Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering
Abstract
1. Introduction
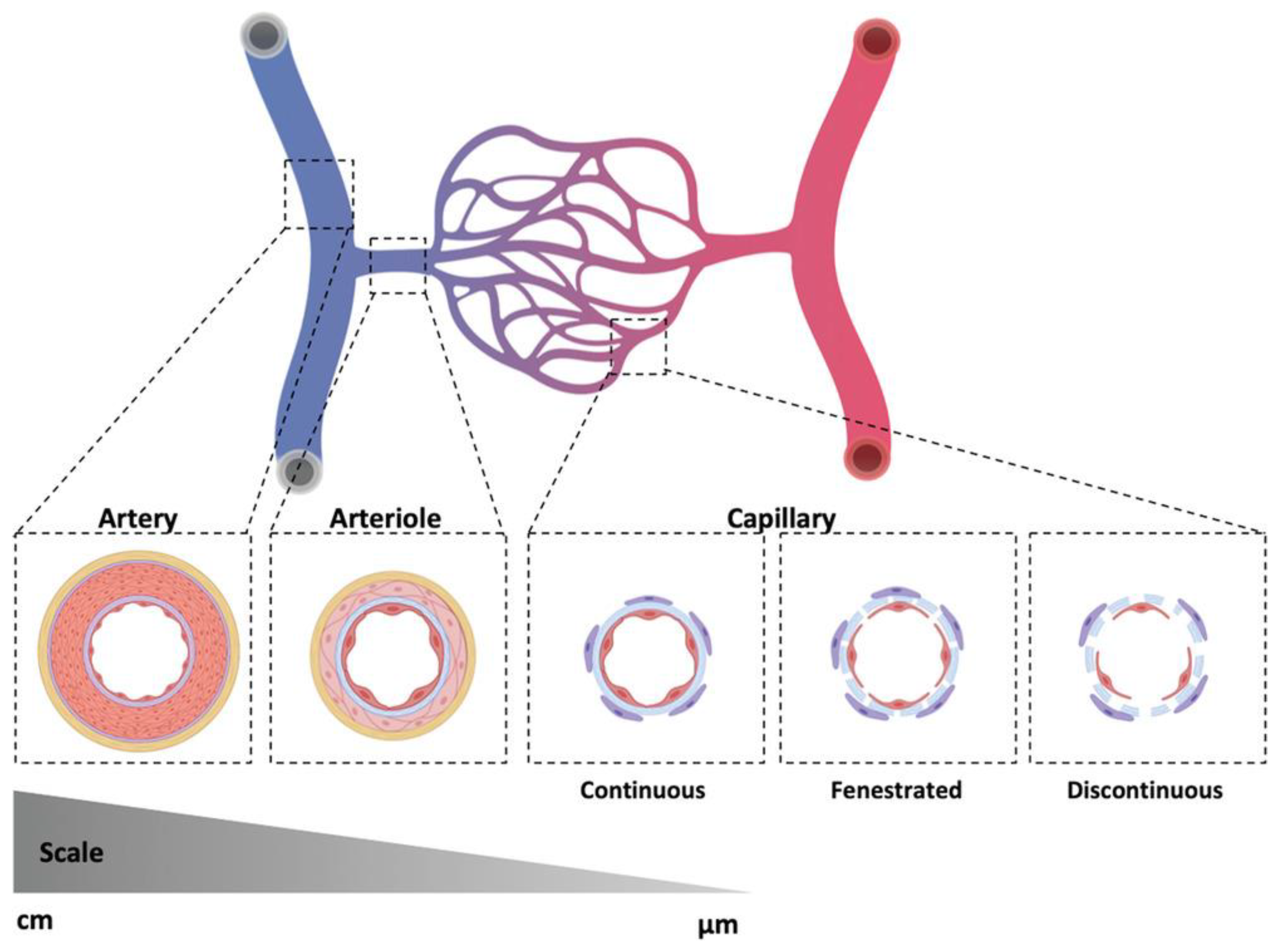
2. Tissue-Engineered Vascular Grafts
2.1. Middle- and Large-Diameter Artificial Grafts
2.2. Small-Diameter Artificial Grafts
2.3. Engineered Vasculature
3. Material Selection
3.1. Synthetic Polymers
3.1.1. Nondegradable Polymers
3.1.2. Biodegradable Polymers
PGA
PLA
PCL
PGS
PEG
3.2. Natural Polymers
3.2.1. Collagen and Gelatin
3.2.2. Elastin
3.2.3. Silk Fibroin
3.2.4. Chitosan
3.2.5. Alginate
4. Fabrication Methods for Tissue-Engineered Vascular Grafts
4.1. Biobased Techniques
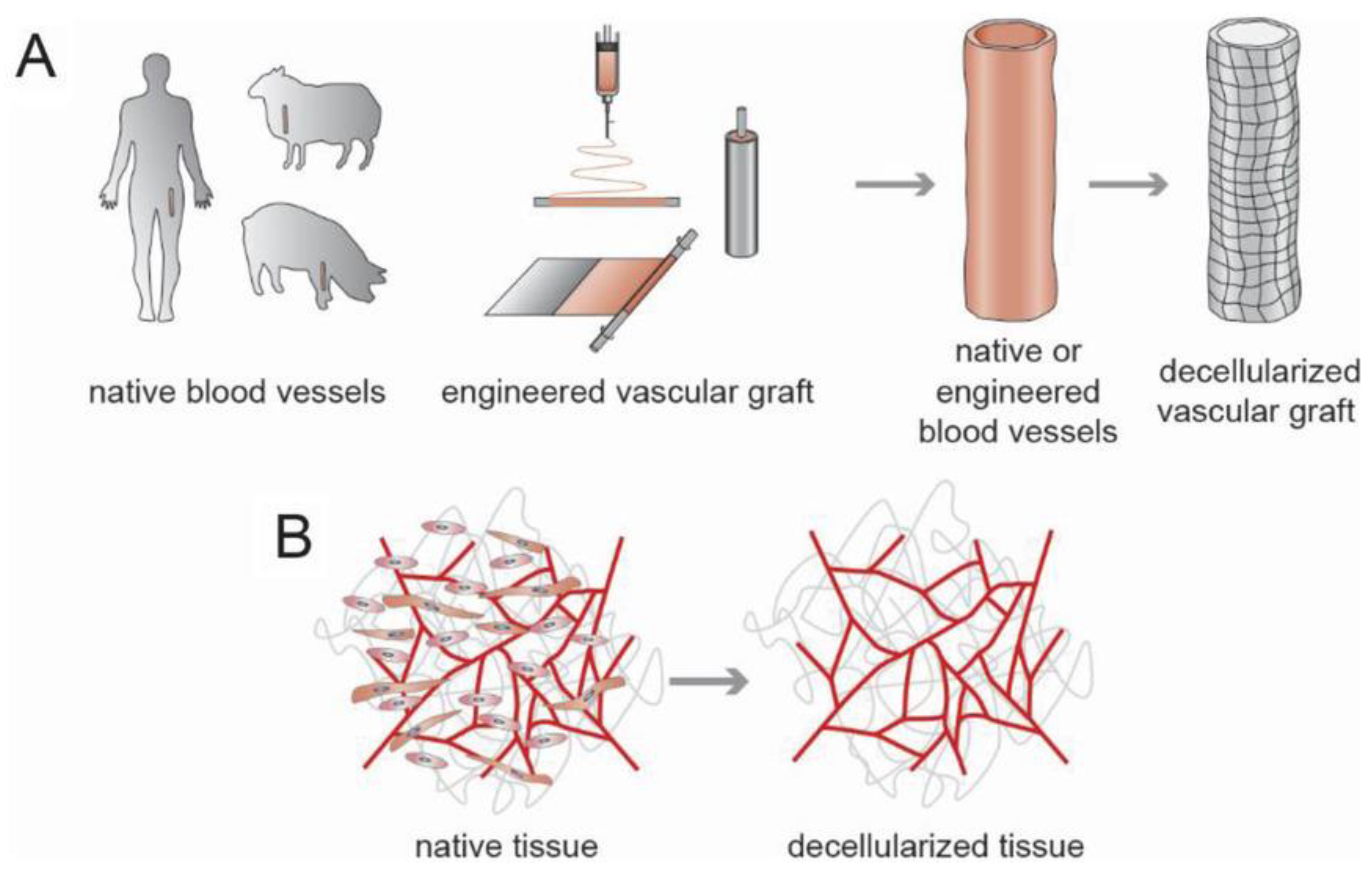
4.1.1. Decellularization
4.1.2. Self-Assembly
4.2. Engineering-Based Techniques
4.2.1. Electrospinning
4.2.2. Molding
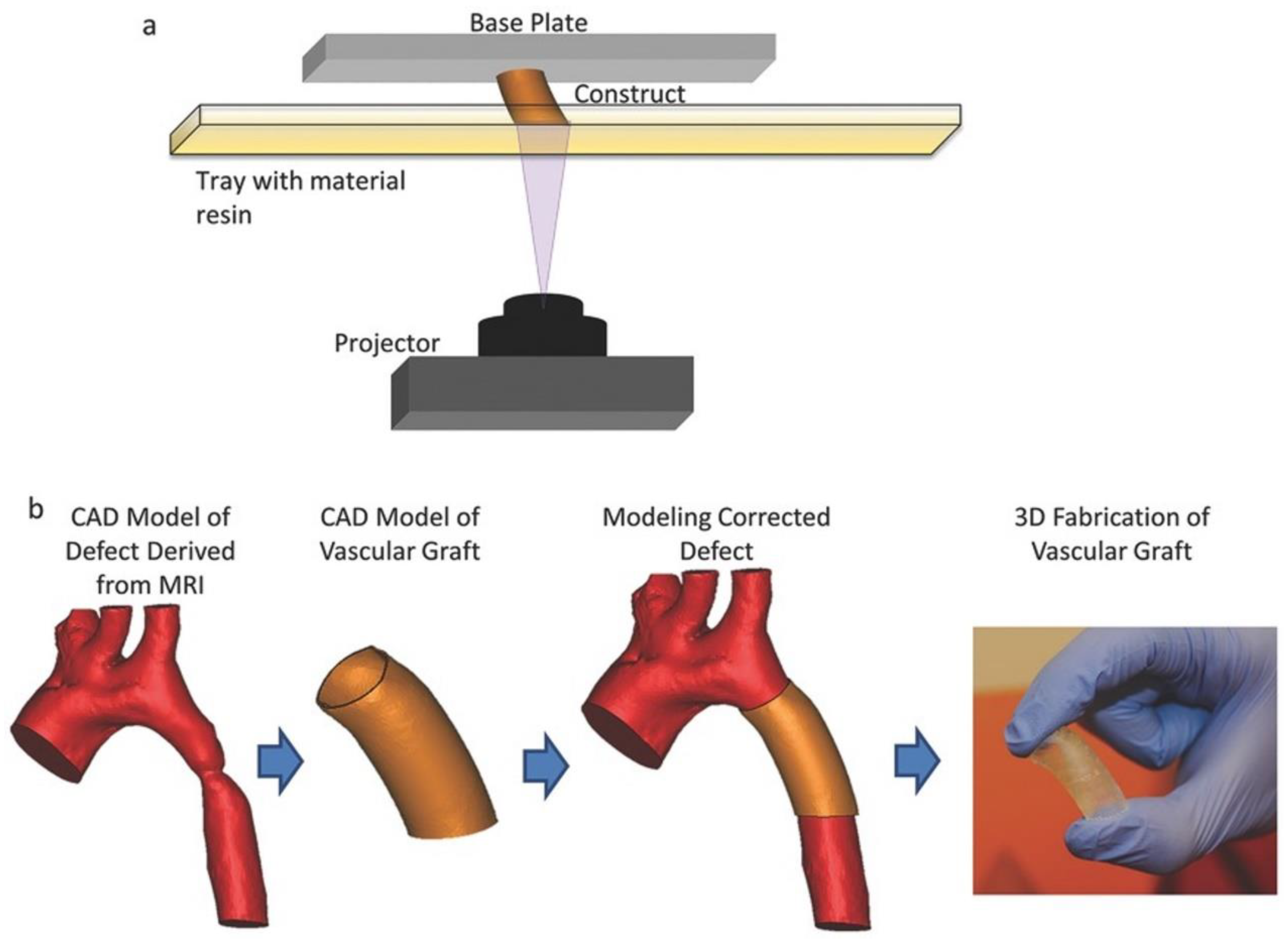
4.2.3. 3D Printing
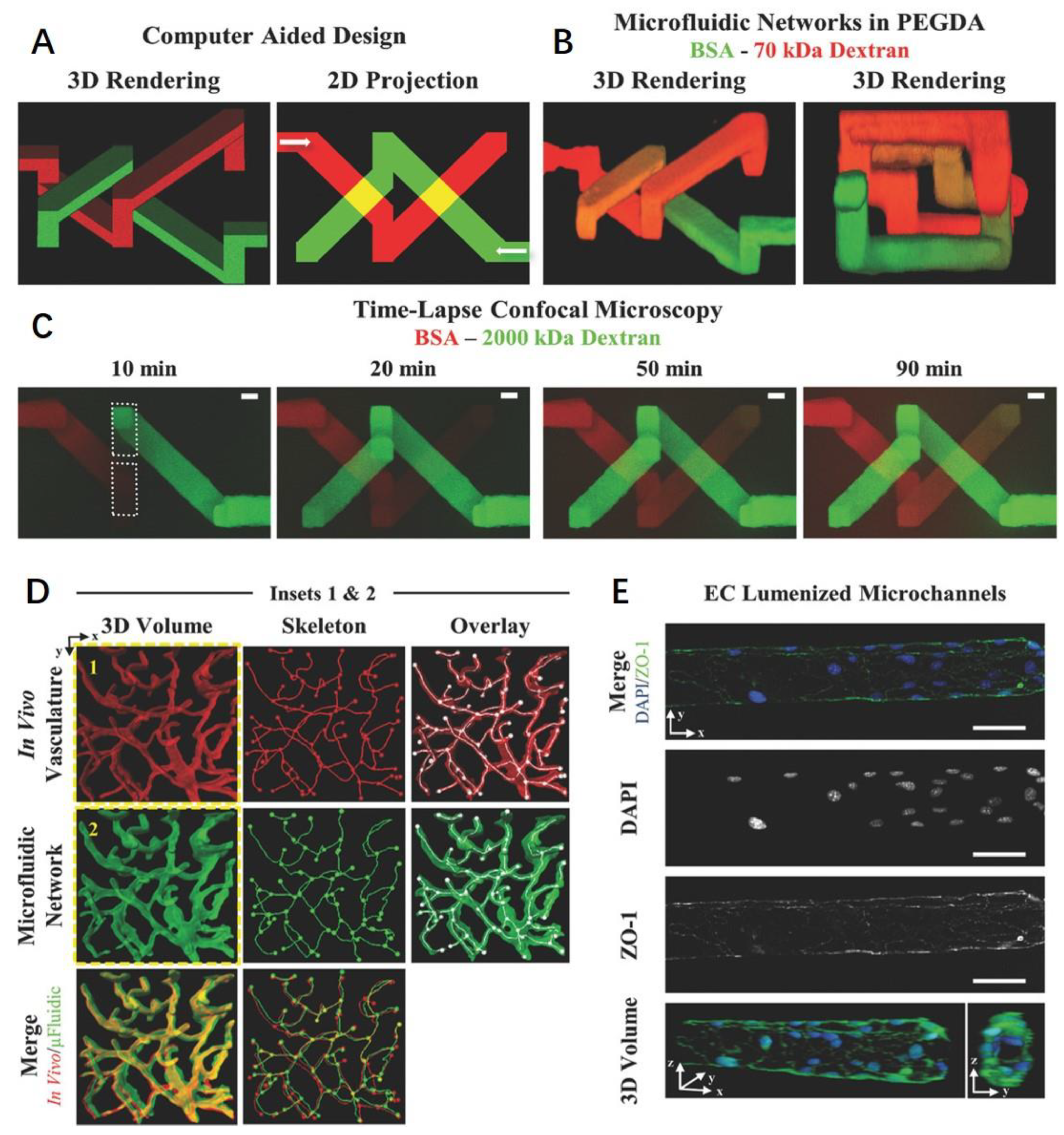
4.2.4. Laser Degradation
5. Vascularized Tissue Engineering
5.1. Peripheral Nerve Tissue Engineering
5.2. Bone Tissue Engineering
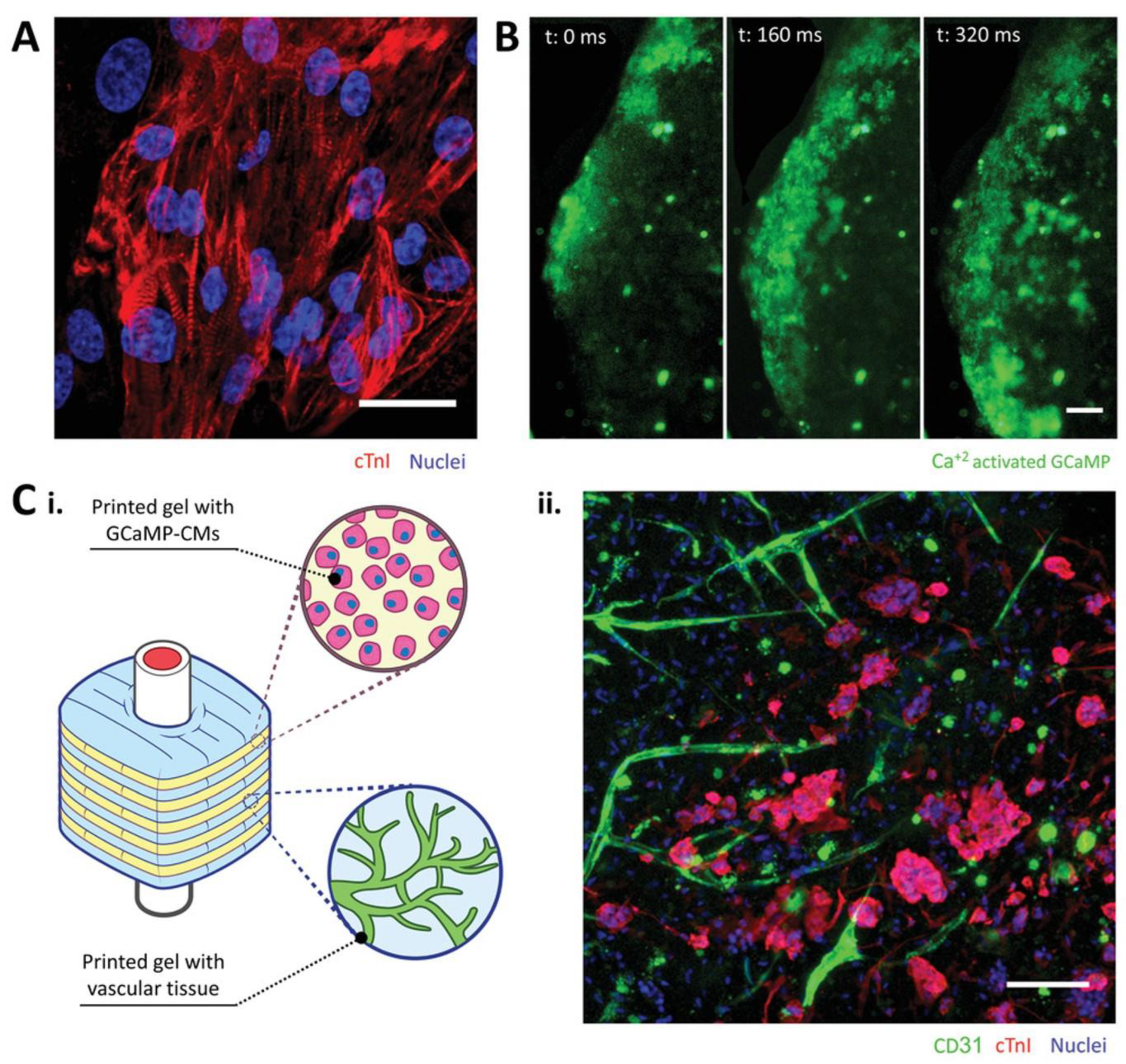
5.3. Heart Tissue Engineering
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, M.T.; Harley, B.A. Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression. Biomaterials 2020, 255, 120207. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [PubMed]
- Putnam, A.J. The instructive role of the vasculature in stem cell niches. Biomater. Sci. 2014, 2, 1562–1573. [Google Scholar] [CrossRef]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From arteries to capillaries: Approaches to engineering human vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef]
- Rafii, S.; Butler, J.M.; Ding, B.-S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.-W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef]
- Crosby, C.O.; Zoldan, J. Mimicking the physical cues of the ECM in angiogenic biomaterials. Regen. Biomater. 2019, 6, 61–73. [Google Scholar] [CrossRef]
- Qadura, M.; Terenzi, D.C.; Verma, S.; Al-Omran, M.; Hess, D.A. Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies. Stem Cells 2018, 36, 161–171. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N. Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar]
- Martins, B.; Ferreira, D.; Neto, C.; Abelha, A.; Machado, J. Data mining for cardiovascular disease prediction. J. Med. Syst. 2021, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [PubMed]
- Zhao, D. Epidemiological features of cardiovascular disease in Asia. JACC Asia 2021, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Perets, A.; Baruch, Y.; Weisbuch, F.; Shoshany, G.; Neufeld, G.; Cohen, S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 65, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Levick, J.R. An Introduction to Cardiovascular Physiology; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Traore, M.A.; George, S.C. Tissue engineering the vascular tree. Tissue Eng. Part B Rev. 2017, 23, 505–514. [Google Scholar] [CrossRef]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar]
- Szklanny, A.A.; Machour, M.; Redenski, I.; Chochola, V.; Goldfracht, I.; Kaplan, B.; Epshtein, M.; Simaan Yameen, H.; Merdler, U.; Feinberg, A. 3D Bioprinting of Engineered Tissue Flaps with Hierarchical Vessel Networks (VesselNet) for Direct Host-To-Implant Perfusion. Adv. Mater. 2021, 33, 2102661. [Google Scholar] [CrossRef]
- Ben-Shaul, S.; Landau, S.; Merdler, U.; Levenberg, S. Mature vessel networks in engineered tissue promote graft–host anastomosis and prevent graft thrombosis. Proc. Natl. Acad. Sci. USA 2019, 116, 2955–2960. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: Beyond creating static networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Mandrycky, C.J.; Howard, C.C.; Rayner, S.G.; Shin, Y.J.; Zheng, Y. Organ-on-a-chip systems for vascular biology. J. Mol. Cell. Cardiol. 2021, 159, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Mar, R.; Van Blitterswijk, C.A.; Mulligan, R.C.; D’Amore, P.A.; et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft—Past, present, and future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Bakey, M.; De Bakey, M. Successful resection of aneurysm of distal aortic arch and replacement by graft. J. Am. Med. Assoc. 1954, 155, 1398. [Google Scholar] [CrossRef] [PubMed]
- Walpoth, B.H.; Bowlin, G.L. The daunting quest for a small diameter vascular graft. Expert Rev. Med. Devices 2005, 2, 647–651. [Google Scholar] [CrossRef]
- Zdrahala, R.J. Small caliber vascular grafts. Part I: State of the art. J. Biomater. Appl. 1996, 10, 309–329. [Google Scholar] [CrossRef]
- Zdrahala, R.J. Small caliber vascular grafts. Part II: Polyurethanes revisited. J. Biomater. Appl. 1996, 11, 37–61. [Google Scholar] [CrossRef]
- Chlupáč, J.; Filova, E.; Bačáková, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58, S119–S139. [Google Scholar] [CrossRef]
- Nevelsteen, A.; Wouters, L.; Suy, R. Aortofemoral Dacron reconstruction for aorto-iliac occlusive disease: A 25-year survey. Eur. J. Vasc. Surg. 1991, 5, 179–186. [Google Scholar] [CrossRef]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef]
- Polo, J.R.; Ligero, J.M.; Diaz-Cartelle, J.; Garcia-Pajares, R.; Cervera, T.; Reparaz, L. Randomized comparison of 6-mm straight grafts versus 6-to 8-mm tapered grafts for brachial-axillary dialysis access. J. Vasc. Surg. 2004, 40, 319–324. [Google Scholar] [CrossRef] [PubMed]
- García-Pajares, R.; Polo, J.R.; Flores, n.; Gonzalez-Tabares, E.; Solís, J.V. Upper arm polytetrafluoroethylene grafts for dialysis access. Analysis of two different graft sizes: 6 mm and 6–8 mm. Vasc. Endovasc. Surg. 2003, 37, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Filipe, E.C.; Santos, M.; Hung, J.; Lee, B.S.; Yang, N.; Chan, A.H.; Ng, M.K.; Rnjak-Kovacina, J.; Wise, S.G. Rapid endothelialization of off-the-shelf small diameter silk vascular grafts. JACC Basic Transl. Sci. 2018, 3, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Tang, J.; Hong, F.F.; Lu, X.; Chen, L. Physicochemical properties and in vitro biocompatibility of three bacterial nanocellulose conduits for blood vessel applications. Carbohydr. Polym. 2020, 239, 116246. [Google Scholar] [CrossRef] [PubMed]
- Dekker, A.; Reitsma, K.; Beugeling, T.; Bantjes, A.; Feijen, J.; Van Aken, W. Adhesion of endothelial cells and adsorption of serum proteins on gas plasma-treated polytetrafluoroethylene. Biomaterials 1991, 12, 130–138. [Google Scholar] [CrossRef]
- Gharamti, A.; Kanafani, Z.A. Vascular graft infections: An update. Infect. Dis. Clin. 2018, 32, 789–809. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Thomsom, J.A.; Turng, L.-S. Promoting endothelial cell affinity and antithrombogenicity of polytetrafluoroethylene (PTFE) by mussel-inspired modification and RGD/heparin grafting. J. Mater. Chem. B 2018, 6, 3475–3485. [Google Scholar] [CrossRef]
- Zhu, A.; Ming, Z.; Jian, S. Blood compatibility of chitosan/heparin complex surface modified ePTFE vascular graft. Appl. Surf. Sci. 2005, 241, 485–492. [Google Scholar] [CrossRef]
- Ku, S.H.; Park, C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef]
- Jiang, Y.-C.; Wang, X.-F.; Xu, Y.-Y.; Qiao, Y.-H.; Guo, X.; Wang, D.-F.; Li, Q.; Turng, L.-S. Polycaprolactone nanofibers containing vascular endothelial growth factor-encapsulated gelatin particles enhance mesenchymal stem cell differentiation and angiogenesis of endothelial cells. Biomacromolecules 2018, 19, 3747–3753. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, H.; Yang, F.; Bei, J.; Wang, S. Preparation and cell affinity of microtubular orientation-structured PLGA (70/30) blood vessel scaffold. Biomaterials 2008, 29, 3128–3136. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.N.; Chronos, N.A.; Kyles, A.E.; Gregory, C.R.; Hoyt, G. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef]
