Valorization of Waste from Argan Seeds for Polyhydroxybutyrate Production Using Bacterial Strains Isolated from Argan Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Dry Biomass and PHB Quantification
2.1.1. Dry Biomass Quantification
2.1.2. PHB Extraction and Purification by Sodium Hypochlorite—Chloroform Dispersion Method
2.1.3. PHB Quantification
2.2. Preparation and Chemical Pretreatment of Argan Seed Pulp
2.3. Cultivation Conditions for Maximum Bacterial Growth and PHB Production
2.3.1. Effect of Temperature and Incubation Time
2.3.2. Effect of pH
2.3.3. Effect of NaCl Concentration
2.3.4. Effect of Nitrogen Sources
2.3.5. Effect of Argan Seed Waste Concentration
2.3.6. Effect of Culture Fermentation Volume
2.4. Characterization of Extracted PHB through Analytical Tools
2.4.1. Preliminary Characterization by UV-Visible Spectrophotometry
2.4.2. Chemical Characterization by Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Dry Biomass and PHB Quantification
3.2. Cultivation Conditions for Maximum Bacterial Growth and PHB Production
3.2.1. Effect of Temperature and Incubation Time
3.2.2. Effect of pH
3.2.3. Effect of NaCl Concentration
3.2.4. Effect of Nitrogen Sources
3.2.5. Effect of Argan Seeds Waste Concentration
3.2.6. Effect of Culture Fermentation Volume
3.2.7. Comparative Analysis of Optimal Growth Conditions and Putative PHB Accumulation of the Species 2D1 and 1B
4. Characterization of Extracted PHB through Analytical Tools
4.1. Preliminary Characterization by UV-Visible Spectrophotometry
4.2. Chemical Characterization by Fourier Transform Infrared Spectroscopy (FTIR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirohi, R.; Prakash Pandey, J.; Kumar Gaur, V.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef]
- Carpine, R.; Olivieri, G.; Hellingwerf, K.J.; Pollio, A.; Marzocchella, A. Industrial production of poly-β-hydroxybutyrate from CO2: Can cyanobacteria meet this challenge? Processes 2020, 8, 323. [Google Scholar] [CrossRef]
