Synthetic Heparan Sulfate Mimetic Polymer Enhances Corneal Nerve Regeneration and Wound Healing after Experimental Laser Ablation Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Corneal Injury by Laser Ablation
2.3. Clinical Course
2.4. Tissue Processing and Microscopy
2.5. Cell Counting
2.6. Whole-Mounted Cornea Immunofluorescence
2.7. Sholl Analysis
2.8. Statistical Analysis
3. Results
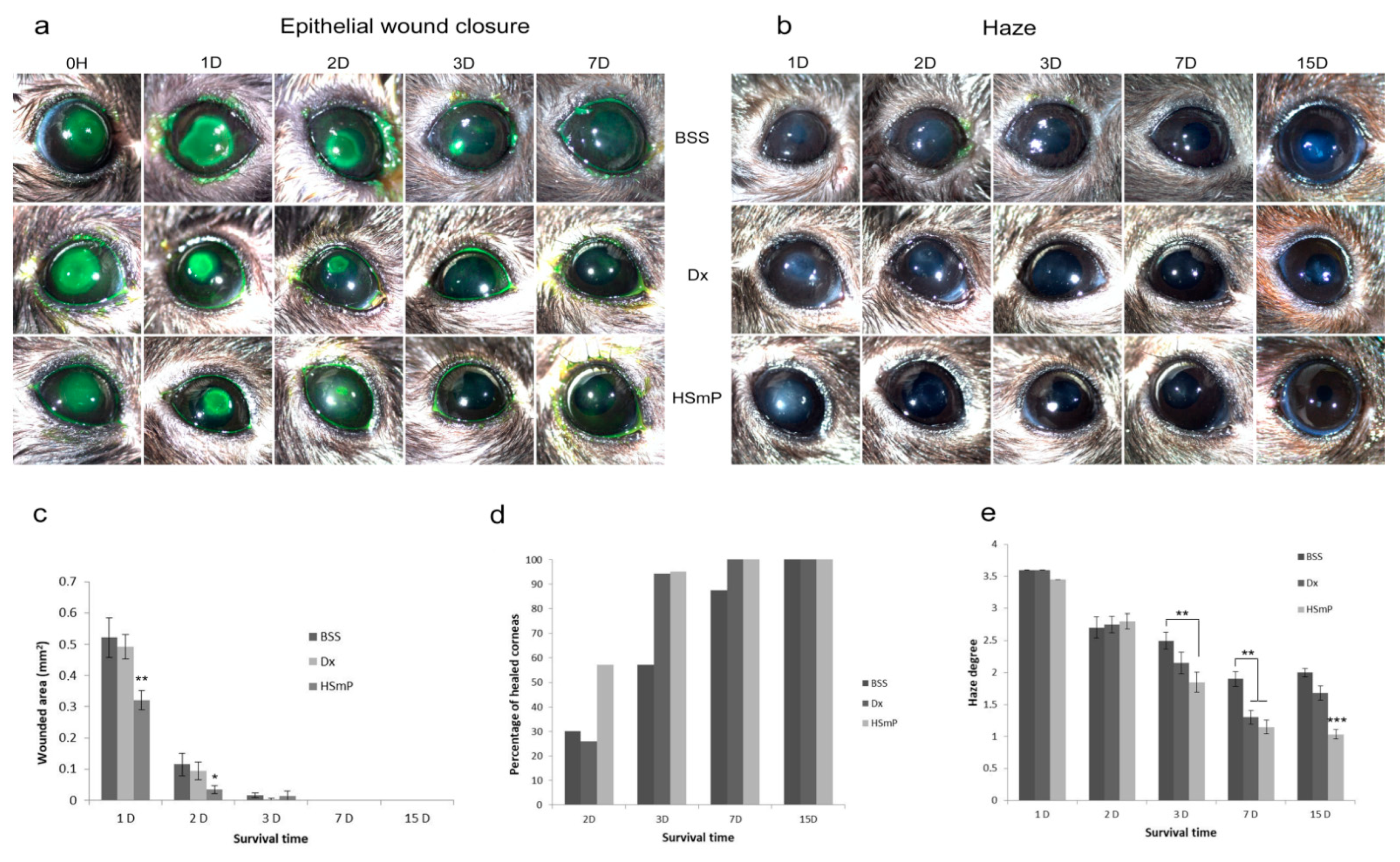
3.1. Wound Healing
3.2. Corneal Transparency
3.3. Histopathological Analysis
3.3.1. Cell Proliferation
3.3.2. Apoptosis
3.3.3. Epithelial Morphology
3.3.4. Myofibroblast Formation
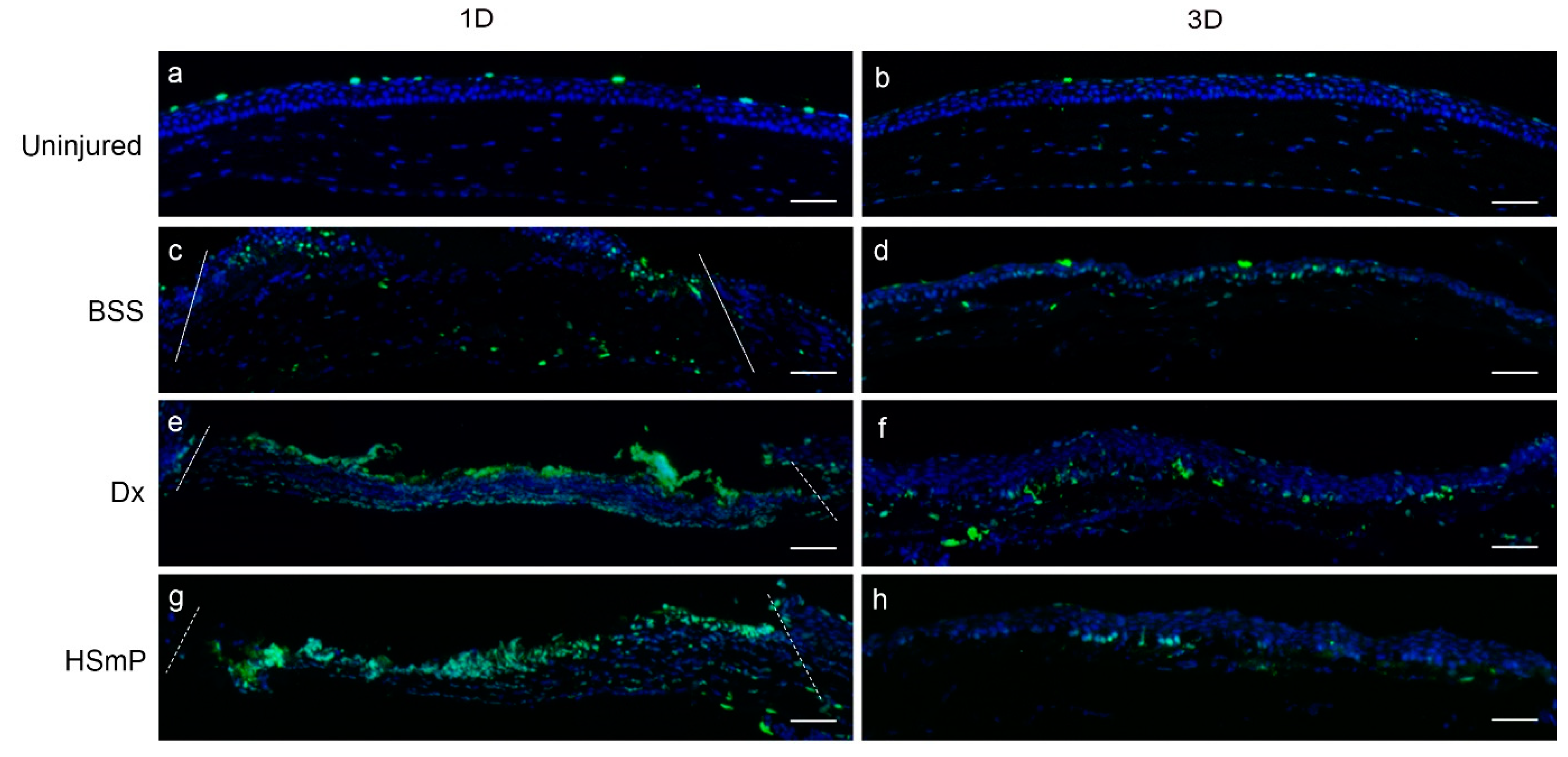
3.3.5. Nerve Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aifa, A.; Gueudry, J.; Portmann, A.; Delcampe, A.; Muraine, M. Topical Treatment with a New Matrix Therapy Agent (RGTA) for the Treatment of Corneal Neurotrophic Ulcers. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8181–8185. [Google Scholar] [CrossRef]
- Zeppieri, M.; Salvetat, M.L.; Beltrami, A.P.; Cesselli, D.; Bergamin, N.; Russo, R.; Cavaliere, F.; Varano, G.P.; Alcalde, I.; Merayo, J.; et al. Human Adipose-Derived Stem Cells for the Treatment of Chemically Burned Rat Cornea: Preliminary Results. Curr. Eye Res. 2013, 38, 451–463. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal Blindness: A Global Perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Dua, H.S.; Gomes, J.A.; Singh, A. Corneal Epithelial Wound Healing. Br. J. Ophthalmol. 1994, 78, 401–408. [Google Scholar] [CrossRef]
- Belmonte, C.; Aracil, A.; Acosta, M.C.; Luna, C.; Gallar, J. Nerves and Sensations from the Eye Surface. Ocul. Surf. 2004, 2, 248–253. [Google Scholar] [CrossRef]
- Gonzalez-Coto, A.F.; Alonso-Ron, C.; Alcalde, I.; Gallar, J.; Meana, Á.; Merayo-Lloves, J.; Belmonte, C. Expression of Cholecystokinin, Gastrin, and Their Receptors in the Mouse Cornea. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1965–1975. [Google Scholar] [CrossRef]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic Keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef]
- Garana, R.M.; Petroll, W.M.; Chen, W.T.; Herman, I.M.; Barry, P.; Andrews, P.; Cavanagh, H.D.; Jester, J. V Radial Keratotomy. II. Role of the Myofibroblast in Corneal Wound Contraction. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3271–3282. [Google Scholar]