- Lee, Y.B.; Shin, Y.M.; Lee, J.-h.; Jun, I.; Kang, J.K.; Park, J.-C.; Shin, H. Polydopamine-mediated immobilization of multiple bioactive molecules for the development of functional vascular graft materials. Biomaterials 2012, 33, 8343–8352. [Google Scholar] [CrossRef]
- Freeman, J.; Chen, A.; Weinberg, R.J.; Okada, T.; Chen, C.; Lin, P.H. Sustained thromboresistant bioactivity with reduced intimal hyperplasia of heparin-bonded polytetrafluoroethylene propaten graft in a chronic canine femoral artery bypass model. Ann. Vasc. Surg. 2018, 49, 295–303. [Google Scholar] [CrossRef]
- Lumsden, A.B.; Morrissey, N.J.; Staffa, R.; Lindner, J.; Janousek, L.; Treska, V.; Stadler, P.; Moursi, M.; Storck, M.; Johansen, K. Randomized controlled trial comparing the safety and efficacy between the FUSION BIOLINE heparin-coated vascular graft and the standard expanded polytetrafluoroethylene graft for femoropopliteal bypass. J. Vasc. Surg. 2015, 61, 703–712.e701. [Google Scholar] [CrossRef]
- Aalders, G.J.; van Vroonhoven, T.J. Polytetrafluoroethylene versus human umbilical vein in above-knee femoropopliteal bypass: Six-year results of a randomized clinical trial. J. Vasc. Surg. 1992, 16, 816–824. [Google Scholar] [CrossRef]
- Rosenthal, D.; Evans, R.; McKinsey, J.; Seagraves, M.; Lamis, P.; Clark, M.; Daniel, W. Prosthetic above-knee femoropopliteal bypass for intermittent claudication. J. Cardiovasc. Surg. 1990, 31, 462–468. [Google Scholar]
- Moore, K.H.; Murphy, H.A.; George, E.M. The glycocalyx: A central regulator of vascular function. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2021, 320, R508–R518. [Google Scholar] [CrossRef] [PubMed]
- van Hinsbergh, V.W. The endothelium: Vascular control of haemostasis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, I.; Fredenburgh, J.; Hirsh, J.; Weitz, J. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering artificial blood vessels from natural materials. Trends Biotechnol. 2021, 40, 693–707. [Google Scholar] [CrossRef]
- Veith, F.J.; Gupta, S.K.; Ascer, E.; White-Flores, S.; Samson, R.H.; Scher, L.A.; Towne, J.B.; Bernhard, V.M.; Bonier, P.; Flinn, W.R. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J. Vasc. Surg. 1986, 3, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Leal, B.B.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular tissue engineering: Polymers and methodologies for small caliber vascular grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- Best, C.A.; Szafron, J.M.; Rocco, K.A.; Zbinden, J.; Dean, E.W.; Maxfield, M.W.; Kurobe, H.; Tara, S.; Bagi, P.S.; Udelsman, B.V. Differential outcomes of venous and arterial tissue engineered vascular grafts highlight the importance of coupling long-term implantation studies with computational modeling. Acta Biomater. 2019, 94, 183–194. [Google Scholar] [CrossRef]
- Seifu, D.G.; Purnama, A.; Mequanint, K.; Mantovani, D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 2013, 10, 410–421. [Google Scholar] [CrossRef]
- Qiu, Y.; Brown, A.C.; Myers, D.R.; Sakurai, Y.; Mannino, R.G.; Tran, R.; Ahn, B.; Hardy, E.T.; Kee, M.F.; Kumar, S. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. USA 2014, 111, 14430–14435. [Google Scholar] [CrossRef]
- Milleret, V.; Hefti, T.; Hall, H.; Vogel, V.; Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 2012, 8, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue engineering at the blood-contacting surface: A review of challenges and strategies in vascular graft development. Adv. Healthc. Mater. 2018, 7, 1701461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mithieux, S.M.; Weiss, A.S. Fabrication techniques for vascular and vascularized tissue engineering. Adv. Healthc. Mater. 2019, 8, 1900742. [Google Scholar] [CrossRef]
- Herbert, S.P.; Stainier, D.Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 551–564. [Google Scholar] [CrossRef]
- Chatelain, L.; Louyriac, E.; Douair, I.; Lu, E.; Tuna, F.; Wooles, A.J.; Gardner, B.M.; Maron, L.; Liddle, S.T. Terminal uranium (V)-nitride hydrogenations involving direct addition or Frustrated Lewis Pair mechanisms. Nat. Commun. 2020, 11, 337. [Google Scholar] [CrossRef]
- Palikuqi, B.; Nguyen, D.-H.T.; Li, G.; Schreiner, R.; Pellegata, A.F.; Liu, Y.; Redmond, D.; Geng, F.; Lin, Y.; Gómez-Salinero, J.M. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 2020, 585, 426–432. [Google Scholar] [CrossRef]
- Landau, S.; Szklanny, A.A.; Yeo, G.C.; Shandalov, Y.; Kosobrodova, E.; Weiss, A.S.; Levenberg, S. Tropoelastin coated PLLA-PLGA scaffolds promote vascular network formation. Biomaterials 2017, 122, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional printed Mg-doped β-TCP bone tissue engineering scaffolds: Effects of magnesium ion concentration on osteogenesis and angiogenesis in vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef]
- Wang, C.; Lai, J.; Li, K.; Zhu, S.; Lu, B.; Liu, J.; Tang, Y.; Wei, Y. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact. Mater. 2021, 6, 137–145. [Google Scholar] [CrossRef]
- Nguyen, D.-H.T.; Stapleton, S.C.; Yang, M.T.; Cha, S.S.; Choi, C.K.; Galie, P.A.; Chen, C.S. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 6712–6717. [Google Scholar] [CrossRef]
- Landau, S.; Ben-Shaul, S.; Levenberg, S. Oscillatory strain promotes vessel stabilization and alignment through fibroblast YAP-mediated mechanosensitivity. Adv. Sci. 2018, 5, 1800506. [Google Scholar] [CrossRef]
- Kim, S.; Chung, M.; Ahn, J.; Lee, S.; Jeon, N.L. Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab Chip 2016, 16, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Niklason, L.E. Vascular tissue engineering: Building perfusable vasculature for implantation. Curr. Opin. Chem. Eng. 2014, 3, 68–74. [Google Scholar] [CrossRef]
- Emerson, D.R.; Cieślicki, K.; Gu, X.; Barber, R.W. Biomimetic design of microfluidic manifolds based on a generalised Murray’s law. Lab Chip 2006, 6, 447–454. [Google Scholar] [CrossRef]
- Czajka, C.A.; Calder, B.W.; Yost, M.J.; Drake, C.J. Implanted scaffold-free prevascularized constructs promote tissue repair. Ann. Plast. Surg. 2015, 74, 371. [Google Scholar] [CrossRef]
- Ercolani, E.; Del Gaudio, C.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: Reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2015, 9, 861–888. [Google Scholar] [CrossRef]
- Braghirolli, D.; Helfer, V.; Chagastelles, P.; Dalberto, T.; Gamba, D.; Pranke, P. Electrospun scaffolds functionalized with heparin and vascular endothelial growth factor increase the proliferation of endothelial progenitor cells. Biomed. Mater. 2017, 12, 025003. [Google Scholar] [CrossRef]
- Mohan, T.; Nagaraj, C.; Nagy, B.M.; Bračicč, M.; Maver, U.; Olschewski, A.; Stana Kleinschek, K.; Kargl, R. Nano-and micropatterned polycaprolactone cellulose composite surfaces with tunable protein adsorption, fibrin clot formation, and endothelial cellular response. Biomacromolecules 2019, 20, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, W.; Cheng, S.; Zhang, W.; Liu, S.; Jiang, X. In vitro evaluation of essential mechanical properties and cell behaviors of a novel polylactic-co-glycolic acid (PLGA)-based tubular scaffold for small-diameter vascular tissue engineering. Polymers 2017, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Chen, X.; Hou, Y.L.; Hou, Y.J.; Tian, S.B.; Chen, Y.H.; Yu, L.; Nie, M.H.; Liu, X.Q. Characterization of different biodegradable scaffolds in tissue engineering. Mol. Med. Rep. 2019, 19, 4043–4056. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.-S. Artificial small-diameter blood vessels: Materials, fabrication, surface modification, mechanical properties, and bioactive functionalities. J. Mater. Chem. B 2020, 8, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 1–28. [Google Scholar]
