Toughening Polylactide Stereocomplex by Injection Molding with Thermoplastic Starch and Chain Extender
Abstract
1. Introduction
- (a)
- The polylactide stereocomplex (ST) would have better thermal stability than PLLA;
- (b)
- Thermoplastic starch would create a tougher polylactide stereocomplex;
- (c)
- The multifunctional epoxide group of a chain extender would reduce the stereocomplex degradation and enhance the properties of ST/TPS blends.
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Material Characterization
2.3.1. Gel Permeation Chromatography
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. X-ray Diffraction Analysis
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Dynamic Mechanical Analysis (DMA)
2.3.7. Heat Resistance Analysis
2.3.8. Tensile Testing
2.3.9. Impact Testing
3. Results
3.1. Injection Molding of Blends
3.2. Thermal Properties
3.3. XRD Analysis
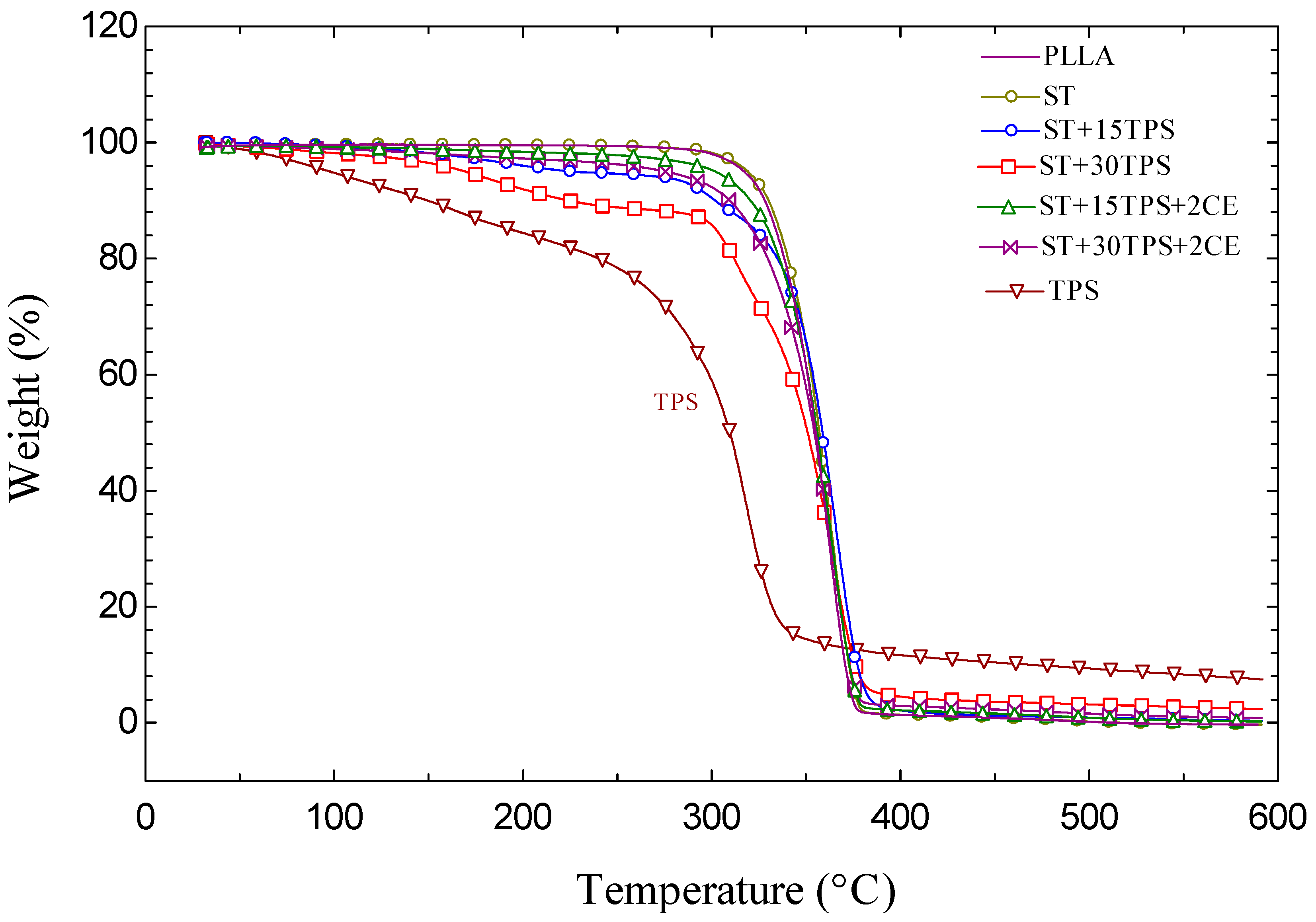
3.4. Thermal Stability
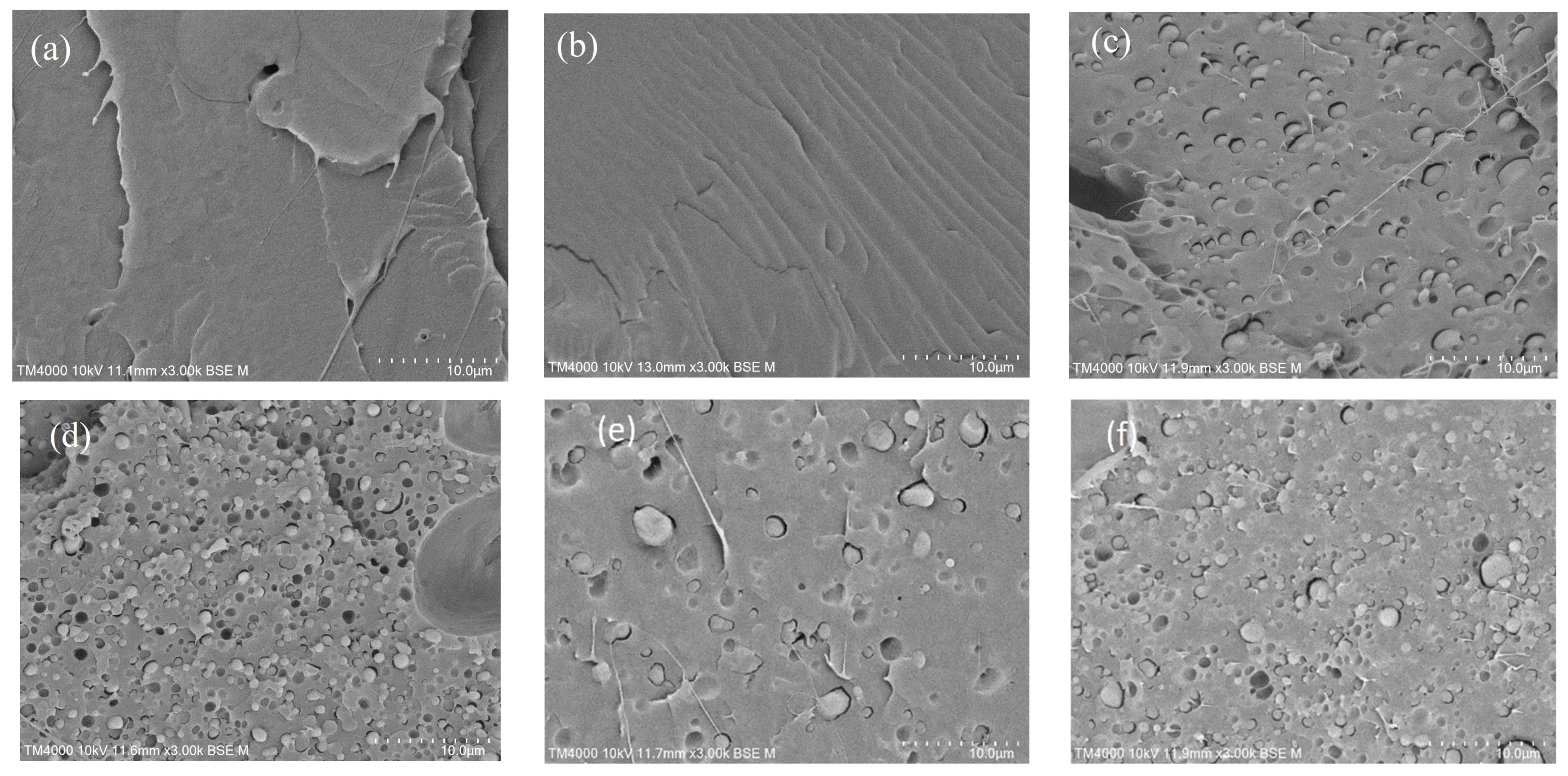
3.5. Morphology
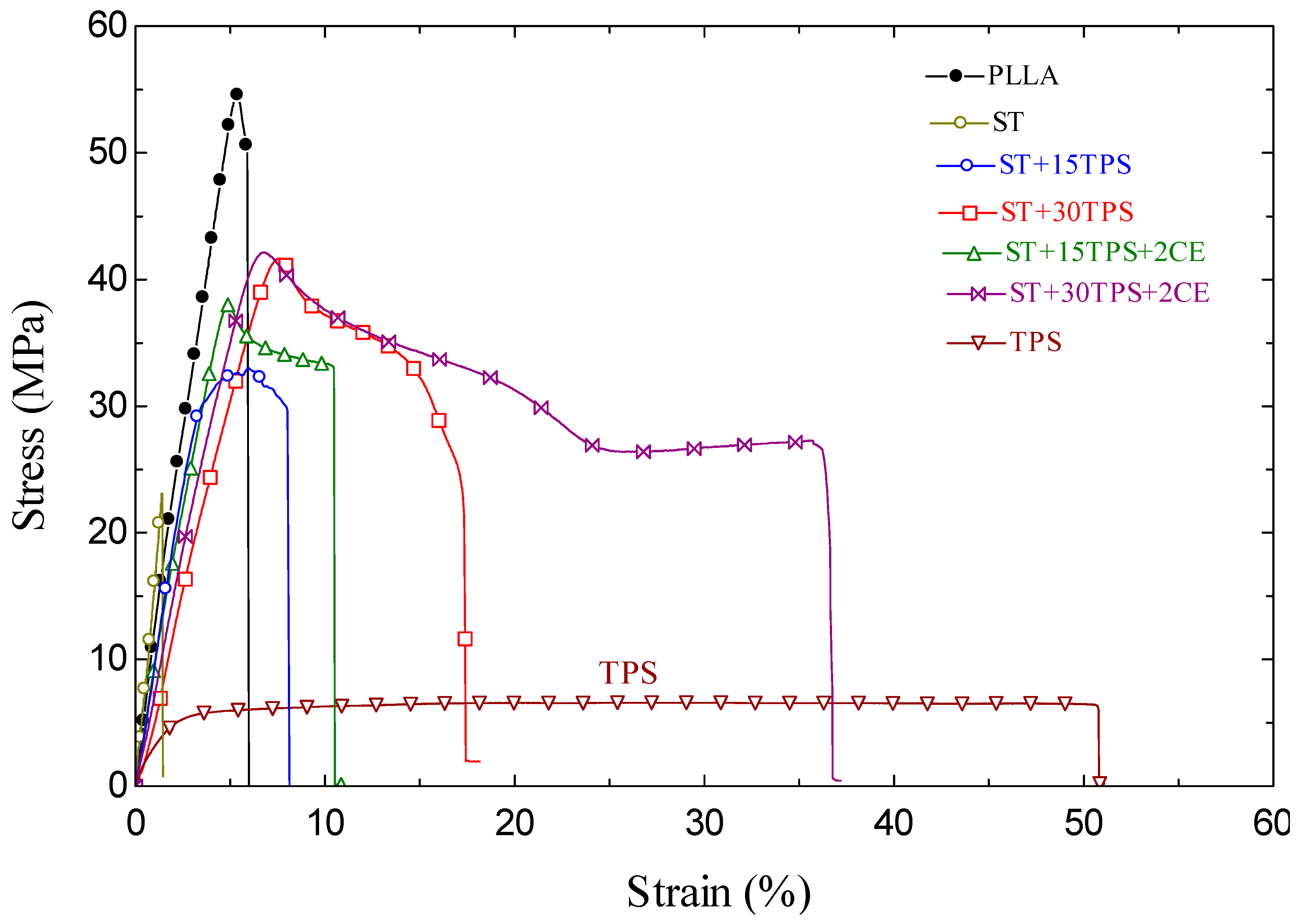
3.6. Mechanical Properties
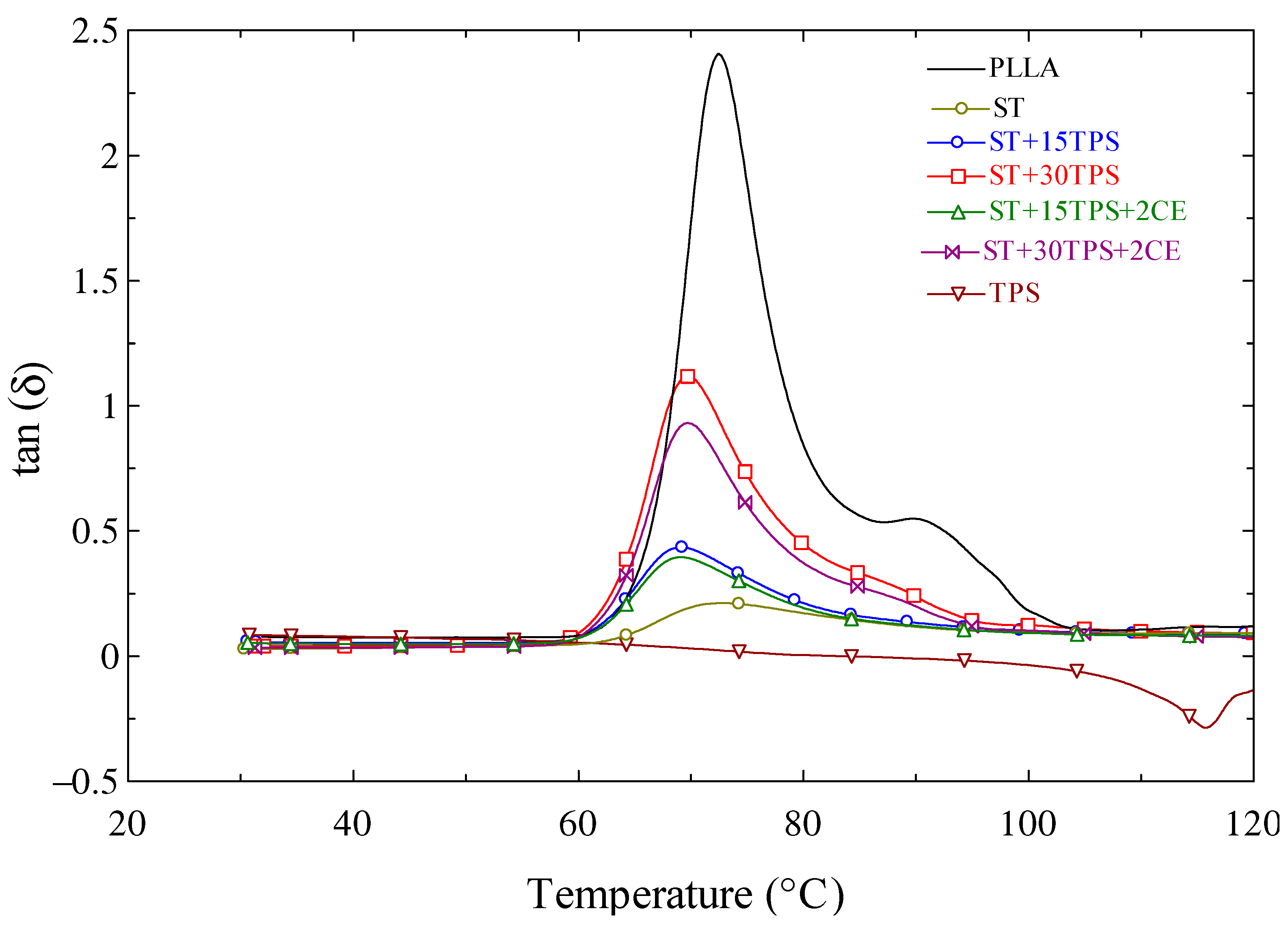
3.7. DMA Analysis
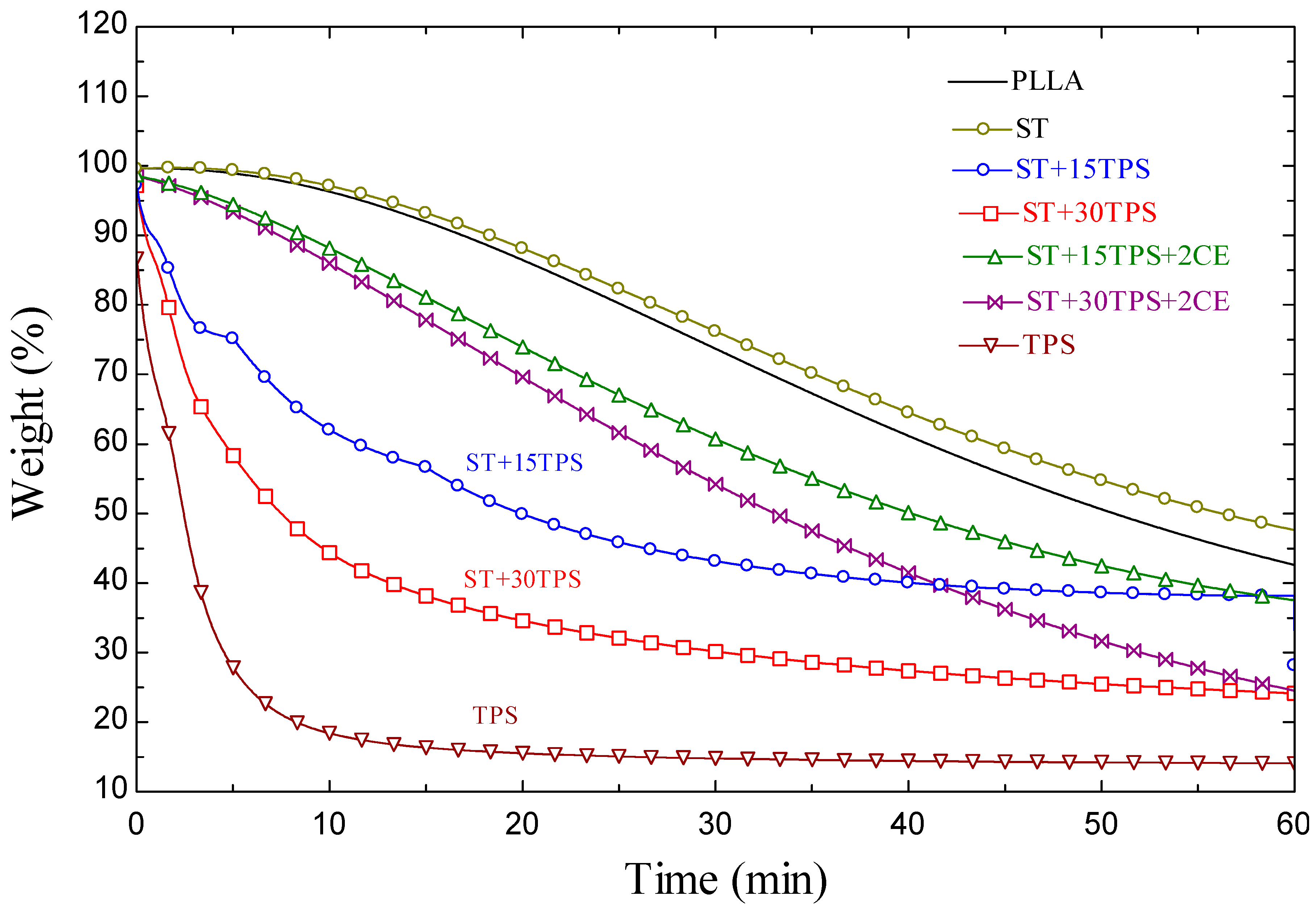
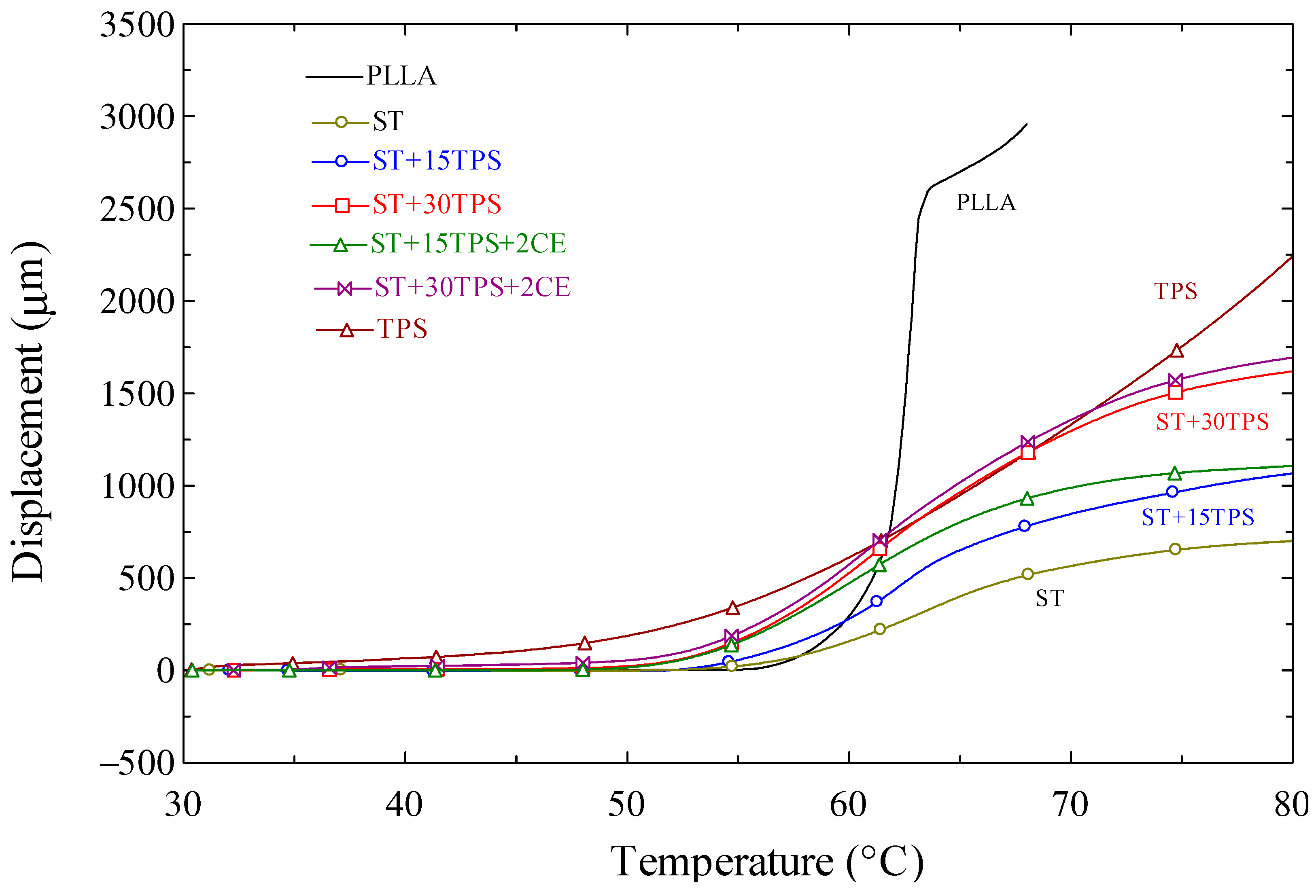
