In Vitro Fermentation of Hyaluronan with Different Molecular Weights by Human Gut Microbiota: Differential Effects on Gut Microbiota Structure and Metabolic Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation for Stool
2.3. In Vitro Fermentation
2.4. HA Degradation Analysis
2.5. SCFAs
2.6. Gut Microbiota Analysis
2.7. Untargeted Metabolomics Analysis
2.8. Statistical Analysis
3. Results
3.1. Degradation of HA
3.2. SCFAs Production during Fermentation
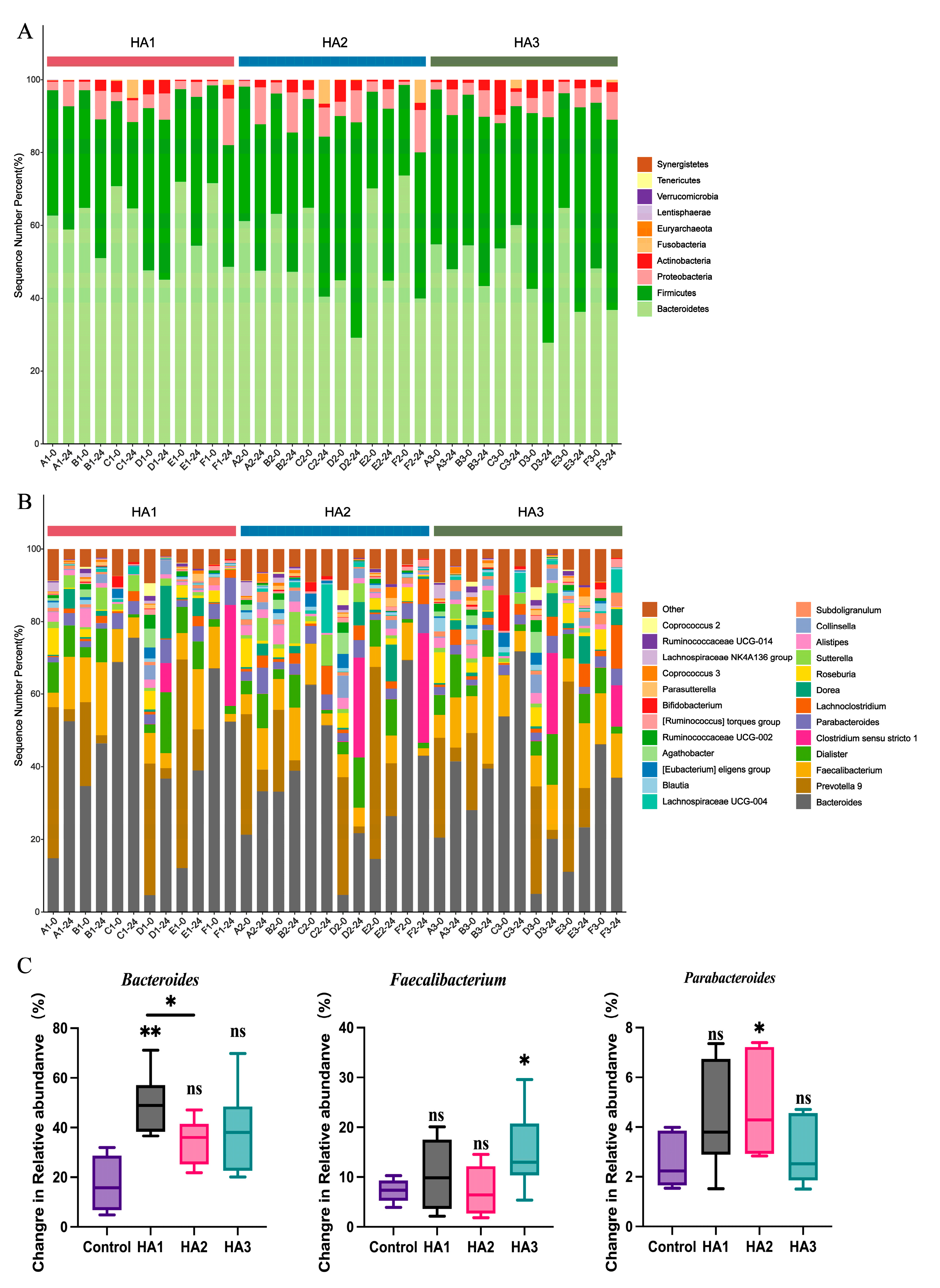
3.3. Changes in Microbial by Fermentation of HA
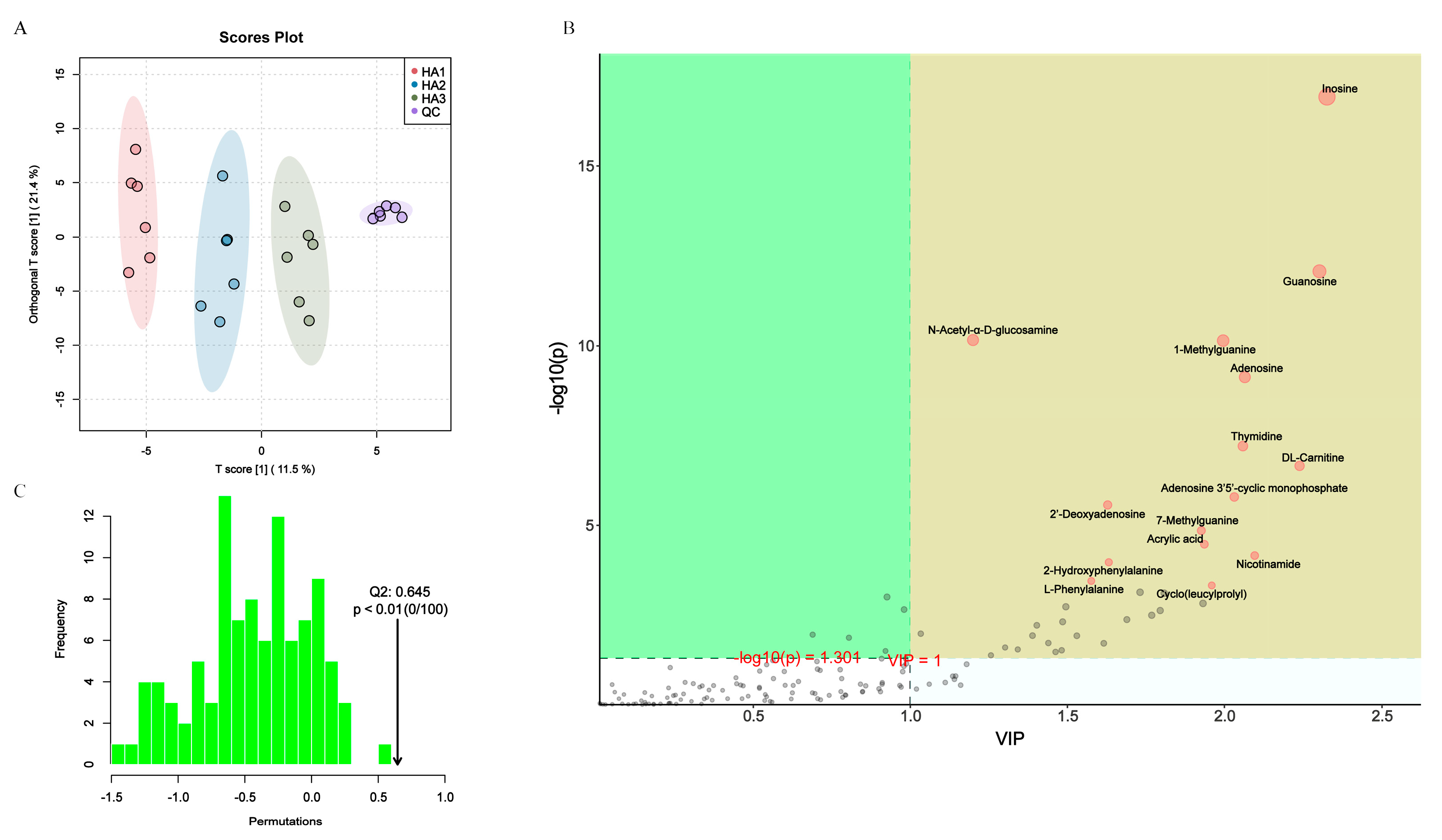
3.4. Changes in the Fecal Metabolism
3.5. Association of Gut Microbiota and Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. The Structure and Function of Hyaluronan: An Overview. Immunol. Cell Biol. 1996, 74, A1–A7. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Ma, Y.; Huang, Y.; Li, S.; Xu, H.; Su, E. Current Advances in the Biosynthesis of Hyaluronic Acid with Variable Molecular Weights. Carbohydr. Polym. 2021, 269, 118320. [Google Scholar] [CrossRef]
- Yao, Z.-Y.; Qin, J.; Gong, J.-S.; Ye, Y.-H.; Qian, J.-Y.; Li, H.; Xu, Z.-H.; Shi, J.-S. Versatile Strategies for Bioproduction of Hyaluronic Acid Driven by Synthetic Biology. Carbohydr. Polym. 2021, 264, 118015. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan Fragments: An Information-Rich System. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Ke, C.; Wang, D.; Sun, Y.; Qiao, D.; Ye, H.; Zeng, X. Immunostimulatory and Antiangiogenic Activities of Low Molecular Weight Hyaluronic Acid. Food Chem. Toxicol. 2013, 58, 401–407. [Google Scholar] [CrossRef]
- Boltje, T.J.; Buskas, T.; Boons, G.-J. Opportunities and Challenges in Synthetic Oligosaccharide and Glycoconjugate Research. Nat. Chem. 2009, 1, 611–622. [Google Scholar] [CrossRef]
