO/PA6 | 422 ± 4 | 486 ± 12 | 24.4 ± 0.2 | 2.28 ± 3.3 × 10−2 |
| DiDopoEDA/PA6 | 429 ± 3 | 566 ± 11 | 24.4 ± 0.2 | 2.03 ± 0.1 × 10−2 |
| Exolit® OP 1230/PA6 | 455 ± 1 | 495 ± 3 | 24.7 ± 0.2 | 4.07 ± 1.8 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Liu, L.; Feng, H.; Li, D.; Xia, Z.; Yang, R. Flame Retardancy of Nylon 6 Fibers: A Review. Polymers 2023, 15, 2161. https://doi.org/10.3390/polym15092161
Guo X, Liu L, Feng H, Li D, Xia Z, Yang R. Flame Retardancy of Nylon 6 Fibers: A Review. Polymers. 2023; 15(9):2161. https://doi.org/10.3390/polym15092161
Chicago/Turabian StyleGuo, Xiaocheng, Linjing Liu, Haisheng Feng, Dinghua Li, Zhonghua Xia, and Rongjie Yang. 2023. "Flame Retardancy of Nylon 6 Fibers: A Review" Polymers 15, no. 9: 2161. https://doi.org/10.3390/polym15092161
APA StyleGuo, X., Liu, L., Feng, H., Li, D., Xia, Z., & Yang, R. (2023). Flame Retardancy of Nylon 6 Fibers: A Review. Polymers, 15(9), 2161. https://doi.org/10.3390/polym15092161