- Hess, F. History of (MICRO) vascular surgery and the development of small-caliber blood vessel prostheses (with some notes on patency rates and re-endothelialization). Microsurgery 1985, 6, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.J.; Maki, T.; Borland, K.M.; Mahoney, M.D.; Solomon, B.A.; Muller, T.E.; Monaco, A.P.; Chick, W.L. Biohybrid artificial pancreas: Long-term implantation studies in diabetic, pancreatectomized dogs. Science 1991, 252, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Catto, V.; Farè, S.; Freddi, G.; Tanzi, M.C. Vascular tissue engineering: Recent advances in small diameter blood vessel regeneration. Int. Sch. Res. Not. 2014, 2014, 923030. [Google Scholar] [CrossRef]
- Geelhoed, W.J.; Moroni, L.; Rotmans, J.I. Utilizing the foreign body response to grow tissue engineered blood vessels in vivo. J. Cardiovasc. Transl. Res. 2017, 10, 167–179. [Google Scholar] [CrossRef]
- Kumar, V.A.; Brewster, L.P.; Caves, J.M.; Chaikof, E.L. Tissue engineering of blood vessels: Functional requirements, progress, and future challenges. Cardiovasc. Eng. Technol. 2011, 2, 137–148. [Google Scholar] [CrossRef]
- Gabriel, M.; Niederer, K.; Becker, M.; Raynaud, C.M.; Vahl, C.-F.; Frey, H. Tailoring novel PTFE surface properties: Promoting cell adhesion and antifouling properties via a wet chemical approach. Bioconjugate Chem. 2016, 27, 1216–1221. [Google Scholar] [CrossRef]
- Chen, L.; He, H.; Wang, M.; Li, X.; Yin, H. Surface coating of polytetrafluoroethylene with extracellular matrix and anti-CD34 antibodies facilitates endothelialization and inhibits platelet adhesion under sheer stress. Tissue Eng. Regen. Med. 2017, 14, 359–370. [Google Scholar] [CrossRef]
- Maurmann, N.; Sperling, L.-E.; Pranke, P. Electrospun and electrosprayed scaffolds for tissue engineering. Cut.-Edge Enabling Technol. Regen. Med. 2018, 1078, 79–100. [Google Scholar]
- Tysoe, O.C.; Justin, A.W.; Brevini, T.; Chen, S.E.; Mahbubani, K.T.; Frank, A.K.; Zedira, H.; Melum, E.; Saeb-Parsy, K.; Markaki, A.E. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 2019, 14, 1884–1925. [Google Scholar] [CrossRef]
- Niklason, L.; Gao, J.; Abbott, W.; Hirschi, K.; Houser, S.; Marini, R.; Langer, R. Functional arteries grown in vitro. Science 1999, 284, 489–493. [Google Scholar] [CrossRef] [PubMed]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Opfermann, J.; Ong, C.S.; Shinoka, T.; Breuer, C.K.; Krieger, A.; Johnson, J.; Hibino, N. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J. Thorac. Cardiovasc. Surg. 2017, 153, 924–932. [Google Scholar] [CrossRef]
- Melchiorri, A.J.; Hibino, N.; Brandes, Z.R.; Jonas, R.A.; Fisher, J.P. Development and assessment of a biodegradable solvent cast polyester fabric small-diameter vascular graft. J. Biomed. Mater. Res. Part A 2014, 102, 1972–1981. [Google Scholar] [CrossRef]
- Niklason, L.E.; Langer, R.S. Advances in tissue engineering of blood vessels and other tissues. Transpl. Immunol. 1997, 5, 303–306. [Google Scholar] [CrossRef]
- Niklason, L.E.; Abbott, W.; Gao, J.; Klagges, B.; Hirschi, K.K.; Ulubayram, K.; Conroy, N.; Jones, R.; Vasanawala, A.; Sanzgiri, S. Morphologic and mechanical characteristics of engineered bovine arteries. J. Vasc. Surg. 2001, 33, 628–638. [Google Scholar] [CrossRef]
- Dahl, S.L.; Kypson, A.P.; Lawson, J.H.; Blum, J.L.; Strader, J.T.; Li, Y.; Manson, R.J.; Tente, W.E.; DiBernardo, L.; Hensley, M.T. Readily available tissue-engineered vascular grafts. Sci. Transl. Med. 2011, 3, 68ra9. [Google Scholar] [CrossRef]
- Dahl, L.; Lawson, J.; Prichard, H.; Manson, R.; Tente, W.; Kypson, A. Abstracts from the emerging science series, April 24, 2013. Circulation 2013, 127, 2071–2072. [Google Scholar]
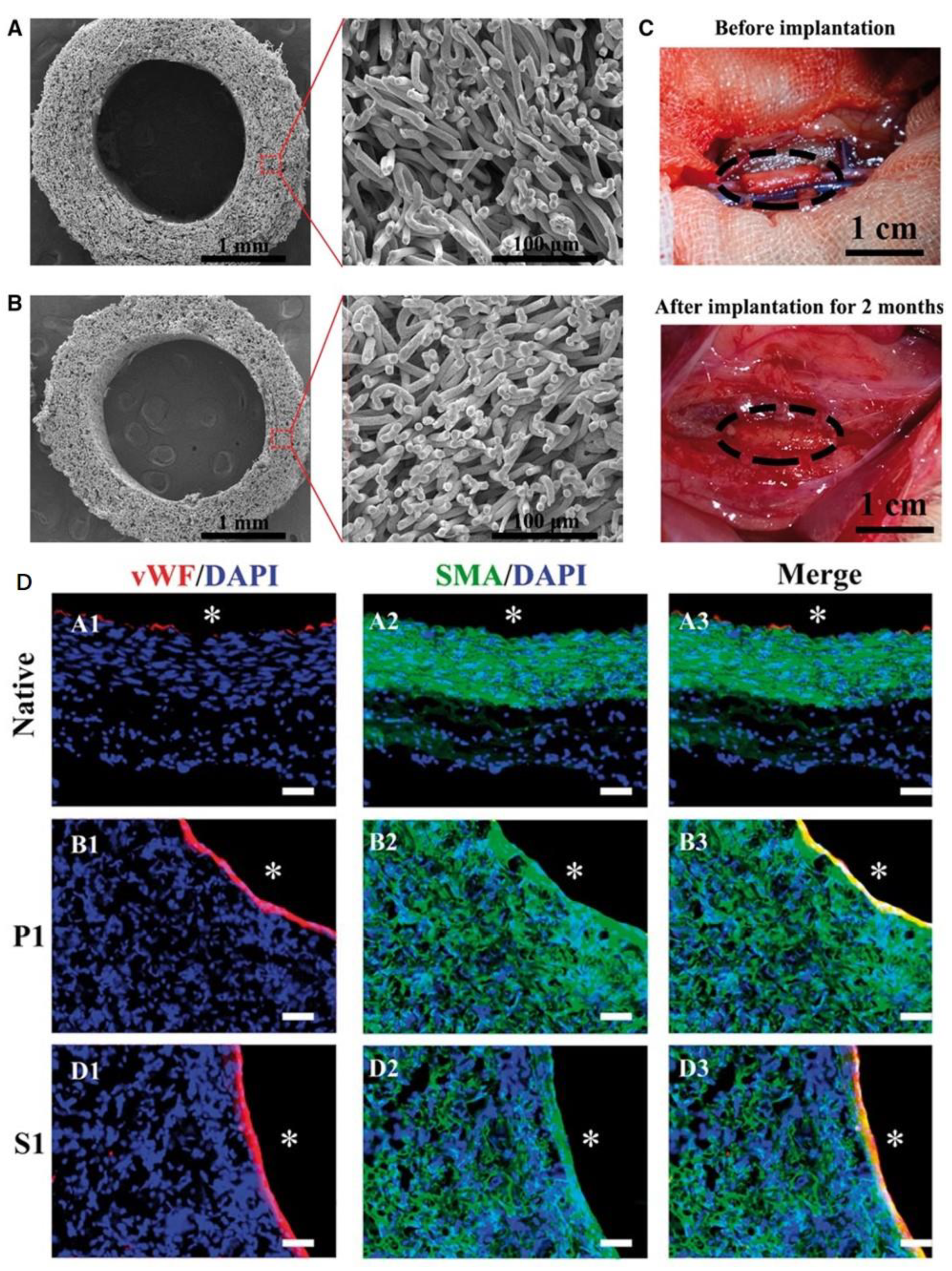
- Lawson, J.H.; Glickman, M.H.; Ilzecki, M.; Jakimowicz, T.; Jaroszynski, A.; Peden, E.K.; Pilgrim, A.J.; Prichard, H.L.; Guziewicz, M.; Przywara, S. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: Two phase 2 single-arm trials. Lancet 2016, 387, 2026–2034. [Google Scholar] [CrossRef]
- Lawson, J.H.; Glickman, M.H.; Jakimowicz, T.; Peden, E.K.; Szmidt, J.; Witkiewicz, W.; Zubilewicz, T.; Niklason, L.E. Bioengineered Human Acellular Vessels for Dialysis Access: Completed Phase 2 Studies. J. Am. Coll. Surg. 2017, 225, e52. [Google Scholar] [CrossRef]
- Lin, C.; Liu, C.; Zhang, L.; Huang, Z.; Zhao, P.; Chen, R.; Pang, M.; Chen, Z.; He, L.; Luo, C. Interaction of iPSC-derived neural stem cells on poly (L-lactic acid) nanofibrous scaffolds for possible use in neural tissue engineering. Int. J. Mol. Med. 2018, 41, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.P.; Vaquette, C.; Liu, X.; Schmitt, J.-F.; Rahouadj, R. Suitability of a PLCL fibrous scaffold for soft tissue engineering applications: A combined biological and mechanical characterisation. J. Biomater. Appl. 2018, 32, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.S.; Cheon, J.Y.; Kim, S.H.; Park, W.H. Small diameter vascular graft with fibroblast cells and electrospun poly (L-lactide-co-ε-caprolactone) scaffolds: Cell Matrix Engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 942–959. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, P.C.; Rial-Hermida, M.I.; Montini-Ballarin, F.; Abraham, G.A.; Concheiro, A.; Alvarez-Lorenzo, C. Surface-modified bioresorbable electrospun scaffolds for improving hemocompatibility of vascular grafts. Mater. Sci. Eng. C 2017, 75, 1115–1127. [Google Scholar] [CrossRef]
- Hashi, C.K.; Zhu, Y.; Yang, G.-Y.; Young, W.L.; Hsiao, B.S.; Wang, K.; Chu, B.; Li, S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl. Acad. Sci. USA 2007, 104, 11915–11920. [Google Scholar] [CrossRef]
- Bertram, U.; Steiner, D.; Poppitz, B.; Dippold, D.; Köhn, K.; Beier, J.P.; Detsch, R.; Boccaccini, A.R.; Schubert, D.W.; Horch, R.E. Vascular tissue engineering: Effects of integrating collagen into a PCL based nanofiber material. BioMed Res. Int. 2017, 2017, 9616939. [Google Scholar] [CrossRef]
- Pektok, E.; Nottelet, B.; Tille, J.-C.; Gurny, R.; Kalangos, A.; Moeller, M.; Walpoth, B.H. Degradation and healing characteristics of small-diameter poly (ε-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, L.; Liang, Q.; Shi, J.; Hou, D.; Tang, D.; Chen, S.; Kong, D.; Wang, S. The grafts modified by heparinization and catalytic nitric oxide generation used for vascular implantation in rats. Regen. Biomater. 2018, 5, 105–114. [Google Scholar] [CrossRef]
- Koosehgol, S.; Ebrahimian-Hosseinabadi, M.; Alizadeh, M.; Zamanian, A. Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater. Sci. Eng. C 2017, 79, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, N.; Johnson, R.; Anderson, R.; Elliott, W.; Tan, W. Highly compliant vascular grafts with gelatin-sheathed coaxially structured nanofibers. Langmuir 2015, 31, 12993–13002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Allen, R.A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly (glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Motlagh, D.; Yang, J.; Lui, K.Y.; Webb, A.R.; Ameer, G.A. Hemocompatibility evaluation of poly (glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials 2006, 27, 4315–4324. [Google Scholar] [CrossRef]