- Kessler, S.P.; Obery, D.R.; Nickerson, K.P.; Petrey, A.C.; McDonald, C.; de la Motte, C.A. Multifunctional Role of 35 Kilodalton Hyaluronan in Promoting Defense of the Intestinal Epithelium. J. Histochem. Cytochem. 2018, 66, 273–287. [Google Scholar] [CrossRef]
- Zheng, L.; Riehl, T.E.; Stenson, W.F. Regulation of Colonic Epithelial Repair in Mice by Toll-Like Receptors and Hyaluronic Acid. Gastroenterology 2009, 137, 2041–2051. [Google Scholar] [CrossRef]
- Kimura, M.; Maeshima, T.; Kubota, T.; Kurihara, H.; Masuda, Y.; Nomura, Y. Absorption of Orally Administered Hyaluronan. J. Med. Food 2016, 19, 1172–1179. [Google Scholar] [CrossRef]
- Pan, L.; Ai, X.; Fu, T.; Ren, L.; Shang, Q.; Li, G.; Yu, G. In Vitro Fermentation of Hyaluronan by Human Gut Microbiota: Changes in Microbiota Community and Potential Degradation Mechanism. Carbohydr. Polym. 2021, 269, 118313. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Su, C.-W.; Ji, Q.; Chen, C.-Y.; Wang, R.; Vijaya Kumar, D.; Lan, J.; Jiao, L.; Shi, H.N. Hyaluronan-Induced Alterations of the Gut Microbiome Protects Mice against Citrobacter Rodentium Infection and Intestinal Inflammation. Gut Microbes 2021, 13, 1972757. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Chen, C.; Fu, X. Digestive Property and Bioactivity of Blackberry Polysaccharides with Different Molecular Weights. J. Agric. Food Chem. 2019, 67, 12428–12440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, L.; Zhai, Q.; Zhao, R.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. In Vitro Fermentation of Heparin by the Human Gut Microbiota: Changes in the Microbiota Community and Metabolic Functions. Food Chem. 2023, 406, 135010. [Google Scholar] [CrossRef]