- Das, S.K.; Eshkalak, S.K.; Chinnappan, A.; Ghosh, R.; Jayathilaka WA, D.M.; Baskar, C.; Ramakrishna, S. Plastic Recycling of Polyethylene Terephthalate (PET) and Polyhydroxybutyrate (PHB)—A Comprehensive Review. Mater. Circ. Econ. 2021, 3, 9. [Google Scholar] [CrossRef]
- Haddadi, M.H.; Asadolahi, R.; Negahdari, B. The bioextraction of bioplastics with focus on polyhydroxybutyrate: A review. Int. J. Environ. Sci. Technol. 2019, 16, 3935–3948. [Google Scholar] [CrossRef]
- Sakthiselvan, P.; Madhumathi, R. Optimization on Microbial Production of Polyhydroxybutyrate ( PHB ): A Review. Int. J. Res. Anal. Rev. 2019, 6, 243–251. [Google Scholar]
- Chekroud, Z.; Djerrab, L.; Rouabhia, A.; Dems, M.A.; Atailia, I.; Djazy, F.; Smadi, M.A. Valorisation of date fruits by-products for the production of biopolymer polyhydroxybutyrate (PHB) using the bacterial strain Bacillus paramycoides. Ital. J. Food Sci. 2022, 34, 48–58. [Google Scholar] [CrossRef]
- Aragosa, A.; Specchia, V.; Frigione, M. Isolation of two bacterial species from argan soil in morocco associated with polyhydroxybutyrate (PHB) accumulation: Current potential and future prospects for the bio-based polymer production. Polymers 2021, 13, 1870. [Google Scholar] [CrossRef]
- Aragosa, A.; Saccomanno, B.; Specchia, V.; Frigione, M. A Novel Sphingomonas sp. Isolated from Argan Soil for the Polyhydroxybutyrate Production from Argan Seeds Waste. Polymers 2023, 15, 512. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, M.H.; Kang, S.J.; Lee, S.Y.; Oh, T.K. Sphingomonas dokdonensis sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 2006, 56, 2165–2169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, X.; Gong, L.; Li, P.; Dai, C.; Shao, W. High poly (β-hydroxybutyrate) production by Pseudomonas fluorescens A2a5 from inexpensive substrates. Enzym. Microb. Technol. 2008, 42, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Soran, H.; Beyatli, Y. Determination of Poly-β-hydroxybutyrate (PHB) production by some Bacillus sp. World J. Microbiol. Biotechnol. 2005, 21, 565–566. [Google Scholar] [CrossRef]