- Khosravi, R.; Best, C.A.; Allen, R.A.; Stowell, C.E.; Onwuka, E.; Zhuang, J.J.; Lee, Y.-U.; Yi, T.; Bersi, M.R.; Shinoka, T. Long-term functional efficacy of a novel electrospun poly (glycerol sebacate)-based arterial graft in mice. Ann. Biomed. Eng. 2016, 44, 2402–2416. [Google Scholar] [CrossRef]
- Alconcel, S.N.; Baas, A.S.; Maynard, H.D. FDA-approved poly (ethylene glycol)–protein conjugate drugs. Polym. Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Hill-West, J.L.; Chowdhury, S.M.; Slepian, M.J.; Hubbell, J.A. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc. Natl. Acad. Sci. USA 1994, 91, 5967–5971. [Google Scholar] [CrossRef]
- West, J.L.; Hubbell, J.A. Photopolymerized hydrogel materials for drug delivery applications. React. Polym. 1995, 25, 139–147. [Google Scholar] [CrossRef]
- Hahn, M.S.; McHale, M.K.; Wang, E.; Schmedlen, R.H.; West, J.L. Physiologic pulsatile flow bioreactor conditioning of poly (ethylene glycol)-based tissue engineered vascular grafts. Ann. Biomed. Eng. 2007, 35, 190–200. [Google Scholar] [CrossRef] [PubMed]
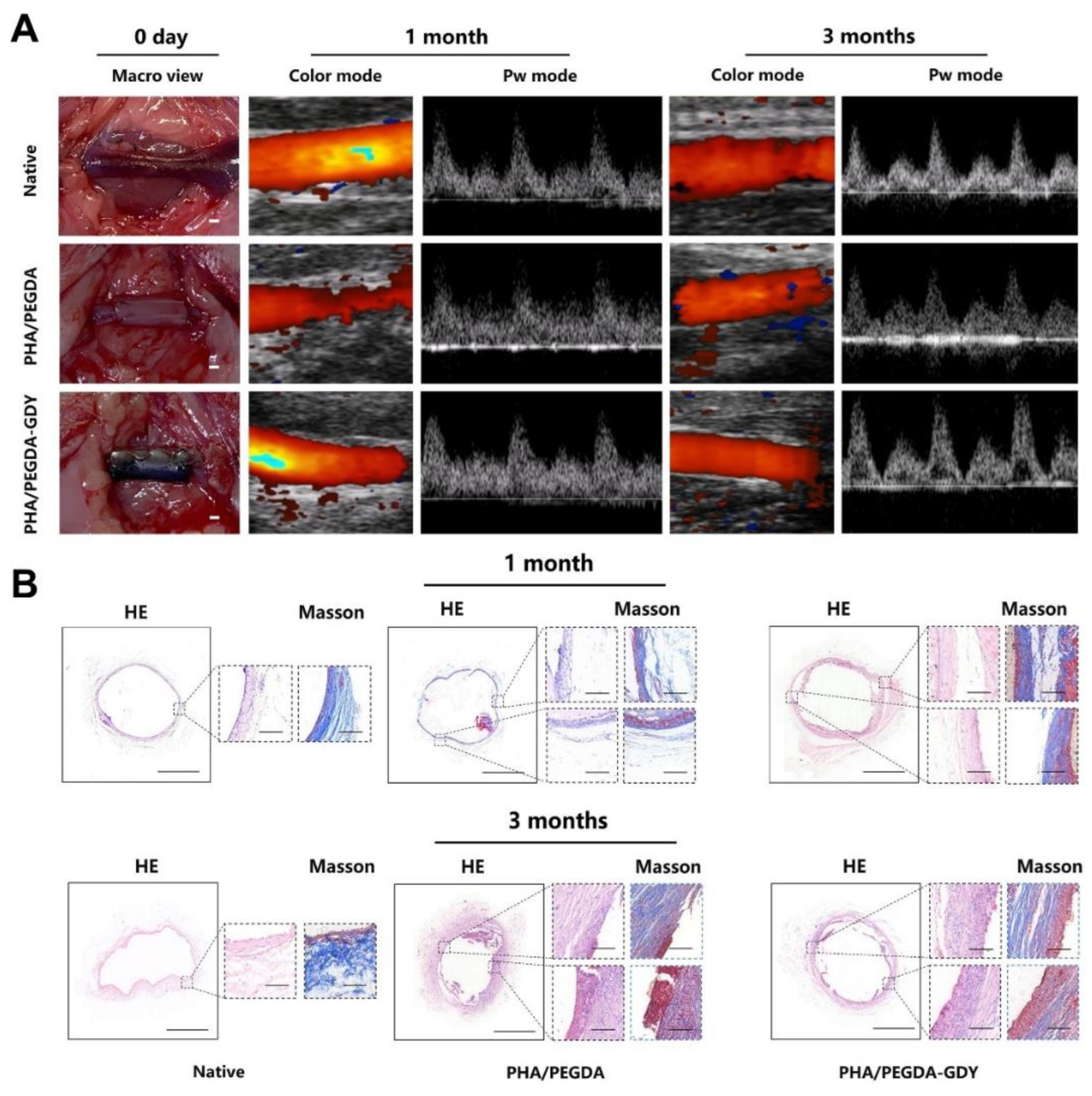
- Hou, J.; Zhang, X.; Wu, Y.; Jie, J.; Wang, Z.; Chen, G.-Q.; Sun, J.; Wu, L.-P. Amphiphilic and fatigue-resistant organohydrogels for small-diameter vascular grafts. Sci. Adv. 2022, 8, eabn5360. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Devalliere, J.; Chen, Y.; Dooley, K.; Yarmush, M.L.; Uygun, B.E. Improving functional re-endothelialization of acellular liver scaffold using REDV cell-binding domain. Acta Biomater. 2018, 78, 151–164. [Google Scholar] [CrossRef]
- Liao, S.; He, Q.; Yang, L.; Liu, S.; Zhang, Z.; Guidoin, R.; Fu, Q.; Xie, X. Toward endothelialization via vascular endothelial growth factor immobilization on cell-repelling functional polyurethanes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 965–977. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.; Han, F.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 2013, 34, 2202–2212. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, K.; Feng, J.; Liu, J.; Huang, R.; Chen, Z.; Yang, J.; Dai, Z.; Chen, Y.; Wang, N. Construction of Small-Diameter Vascular Graft by Shape-Memory and Self-Rolling Bacterial Cellulose Membrane. Adv. Healthc. Mater. 2017, 6, 1601343. [Google Scholar] [CrossRef]
- Huynh, T.; Abraham, G.; Murray, J.; Brockbank, K.; Hagen, P.-O.; Sullivan, S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat. Biotechnol. 1999, 17, 1083–1086. [Google Scholar] [CrossRef]
- Damink, L.O.; Dijkstra, P.; Van Luyn, M.; Van Wachem, P.; Nieuwenhuis, P.; Feijen, J. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials 1996, 17, 679–684. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Nicolescu, C.T.; Marinelli, J.T.; Tien, J. Generation, endothelialization, and microsurgical suture anastomosis of strong 1-mm-diameter collagen tubes. Tissue Eng. Part A 2017, 23, 335–344. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, Y.; Celik, H.; Akkus, O.; Bernacki, S.H.; King, M.W. Engineering small-caliber vascular grafts from collagen filaments and nanofibers with comparable mechanical properties to native vessels. Biofabrication 2019, 11, 035020. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, Y.; Lekakou, C.; Labeed, F.; Tomlins, P. Fabrication and characterisation of biomimetic, electrospun gelatin fibre scaffolds for tunica media-equivalent, tissue engineered vascular grafts. Mater. Sci. Eng. C 2016, 61, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, J.; Cui, H.; Chen, H.; Wang, Y.; Du, X. Programmed shape-morphing scaffolds enabling facile 3D endothelialization. Adv. Funct. Mater. 2018, 28, 1801027. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Yu, C.; Zhang, Y.S. Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Bio-Des. Manuf. 2018, 1, 215–224. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Liang, Q.; Gao, F.; Zeng, Z.; Yang, J.; Wu, M.; Gao, C.; Cheng, D.; Pan, H.; Liu, W.; Ruan, C. Coaxial scale-up printing of diameter-tunable biohybrid hydrogel microtubes with high strength, perfusability, and endothelialization. Adv. Funct. Mater. 2020, 30, 2001485. [Google Scholar] [CrossRef]
- Gong, C.; Wang, H.; Liu, P.; Guo, T. Impact of intraoperative vascular occlusion during liver surgery on long-term outcomes: A systematic review and meta-analysis. Int. J. Surg. 2017, 44, 110–116. [Google Scholar] [CrossRef]
- Nguyen, T.-U.; Shojaee, M.; Bashur, C.A.; Kishore, V. Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering. Biofabrication 2018, 11, 015007. [Google Scholar] [CrossRef]
- XIONG, D.-s. Synthesis and properties of physically crosslinked poly (vinyl alcohol) hydrogels. J. China Univ. Min. Technol. 2008, 18, 271–274. [Google Scholar]
- Li, D.Y.; Brooke, B.; Davis, E.C.; Mecham, R.P.; Sorensen, L.K.; Boak, B.B.; Eichwald, E.; Keating, M.T. Elastin is an essential determinant of arterial morphogenesis. Nature 1998, 393, 276–280. [Google Scholar] [CrossRef]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-recombinant-Elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 2020, 32, 2003915. [Google Scholar] [CrossRef]
- Golińska, P. Biopolymer-based nanofilms: Utility and toxicity. In Biopolymer-Based Nano Films; Elsevier: Amsterdam, The Netherlands, 2021; pp. 353–385. [Google Scholar]
- Lovett, M.; Eng, G.; Kluge, J.; Cannizzaro, C.; Vunjak-Novakovic, G.; Kaplan, D.L. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 2010, 6, 217–224. [Google Scholar] [CrossRef]
- Catto, V.; Farè, S.; Cattaneo, I.; Figliuzzi, M.; Alessandrino, A.; Freddi, G.; Remuzzi, A.; Tanzi, M.C. Small diameter electrospun silk fibroin vascular grafts: Mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Mater. Sci. Eng. C 2015, 54, 101–111. [Google Scholar] [CrossRef]
- Marcolin, C.; Draghi, L.; Tanzi, M.; Faré, S. Electrospun silk fibroin–gelatin composite tubular matrices as scaffolds for small diameter blood vessel regeneration. J. Mater. Sci. Mater. Med. 2017, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Achilli, M.; Alessandrino, A.; Freddi, G.; Tanzi, M.C.; Farè, S.; Mantovani, D. Collagen-reinforced electrospun silk fibroin tubular construct as small calibre vascular graft. Macromol. Biosci. 2012, 12, 1566–1574. [Google Scholar] [CrossRef]
- Liu, S.; Dong, C.; Lu, G.; Lu, Q.; Li, Z.; Kaplan, D.L.; Zhu, H. Bilayered vascular grafts based on silk proteins. Acta Biomater. 2013, 9, 8991–9003. [Google Scholar] [CrossRef]
- Cattaneo, I.; Figliuzzi, M.; Azzollini, N.; Catto, V.; Farè, S.; Tanzi, M.C.; Alessandrino, A.; Freddi, G.; Remuzzi, A. In vivo regeneration of elastic lamina on fibroin biodegradable vascular scaffold. Int. J. Artif. Organs 2013, 36, 166–174. [Google Scholar] [CrossRef]
- Enomoto, S.; Sumi, M.; Kajimoto, K.; Nakazawa, Y.; Takahashi, R.; Takabayashi, C.; Asakura, T.; Sata, M. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J. Vasc. Surg. 2010, 51, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Fowler, G.C. Pfenninger and Fowler’s Procedures for Primary Care E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Winn, H.R. Youmans and Winn Neurological Surgery; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Zhu, A.P.; Zhao, F.; Fang, N. Regulation of vascular smooth muscle cells on poly (ethylene terephthalate) film by O-carboxymethylchitosan surface immobilization. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 86, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; Liu, Y.; McGuinness, G.B.; Cahill, P.A. Characterization of poly (vinyl alcohol)/chitosan hydrogels as vascular tissue engineering scaffolds. In Macromolecular Symposia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 106–110. [Google Scholar]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.V.; Matthew, H.W. Vascular cell responses to polysaccharide materials: In vitro and in vivo evaluations. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, D.; Duan, Z.; Xue, W.; Shang, L.; Chen, F.; Luo, Y. Initial investigation of novel human-like collagen/chitosan scaffold for vascular tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Aussel, A.; Montembault, A.; Malaise, S.; Foulc, M.P.; Faure, W.; Cornet, S.; Aid, R.; Chaouat, M.; Delair, T.; Letourneur, D. In vitro mechanical property evaluation of chitosan-based hydrogels intended for vascular graft development. J. Cardiovasc. Transl. Res. 2017, 10, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Yang, C.; Zhong, W.; Lu, Z.; Li, X.; Shi, X.; Wang, D. Wire templated electrodeposition of vessel-like structured chitosan hydrogel by using a pulsed electrical signal. Soft Matter 2020, 16, 9471–9478. [Google Scholar] [CrossRef] [PubMed]