3.8. Heat Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeidlou, S.; Huneault, M.A.; Li, H.; Sammut, P.; Park, C.B. Evidence of a dual network/spherulitic crystalline morphology in PLA stereocomplexes. Polymer 2012, 53, 5816–5824. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Bian, X.; Feng, L.; Xiang, S.; Sun, B.; Chen, Z.; Li, G.; Chen, X. Melt stereocomplexation from poly (L-lactic acid) and poly (D-lactic acid) with different optical purity. Polym. Degrad. Stab. 2013, 98, 844–852. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D.; Turng, L.-S.; Veang-in, O. Injection molding and characterization of polylactide stereocomplex. Polym. Degrad. Stab. 2015, 120, 290–299. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Effect of nucleation and plasticization on the stereocomplex formation between enantiomeric poly (lactic acid) s. Polymer 2013, 54, 5762–5770. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A review of research and application of polylactic acid composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly (lactic acid)(PLA)/thermoplastic starch (TPS) blends. Polym.-Plast. Tech. Mat. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Ferri, J.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Fenollar, O.; Balart, R. Poly (lactic acid) formulations with improved toughness by physical blending with thermoplastic starch. J. Appl. Polym. Sci. 2018, 135, 45751. [Google Scholar] [CrossRef]
- Anderson, K.S.; Lim, S.H.; Hillmyer, M.A. Toughening of polylactide by melt blending with linear low-density polyethylene. J. Appl. Polym. Sci. 2003, 89, 3757–3768. [Google Scholar] [CrossRef]