- Zieske, J.D.; Guimaraes, S.R.; Hutcheon, A.E. Kinetics of Keratocyte Proliferation in Response to Epithelial Debridement. Exp. Eye Res. 2001, 72, 33–39. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.C.; Merayo-Lloves, J.; Blanco-Mezquita, T.; Mar-Sardana, S. Wound Healing Following Refractive Surgery in Hens. Exp. Eye Res. 2006, 83, 728–735. [Google Scholar] [CrossRef]
- Del Pero, R.A.; Gigstad, J.E.; Roberts, A.D.; Klintworth, G.K.; Martin, C.A.; L’Esperance, F.A., Jr.; Taylor, D.M. A Refractive and Histopathologic Study of Excimer Laser Keratectomy in Primates. Am. J. Ophthalmol. 1990, 109, 419–429. [Google Scholar] [CrossRef]
- Fini, M.E.; Stramer, B.M. How the Cornea Heals: Cornea-Specific Repair Mechanisms Affecting Surgical Outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Corneal Stromal Wound Healing in Refractive Surgery: The Role of Myofibroblasts. Prog. Retin. Eye Res. 1999, 18, 311–356. [Google Scholar] [CrossRef]
- Okada, Y.; Reinach, P.S.; Kitano, A.; Shirai, K.; Kao, W.W.-Y.; Saika, S. Neurotrophic Keratopathy; Its Pathophysiology and Treatment. Histol. Histopathol. 2010, 25, 771–780. [Google Scholar] [CrossRef]
- Araki, K.; Ohashi, Y.; Kinoshita, S.; Hayashi, K.; Kuwayama, Y.; Tano, Y. Epithelial Wound Healing in the Denervated Cornea. Curr. Eye Res. 1994, 13, 203–211. [Google Scholar] [CrossRef]
- Gallar, J.; Pozo, M.A.; Rebollo, I.; Belmonte, C. Effects of Capsaicin on Corneal Wound Healing. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1968–1974. [Google Scholar]
- Bech, F.; González-González, O.; Artime, E.; Serrano, J.; Alcalde, I.; Gallar, J.; Merayo-Lloves, J.; Belmonte, C. Functional and Morphologic Alterations in Mechanical, Polymodal, and Cold Sensory Nerve Fibers of the Cornea Following Photorefractive Keratectomy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2281–2292. [Google Scholar] [CrossRef]
- Fini, M.E. Keratocyte and Fibroblast Phenotypes in the Repairing Cornea. Prog. Retin. Eye Res. 1999, 18, 529–551. [Google Scholar] [CrossRef]
- Edwards, T.J.; Hammarlund, M. Syndecan Promotes Axon Regeneration by Stabilizing Growth Cone Migration. Cell Rep. 2014, 8, 272–283. [Google Scholar] [CrossRef][Green Version]