- Angra, V.; Sehgal, R.; Kaur, M.; Gupta, R. Commercialization of bionanocomposites. In Bionanocomposites in Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 587–610. [Google Scholar]
- Tahmasebifar, A.; Yilmaz, B.; Baran, E.T. Polysaccharide-based 3D bioprinter inks for tissue engineering. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 207–242. [Google Scholar]
- Gao, G.; Kim, H.; Kim, B.S.; Kong, J.S.; Lee, J.Y.; Park, B.W.; Chae, S.; Kim, J.; Ban, K.; Jang, J. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. 2019, 6, 041402. [Google Scholar] [CrossRef]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.S.; Kim, B.S.; Choi, Y.J.; Jang, W.B.; Hong, Y.J.; Kwon, S.M. Tissue engineered bio-blood-vessels constructed using a tissue-specific bioink and 3D coaxial cell printing technique: A novel therapy for ischemic disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Gao, G.; Park, J.Y.; Kim, B.S.; Jang, J.; Cho, D.W. Coaxial cell printing of freestanding, perfusable, and functional in vitro vascular models for recapitulation of native vascular endothelium pathophysiology. Adv. Healthc. Mater. 2018, 7, 1801102. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Anfang, R.; Anandappa, A.; Costa, J.; Javidfar, J.; Wobma, H.M.; Singh, G.; Freytes, D.O.; Bacchetta, M.D.; Sonett, J.R. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. 2013, 96, 1046–1056. [Google Scholar] [CrossRef]
- Soffer-Tsur, N.; Shevach, M.; Shapira, A.; Peer, D.; Dvir, T. Optimizing the biofabrication process of omentum-based scaffolds for engineering autologous tissues. Biofabrication 2014, 6, 035023. [Google Scholar] [CrossRef]
- DeQuach, J.A.; Yuan, S.H.; Goldstein, L.S.; Christman, K.L. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng. Part A 2011, 17, 2583–2592. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Dai, E.; Hawes, M.; Liu, L.; Chaudhuri, O.; Haller, C.A.; Mooney, D.J.; Chaikof, E.L. Evaluation of a bioengineered construct for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Conklin, B.; Richter, E.; Kreutziger, K.; Zhong, D.-S.; Chen, C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002, 24, 173–183. [Google Scholar] [CrossRef]
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of vascular graft studies in large animal models. Tissue Eng. Part B Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Dimitrievska, S.; Cai, C.; Weyers, A.; Balestrini, J.L.; Lin, T.; Sundaram, S.; Hatachi, G.; Spiegel, D.A.; Kyriakides, T.R.; Miao, J. Click-coated, heparinized, decellularized vascular grafts. Acta Biomater. 2015, 13, 177–187. [Google Scholar] [CrossRef]
- Jiang, B.; Suen, R.; Wang, J.-J.; Zhang, Z.J.; Wertheim, J.A.; Ameer, G.A. Vascular scaffolds with enhanced antioxidant activity inhibit graft calcification. Biomaterials 2017, 144, 166–175. [Google Scholar] [CrossRef]
- Borschel, G.H.; Huang, Y.-C.; Calve, S.; Arruda, E.M.; Lynch, J.B.; Dow, D.E.; Kuzon, W.M.; Dennis, R.G.; Brown, D.L. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005, 11, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Tillman, B.W.; Yazdani, S.K.; Neff, L.P.; Corriere, M.A.; Christ, G.J.; Soker, S.; Atala, A.; Geary, R.L.; Yoo, J.J. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J. Vasc. Surg. 2012, 56, 783–793. [Google Scholar] [CrossRef]
- Kristofik, N.J.; Qin, L.; Calabro, N.E.; Dimitrievska, S.; Li, G.; Tellides, G.; Niklason, L.E.; Kyriakides, T.R. Improving in vivo outcomes of decellularized vascular grafts via incorporation of a novel extracellular matrix. Biomaterials 2017, 141, 63–73. [Google Scholar] [CrossRef]
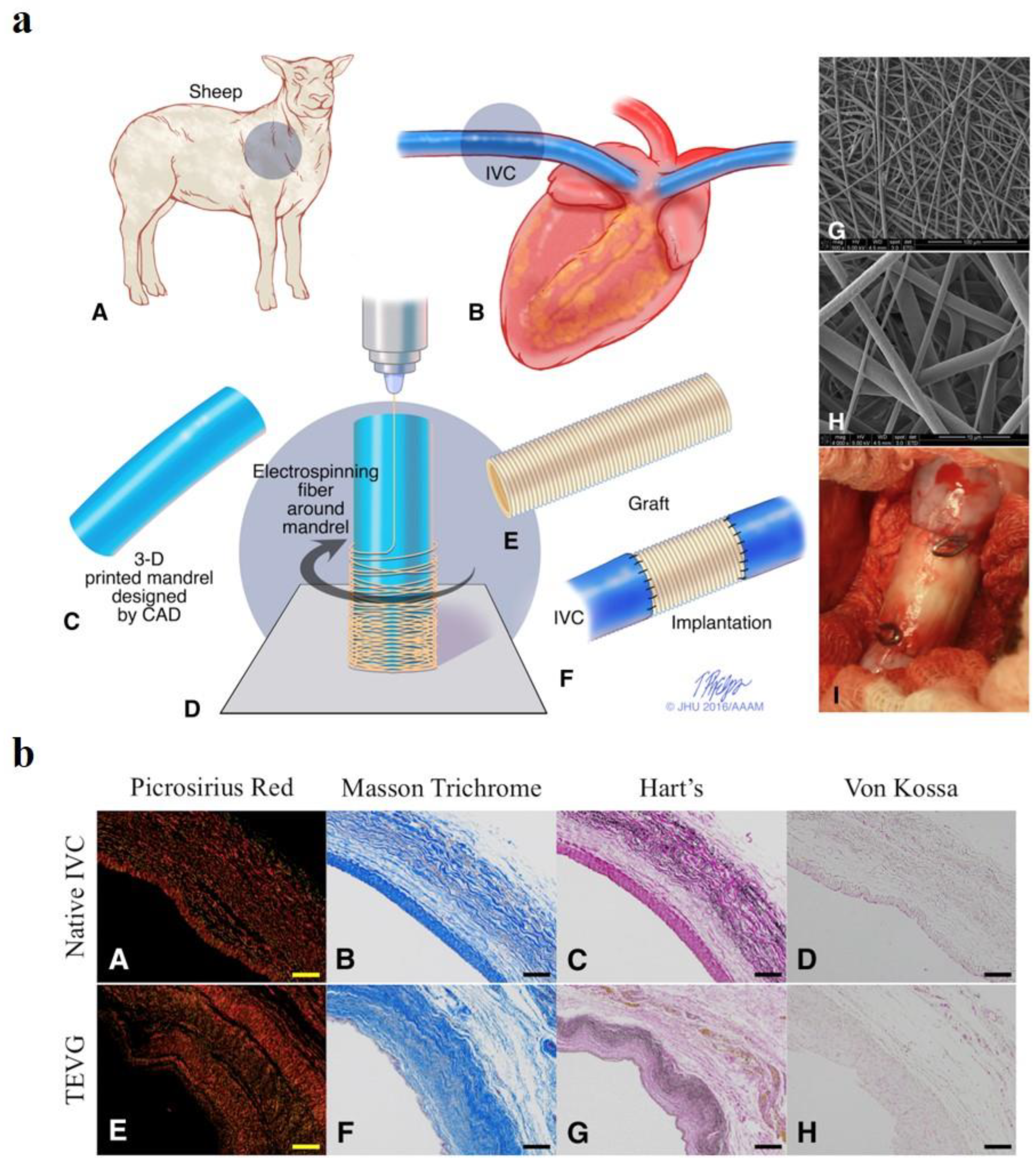
- Syedain, Z.; Reimer, J.; Lahti, M.; Berry, J.; Johnson, S.; Bianco, R.; Tranquillo, R.T. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat. Commun. 2016, 7, 12951. [Google Scholar] [CrossRef]
- Sparks, C.H. Die-grown reinforced arterial grafts: Observations on long-term animal grafts and clinical experience. Ann. Surg. 1970, 172, 787. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, W.; van der Bogt, K.; Rothuizen, T.; Damanik, F.; Hamming, J.; Mota, C.; van Agen, M.; de Boer, H.; Restrepo, M.T.; Hinz, B. A novel method for engineering autologous non-thrombogenic in situ tissue-engineered blood vessels for arteriovenous grafting. Biomaterials 2020, 229, 119577. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, M.; Madeddu, P. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front. Bioeng. Biotechnol. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Moschouris, K.; Firoozi, N.; Kang, Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen. Med. 2016, 11, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Kojima, K.; Kodama, S.; Paz, A.C.; Chambers, M.; Umezu, M.; Vacanti, C.A. Bioengineered three-layered robust and elastic artery using hemodynamically-equivalent pulsatile bioreactor. Circulation 2008, 118, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- L’heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar]
- McAllister, T.N.; Maruszewski, M.; Garrido, S.A.; Wystrychowski, W.; Dusserre, N.; Marini, A.; Zagalski, K.; Fiorillo, A.; Avila, H.; Manglano, X. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: A multicentre cohort study. Lancet 2009, 373, 1440–1446. [Google Scholar] [CrossRef]
- Yuan, B.; Jin, Y.; Sun, Y.; Wang, D.; Sun, J.; Wang, Z.; Zhang, W.; Jiang, X. A strategy for depositing different types of cells in three dimensions to mimic tubular structures in tissues. Adv. Mater. 2012, 24, 890–896. [Google Scholar] [CrossRef]
- Rayatpisheh, S.; Heath, D.E.; Shakouri, A.; Rujitanaroj, P.-O.; Chew, S.Y.; Chan-Park, M.B. Combining cell sheet technology and electrospun scaffolding for engineered tubular, aligned, and contractile blood vessels. Biomaterials 2014, 35, 2713–2719. [Google Scholar] [CrossRef]
- Xing, Q.; Qian, Z.; Tahtinen, M.; Yap, A.H.; Yates, K.; Zhao, F. Aligned nanofibrous cell-derived extracellular matrix for anisotropic vascular graft construction. Adv. Healthc. Mater. 2017, 6, 1601333. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Sundaram, M.N.; Vijayan, P.; Nair, S.V.; Jayakumar, R. Engineering poly (hydroxy butyrate-co-hydroxy valerate) based vascular scaffolds to mimic native artery. Int. J. Biol. Macromol. 2018, 109, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Caves, J.M.; Haller, C.A.; Dai, E.; Liu, L.; Grainger, S.; Chaikof, E.L. Acellular vascular grafts generated from collagen and elastin analogs. Acta Biomater. 2013, 9, 8067–8074. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Forgacs, G. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef]