- Su, Z.; Li, Q.; Liu, Y.; Hu, G.-H.; Wu, C. Compatibility and phase structure of binary blends of poly (lactic acid) and glycidyl methacrylate grafted poly (ethylene octane). Eur. Polym. J. 2009, 45, 2428–2433. [Google Scholar] [CrossRef]
- Ishida, S.; Nagasaki, R.; Chino, K.; Dong, T.; Inoue, Y. Toughening of poly (L-lactide) by melt blending with rubbers. J. Appl. Polym. Sci. 2009, 113, 558–566. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of chain extension on the properties of PLA/TPS blends. J. Appl. Polym. Sci. 2011, 122, 134–141. [Google Scholar] [CrossRef]
- Averous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. macromol. Sci. Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Koh, J.J.; Zhang, X.; He, C. Fully biodegradable Poly (lactic acid)/Starch blends: A review of toughening strategies. Int. J. Biol. Macromol. 2018, 109, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Petinakis, E.; Liu, X.; Yu, L.; Way, C.; Sangwan, P.; Dean, K.; Bateman, S.; Edward, G. Biodegradation and thermal decomposition of poly (lactic acid)-based materials reinforced by hydrophilic fillers. Polym. Degrad. Stab. 2010, 95, 1704–1707. [Google Scholar] [CrossRef]