- Coles, C.H.; Shen, Y.; Tenney, A.P.; Siebold, C.; Sutton, G.C.; Lu, W.; Gallagher, J.T.; Jones, E.Y.; Flanagan, J.G.; Aricescu, A.R. Proteoglycan-Specific Molecular Switch for RPTPσ Clustering and Neuronal Extension. Science 2011, 332, 484–488. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth Factors: Importance in Wound Healing and Maintenance of Transparency of the Cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Klenkler, B.; Sheardown, H. Growth Factors in the Anterior Segment: Role in Tissue Maintenance, Wound Healing and Ocular Pathology. Exp. Eye Res. 2004, 79, 677–688. [Google Scholar] [CrossRef]
- Wilson, S.E.; He, Y.G.; Weng, J.; Zieske, J.D.; Jester, J.V.; Schultz, G.S. Effect of Epidermal Growth Factor, Hepatocyte Growth Factor, and Keratinocyte Growth Factor, on Proliferation, Motility and Differentiation of Human Corneal Epithelial Cells. Exp. Eye Res. 1994, 59, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Georges, S.; Heymann, D.; Padrines, M. Modulatory Effects of Proteoglycans on Proteinase Activities. Methods Mol. Biol. 2012, 836, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Malavé, C.; Villegas, G.M.; Hernández, M.; Martínez, J.C.; Castillo, C.; Suárez de Mata, Z.; Villegas, R. Role of Glypican-1 in the Trophic Activity on PC12 Cells Induced by Cultured Sciatic Nerve Conditioned Medium: Identification of a Glypican-1-Neuregulin Complex. Brain Res. 2003, 983, 74–83. [Google Scholar] [CrossRef]
- Noecker, R. Effects of Common Ophthalmic Preservatives on Ocular Health. Adv. Ther. 2001, 18, 205–215. [Google Scholar] [CrossRef]
- Tripathi, B.J.; Tripathi, R.C. Cytotoxic Effects of Benzalkonium Chloride and Chlorobutanol on Human Corneal Epithelial Cells in Vitro. Lens Eye Toxic Res. 1989, 6, 395–403. [Google Scholar]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in Ophthalmology: Drug Delivery Innovations, Pharmacology, Clinical Applications, and Future Perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef]
- Geerling, G.; Maclennan, S.; Hartwig, D. Autologous Serum Eye Drops for Ocular Surface Disorders. Br. J. Ophthalmol. 2004, 88, 1467–1474. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; Alcalde, I.; Merayo-Lloves, J.; Orive, G. Plasma Rich in Growth Factors (PRGF-Endoret) Stimulates Corneal Wound Healing and Reduces Haze Formation after PRK Surgery. Exp. Eye Res. 2013, 115, 153–161. [Google Scholar] [CrossRef]