- Kim, B.S. Production of poly (3-hydroxybutyrate) from inexpensive substrates. Enzym. Microb. Technol. 2000, 27, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Vijayendra, S.V.N.; Rastogi, N.K.; Shamala, T.R.; Kumar, P.K.A.; Kshama, L.; Joshi, G.J. Optimization of polyhydroxybutyrate production by Bacillus sp. CFR 256 with corn steep liquor as a nitrogen source. Indian J. Microbiol. 2007, 47, 170–175. [Google Scholar] [CrossRef]
- Kek, Y.K.; Chang, C.W.; Amirul, A.A.; Sudesh, K. Heterologous expression of Cupriavidus sp. USMAA2-4 PHA synthase gene in PHB−4 mutant for the production of poly (3-hydroxybutyrate) and its copolymers. World J. Microbiol. Biotechnol. 2010, 26, 1595–1603. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; Almeida, M.C.M.D.; Grandfils, C.; Da Fonseca, M.M.R. Poly (3-hydroxybutyrate) production by Cupriavidusnecator using waste glycerol. Process Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Chiellini, E.; Fernandez, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.H.; Shen, C.Y.; You, M.L.; Xiao, J.F.; Chen, G.Q. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3 -hydroxyalkanoates scaffolds into nerve cells. Biomaterials 2010, 31, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanarayanana, G.; Kiran, G.S.; Selvin, J.; Saibaba, G. Optimization of polyhydroxybutyrate production by marine Bacillus megaterium MSBN04 under solid state culture. Int. J. Biol. Macromol. 2013, 60, 253–261. [Google Scholar] [CrossRef]
- Rajendran, R.; Mekala, M.; Suganya, K. PHB production by Bacillus species using the cheap substrate groundnut oil cake. Int. J. Pharma Biosci. 2013, 4, 1006–1015. [Google Scholar]
- Harhar, H.; Gharby, S.; Kartah, B.; Pioch, D.; Guillaume, D.; Charrouf, Z. Effect of harvest date of Argania spinosa fruits on Argan oil quality. Ind. Crops Prod. 2014, 56, 156–159. [Google Scholar] [CrossRef]
- Chakhchar, A.; Ben Salah, I.; El Kharrassi, Y.; Filali-Maltouf, A.; El Modafar, C.; Lamaoui, M. Agro-Fruit-Forest Systems Based on Argan Tree in Morocco: A Review of Recent Results. Front. Plant Sci. 2022, 12, 783615. [Google Scholar] [CrossRef]