- Kelm, J.M.; Lorber, V.; Snedeker, J.G.; Schmidt, D.; Broggini-Tenzer, A.; Weisstanner, M.; Odermatt, B.; Mol, A.; Zünd, G.; Hoerstrup, S.P. A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J. Biotechnol. 2010, 148, 46–55. [Google Scholar] [CrossRef]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef]
- Andrique, L.; Recher, G.; Alessandri, K.; Pujol, N.; Feyeux, M.; Bon, P.; Cognet, L.; Nassoy, P.; Bikfalvi, A. A model of guided cell self-organization for rapid and spontaneous formation of functional vessels. Sci. Adv. 2019, 5, eaau6562. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Zargham, S.; Bazgir, S.; Tavakoli, A.; Rashidi, A.S.; Damerchely, R. The effect of flow rate on morphology and deposition area of electrospun nylon 6 nanofiber. J. Eng. Fibers Fabr. 2012, 7, 155892501200700414. [Google Scholar] [CrossRef]
- Mokhtari, F.; Latifi, M.; Shamshirsaz, M. Electrospinning/electrospray of polyvinylidene fluoride (PVDF): Piezoelectric nanofibers. J. Text. Inst. 2016, 107, 1037–1055. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Jian, H.Y.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.M.; Kleinmeyer, J.D.; Hirvonen, J.K.; Tan, N.B. Controlled deposition of electrospun poly (ethylene oxide) fibers. Polymer 2001, 42, 8163–8170. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Dhanasopon, A.P.; Rofail, F.; Smith, H.; Wu, B.M.; Shemin, R.; Beygui, R.E.; MacLellan, W.R. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 2008, 29, 2907–2914. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, Y.; Wang, Z.; Wang, J.; Zhang, C.; Li, C.; Kong, D. The regeneration of macro-porous electrospun poly (ε-caprolactone) vascular graft during long-term in situ implantation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1618–1627. [Google Scholar] [CrossRef]
- Fiqrianti, I.A.; Widiyanti, P.; Manaf, M.A.; Savira, C.Y.; Cahyani, N.R.; Bella, F.R. Poly-L-lactic acid (PLLA)-chitosan-collagen electrospun tube for vascular graft application. J. Funct. Biomater. 2018, 9, 32. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Dempsey, D.K.; Cosgriff-Hernandez, E. Electrospun vascular grafts with improved compliance matching to native vessels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 313–323. [Google Scholar] [CrossRef]
- Hong, Y.; Ye, S.-H.; Nieponice, A.; Soletti, L.; Vorp, D.A.; Wagner, W.R. A small diameter, fibrous vascular conduit generated from a poly (ester urethane) urea and phospholipid polymer blend. Biomaterials 2009, 30, 2457–2467. [Google Scholar] [CrossRef]
- Han, J.; Lazarovici, P.; Pomerantz, C.; Chen, X.; Wei, Y.; Lelkes, P.I. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 2011, 12, 399–408. [Google Scholar] [CrossRef]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, J.J.; Lim, G.J.; Atala, A.; Stitzel, J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Lee, M.-K.; Park, C.; Jung, H.-D.; Kim, H.-E.; Jang, T.-S. Fabrication of strong, bioactive vascular grafts with PCL/collagen and PCL/silica bilayers for small-diameter vascular applications. Mater. Des. 2019, 181, 108079. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, X.; Wei, Y.; Zhang, Q.; Wang, T.; Zhu, M.; Li, W.; Huang, R.; Liu, R.; Chen, J. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci. Rep. 2017, 7, 3615. [Google Scholar] [CrossRef]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules 2008, 9, 2097–2103. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Feng, Y.; Yang, Y.; Wei, Z.; Zhao, W.; Zhao, C. A chitosan modified asymmetric small-diameter vascular graft with anti-thrombotic and anti-bacterial functions for vascular tissue engineering. J. Mater. Chem. B 2020, 8, 568–577. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, B.; Wang, Y.; Yin, A.; Huang, C.; Wang, S.; Mo, X. Electrospun poly (l-lactide-co-caprolactone)–collagen–chitosan vascular graft in a canine femoral artery model. J. Mater. Chem. B 2015, 3, 5760–5768. [Google Scholar] [CrossRef]
- Yin, A.; Zhang, K.; McClure, M.J.; Huang, C.; Wu, J.; Fang, J.; Mo, X.; Bowlin, G.L.; Al-Deyab, S.S.; El-Newehy, M. Electrospinning collagen/chitosan/poly (L-lactic acid-co-ϵ-caprolactone) to form a vascular graft: Mechanical and biological characterization. J. Biomed. Mater. Res. Part A 2013, 101, 1292–1301. [Google Scholar] [CrossRef]
- Torre-Muruzabal, A.; Daelemans, L.; Van Assche, G.; De Clerck, K.; Rahier, H. Creation of a nanovascular network by electrospun sacrificial nanofibers for self-healing applications and its effect on the flexural properties of the bulk material. Polym. Test. 2016, 54, 78–83. [Google Scholar] [CrossRef]
- Gualandi, C.; Zucchelli, A.; Fernández Osorio, M.; Belcari, J.; Focarete, M.L. Nanovascularization of polymer matrix: Generation of nanochannels and nanotubes by sacrificial electrospun fibers. Nano Lett. 2013, 13, 5385–5390. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Wang, X.; Faley, S.; Baer, B.; Balikov, D.A.; Sung, H.J.; Bellan, L.M. Development of 3D microvascular networks within gelatin hydrogels using thermoresponsive sacrificial microfibers. Adv. Healthc. Mater. 2016, 5, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Kuiper, J. Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Karimi, A.; Navidbakhsh, M.; Shojaei, A.; Faghihi, S. Measurement of the uniaxial mechanical properties of healthy and atherosclerotic human coronary arteries. Mater. Sci. Eng. C 2013, 33, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D.; Black, R.A.; Vito, R.P.; Nerem, R.M. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann. Biomed. Eng. 2000, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Aper, T.; Wilhelmi, M.; Gebhardt, C.; Hoeffler, K.; Benecke, N.; Hilfiker, A.; Haverich, A. Novel method for the generation of tissue-engineered vascular grafts based on a highly compacted fibrin matrix. Acta Biomater. 2016, 29, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Stolz, D.B.; Wang, Y. Substantial expression of mature elastin in arterial constructs. Proc. Natl. Acad. Sci. USA 2011, 108, 2705–2710. [Google Scholar] [CrossRef]
- Nieponice, A.; Soletti, L.; Guan, J.; Hong, Y.; Gharaibeh, B.; Maul, T.M.; Huard, J.; Wagner, W.R.; Vorp, D.A. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng. Part A 2010, 16, 1215–1223. [Google Scholar] [CrossRef]
- Heath, D.E.; Kang, G.C.; Cao, Y.; Poon, Y.F.; Chan, V.; Chan-Park, M.B. Biomaterials patterned with discontinuous microwalls for vascular smooth muscle cell culture: Biodegradable small diameter vascular grafts and stable cell culture substrates. J. Biomater. Sci. Polym. Ed. 2016, 27, 1477–1494. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kingston, B.R.; Chan, W.C. Transcribing in vivo blood vessel networks into in vitro perfusable microfluidic devices. Adv. Mater. Technol. 2020, 5, 2000103. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. TRENDS Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Harrysson, O.L.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Busari, H.; Seims, K.B.; Schwarzenberg, P.; Dailey, H.L.; Chow, L.W. 3D printing with peptide–polymer conjugates for single-step fabrication of spatially functionalized scaffolds. Biomater. Sci. 2019, 7, 4237–4247. [Google Scholar] [CrossRef] [PubMed]
- Kabirian, F.; Ditkowski, B.; Zamanian, A.; Hoylaerts, M.F.; Mozafari, M.; Heying, R. Controlled NO-release from 3D-printed small-diameter vascular grafts prevents platelet activation and bacterial infectivity. ACS Biomater. Sci. Eng. 2019, 5, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, X.; Khademhosseini, A.; Zhang, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 2018, 10, 024102. [Google Scholar] [CrossRef]
