Photooxidative Behavior of Polystyrene Nanocomposites Filled with Two-Dimensional Molybdenum Disulfide
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Sample Preparation
2.2. Production of PS/MoS2 Nanocomposites
2.3. Accelerated Laboratory Weathering
2.4. Characterization Techniques
2.4.1. UV-Vis Spectroscopy
2.4.2. Fourier Transform Infrared Spectroscopy
2.4.3. Size Exclusion Chromatography
3. Results and Discussion
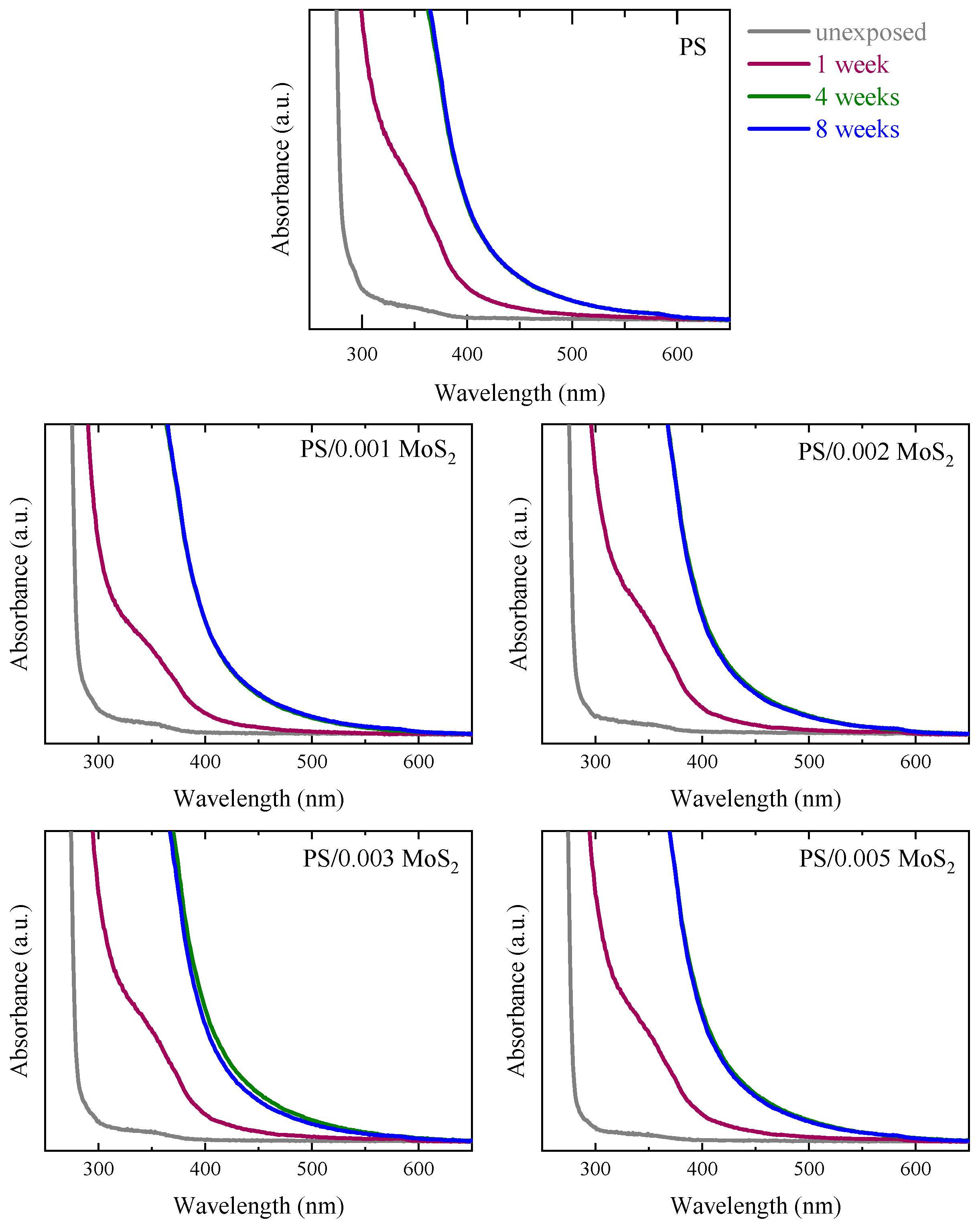
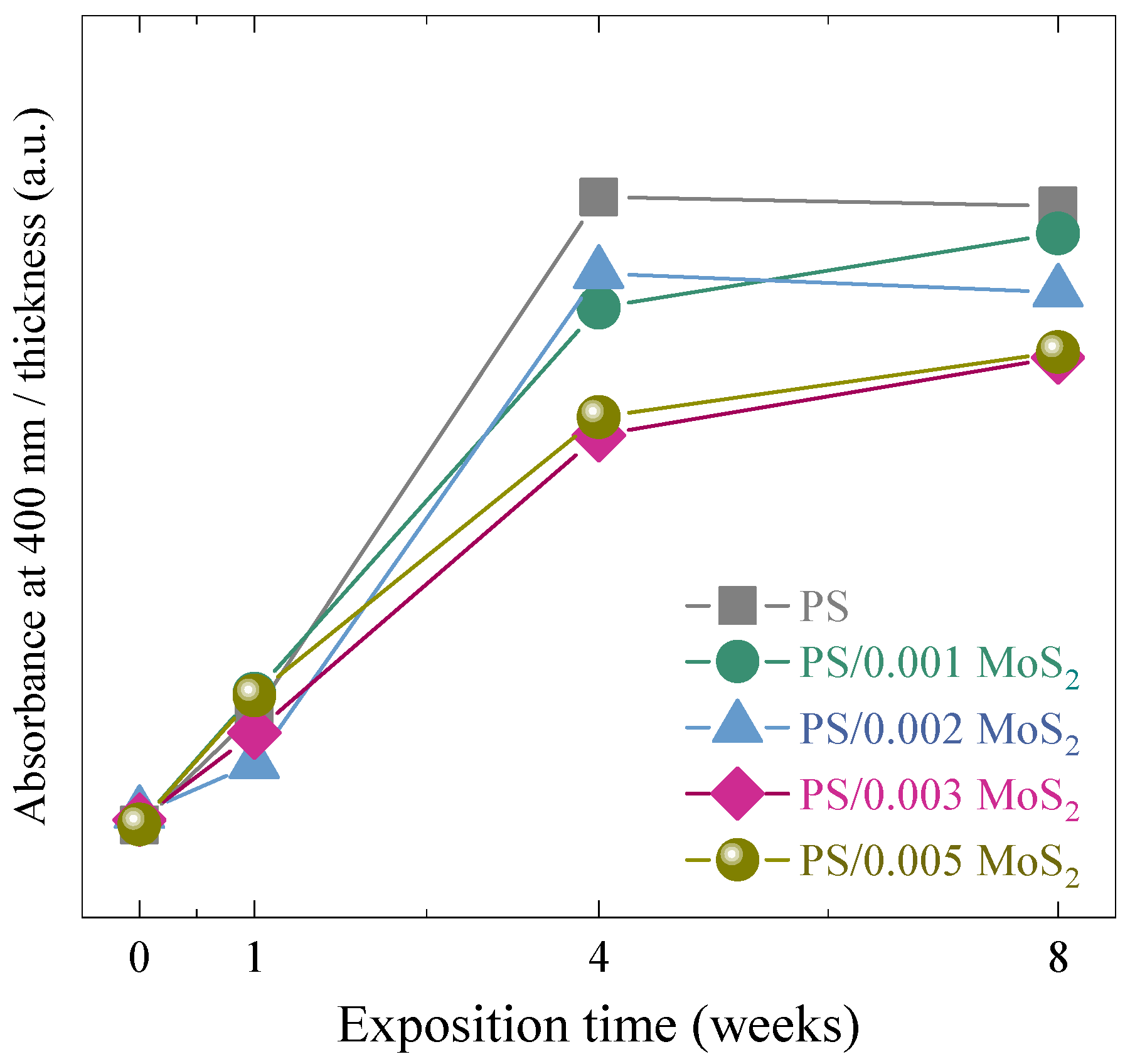
3.1. UV-Vis Spectroscopy
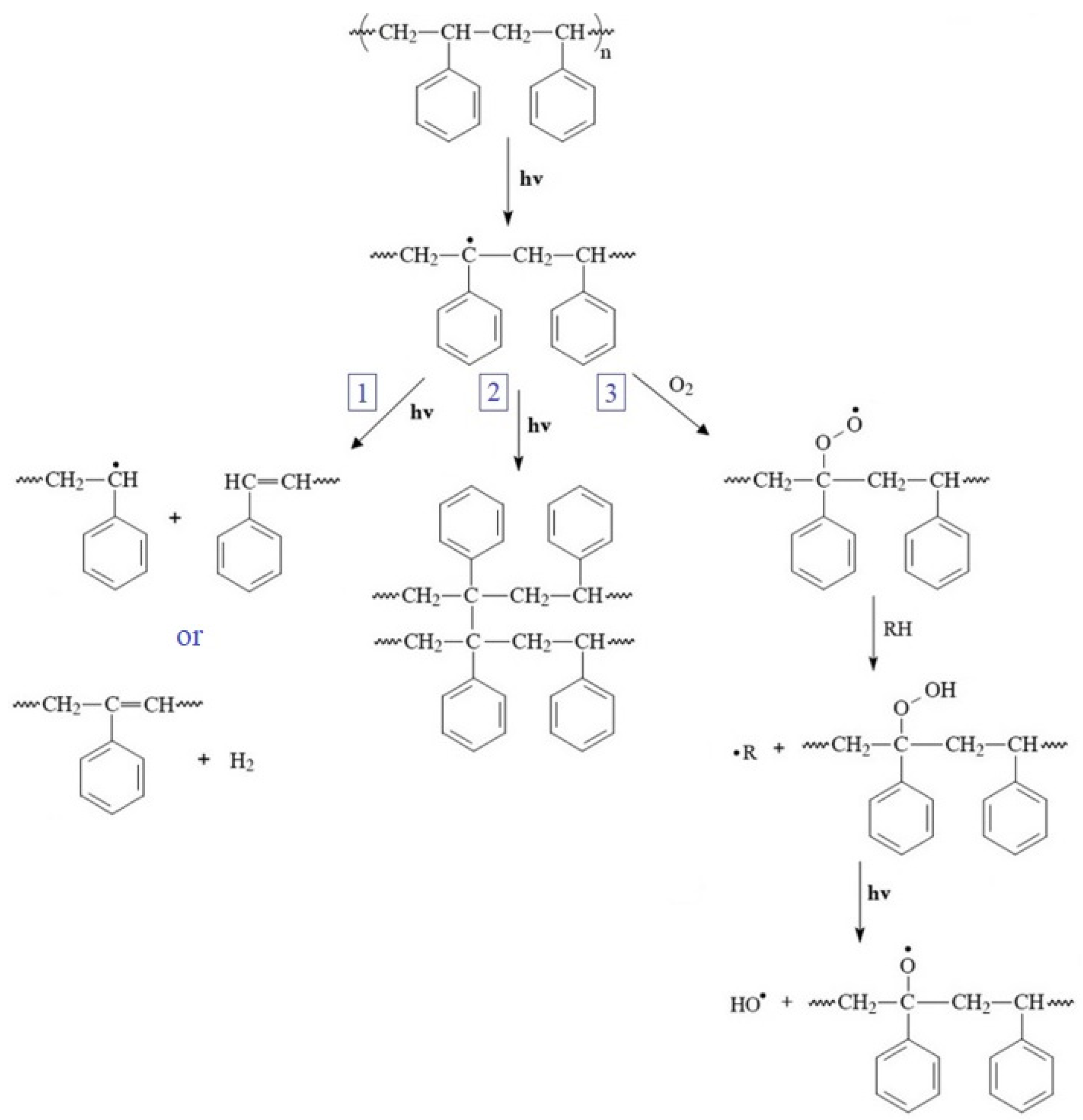
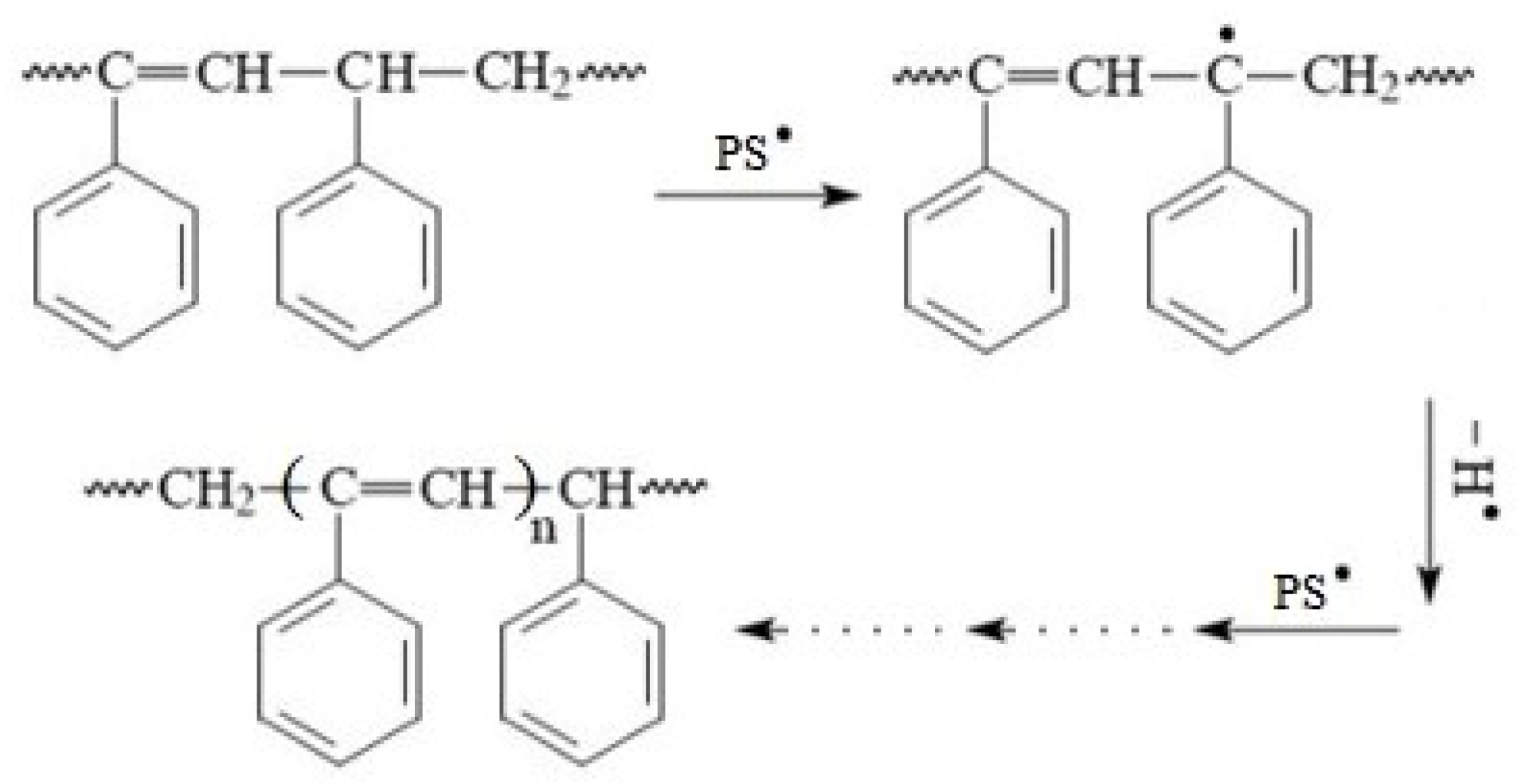
3.2. FTIR
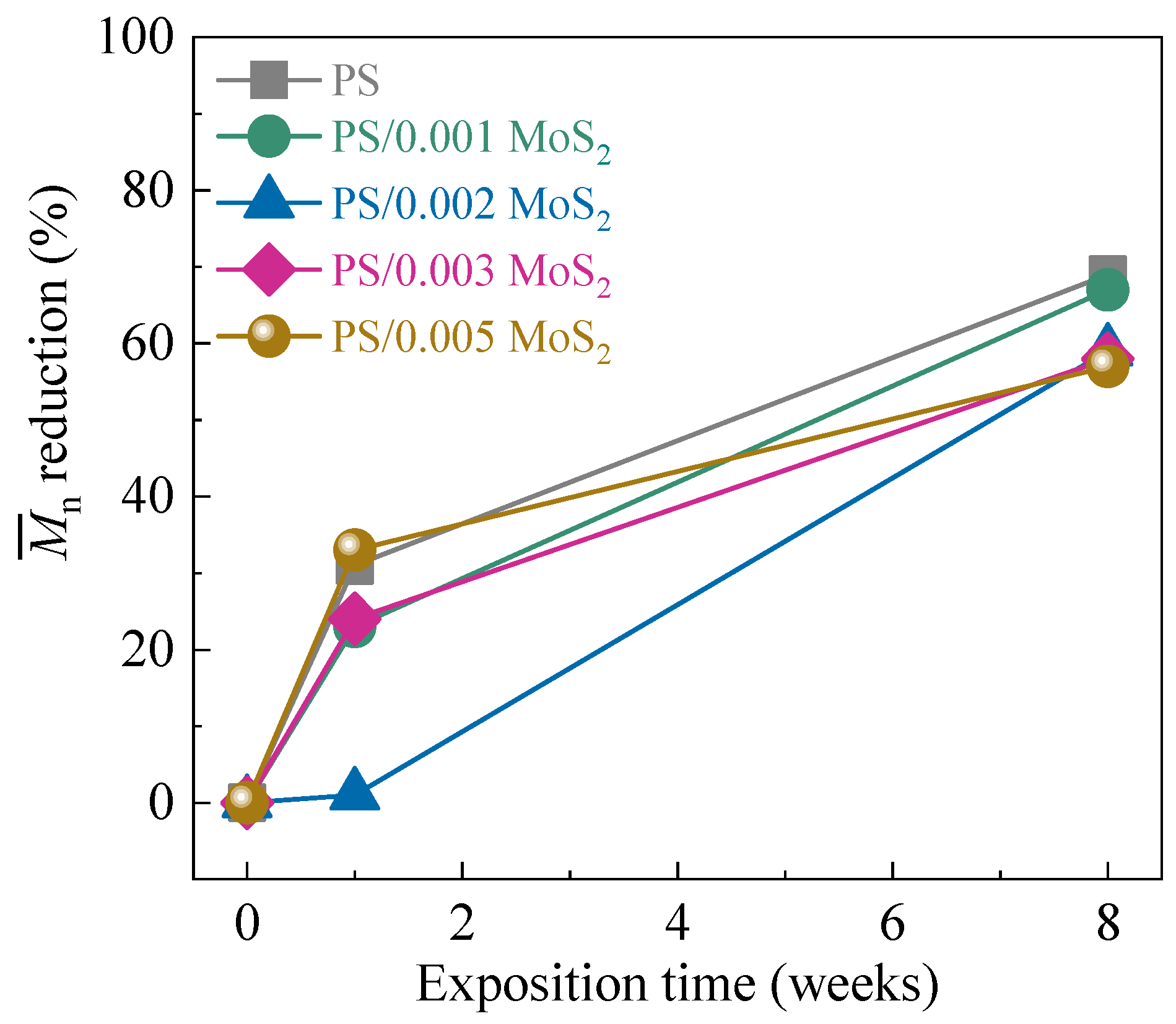
3.3. Molecular Weight Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, W.; Hou, J.; Li, T.; Li, P.; Wang, Y.; Liu, G.; Wang, K. Enhanced Visible Light Photochemical Activity and Stability of MoS2/Cu2O Nanocomposites by Tunable Heterojunction. Mater. Today Commun. 2020, 23, 100933. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Singh, K.; Kumar, M.; Kumar, A. A Synoptic Review of MoS2: Synthesis to Applications. Superlattices Microstruct. 2019, 128, 274–297. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Colas, G.; Saulot, A.; Regis, E.; Berthier, Y. Investigation of Crystalline and Amorphous MoS2 Based Coatings: Towards Developing New Coatings for Space Applications. Wear 2015, 330–331, 448–460. [Google Scholar] [CrossRef]
- Sorrentino, A.; Altavilla, C.; Merola, M.; Senatore, A.; Ciambelli, P.; Iannace, S. Nanosheets of MoS2-Oleylamine as Hybrid Filler for Self-Lubricating Polymer Composites: Thermal, Tribological, and Mechanical Properties. Polym. Compos. 2015, 36, 1124–1134. [Google Scholar] [CrossRef]