- Anitua, E.; de la Fuente, M.; Alcalde, I.; Sanchez, C.; Merayo-Lloves, J.; Muruzabal, F. Development and Optimization of Freeze-Dried Eye Drops Derived from Plasma Rich in Growth Factors Technology. Transl. Vis. Sci. Technol. 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Papy-Garcia, D.; Barbier-Chassefière, V.; Rouet, V.; Kerros, M.-E.; Klochendler, C.; Tournaire, M.-C.; Barritault, D.; Caruelle, J.-P.; Petit, E. Nondegradative Sulfation of Polysaccharides. Synthesis and Structure Characterization of Biologically Active Heparan Sulfate Mimetics. Macromolecules 2005, 38, 4647–4654. [Google Scholar] [CrossRef]
- Ledoux, D.; Merciris, D.; Barritault, D.; Caruelle, J.P. Heparin-like Dextran Derivatives as Well as Glycosaminoglycans Inhibit the Enzymatic Activity of Human Cathepsin, G. FEBS Lett. 2003, 537, 23–29. [Google Scholar] [CrossRef]
- Barbosa, I.; Morin, C.; Garcia, S.; Duchesnay, A.; Oudghir, M.; Jenniskens, G.; Miao, H.Q.; Guimond, S.; Carpentier, G.; Cebrian, J.; et al. A Synthetic Glycosaminoglycan Mimetic (RGTA) Modifies Natural Glycosaminoglycan Species during Myogenesis. J. Cell Sci. 2005, 118, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Cejkova, J.; Olmiere, C.; Cejka, C.; Trosan, P.; Holan, V. The Healing of Alkali-Injured Cornea Is Stimulated by a Novel Matrix Regenerating Agent (RGTA, CACICOL20): A Biopolymer Mimicking Heparan Sulfates Reducing Proteolytic, Oxidative and Nitrosative Damage. Histol. Histopathol. 2013, 29, 457–478. [Google Scholar]
- Meddahi, A.; Bree, F.; Papy-Garcia, D.; Gautron, J.; Barritault, D.; Caruelle, J.P. Pharmacological Studies of RGTA(11), a Heparan Sulfate Mimetic Polymer, Efficient on Muscle Regeneration. J. Biomed. Mater. Res. 2002, 62, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Mestries, P.; Alexakis, C.; Papy-Garcia, D.; Duchesnay, A.; Barritault, D.; Caruelle, J.P.; Kern, P. Specific RGTA Increases Collagen V Expression by Cultured Aortic Smooth Muscle Cells via Activation and Protection of Transforming Growth Factor-Beta1. Matrix Biol. 2001, 20, 171–181. [Google Scholar] [CrossRef]
- Chebbi, C.K.; Kichenin, K.; Amar, N.; Nourry, H.; Warnet, J.M.; Barritault, D.; Baudouin, C. Pilot study of a new matrix therapy agent (RGTA OTR4120) in treatment-resistant corneal ulcers and corneal dystrophy. J. Fr. Ophtalmol. 2008, 31, 465–471. [Google Scholar]