- Gao, Q.; He, Y.; Fu, J.-z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Xu, L.; Varkey, M.; Jorgensen, A.; Ju, J.; Jin, Q.; Park, J.H.; Fu, Y.; Zhang, G.; Ke, D.; Zhao, W. Bioprinting small diameter blood vessel constructs with an endothelial and smooth muscle cell bilayer in a single step. Biofabrication 2020, 12, 045012. [Google Scholar] [CrossRef]
- Jakab, K.; Norotte, C.; Marga, F.; Murphy, K.; Vunjak-Novakovic, G.; Forgacs, G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010, 2, 022001. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chai, W.; Huang, Y.; Markwald, R.R. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol. Bioeng. 2012, 109, 3152–3160. [Google Scholar] [CrossRef]
- Duan, B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Melchiorri, A.J.; Hibino, N.; Best, C.; Yi, T.; Lee, Y.; Kraynak, C.; Kimerer, L.K.; Krieger, A.; Kim, P.; Breuer, C.K. 3D-printed biodegradable polymeric vascular grafts. Adv. Healthc. Mater. 2016, 5, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Heintz, K.A.; Bregenzer, M.E.; Mantle, J.L.; Lee, K.H.; West, J.L.; Slater, J.H. Fabrication of 3D biomimetic microfluidic networks in hydrogels. Adv. Healthc. Mater. 2016, 5, 2153–2160. [Google Scholar] [CrossRef]
- Oujja, M.; Pérez, S.; Fadeeva, E.; Koch, J.; Chichkov, B.; Castillejo, M. Three dimensional microstructuring of biopolymers by femtosecond laser irradiation. Appl. Phys. Lett. 2009, 95, 263703. [Google Scholar] [CrossRef]
- Hribar, K.C.; Meggs, K.; Liu, J.; Zhu, W.; Qu, X.; Chen, S. Three-dimensional direct cell patterning in collagen hydrogels with near-infrared femtosecond laser. Sci. Rep. 2015, 5, 17203. [Google Scholar] [CrossRef] [PubMed]
- Applegate, M.B.; Coburn, J.; Partlow, B.P.; Moreau, J.E.; Mondia, J.P.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc. Natl. Acad. Sci. USA 2015, 112, 12052–12057. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Lutolf, M.P. In situ patterning of microfluidic networks in 3D cell-laden hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Berkovitch, Y.; Yelin, D.; Seliktar, D. Photo-patterning PEG-based hydrogels for neuronal engineering. Eur. Polym. J. 2015, 72, 473–483. [Google Scholar] [CrossRef]
- Arakawa, C.K.; Badeau, B.A.; Zheng, Y.; DeForest, C.A. Multicellular vascularized engineered tissues through user-programmable biomaterial photodegradation. Adv. Mater. 2017, 29, 1703156. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.-N.; Armbruster, C.; Carrel, T.; Tevaearai, H.T. Current state of the art in myocardial tissue engineering. Tissue Eng. 2007, 13, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Shipley, R.J.; Phillips, J.B. Vascularization strategies for peripheral nerve tissue engineering. Anat. Rec. 2018, 301, 1657–1667. [Google Scholar] [CrossRef]
- Best, T.J.; Mackinnon, S.E. Peripheral nerve revascularization: A current literature review. J. Reconstr. Microsurg. 1994, 10, 193–204. [Google Scholar] [CrossRef]
- Appenzeller, O.; Dhital, K.K.; Cowen, T.; Burnstock, G. The nerves to blood vessels supplying blood to nerves: The innervation of vasa nervorum. Brain Res. 1984, 304, 383–386. [Google Scholar] [CrossRef]
- Smith, D.R.; Kobrine, A.I.; Rizzoli, H.V. Absence of autoregulation in peripheral nerve blood flow. J. Neurol. Sci. 1977, 33, 347–352. [Google Scholar] [CrossRef]
- Zochodne, D.W. Nerve and ganglion blood flow in diabetes: An appraisal. Int. Rev. Neurobiol. 2002, 50, 161–202. [Google Scholar] [PubMed]
- Low, P.A.; Lagerlund, T.D.; McManis, P.G. Nerve blood flow and oxygen delivery in normal, diabetic, and ischemic neuropathy. Int. Rev. Neurobiol. 1989, 31, 355–438. [Google Scholar] [PubMed]
- Kokaia, Z.; Lindvall, O. Neurogenesis after ischaemic brain insults. Curr. Opin. Neurobiol. 2003, 13, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, C.; Rafii, S.; Rafii, D.; Shahar, A.; Goldman, S.A. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell. Neurosci. 1999, 13, 450–464. [Google Scholar] [CrossRef]
- Isahara, K.; Yamamoto, M. The interaction of vascular endothelial cells and dorsal root ganglion neurites is mediated by vitronectin and heparan sulfate proteoglycans. Dev. Brain Res. 1995, 84, 164–178. [Google Scholar] [CrossRef]
- Strange, F.S.C. An operation for nerve pedicle grafting. Preliminary communication. Br. J. Surg. 1947, 34, 423–425. [Google Scholar] [CrossRef]
- Koshima, I.; Harii, K. Experimental study of vascularized nerve grafts: Multifactorial analyses of axonal regeneration of nerves transplanted into an acute burn wound. J. Hand Surg. 1985, 10, 64–72. [Google Scholar] [CrossRef]
- Kanaya, F.; Firrell, J.; Tsai, T.-M.; Breidenbach, W.C. Functional results of vascularized versus nonvascularized nerve grafting. Plast. Reconstr. Surg. 1992, 89, 924–930. [Google Scholar] [CrossRef]
- Ozcan, G.; Shenaq, S.; Spira, M. Vascularized nerve tube: An experimental alternative for vascularized nerve grafts over short gaps. J. Reconstr. Microsurg. 1993, 9, 405–413. [Google Scholar] [CrossRef]
- Iijima, Y.; Ajiki, T.; Murayama, A.; Takeshita, K. Effect of artificial nerve conduit vascularization on peripheral nerve in a necrotic bed. Plast. Reconstr. Surg. Glob. Open 2016, 4, e665. [Google Scholar] [CrossRef]
- Kakinoki, R.; Nishijima, N.; Ueba, Y.; Oka, M.; Yamamuro, T.; Nakamura, T. Nerve regeneration over a 25 mm gap in rat sciatic nerves using tubes containing blood vessels: The possibility of clinical application. Int. Orthop. 1997, 21, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It takes two to tango: Coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Byambaa, B.; Annabi, N.; Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Jia, W.; Kazemzadeh-Narbat, M.; Shin, S.R.; Tamayol, A.; Khademhosseini, A. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv. Healthc. Mater. 2017, 6, 1700015. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, S.; Qu, X.; Hu, Y.; Zhang, X.; Wang, T.; Wei, F. BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochim Biophys. Sin. 2011, 43, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in bone tissue engineering constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Casanova, M.R.; Oliveira, C.; Fernandes, E.M.; Reis, R.L.; Silva, T.H.; Martins, A.; Neves, N.M. Spatial immobilization of endogenous growth factors to control vascularization in bone tissue engineering. Biomater. Sci. 2020, 8, 2577–2589. [Google Scholar] [CrossRef]
- Divband, B.; Samiei, M.; Davaran, S.; Roshangar, L.; Shahi, S.; Aghazadeh, M. Synthesis and in vitro Evaluation of Thermosensitive PLA-gP (HEM-co-NIPAAM) Hydrogel Used for Delivery of VEGF. Biointerface Res. Appl. Chem. 2020, 11, 8043–8051. [Google Scholar]
- Mitra, D.; Whitehead, J.; Yasui, O.W.; Leach, J.K. Bioreactor culture duration of engineered constructs influences bone formation by mesenchymal stem cells. Biomaterials 2017, 146, 29–39. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Y.; Basuthakur, P.; Patra, C.R.; Ramakrishna, S.; Liu, Y.; Thomas, V.; Nanda, H.S. Fibro-porous PLLA/gelatin composite membrane doped with cerium oxide nanoparticles as bioactive scaffolds for future angiogenesis. J. Mater. Chem. B 2020, 8, 9110–9120. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, S.; Ji, X.; Zhou, Y.; Zhang, Y.; Li, Q.; Tan, C.; Peng, F.; Zhang, Y.; Huang, W. Immobilizing magnesium ions on 3D printed porous tantalum scaffolds with polydopamine for improved vascularization and osteogenesis. Mater. Sci. Eng. C 2020, 117, 111303. [Google Scholar] [CrossRef]