- Furlan, K.P.; de Mello, J.D.B.; Klein, A.N. Self-Lubricating Composites Containing MoS2: A Review. Tribol. Int. 2018, 120, 280–298. [Google Scholar] [CrossRef]
- Bai, J.; Song, J.; Wei, J. Tribological and Mechanical Properties of MoS2 Enhanced Polyamide 12 for Selective Laser Sintering. J. Mater. Process. Technol. 2019, 264, 382–388. [Google Scholar] [CrossRef]
- Shao, N.; Wang, J.; Wang, D.; Corvini, P. Preparation of Three-Dimensional Ag3PO4/TiO2@MoS2 for Enhanced Visible-Light Photocatalytic Activity and Anti-Photocorrosion. Appl. Catal. B 2017, 203, 964–978. [Google Scholar] [CrossRef]
- Gong, C.; Hu, K.; Wang, X.; Wangyang, P.; Yan, C.; Chu, J.; Liao, M.; Dai, L.; Zhai, T.; Wang, C.; et al. 2D Nanomaterial Arrays for Electronics and Optoelectronics. Adv. Funct. Mater. 2018, 28, 1706559. [Google Scholar] [CrossRef]
- Phan, T.T.T.; Nguyen, T.T.H.; Huu, H.T.; Truong, T.T.; Nguyen, L.T.; Nguyen, V.T.; Tran, V.A.; Nguyen, T.L.; Nguyen, H.L.; Vo, V. Hydrothermal Synthesis of MoS2/RGO Heterostructures for Photocatalytic Degradation of Rhodamine B under Visible Light. J. Nanomater. 2021, 2021, 9941202. [Google Scholar] [CrossRef]
- Akshatha, S.R.; Sreenivasa, S.; Parashuram, L.; Raghu, M.S.; Kumar, K.Y.; Rao, T.M.C. Visible-Light-Induced Photochemical Hydrogen Evolution and Degradation of Crystal Violet Dye by Interwoven Layered MoS2/Wurtzite ZnS Heterostructure Photocatalyst. Chemistry Select 2020, 5, 6918–6926. [Google Scholar] [CrossRef]
- Ghim, D.; Chou, P.I.; Chae, S.H.; Jun, Y.S. Effects of MoS2 Layer Thickness on Its Photochemically Driven Oxidative Dissolution. Environ. Sci. Technol. 2021, 55, 13759–13769. [Google Scholar] [CrossRef]