- Garcia-Filipe, S.; Barbier-Chassefiere, V.; Alexakis, C.; Huet, E.; Ledoux, D.; Kerros, M.E.; Petit, E.; Barritault, D.; Caruelle, J.P.; Kern, P. RGTA OTR4120, a Heparan Sulfate Mimetic, is a Possible Long-Term Active Agent to Heal Burned Skin. J. Biomed. Mater. Res. A 2007, 80, 75–84. [Google Scholar] [CrossRef]
- Groah, S.L.; Libin, A.; Spungen, M.; Nguyen, K.L.; Woods, E.; Nabili, M.; Ramella-Roman, J.; Barritault, D. Regenerating Matrix-Based Therapy for Chronic Wound Healing: A Prospective within-Subject Pilot Study. Int. Wound J. 2011, 8, 85–95. [Google Scholar] [CrossRef]
- Zakine, G.; Barbier, V.; Garcia-Filipe, S.; Luboinski, J.; Papy-Garcia, D.; Chachques, J.C.; Carpentier, A.; Barritault, D. Matrix Therapy with RGTA OTR4120 Improves Healing Time and Quality in Hairless Rats with Deep Second-Degree Burns. Plast Reconstr. Surg. 2011, 127, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Akal, A.; Ulas, T.; Goncu, T.; Guldur, M.E.; Kocarslan, S.; Taskin, A.; Sezen, H.; Ozkan, K.; Yilmaz, O.F.; Buyukhatipoglu, H. Evaluating the Safety of Intracameral Bevacizumab Application Using Oxidative Stress and Apoptotic Parameters in Corneal Tissue. Int. J. Ophthalmol. 2015, 8, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Wang, C.; Tukler-Henriksson, J.; Pflugfelder, S.C.; Camodeca, C.; Nuti, E.; Rossello, A.; Li, D.-Q.; de Paiva, C.S. MMP-8 Is Critical for Dexamethasone Therapy in Alkali-Burned Corneas Under Dry Eye Conditions. J. Cell. Physiol. 2016, 231, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Pelegrino, F.S.A.; Henriksson, J.T.; Pflugfelder, S.C.; Volpe, E.A.; Li, D.-Q.; de Paiva, C.S. Differential Effects of Dexamethasone and Doxycycline on Inflammation and MMP Production in Murine Alkali-Burned Corneas Associated with Dry Eye. Ocul. Surf. 2016, 14, 242–254. [Google Scholar] [CrossRef]
- Zeppieri, M.; Salvetat, M.L.; Beltrami, A.; Cesselli, D.; Russo, R.; Alcalde, I.; Merayo-Lloves, J.; Brusini, P.; Parodi, P.C. Adipose Derived Stem Cells for Corneal Wound Healing after Laser Induced Corneal Lesions in Mice. J. Clin. Med. 2017, 6, 115. [Google Scholar] [CrossRef]
- Alcalde, I.; Íñigo-Portugués, A.; Carreño, N.; Riestra, A.C.; Merayo-Lloves, J.M. Effects of New Biomimetic Regenerating Agents on Corneal Wound Healing in an Experimental Model of Post-Surgical Corneal Ulcers. Arch. Soc. Española Oftalmol. Engl. Ed. 2015, 90, 467–474. [Google Scholar] [CrossRef]