- Acioli-Moura, R.; Sun, X.S. Thermal degradation and physical aging of poly (lactic acid) and its blends with starch. Polym. Eng. Sci. 2008, 48, 829–836. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, L.; Ma, S.; Yang, Y.; Zhang, C.; Tang, Z.; Zhu, J. Effect of castor oil enrichment layer produced by reaction on the properties of PLA/HDI-g-starch blends. Carbohydr. Polym. 2013, 94, 235–243. [Google Scholar] [CrossRef]
- Randall, J.R.; Cink, K.; Smith, J.C. Branching Polylactide by Reacting OH or COOH Polylactide with Epoxide Acrylate (co) Polymer. U.S. Patent US7566753B2, 28 July 2009. [Google Scholar]
- Villalobos, M.; Awojulu, A.; Greeley, T.; Turco, G.; Deeter, G. Oligomeric chain extenders for economic reprocessing and recycling of condensation plastics. Energy 2006, 31, 3227–3234. [Google Scholar] [CrossRef]
- Baimark, Y.; Srihanam, P. Influence of chain extender on thermal properties and melt flow index of stereocomplex PLA. Polym. Test. 2015, 45, 52–57. [Google Scholar] [CrossRef]
- Lendvai, L.; Brenn, D. Mechanical, morphological and thermal characterization of compatibilized poly (lactic acid)/thermoplastic starch blends. Acta Tech. Jaurinen. 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Najafi, N.; Heuzey, M.; Carreau, P.; Wood-Adams, P.M. Control of thermal degradation of polylactide (PLA)-clay nanocomposites using chain extenders. Polym. Degrad. Stab. 2012, 97, 554–565. [Google Scholar] [CrossRef]