- El Abbassi, A.; Khalid, N.; Zbakh, H.; Ahmad, A. Physicochemical Characteristics, Nutritional Properties, and Health Benefits of Argan Oil: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Hand, S.; Gill, J.; Chu, K.H. Phage-based extraction of polyhydroxybutyrate (PHB) produced from synthetic crude glycerol. Sci. Total Environ. 2016, 557–558, 317–321. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Madhumathi, R.; Muthukumar, K.; Velan, M. Optimization of Polyhydroxybutyrate Production by Bacillus safensis EBT1. Clean-Soil Air Water 2016, 44, 1066–1074. [Google Scholar] [CrossRef]
- Bhuwal, A.K.; Singh, G.; Aggarwal, N.K.; Goyal, V.; Yadav, A. Isolation and screening of polyhydroxyalkanoates producing bacteria from pulp, paper, and cardboard industry wastes. Int. J. Biomater. 2013, 2013, 752821. [Google Scholar] [CrossRef] [PubMed]
- Law, J.H.; Slepecky, R.I. Assay of Poly-b-Hydroxybutyric Acid. J. Bacteriol. 1961, 82, 33–36. [Google Scholar] [CrossRef]
- Chandani Devi, N.; Mazumder, P.B.; Bhattacharjee, A. Statistical Optimization of Polyhydroxybutyrate Production by Bacillus Pumilus H9 Using Cow Dung as a Cheap Carbon Source by Response Surface Methodology. J. Polym. Env. 2018, 26, 3159–3167. [Google Scholar] [CrossRef]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Sukruansuwan, V.; Napathorn, S.C. Use of agro-industrial residue from the canned pineapple industry for polyhydroxybutyrate production by Cupriavidus necator strain A-04. Biotechnol. Biofuels 2018, 11, 202. [Google Scholar] [CrossRef]
- Penkhrue, W.; Jendrossek, D.; Khanongnuch, C.; Pathomareeid, W.; Aizawa, T.; Behrens, R.L.; Lumyongid, S. Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS ONE 2020, 15, e0230443. [Google Scholar] [CrossRef]
- Biradar, G.G.; Shivasharana, C.T.; Kaliwal, B.B. Isolation and characterization of polyhydroxybutyrate (PHB) producing Bacillus species from agricultural soil. Pelagia Res. Libr. Eur. J. Exp. Biol. 2015, 5, 58–65. [Google Scholar]
- Mayeli, N.; Motamedi, H.; Heidarizadeh, F. Production of polyhydroxybutyrate by Bacillus axaraqunsis BIPC01 using petrochemical wastewater as carbon source. Braz. Arch. Biol. Technol. 2015, 58, 643–650. [Google Scholar] [CrossRef]