- Reimondez-Troitiño, S.; Alcalde, I.; Csaba, N.; Íñigo-Portugués, A.; de la Fuente, M.; Bech, F.; Riestra, A.C.; Merayo-Lloves, J.; Alonso, M.J. Polymeric Nanocapsules: A Potential New Therapy for Corneal Wound Healing. Drug Deliv. Transl. Res. 2016, 6, 708–721. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development. Test No. 405: Acute Eye Irritation/Corrosion; OECD: Paris, France, 2021; p. 12. [Google Scholar]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjöström, P.J.; van Meyel, D.J. Neuronal Morphometry Directly from Bitmap Images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef]
- Azar, D.T.; Pluznik, D.; Jain, S.; Khoury, J.M. Gelatinase B and A Expression after Laser in Situ Keratomileusis and Photorefractive Keratectomy. Arch. Ophthalmol. 1998, 116, 1206–1208. [Google Scholar] [CrossRef][Green Version]
- Kato, T.; Chang, J.H.; Azar, D.T. Expression of Type XVIII Collagen during Healing of Corneal Incisions and Keratectomy Wounds. Invest. Ophthalmol. Vis. Sci. 2003, 44, 78–85. [Google Scholar] [CrossRef]
- Mohan, R.R.; Stapleton, W.M.; Sinha, S.; Netto, M.V.; Wilson, S.E. A Novel Method for Generating Corneal Haze in Anterior Stroma of the Mouse Eye with the Excimer Laser. Exp. Eye Res. 2008, 86, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Santhiago, M.R.; Barbosa, F.L.; Agrawal, V.; Singh, N.; Ambati, B.K.; Wilson, S.E. Effect of TGFbeta and PDGF-B Blockade on Corneal Myofibroblast Development in Mice. Exp. Eye Res. 2011, 93, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Netto, M.V.; Mohan, R.R.; Sinha, S.; Sharma, A.; Dupps, W.; Wilson, S.E. Stromal Haze, Myofibroblasts, and Surface Irregularity after PRK. Exp. Eye Res. 2006, 82, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Sumioka, T.; Okada, Y.; Reinach, P.S.; Shirai, K.; Miyajima, M.; Yamanaka, O.; Saika, S. Impairment of Corneal Epithelial Wound Healing in a TRPV1-Deficient Mouse. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3295–3302. [Google Scholar] [CrossRef]
- Barbier-Chassefiere, V.; Garcia-Filipe, S.; Yue, X.L.; Kerros, M.E.; Petit, E.; Kern, P.; Saffar, J.L.; Papy-Garcia, D.; Caruelle, J.P.; Barritault, D. Matrix Therapy in Regenerative Medicine, a New Approach to Chronic Wound Healing. J. Biomed. Mater. Res. A 2009, 90, 641–647. [Google Scholar] [CrossRef]