- Cailloux, J.; Santana, O.; Franco-Urquiza, E.; Bou, J.; Carrasco, F.; Maspoch, M.L. Sheets of branched poly (lactic acid) obtained by one-step reactive extrusion–calendering process: Physical aging and fracture behavior. J. Mater. Sci. 2014, 49, 4093–4107. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, X.; Liu, Q.; Hrymak, A. The effect of polymeric chain extenders on physical properties of thermoplastic starch and polylactic acid blends. J. Polym. Environ. 2012, 20, 315–325. [Google Scholar] [CrossRef]
- Przybytek, A.; Sienkiewicz, M.; Kucińska-Lipka, J.; Janik, H. Preparation and characterization of biodegradable and compostable PLA/TPS/ESO compositions. Ind. Crops Prod. 2018, 122, 375–383. [Google Scholar] [CrossRef]
- Sarasua, J.; Arraiza, A.L.; Balerdi, P.; Maiza, I. Crystallinity and mechanical properties of optically pure polylactides and their blends. Polym. Eng. Sci. 2005, 45, 745–753. [Google Scholar] [CrossRef]
- Veang-in, O.; Srithep, Y.; Morris, J.; Pholharn, D. Characterization of Polymer Composites between Stereocomplex Polylactide Blends with Poly (methyl methacrylate). Mater. Sci. 2022, 28, 474–481. [Google Scholar] [CrossRef]
- Balla, B.; Bartos, A.; Kun, D.; Csiszar, E.; Moczo, J.; Fekete, E. Improving mechanical and water sorption properties of thermoplastic starch by incorporating chitosan filler. Polym. Test. 2021, 101, 107278. [Google Scholar] [CrossRef]
- Najafi, N.; Heuzey, M.; Carreau, P. Polylactide (PLA)-clay nanocomposites prepared by melt compounding in the presence of a chain extender. Compos. Sci. Technol. 2012, 72, 608–615. [Google Scholar] [CrossRef]