- Goodman, A.L.; Kallstrom, G.; Faith, J.J.; Reyes, A.; Moore, A.; Dantas, G.; Gordon, J.I. Extensive Personal Human Gut Microbiota Culture Collections Characterized and Manipulated in Gnotobiotic Mice. Proc. Natl. Acad. Sci. USA 2011, 108, 6252–6257. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A Modified Uronic Acid Carbazole Reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the Role of 5-Hydroxytryptophan Synthesis Regulation Alleviates the Symptom of Depression and Related Microbiota Dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut Microbiota-Derived Inosine from Dietary Barley Leaf Supplementation Attenuates Colitis through PPARγ Signaling Activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef]
- Kawada, C.; Yoshida, T.; Yoshida, H.; Matsuoka, R.; Sakamoto, W.; Odanaka, W.; Sato, T.; Yamasaki, T.; Kanemitsu, T.; Masuda, Y.; et al. Ingested Hyaluronan Moisturizes Dry Skin. Nutr. J. 2014, 13, 70. [Google Scholar] [CrossRef]
- Oe, M.; Tashiro, T.; Yoshida, H.; Nishiyama, H.; Masuda, Y.; Maruyama, K.; Koikeda, T.; Maruya, R.; Fukui, N. Oral Hyaluronan Relieves Knee Pain: A Review. Nutr. J. 2016, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Nogal, A.; Valdes, A.M.; Menni, C. The Role of Short-Chain Fatty Acids in the Interplay between Gut Microbiota and Diet in Cardio-Metabolic Health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Bajic, D.; Niemann, A.; Hillmer, A.-K.; Mejias-Luque, R.; Bluemel, S.; Docampo, M.; Funk, M.C.; Tonin, E.; Boutros, M.; Schnabl, B.; et al. Gut Microbiota-Derived Propionate Regulates the Expression of Reg3 Mucosal Lectins and Ameliorates Experimental Colitis in Mice. J. Crohn’s Colitis 2020, 14, 1462–1472. [Google Scholar] [CrossRef]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, J.; Yi, J.; Wu, X.; Ma, Y.; Li, R. Pectin and Homogalacturonan with Small Molecular Mass Modulate Microbial Community and Generate High SCFAs via in Vitro Gut Fermentation. Carbohydr. Polym. 2021, 269, 118326. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Chengxiao, Y.; Dongmei, W.; Kai, Z.; Hou, L.; Xiao, H.; Ding, T.; Liu, D.; Ye, X.; Linhardt, R.J.; Chen, S. Challenges of Pectic Polysaccharides as a Prebiotic from the Perspective of Fermentation Characteristics and Anti-Colitis Activity. Carbohydr. Polym. 2021, 270, 118377. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Tomioka, S.; Seki, N.; Sugiura, Y.; Akiyama, M.; Uchiyama, J.; Yamaguchi, G.; Yakabe, K.; Ejima, R.; Hattori, K.; Kimizuka, T.; et al. Cooperative Action of Gut-Microbiota-Accessible Carbohydrates Improves Host Metabolic Function. Cell Rep. 2022, 40, 111087. [Google Scholar] [CrossRef]
- Aoki, R.; Onuki, M.; Hattori, K.; Ito, M.; Yamada, T.; Kamikado, K.; Kim, Y.-G.; Nakamoto, N.; Kimura, I.; Clarke, J.M.; et al. Commensal Microbe-Derived Acetate Suppresses NAFLD/NASH Development via Hepatic FFAR2 Signalling in Mice. Microbiome 2021, 9, 188. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides Produces Acetate to Alleviate Heparanase-Exacerbated Acute Pancreatitis through Reducing Neutrophil Infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef]
- Fu, Y.-P.; Peng, X.; Zhang, C.-W.; Jiang, Q.-X.; Li, C.-Y.; Paulsen, B.S.; Rise, F.; Huang, C.; Feng, B.; Li, L.-X.; et al. Salvia Miltiorrhiza Polysaccharide and Its Related Metabolite 5-Methoxyindole-3-Carboxaldehyde Ameliorate Experimental Colitis by Regulating Nrf2/Keap1 Signaling Pathway. Carbohydr. Polym. 2023, 306, 120626. [Google Scholar] [CrossRef]
- Ghaffarzadegan, T.; Marungruang, N.; Fåk, F.; Nyman, M. Molecular Properties of Guar Gum and Pectin Modify Cecal Bile Acids, Microbiota, and Plasma Lipopolysaccharide-Binding Protein in Rats. PLoS ONE 2016, 11, e0157427. [Google Scholar] [CrossRef]
- Regulation of Gut Microbiota and Intestinal Metabolites by Poria Cocos Oligosaccharides Improves Glycolipid Metabolism Disturbance in High-Fat Diet-Fed Mice—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35472435/ (accessed on 5 March 2023).