- Pal-Ghosh, S.; Pajoohesh-Ganji, A.; Tadvalkar, G.; Kyne, B.M.; Guo, X.; Zieske, J.D.; Stepp, M.A. Topical Mitomycin-C Enhances Subbasal Nerve Regeneration and Reduces Erosion Frequency in the Debridement Wounded Mouse Cornea. Exp. Eye Res. 2016, 146, 361–369. [Google Scholar] [CrossRef]
- Wilson, S.E.; Mohan, R.R.; Ambrosio, R., Jr.; Hong, J.; Lee, J. The Corneal Wound Healing Response: Cytokine-Mediated Interaction of the Epithelium, Stroma, and Inflammatory Cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Jester, J.V.; Barry, P.A.; Lind, G.J.; Petroll, W.M.; Garana, R.; Cavanagh, H.D. Corneal Keratocytes: In Situ and in Vitro Organization of Cytoskeletal Contractile Proteins. Investig. Ophthalmol. Vis. Sci. 1994, 35, 730–743. [Google Scholar]
- Barritault, D.; Caruelle, J.-P. Regenerating agents (RGTAs): A new therapeutic approach. Ann. Pharm. Fr. 2006, 64, 135–144. [Google Scholar] [CrossRef]
- Felipe, C.D.; Gonzalez, G.G.; Gallar, J.; Belmonte, C. Quantification and Immunocytochemical Characteristics of Trigeminal Ganglion Neurons Projecting to the Cornea: Effect of Corneal Wounding. Eur. J. Pain 1999, 3, 31–39. [Google Scholar] [CrossRef]
- Gallar, J.; Tervo, T.M.T.; Neira, W.; Holopainen, J.M.; Lamberg, M.E.; Miñana, F.; Acosta, M.C.; Belmonte, C. Selective Changes in Human Corneal Sensation Associated with Herpes Simplex Virus Keratitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4516–4522. [Google Scholar] [CrossRef]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M.T. Corneal Nerves: Structure, Contents and Function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Baldwin, H.C.; Marshall, J. Growth Factors in Corneal Wound Healing Following Refractive Surgery: A Review. Acta Ophthalmol. Scand. 2002, 80, 238–247. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, M.P.; Alio, J.L.; Arnalich-Montiel, F.; Fuentes-Julian, S.; de Benito-Llopis, L.; Amparo, F.; Bataille, L. Cornea and Ocular Surface Treatment. Curr. Stem Cell Res. Ther. 2010, 5, 195–204. [Google Scholar] [CrossRef] [PubMed]
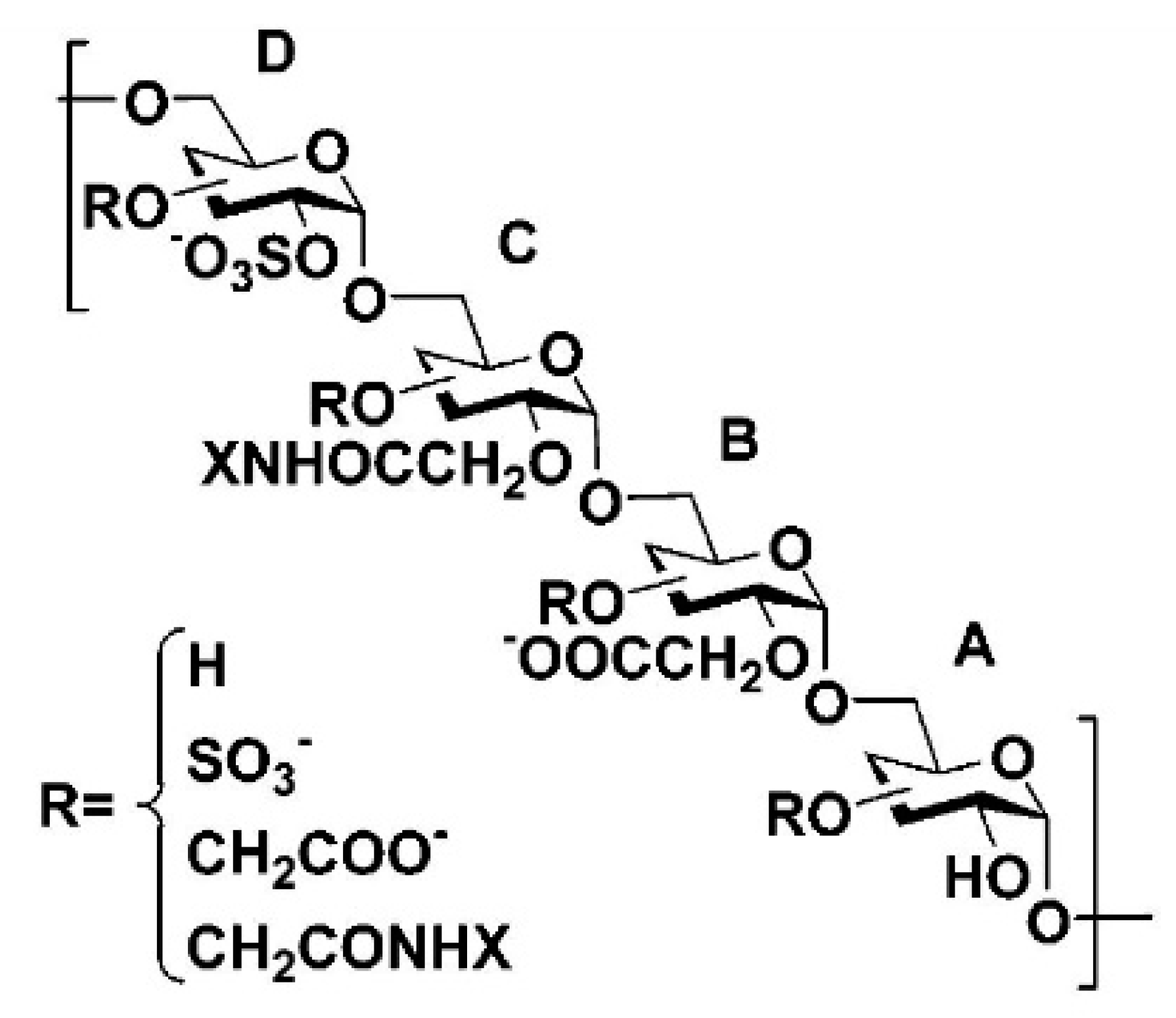




| Group | Corneal Thickness (in µm ± SEM) | Myofibroblasts (Number of α-SMA+ Cells ± SEM) | Intraepithelial Nerve Density (β-Tubulin III+ Fibers/mm ± SEM) |