- Grause, G.; Chien, M.F.; Inoue, C. Changes during the Weathering of Polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- Yagoubi, W.; Abdelhafidi, A.; Sebaa, M.; Chabira, S.F. Identification of Carbonyl Species of Weathered LDPE Films by Curve Fitting and Derivative Analysis of IR Spectra. Polym. Test 2015, 44, 37–48. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, Z.; Liu, Y.; Lai, B.; Ong, W.-J.; Wang, S.; Duan, X. Recent Advances in Molybdenum Disulfide-Based Advanced Oxidation Processes. Environ. Funct. Mater. 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-Term Phototransformation of Microplastics under Simulated Sunlight Irradiation in Aquatic Environments: Roles of Reactive Oxygen Species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef]
- Strohriegl, P.; Senker, J.; Meides, N.; Menzel, T.; Poetzschner, B.; Löder, M.G.J.; Mansfeld, U.; Altstaedt, V. Reconstructing the Environmental Degradation of Polystyrene by Accelerated Weathering. Environ. Sci. Technol. 2021, 55, 7930–7938. [Google Scholar] [CrossRef]
- Garg, S.; Rose, A.L.; Waite, T.D. Photochemical Production of Superoxide and Hydrogen Peroxide from Natural Organic Matter. Geochim. Cosmochim. Acta 2011, 75, 4310–4320. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, L.; Yang, Q.; Zupanic, A.; Xu, Q.; Jiang, L. Using MoS2 Nanomaterials to Generate or Remove Reactive Oxygen Species: A Review. ACS Appl. Nano Mater. 2021, 4, 7523–7537. [Google Scholar] [CrossRef]
- Chen, T.; Zou, H.; Wu, X.; Liu, C.; Situ, B.; Zheng, L.; Yang, G. Nanozymatic Antioxidant System Based on MoS2 Nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 12453–12462. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, Q.; Wang, L.; Liu, B.; Wang, K.; Wang, Z. Dual Roles of MoS2 Nanosheets in Advanced Oxidation Processes: Activating Permonosulfate and Quenching Radicals. Chem. Eng. J. 2022, 440, 135866. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental Applications of 2D Molybdenum Disulfide (MoS2) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.M.; Silva, G.C.; Fechine, G.J.M. Effect of Exfoliation Medium on the Morphology of Multi-Layer Graphene Oxide and Its Importance for Poly(Ethylene Terephthalate) Based Nanocomposites. Polym. Test 2020, 90, 106742. [Google Scholar] [CrossRef]
- Sethulekshmi, A.S.; Jayan, J.S.; Saritha, A.; Joseph, K. Insights into the Reinforcibility and Multifarious Role of WS2 in Polymer Matrix. J Alloys Compd. 2021, 876, 160107. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Jia, Q.; Zhang, Y.; Wu, G.; Ji, T. Improved Thermal Conductivity and Dielectric Properties of HBN/PTFE Composites via Surface Treatment by Silane Coupling Agent. Compos. B Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Batista, N.L.; Helal, E.; Kurusu, R.S.; Moghimian, N.; David, E.; Demarquette, N.R.; Hubert, P. Mass-Produced Graphene—HDPE Nanocomposites: Thermal, Rheological, Electrical, and Mechanical Properties. Polym. Eng. Sci. 2019, 59, 675–682. [Google Scholar] [CrossRef]
- Zhou, K.; Jiang, S.; Bao, C.; Song, L.; Wang, B.; Tang, G.; Hu, Y.; Gui, Z. Preparation of Poly(Vinyl Alcohol) Nanocomposites with Molybdenum Disulfide (MoS2): Structural Characteristics and Markedly Enhanced Properties. RSC Adv. 2012, 2, 11695–11703. [Google Scholar] [CrossRef]
- Fechine, G.J.M.; Rabello, M.S.; Souto-Maior, R.M. The Effect of Ultraviolet Stabilizers on the Photodegradation of Poly(Ethylene Terephthalate). Polym. Degrad. Stab. 2002, 75, 153–159. [Google Scholar] [CrossRef]