- Saleem, F.; Aslam, R.; Saleem, Y.; Naz, S.; Syed, Q.; Munir, N.; Khurshid, N.; Shakoori, A.R. Analysis and evaluation of growth parameters for optimum production of polyhydroxybutyrate (PHB) by Bacillus thuringiensis strain CMBL-BT-6. Pak. J. Zool. 2014, 46, 1337–1344. [Google Scholar]
- Sayyed, R.Z.; Gangurde, N.S. Poly-β-hydroxybutyrate production by Pseudomonas sp. RZS 1 under aerobic and semi-aerobic condition. Indian J. Exp. Biol. 2010, 48, 942–947. [Google Scholar]
- Giedraityte, G.; Kalediene, L. Purification and characterization of polyhydroxybutyrate produced from thermophilic Geobacillus sp. AY 946034 strain. Chemija 2015, 26, 38–45. [Google Scholar]
- Reddy, A.R.; Kumar, R.B.; Prabhakar, K.V. Isolation and Identification of PolyHydroxyButyrate (PHB) producing bacteria from Sewage sample. Res. J. Pharm. Technol. 2017, 10, 1065. [Google Scholar] [CrossRef]
- Nehra, K.; Chhabra, N.; Sidhu, P.K.; Lathwal, P.; Rana, J.S. Molecular identification and characterization of poly-β-hydroxybutyrate (PHB) producing bacteria isolated from contaminated soils. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015, 17, 1055–1064. [Google Scholar]
- Bharti, S.N.; Swetha, G. Need for Bioplastics and Role of Biopolymer PHB: A Short Review. J. Pet. Environ. Biotechnol. 2016, 7, 2. [Google Scholar]
- Karim, F.; Mumtaz, T.; Fakhruddin, A.N.M. Isolation and screening of biopolyester producing bacteria from compost samples in Bangladesh. J. Biosci. Biotechnol. 2018, 7, 23–29. [Google Scholar]
- Thapa, C.; Shakya, P.; Shrestha, R.; Pal, S.; Manandhar, P. Isolation of Polyhydroxybutyrate (PHB) Producing Bacteria, Optimization of Culture Conditions for PHB production, Extraction and Characterization of PHB. Nepal J. Biotechnol. 2019, 6, 62–68. [Google Scholar] [CrossRef]
- Panigrahi, S.; Badveli, U. Screening, Isolation and Quantification of PHB-Producing Soil Bacteria. Int. J. Eng. Sci. Invent. 2013, 2, 1–6. [Google Scholar]
- Rodriguez-Contreras, A.; Koller, M.; de Sousa Dias, M.M.; Calafell, M.; Braunegg, G.; Marqués-Calvo, M.S. Novel poly[(R)-3-hydroxybutyrate]-producing bacterium isolated from a bolivian hypersaline lake. Food Technol. Biotechnol. 2013, 51, 123–130. [Google Scholar]