- Srithep, Y.; Nealey, P.; Turng, L.S. Effects of annealing time and temperature on the crystallinity and heat resistance behavior of injection-molded poly (lactic acid). Polym. Eng. Sci. 2013, 53, 580–588. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D.; Turng, L.-S.; Veang-in, O.; Morris, J. Effects of nucleation and stereocomplex formation of poly (lactic acid). J. Polym. Eng. 2016, 36, 673–679. [Google Scholar]
- Li, H.; Huneault, M. Crystallization of PLA/thermoplastic starch blends. Int. Polym. Process. 2008, 23, 412–418. [Google Scholar]
- Tsuji, H.; Fukui, I. Enhanced thermal stability of poly (lactide) s in the melt by enantiomeric polymer blending. Polymer 2003, 44, 2891–2896. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, C.; Gao, L.; Jiao, L.; Xu, H.; Guo, W. Physical and degradation properties of binary or ternary blends composed of poly (lactic acid), thermoplastic starch and GMA grafted POE. Polym. Degrad. Stab. 2011, 96, 175–182. [Google Scholar]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar]
- Nyambo, C.; Mohanty, A.K.; Misra, M. Polylactide-based renewable green composites from agricultural residues and their hybrids. Biomacromolecules 2010, 11, 1654–1660. [Google Scholar] [CrossRef]





| Sample | PLLA (wt%) | PDLA (wt%) | TPS (wt%) |
|---|---|---|---|
| PLLA | 100 | 0 | 0 |
| TPS | 0 | 0 | 100 |