- Oliveira, C.F.P.; Carastan, D.J.; Demarquette, N.R.; Fechine, G.J.M. Photooxidative Behavior of Polystyrene–Montmorillonite Nanocomposites. Polym. Eng. Sci. 2008, 48, 1511–1517. [Google Scholar] [CrossRef]
- Afshar, M.; Morshedian, J.; Ahmadi, S. Radiation Attenuation Capability and Flow Characteristics of HDPE Composite Loaded with W, MoS2, and B4C. Polym. Compos. 2017, 40, 149–158. [Google Scholar] [CrossRef]
- Teijido, R.; Ruiz-Rubio, L.; Echaide, A.G.; Vilas-Vilela, J.L.; Lanceros-Mendez, S.; Zhang, Q. State of the Art and Current Trends on Layered Inorganic-Polymer Nanocomposite Coatings for Anticorrosion and Multi-Functional Applications. Prog. Org. Coat 2022, 163, 106684. [Google Scholar] [CrossRef]
- Rodriguez, C.L.C.; Nunes, M.A.B.S.; Garcia, P.S.; Fechine, G.J.M. Molybdenum Disulfide as a Filler for a Polymeric Matrix at an Ultralow Content: Polystyrene Case. Polym. Test 2021, 93, 106882. [Google Scholar] [CrossRef]
- Muñoz, P.A.R.; de Oliveira, C.F.P.; Amurin, L.G.; Rodriguez, C.L.C.; Nagaoka, D.A.; Tavares, M.I.B.; Domingues, S.H.; Andrade, R.J.E.; Fechine, G.J.M. Novel Improvement in Processing of Polymer Nanocomposite Based on 2D Materials as Fillers. Express. Polym. Lett. 2018, 12, 930–945. [Google Scholar] [CrossRef]
- Trinh, D.V.; Linton, R.C.; Vaughn, J.A.; Finckenor, M.M.; Van De Mark, M.R. Solar Simulation Photodegradation of Polystyrene: Phthalocyanine Pigments as Inhibitor of the Photodegradation Process. Polym. Degrad. Stab. 1994, 46, 325–331. [Google Scholar] [CrossRef]