- Ayub, N.D.; Pettinari, M.J.; Ruiz, J.A.; López, N.I. A polyhydroxybutyrate-producing Pseudomonas sp. isolated from antarctic environments with high stress resistance. Curr. Microbiol. 2004, 49, 170–174. [Google Scholar] [CrossRef]
- Naranjo, J.M.; Posada, J.A.; Higuita, J.C.; Cardona, C.A. Valorisation of glycerol through the production of biopolymers: The PHB case using Bacillus megaterium. Bioresour. Technol. 2013, 133, 38–44. [Google Scholar] [CrossRef]
- Mechqoq, H.; El Yaagoubi, M.; El Hamdaoui, A.; Momchilova, S.; Guedes da Silva Almeida, J.R.; Msanda, F.; El Aouad, N. Ethnobotany, phytochemistry and biological properties of Argan tree (Argania spinosa (L.) Skeels) (Sapotaceae)—A review. J. Ethnopharmacol. 2021, 281, 114528. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.A.; Junqueira, T.L.; Watanabe MD, B.; Bonomi, A.; Quines, L.K.; Schmidell, W.; de Aragao, G.M.F. Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem. Eng. J. 2019, 146, 97–104. [Google Scholar] [CrossRef]
- Aramvash, A.; Akbari Shahabi, Z.; Dashti Aghjeh, S.; Ghafari, M.D. Statistical physical and nutrient optimization of bioplastic polyhydroxybutyrate production by Cupriavidus necator. Int. J. Environ. Sci. Technol. 2015, 12, 2307–2316. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alrumman, S.A.; Otaif, K.A.; Alamri, S.A.; Mostafa, M.S.; Sahlabji, T. Production and characterization of bioplastic by polyhydroxybutyrate accumulating Erythrobacter aquimaris isolated from mangrove rhizosphere. Molecules 2020, 25, 179. [Google Scholar] [CrossRef]
- Hagagy, N.; Saddiq, A.A.; Tag, H.M.; Selim, S.; AbdElgawad, H.; Martínez-Espinosa, R.M. Characterization of Polyhydroxybutyrate, PHB, Synthesized by Newly Isolated Haloarchaea Halolamina spp. Molecules 2022, 27, 7366. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, N.; Ozdemir, G. Poly-β-hydroxybutyrate ( PHB ) production from domestic wastewater using Enterobacter aerogenes. Afr. J. Microbiol. Res. 2011, 5, 690–702. [Google Scholar]
- Alshehrei, F. Production of polyhydroxybutyrate (PHB) by bacteria isolated from soil of Saudi Arabia. J. Pure Appl. Microbiol. 2019, 13, 897–904. [Google Scholar] [CrossRef]