|---|---|---|---|
| Uninjured | 105.47 ± 1.60 | 0 | 110.61 ± 4.89 |
| BSS | 51.95 ± 2.59 | 11.8 ± 1.49 | 33.26 ± 3.78 |
| Dx | 61.16 ± 5.23 | 7.92 ± 0.45 | 37.32 ± 2.83 |
| HSmP | 98.94 ± 1.62 | 4.92 ± 0.60 | 60.49 ± 8.05 |
| Group | Proliferating Cells (Ki67+ Cells/mm ± SEM) | Apoptotic Cells (TUNEL+ Cells/mm ± SEM) | ||||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D7 | D1 | D2 | D3 | D7 | |
| Uninjured | - | - | - | 48.06 ± 4.09 | - | - | - | 18.01 ± 0.72 |
| BSS | 272.29 ± 8.13 | 77.42 ± 2.72 | 65.30 ± 5.34 | 47.36 ± 1.73 | 256.03 ± 12.49 | 42.35 ± 2.62 | 31.60 ± 4.54 | 15.90 ± 0.75 |
| Dx | 273.16 ± 6.96 | 55.11 ± 1.73 | 77.52 ± 2.97 | 58.07 ± 1.84 | 169.51 ± 16.82 | 62.67 ± 3.00 | 63.99 ± 26.86 | 18.44 ± 2.09 |
| HSmP | 204.57 ± 17.89 | 44.70 ± 2.32 | 44.15 ± 6.24 | 51.65 ± 4.14 | 143.97 ± 25.57 | 61.15 ± 25.93 | 31.53 ± 8.73 | 16.50 ± 2.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcalde, I.; Sánchez-Fernández, C.; Del Olmo-Aguado, S.; Martín, C.; Olmiere, C.; Artime, E.; Quirós, L.M.; Merayo-Lloves, J. Synthetic Heparan Sulfate Mimetic Polymer Enhances Corneal Nerve Regeneration and Wound Healing after Experimental Laser Ablation Injury in Mice. Polymers 2022, 14, 4921. https://doi.org/10.3390/polym14224921
Alcalde I, Sánchez-Fernández C, Del Olmo-Aguado S, Martín C, Olmiere C, Artime E, Quirós LM, Merayo-Lloves J. Synthetic Heparan Sulfate Mimetic Polymer Enhances Corneal Nerve Regeneration and Wound Healing after Experimental Laser Ablation Injury in Mice. Polymers. 2022; 14(22):4921. https://doi.org/10.3390/polym14224921
Chicago/Turabian StyleAlcalde, Ignacio, Cristina Sánchez-Fernández, Susana Del Olmo-Aguado, Carla Martín, Céline Olmiere, Enol Artime, Luis M. Quirós, and Jesús Merayo-Lloves. 2022. "Synthetic Heparan Sulfate Mimetic Polymer Enhances Corneal Nerve Regeneration and Wound Healing after Experimental Laser Ablation Injury in Mice" Polymers 14, no. 22: 4921. https://doi.org/10.3390/polym14224921
APA StyleAlcalde, I., Sánchez-Fernández, C., Del Olmo-Aguado, S., Martín, C., Olmiere, C., Artime, E., Quirós, L. M., & Merayo-Lloves, J. (2022). Synthetic Heparan Sulfate Mimetic Polymer Enhances Corneal Nerve Regeneration and Wound Healing after Experimental Laser Ablation Injury in Mice. Polymers, 14(22), 4921. https://doi.org/10.3390/polym14224921