- Gardette, J.-L.; Mailhot, B.; Lemaire, J. Photooxidation Mechanisms of Styrenic Polymers. Polym. Degrad. Stab. 1995, 48, 457–470. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and Thermal-Oxidation of Polyethylene: Comparison of Mechanisms and Influence of Unsaturation Content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Millan, M.D.; Locklin, J.; Fulghum, T.; Baba, A.; Advincula, R.C. Polymer Thin Film Photodegradation and Photochemical Crosslinking: FT-IR Imaging, Evanescent Waveguide Spectroscopy, and QCM Investigations. Polymer 2005, 46, 5556–5568. [Google Scholar] [CrossRef]
- Fechine, G.J.M.; Christensen, P.A.; Egerton, T.A.; White, J.R. Evaluation of Poly(Ethylene Terephthalate) Photostabilisation Using FTIR Spectrometry of Evolved Carbon Dioxide. Polym. Degrad. Stab. 2009, 94, 234–239. [Google Scholar] [CrossRef]
- Rodriguez, C.L.C.; Muñoz, P.A.R.; Donato, K.Z.; Seixas, L.; Donato, R.K.; Fechine, G.J.M. Understanding the Unorthodox Stabilization of Liquid Phase Exfoliated Molybdenum Disulfide (MoS2) in Water Medium. Phys. Chem. Chem. Phys. 2020, 22, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Rabek, J.F. Polymer Photodegradation, 1st ed.; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Kaczmarek, H.; Kaminâ Ska, A.; Van Herk, A. Photooxidative Degradation of Poly(Alkyl Methacrylate)s. Eur. Polym. J. 2000, 36, 767–777. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Kamińska, A.; Światek, M.; Sanyal, S. Photoinitiated Degradation of Polystyrene in the Presence of Low-Molecular Organic Compounds. Eur. Polym. J. 2000, 36, 1167–1173. [Google Scholar] [CrossRef]
- Martínez-Pardo, M.E.; Cardoso, J.; Vázquez, H.; Aguilar, M. Characterization of MeV Proton Irradiated PS and LDPE Thin Films. Nucl. Instrum. Methods Phys. Res. B 1997, 140, 325–340. [Google Scholar] [CrossRef]
- Hardesty, J.H.; Attili, B. Spectrophotometry and the Beer-Lambert Law: An important Analytical Technique in Chemistry. Collin College, Department of Chemistry. 2010. Available online: http://vfsilesieux.free.fr/1Seuro/BeerLaw.pdf (accessed on 12 October 2022).