- Guevara-Martínez, M.; Gällnö, K.S.; Sjöberg, G.; Jarmander, J.; Perez-Zabaleta, M.; Quillaguamán, J.; Larsson, G. Regulating the production of (R)-3-hydroxybutyrate in escherichia coli by N or P limitation. Front. Microbiol. 2015, 6, 844. [Google Scholar] [CrossRef] [PubMed]
- Qamouche, K.; Chetaine, A.; El Yahyaoui, A.; Moussaif, A.; Fröhlich, P.; Bertau, M.; Haneklaus, N. Uranium and other heavy metal sorption from Moroccan phosphoric acid with argan nutshell sawdust. Miner. Eng. 2021, 171, 2019–2022. [Google Scholar] [CrossRef]
- Zbair, M.; Bottlinger, M.; Ainassaari, K.; Ojala, S.; Stein, O.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Hydrothermal Carbonization of Argan Nut Shell: Functional Mesoporous Carbon with Excellent Performance in the Adsorption of Bisphenol A and Diuron. Waste Biomass Valorization 2020, 11, 1565–1584. [Google Scholar] [CrossRef]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S. Synthesis of hard carbon from argan shells for Na-ion batteries. J. Mater. Chem. A 2017, 5, 9917–9928. [Google Scholar] [CrossRef]
- Coopérative Feminine Amagour Argan. Available online: https://coopmaroc.com/fr/cooperative/cooperative-feminine-amagour-argan (accessed on 5 February 2023).
- Lathwal, P.; Nehra, K.; Singh, M.; Rana, J.S. Characterization of Novel and Efficient Poly-3-hydroxybutyrate (PHB) Producing Bacteria Isolated from Rhizospheric Soils. J. Polym. Environ. 2018, 26, 3437–3450. [Google Scholar] [CrossRef]
- Sabarinathan, D.; Chandrika, S.P.; Venkatraman, P.; Easwaran, M.; Sureka, C.S.; Preethi, K. Production of polyhydroxybutyrate (PHB) from Pseudomonas plecoglossicida and its application towards cancer detection. Inform. Med. Unlocked 2018, 11, 61–67. [Google Scholar] [CrossRef]
- Ramezani, M.; Amoozegar, M.A.; Ventosa, A. Screening and comparative assay of poly-hydroxyalkanoates produced by bacteria isolated from the Gavkhooni Wetland in Iran and evaluation of poly-β-hydroxybutyrate production by halotolerant bacterium Oceanimonas sp. GK1. Ann. Microbiol. 2015, 65, 517–526. [Google Scholar] [CrossRef]