- Shi, C.-X.; Zhao, M.-X.; Shu, X.-D.; Xiong, X.-Q.; Wang, J.-J.; Gao, X.-Y.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. β-Aminoisobutyric Acid Attenuates Hepatic Endoplasmic Reticulum Stress and Glucose/Lipid Metabolic Disturbance in Mice with Type 2 Diabetes. Sci. Rep. 2016, 6, 21924. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Abbey-Toby, A.; Igoudjil, A.; Lettéron, P.; Fromenty, B. β-Aminoisobutyric Acid Prevents Diet-Induced Obesity in Mice with Partial Leptin Deficiency. Obesity 2008, 16, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ghali, S.; Xu, C.; Mussatto, C.C.; Ortiz, C.; Lee, E.C.; Tran, D.H.; Jacobs, J.P.; Lagishetty, V.; Faull, K.F.; et al. Ceragenin CSA13 Reduces Clostridium Difficile Infection in Mice by Modulating the Intestinal Microbiome and Metabolites. Gastroenterology 2018, 154, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.N.; Brannan, L.; Guri, A.J.; Lu, P.; Hontecillas, R.; Bassaganya-Riera, J.; Bevan, D.R. Dietary α-Eleostearic Acid Ameliorates Experimental Inflammatory Bowel Disease in Mice by Activating Peroxisome Proliferator-Activated Receptor-γ. PLoS ONE 2011, 6, e24031. [Google Scholar] [CrossRef]
- Tao, J.-H.; Duan, J.-A.; Zhang, W.; Jiang, S.; Guo, J.-M.; Wei, D.-D. Polysaccharides From Chrysanthemum Morifolium Ramat Ameliorate Colitis Rats via Regulation of the Metabolic Profiling and NF-κ B/TLR4 and IL-6/JAK2/STAT3 Signaling Pathways. Front. Pharmacol. 2018, 9, 746. [Google Scholar] [CrossRef]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin Alleviates Inflammation and Modulates Gut Microbiota in Ulcerative Colitis Rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef]
- Niu, W.; Dong, Y.; Fu, Z.; Lv, J.; Wang, L.; Zhang, Z.; Huo, J.; Ju, J. Effects of Molecular Weight of Chitosan on Anti-Inflammatory Activity and Modulation of Intestinal Microflora in an Ulcerative Colitis Model. Int. J. Biol. Macromol. 2021, 193, 1927–1936. [Google Scholar] [CrossRef]



| Volunteers | Gender | Age | BMI |
|---|---|---|---|
| A | Female | 24 | 21.5 |
| B | Female | 26 | 22.7 |
| C | Female | 25 | 20.2 |
| D | Male | 24 | 21.8 |
| E | Male | 30 | 21.8 |
| F | Male | 22 | 23.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Zhang, C.; Yu, L.; Zhang, C.; Zhao, J.; Narbad, A.; Zhai, Q.; Tian, F. In Vitro Fermentation of Hyaluronan with Different Molecular Weights by Human Gut Microbiota: Differential Effects on Gut Microbiota Structure and Metabolic Function. Polymers 2023, 15, 2103. https://doi.org/10.3390/polym15092103
Zhao R, Zhang C, Yu L, Zhang C, Zhao J, Narbad A, Zhai Q, Tian F. In Vitro Fermentation of Hyaluronan with Different Molecular Weights by Human Gut Microbiota: Differential Effects on Gut Microbiota Structure and Metabolic Function. Polymers. 2023; 15(9):2103. https://doi.org/10.3390/polym15092103
Chicago/Turabian StyleZhao, Ruohan, Chuan Zhang, Leilei Yu, Chengcheng Zhang, Jianxin Zhao, Arjan Narbad, Qixiao Zhai, and Fengwei Tian. 2023. "In Vitro Fermentation of Hyaluronan with Different Molecular Weights by Human Gut Microbiota: Differential Effects on Gut Microbiota Structure and Metabolic Function" Polymers 15, no. 9: 2103. https://doi.org/10.3390/polym15092103
APA StyleZhao, R., Zhang, C., Yu, L., Zhang, C., Zhao, J., Narbad, A., Zhai, Q., & Tian, F. (2023). In Vitro Fermentation of Hyaluronan with Different Molecular Weights by Human Gut Microbiota: Differential Effects on Gut Microbiota Structure and Metabolic Function. Polymers, 15(9), 2103. https://doi.org/10.3390/polym15092103