- Pilař, J.; Michálková, D.; Šeděnková, I.; Pfleger, J.; Pospíšil, J. NOR and Nitroxide-Based HAS in Accelerated Photooxidation of Carbon-Chain Polymers; Comparison with Secondary HAS: An ESRI and ATR FTIR Study. Polym Degrad Stab 2011, 96, 847–862. [Google Scholar] [CrossRef]
- Salvalaggio, M.; Bagatin, R.; Fornaroli, M.; Fanutti, S.; Palmery, S.; Battistel, E. Multi-Component Analysis of Low-Density Polyethylene Oxidative Degradation. Polym Degrad Stab 2006, 91, 2775–2785. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Q.; Jiang, W.; Wang, L.; Xie, H.; Kalogerakis, N.; Ma, Y.; Ji, R. A Carbon-14 Radiotracer-Based Study on the Phototransformation of Polystyrene Nanoplastics in Water: Versus in Air. Environ Sci Nano. 2019, 6, 2907–2917. [Google Scholar] [CrossRef]
- Yang, X.; Ding, X. Prediction of Outdoor Weathering Performance of Polypropylene Filaments by Accelerated Weathering Tests. Geotext. Geomembr. 2006, 24, 103–109. [Google Scholar] [CrossRef]
- Pinto, G.M.; Cremonezzi, J.M.O.; Ribeiro, H.; Andrade, R.J.E.; Demarquette, N.R.; Fechine, G.J.M. From two-dimensional materials to polymer nanocomposites with emerging multifunctional applications: A critical review. Polym. Compos. 2023, 44, 1438–1470. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Gorrasi, G.; Vittoria, V. Effect of Carbon Nanotubes on the Photo-Oxidative Durability of Syndiotactic Polypropylene. Polym. Degrad. Stab. 2010, 95, 1614–1626. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef]

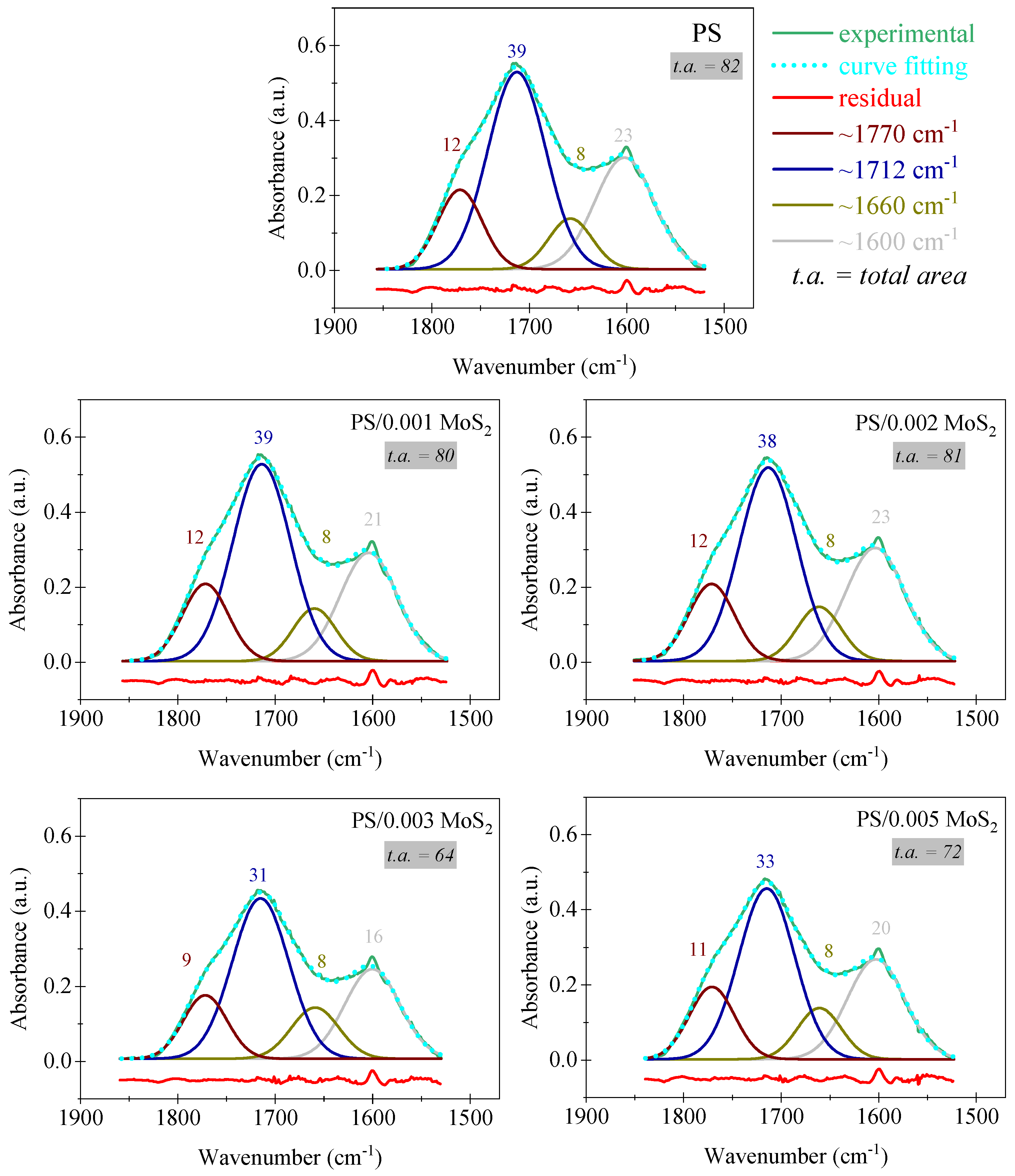

| Unexposed | 4 Weeks | 8 Weeks | |
|---|---|---|---|
| PS |  |  |  |