- Stähli, C.; James-Bhasin, M.; Hoppe, A.; Boccaccini, A.R.; Nazhat, S.N. Effect of ion release from Cu-doped 45S5 Bioglass® on 3D endothelial cell morphogenesis. Acta Biomater. 2015, 19, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W. Accelerated bone regeneration by MOF modified multifunctional membranes through enhancement of osteogenic and angiogenic performance. Adv. Healthc. Mater. 2021, 10, 2001369. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, M.; Zhou, X.; Shao, Y.; Li, J.; Wang, L.; Chu, C.; Xue, F.; Yao, Q.; Bai, J. 3D-printed Mg-incorporated PCL-based scaffolds: A promising approach for bone healing. Mater. Sci. Eng. C 2021, 129, 112372. [Google Scholar] [CrossRef]
- Li, R.; Zhou, C.; Chen, J.; Luo, H.; Li, R.; Chen, D.; Zou, X.; Wang, W. Synergistic osteogenic and angiogenic effects of KP and QK peptides incorporated with an injectable and self-healing hydrogel for efficient bone regeneration. Bioact. Mater. 2022, 18, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.; Cao, L.; Zhang, X.; Wang, J.; Liu, C. Facilitated vascularization and enhanced bone regeneration by manipulation hierarchical pore structure of scaffolds. Mater. Sci. Eng. C 2020, 110, 110622. [Google Scholar] [CrossRef] [PubMed]
- Markou, M.; Kouroupis, D.; Badounas, F.; Katsouras, A.; Kyrkou, A.; Fotsis, T.; Murphy, C.; Bagli, E. Tissue engineering using vascular organoids from human pluripotent stem cell derived mural cell phenotypes. Front. Bioeng. Biotechnol. 2020, 8, 278. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Athirasala, A.; Gordon, R.; Zhang, L.; Bergan, R.; Keene, D.R.; Jones, J.M.; Xie, H.; Chen, Z.; Tao, J. Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nat. Commun. 2019, 10, 3520. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2019, 24, 41–56. [Google Scholar] [CrossRef]
- Piard, C.; Jeyaram, A.; Liu, Y.; Caccamese, J.; Jay, S.M.; Chen, Y.; Fisher, J. 3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance. Biomaterials 2019, 222, 119423. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Luxán, G.; Dimmeler, S. The vasculature: A therapeutic target in heart failure? Cardiovasc. Res. 2022, 118, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, D.L. Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev. 2003, 83, 59–115. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Karra, R.; Dickson, A.L.; Poss, K.D. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife 2015, 4, e05871. [Google Scholar] [CrossRef]
- D’Uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef]
- Scimia, M.C.; Hurtado, C.; Ray, S.; Metzler, S.; Wei, K.; Wang, J.; Woods, C.E.; Purcell, N.H.; Catalucci, D.; Akasaka, T. APJ acts as a dual receptor in cardiac hypertrophy. Nature 2012, 488, 394–398. [Google Scholar] [CrossRef]
- Wang, W.; McKinnie, S.M.; Patel, V.B.; Haddad, G.; Wang, Z.; Zhabyeyev, P.; Das, S.K.; Basu, R.; McLean, B.; Kandalam, V. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: Therapeutic potential of synthetic Apelin analogues. J. Am. Heart Assoc. 2013, 2, e000249. [Google Scholar] [CrossRef]
- Narmoneva, D.A.; Vukmirovic, R.; Davis, M.E.; Kamm, R.D.; Lee, R.T. Endothelial cells promote cardiac myocyte survival and spatial reorganization: Implications for cardiac regeneration. Circulation 2004, 110, 962–968. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Prevascularization in tissue engineering: Current concepts and future directions. Biotechnol. Adv. 2016, 34, 112–121. [Google Scholar] [CrossRef]
- Rodrigues, I.C.P.; Kaasi, A.; Maciel Filho, R.; Jardini, A.L.; Gabriel, L.P. Cardiac tissue engineering: Current state-of-the-art materials, cells and tissue formation. Einstein 2018, 16, eRB4538. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Marsh, P.; Schmiess-Heine, L.; Burke, P.J.; Lee, A.; Lee, J.; Cao, H. Cardiac tissue engineering: State-of-the-art methods and outlook. J. Biol. Eng. 2019, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, L.; Korff, T. Spheroid-based in vitro angiogenesis model. In Angiogenesis Protocols; Springer: Berlin/Heidelberg, Germany, 2016; pp. 167–177. [Google Scholar]
- Basagiannis, D.; Zografou, S.; Murphy, C.; Fotsis, T.; Morbidelli, L.; Ziche, M.; Bleck, C.; Mercer, J.; Christoforidis, S. VEGF induces signalling and angiogenesis by directing VEGFR2 internalisation through macropinocytosis. J. Cell Sci. 2016, 129, 4091–4104. [Google Scholar] [CrossRef] [PubMed]
- Raica, M.; Cimpean, A.M. Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Cai, W.-W.; Gong, L.-L.; Wang, X.; Zhu, X.-X.; Wan, M.-Y.; Wang, P.-Y.; Qiu, L.-Y. FGF-2-mediated FGFR1 signaling in human microvascular endothelial cells is activated by vaccarin to promote angiogenesis. Biomed. Pharmacother. 2017, 95, 144–152. [Google Scholar] [CrossRef]
- Patel, N.G.; Zhang, G. Responsive systems for cell sheet detachment. Organogenesis 2013, 9, 93–100. [Google Scholar] [CrossRef]
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-free 3-D cell sheet technique bridges the gap between 2-D cell culture and animal models. Int. J. Mol. Sci. 2019, 20, 4926. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
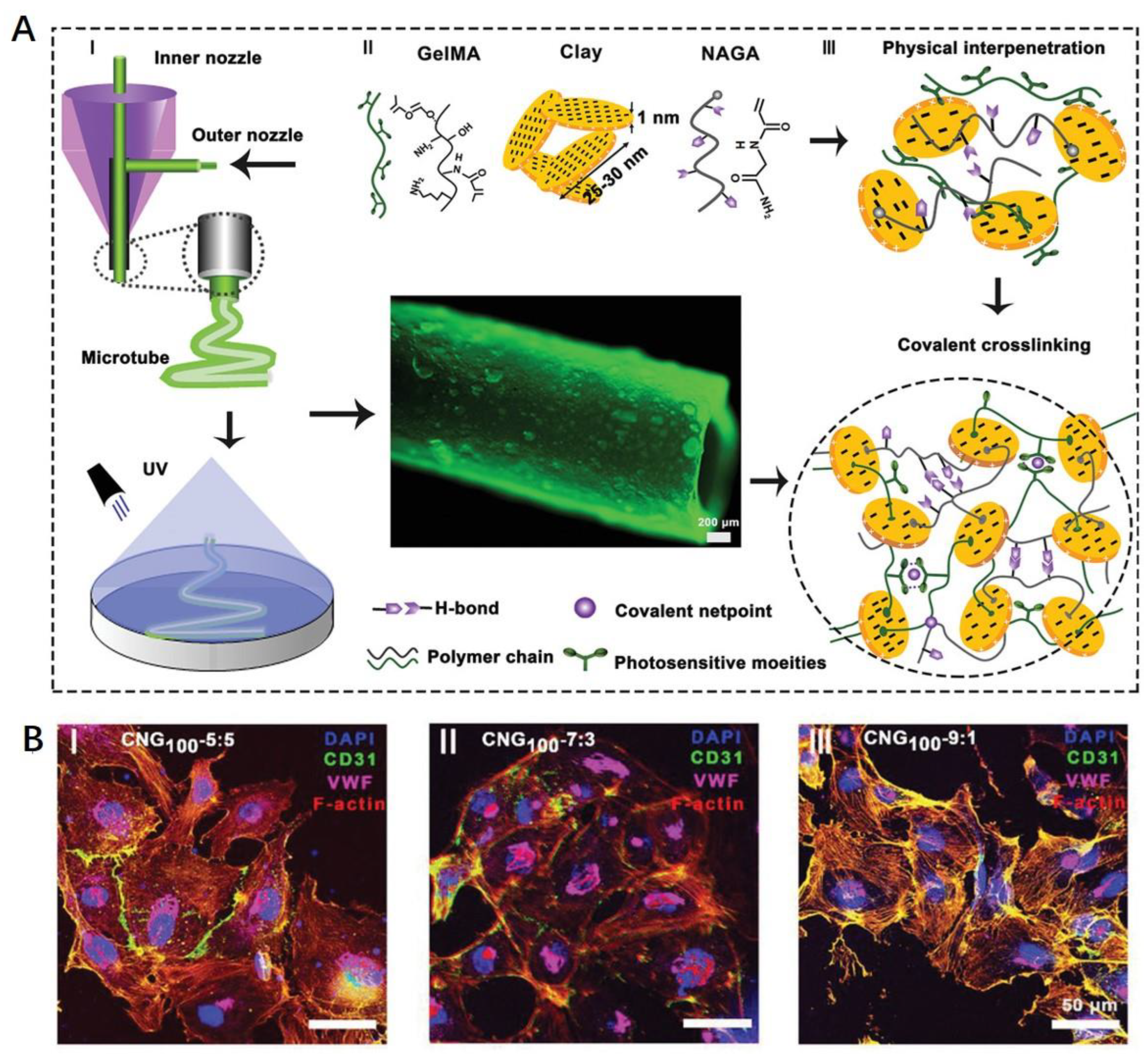
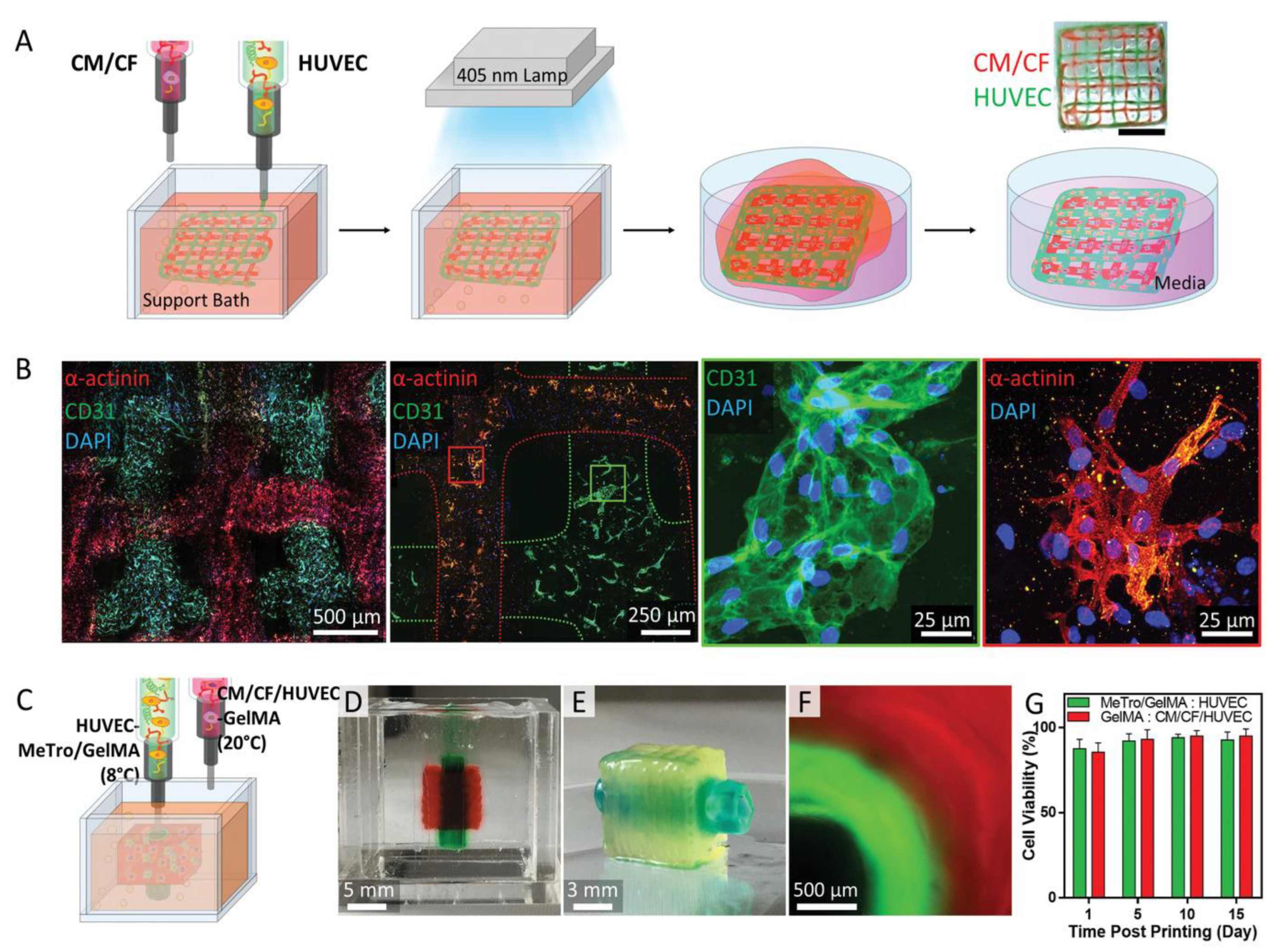
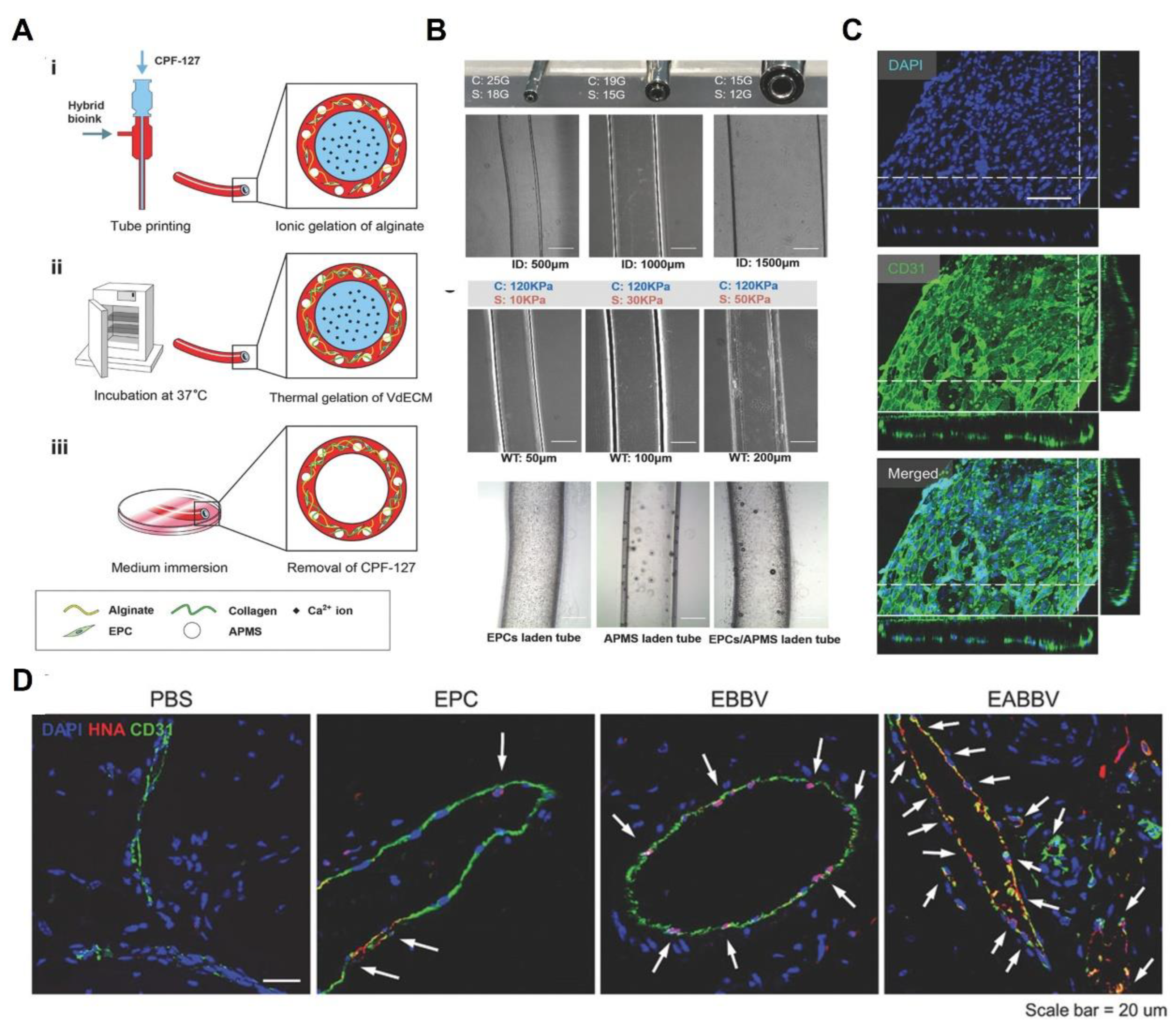


| Tissue | Strategy | General Functions | Examples of Studies | References |
|---|---|---|---|---|
| Peripheral nerve | Autologous grafts | Enhanced regeneration in nerve length, size, and sciatic function index (SFI) | Ulnar nerve with its blood supply | [264–266] |
| Preimplantment of nerve grafts to obtain microcirculation network | Enhanced axon diameter, myelin thickness, and the number of myelinated axons | Vascularized amnion tube obtained through embedding between the femoral artery and vein | [267,268] | |
| Blood-vessel-including tabulation | Regeneration across the nerve gap | Native blood vessel wrapped with the nerve conduit | [269] | |
| Bone | Ion doping | Promoted angiogenesis through HIF-1α/VEGF signaling pathway | Mg-doped tantalum scaffold | [277–282] |
| Adding growth factors | Improved neovascularization and synergistic osteogenesis | GelMA-based hydrogel with angiogenic peptide QK | [283] | |
| Altering topography | Promoted proliferation and differentiation of osteoblasts and ECs; increased bone–matrix interface strength and stimulate mineralization | Pore-structured scaffolds based on poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) | [284] | |
| Coculturing of cells | Enhanced capillary and bone formation | BMSCs/ECs coculture | [285–288] | |
| Heart | Coculturing of cells | Promoted vasculature formation | Endothelial cells cultured with cardiomyocytes | [297–300] |
| Adding growth factors | Enhanced vascularization | VEGF | [301–303] | |
| Microvascular tubes fabricated with cell sheet | Formation of well-perfused microchannels | Coculturing ECs with cardiac cell sheets in a collagen hydrogel | [304,305] | |
| Prevascularization through 3D printing | Integration to the host’s vasculature | A hierarchical vasculature supporting in vitro functionality of cardiomyocytes | [20,306,307] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, D.; Wu, L.-P.; Zhao, M. Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering. Polymers 2023, 15, 2015. https://doi.org/10.3390/polym15092015
Chen J, Zhang D, Wu L-P, Zhao M. Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering. Polymers. 2023; 15(9):2015. https://doi.org/10.3390/polym15092015
Chicago/Turabian StyleChen, Jun, Di Zhang, Lin-Ping Wu, and Ming Zhao. 2023. "Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering" Polymers 15, no. 9: 2015. https://doi.org/10.3390/polym15092015
APA StyleChen, J., Zhang, D., Wu, L.-P., & Zhao, M. (2023). Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering. Polymers, 15(9), 2015. https://doi.org/10.3390/polym15092015