| ST | 50 | 50 | 0 |
| ST + 15%TPS | 42.5 | 42.5 | 15 |
| ST + 30%TPS | 35 | 35 | 30 |
| ST + 15%TPS + 2%CE | 41.5 | 41.5 | 15 |
| ST + 30%TPS + 2%CE | 34 | 34 | 30 |
| Sample | Tg (°C) | Cold Crystallization | Melting Homocrystal (hc) | Melting Stereocomplex Crystal (st) | %Xc a | %Xst b | |||
|---|---|---|---|---|---|---|---|---|---|
| Tcc (°C) | ∆Hcc (J/g) | Tm,hc (°C) | ∆Hm,hc (J/g) | Tm,st (°C) | ∆Hm,st (J/g) | ||||
| PLLA | 61.8 | 87.4 | 22.2 | 173.4 | 49.5 | - | - | 29.0 | - |
| ST | 56.3 | 74.9 | 2.6 | - | - | 224.1 | 71.6 | 48.2 | 48.2 |
| ST + 15TPS | 58.5 | 79.9 | 7.2 | 164.9 | 3.0 | 225.4 | 61.6 | 48.3 | 46.1 |
| ST + 30TPS | 61.4 | 84.5 | 7.0 | 165.8 | 7.3 | 228.0 | 48.9 | 51.8 | 45.1 |
| ST + 15TPS + 2CE | 59.1 | 85.8 | 9.7 | 167.1 | 6.0 | 228.9 | 51.2 | 40.8 | 36.5 |
| ST + 30TPS + 2CE | 58.0 | 86.0 | 16.9 | 165.8 | 7.4 | 214.9 | 42.9 | 35.4 | 30.2 |
| TPS | - | - | - | 84.9 | 158.2 | - | - | ||
| Sample | T30% (min) |
|---|---|
| PLLA | 32.9 |
| ST | 35.2 |
| ST + 15TPS | 6.7 |
| ST + 30TPS | 2.6 |
| ST + 15TPS + 2CE | 22.7 |
| ST + 30TPS + 2CE | 19.9 |
| TPS | 0.7 |
| Sample | Ultimate Tensile Strength (MPa) | Tensile Modulus (MPa) | Strain at Break (%) | Impact Strength (kJ/m2) |
|---|---|---|---|---|
| PLLA | 54.9 ± 3.2 | 2734 ± 217 | 6.0 ± 0.3 | 1.4 ± 0.08 |
| ST | 22.9 ± 1.7 | 2866 ± 234 | 1.5 ± 0.2 | 0.9 ± 0.05 |
| ST + 15TPS | 32.6 ± 2.6 | 1993 ± 147 | 8.2 ± 0.5 | 2.1 ± 0.1 |