| PS/0.001 MoS2 |  |  |  |
| PS/0.002 MoS2 |  |  |  |
| PS/0.003 MoS2 |  |  |  |
| PS/0.005 MoS2 |  |  |  |
| System | Irradiation Time (Weeks) | (Da) | (Da) | / |
|---|---|---|---|---|
| PS | 0 | 73,033 | 169,530 | 2.3 |
| 1 | 50,615 | 127,206 | 2.5 | |
| 8 | 22,871 | 76,998 | 3.4 | |
| PS/MoS2 0.001 | 0 | 72,563 | 168,947 | 2.3 |
| 1 | 56,024 | 139,200 | 2.5 | |
| 8 | 24,156 | 72,920 | 3.0 | |
| PS/MoS2 0.002 | 0 | 71,499 | 161,681 | 2.3 |
| 1 | 72,247 | 148,225 | 2.1 | |
| 8 | 29,438 | 97,328 | 3.3 | |
| PS/MoS2 0.003 | 0 | 77,421 | 170,730 | 2.2 |
| 1 | 58,888 | 149,415 | 2.5 | |
| 8 | 32,387 | 109,711 | 3.4 | |
| PS/MoS2 0.005 | 0 | 75,750 | 161,050 | 2.1 |
| 1 | 51,002 | 131,176 | 2.6 | |
| 8 | 32,817 | 89,719 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo, A.L.; Rodriguez, C.L.C.; Fechine, G.J.M. Photooxidative Behavior of Polystyrene Nanocomposites Filled with Two-Dimensional Molybdenum Disulfide. Polymers 2023, 15, 2099. https://doi.org/10.3390/polym15092099
Angulo AL, Rodriguez CLC, Fechine GJM. Photooxidative Behavior of Polystyrene Nanocomposites Filled with Two-Dimensional Molybdenum Disulfide. Polymers. 2023; 15(9):2099. https://doi.org/10.3390/polym15092099
Chicago/Turabian StyleAngulo, Aurianny Lima, Camila Laura Celis Rodriguez, and Guilhermino José Macedo Fechine. 2023. "Photooxidative Behavior of Polystyrene Nanocomposites Filled with Two-Dimensional Molybdenum Disulfide" Polymers 15, no. 9: 2099. https://doi.org/10.3390/polym15092099
APA StyleAngulo, A. L., Rodriguez, C. L. C., & Fechine, G. J. M. (2023). Photooxidative Behavior of Polystyrene Nanocomposites Filled with Two-Dimensional Molybdenum Disulfide. Polymers, 15(9), 2099. https://doi.org/10.3390/polym15092099