| ST + 30TPS | 41.2 ± 3.4 | 1751 ± 132 | 17.5 ± 2.4 | 4.2 ± 0.2 |
| ST + 15TPS + 2CE | 38.2 ± 2.5 | 22,340 ± 202 | 10.5 ± 0.8 | 3.2 ± 0.2 |
| ST + 30TPS + 2CE | 41.7 ± 2.5 | 1939 ± 157 | 36.7 ± 2.7 | 8.1 ± 0.4 |
| TPS | 6.7 ± 0.3 | 738 ± 54 | 50.8 ± 4.2 | NB 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srithep, Y.; Pholharn, D.; Worajittiphon, P.; Sriprateep, K.; Veang-in, O.; Morris, J. Toughening Polylactide Stereocomplex by Injection Molding with Thermoplastic Starch and Chain Extender. Polymers 2023, 15, 2055. https://doi.org/10.3390/polym15092055
Srithep Y, Pholharn D, Worajittiphon P, Sriprateep K, Veang-in O, Morris J. Toughening Polylactide Stereocomplex by Injection Molding with Thermoplastic Starch and Chain Extender. Polymers. 2023; 15(9):2055. https://doi.org/10.3390/polym15092055
Chicago/Turabian StyleSrithep, Yottha, Dutchanee Pholharn, Patnarin Worajittiphon, Keartisak Sriprateep, Onpreeya Veang-in, and John Morris. 2023. "Toughening Polylactide Stereocomplex by Injection Molding with Thermoplastic Starch and Chain Extender" Polymers 15, no. 9: 2055. https://doi.org/10.3390/polym15092055
APA StyleSrithep, Y., Pholharn, D., Worajittiphon, P., Sriprateep, K., Veang-in, O., & Morris, J. (2023). Toughening Polylactide Stereocomplex by Injection Molding with Thermoplastic Starch and Chain Extender. Polymers, 15(9), 2055. https://doi.org/10.3390/polym15092055