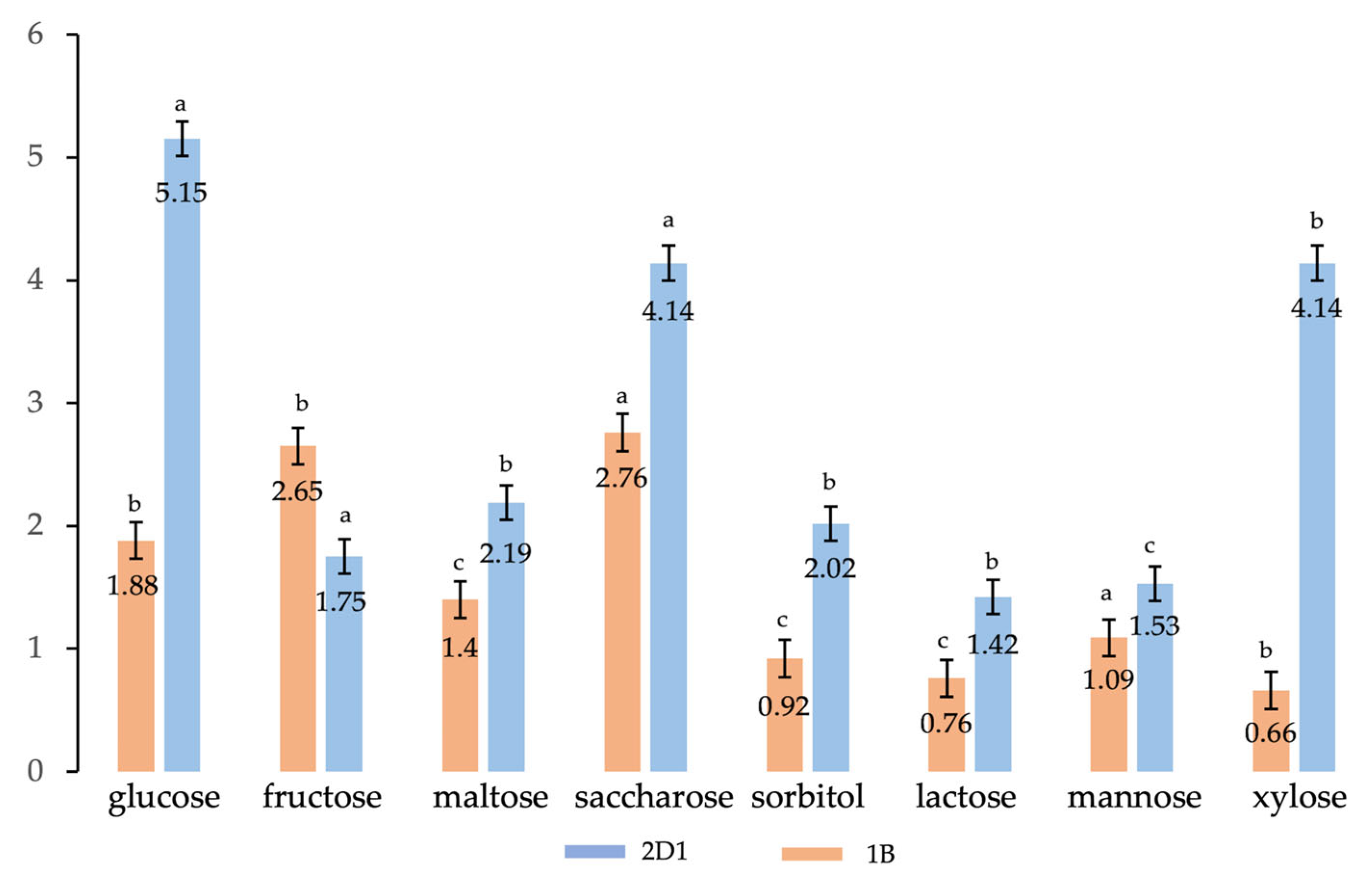


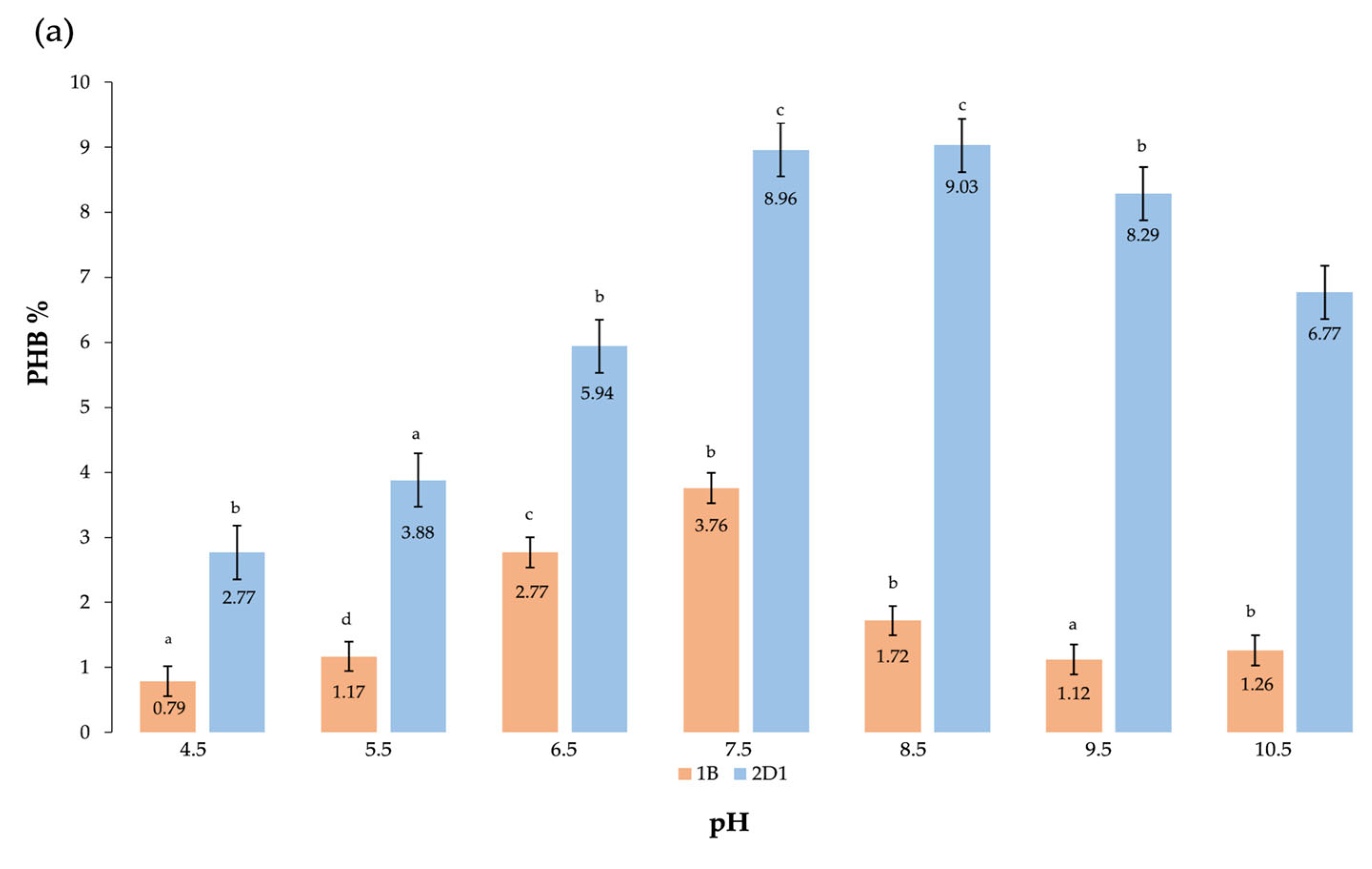
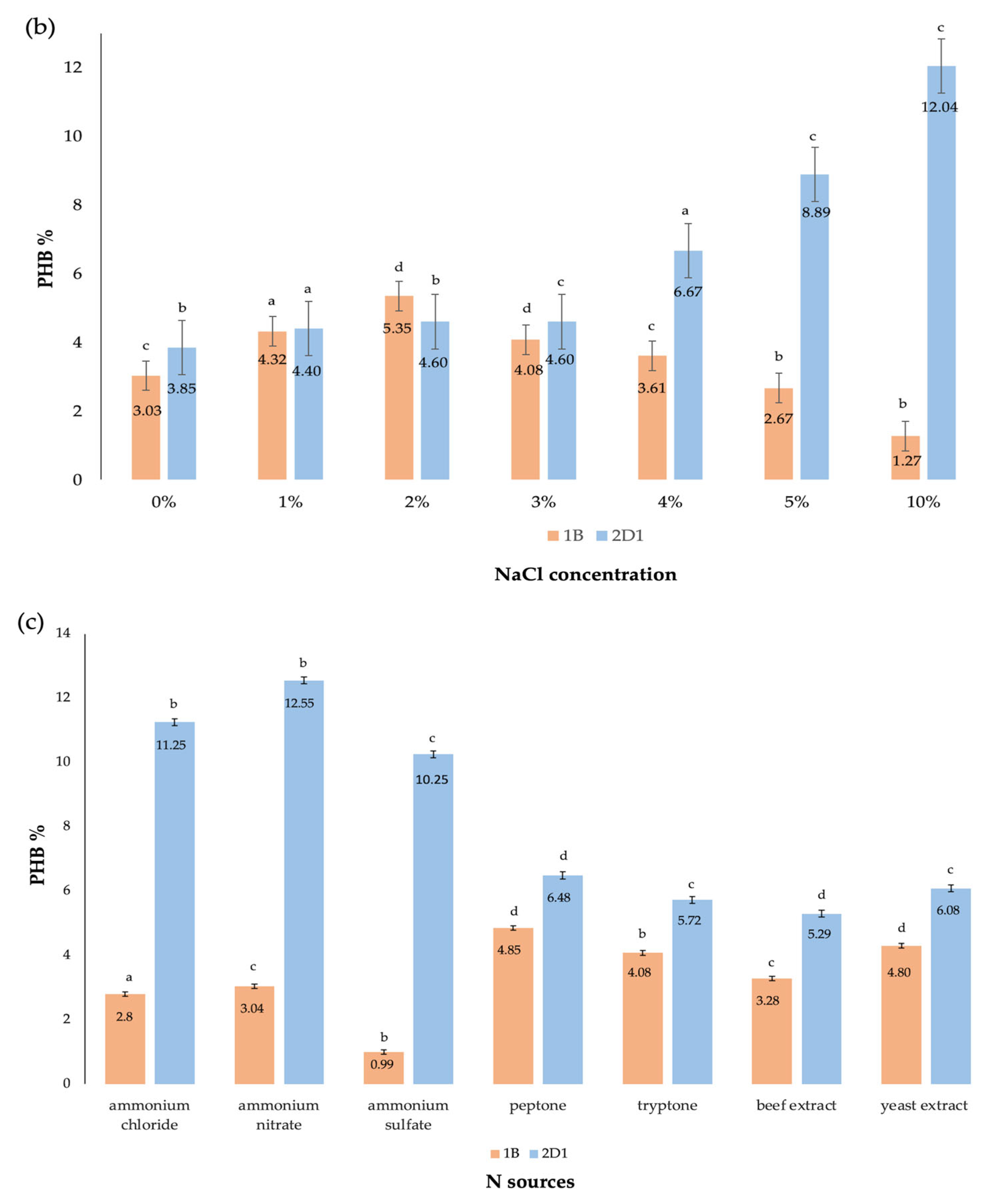

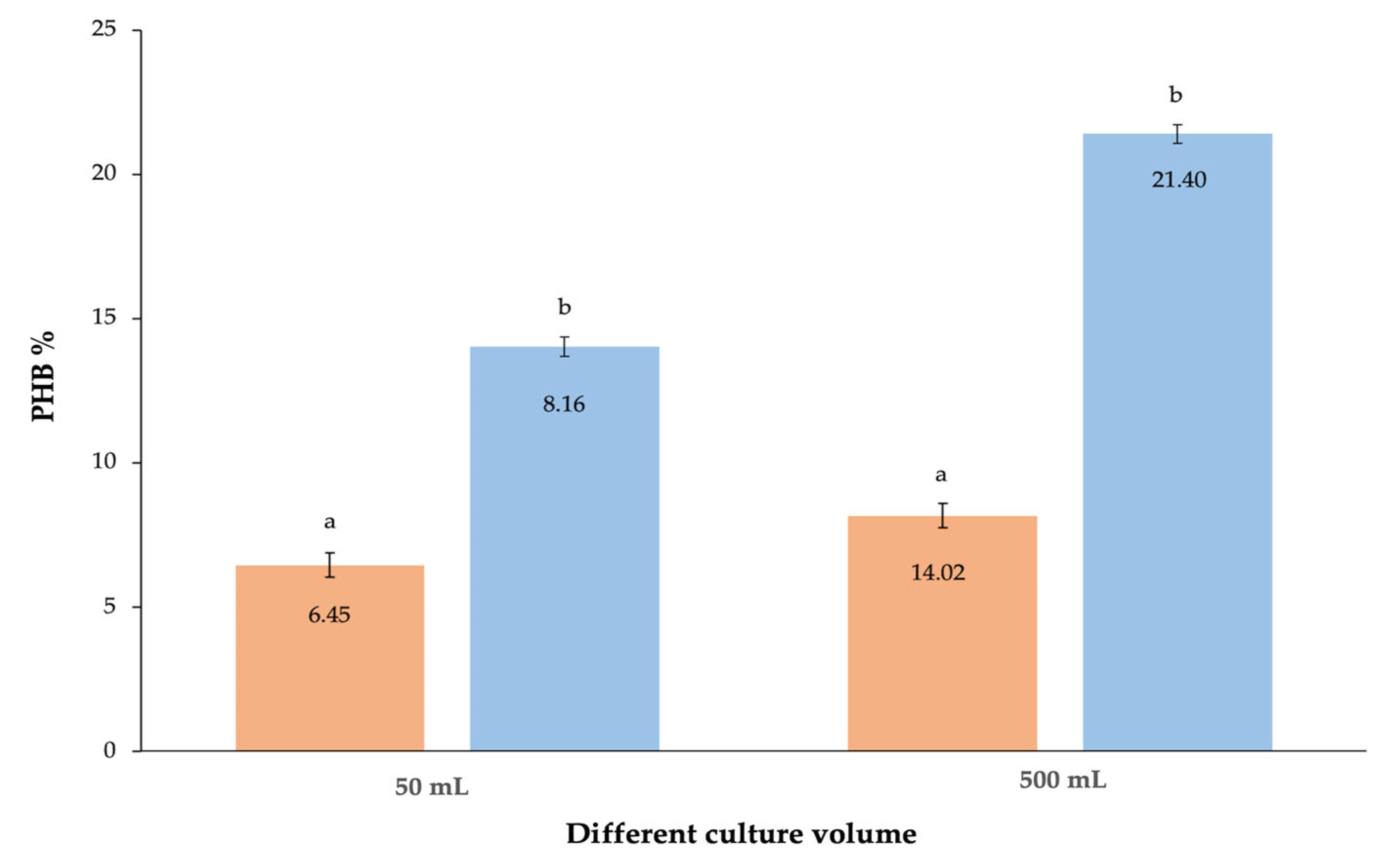



| Carbon Source * | Residual Biomass * g/L | DCW * g/L | Bio-Based Polymer * g/L | Bio-Based Polymer % |
|---|---|---|---|---|
| Glucose | 17.50 | 18.45 ± 0.13 c | 0.95 ± 0.12 c | 5.15 |
| Fructose | 19.01 | 19.35 ± 0.23 b | 0.34 ± 0.20 b | 1.75 |
| Maltose | 17.43 | 17.82 ± 0.15 b | 0.39 ± 0.17 b | 2.19 |
| Saccharose | 20.37 | 21.25 ± 0.11 c | 0.88 ± 0.13 c | 4.14 |
| Sorbitol | 14.99 | 15.30 ± 0.15 c | 0.31 ± 0.10 c | 2.02 |
| Lactose | 13.80 | 14.00 ± 0.20 b | 0.20 ± 0.17 b | 1.42 |
| Mannose | 14.83 | 15.06 ± 0.19 b | 0.23 ± 0.17 b | 1.53 |
| Xylose | 19.22 | 20.05 ± 0.10 c | 0.83 ± 0.08 d | 4.14 |
| Bacterial Strain * | Residual Biomass * g/L | DCW * g/L | Bio-Based Polymer * g/L | Bio-Based Polymer % |
|---|---|---|---|---|
| 2D1 | 18.57 | 20.45 ± 0.29 a | 1.88 ± 0.35 a | 9.19 |
| 1B | 18.08 | 18.65 ± 0.11 c | 0.57 ± 0.20 b | 3.06 |
| Growth Conditions | Selected Optimal Conditions | DCW g/L | Bio-Based Polymer g/L | Bio-Based Polymer % |
|---|---|---|---|---|
| Temperature * | 36°–38 °C | ND | ND | ND |
| Incubation time * | 48 h | 21.21 ± 0.21 b | 1.92± 0.19 b | 9.03 |
| pH * | 7.5–8.5 | 22.67 ± 0.17 c | 2.04 ± 0.13 c | 8.99 |
| NaCl * | 10% | 23.75 ± 0.19 b | 2.86 ± 0.13 c | 12.04 |
| N sources * (0.25%) | Ammonium nitrate | 23.58 ± 0.30 a | 2.96 ± 0.25 b | 12.55 |
| Argan seed waste | 3% | 23.25 ± 0.25 b | 3.26 ± 0.20 b | 14.02 |
| MSM volume ** | 500 mL | 27.61 ± 0.09 d | 5.91 ± 0.16 c | 21.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragosa, A.; Specchia, V.; Frigione, M. Valorization of Waste from Argan Seeds for Polyhydroxybutyrate Production Using Bacterial Strains Isolated from Argan Soils. Polymers 2023, 15, 1972. https://doi.org/10.3390/polym15081972
Aragosa A, Specchia V, Frigione M. Valorization of Waste from Argan Seeds for Polyhydroxybutyrate Production Using Bacterial Strains Isolated from Argan Soils. Polymers. 2023; 15(8):1972. https://doi.org/10.3390/polym15081972
Chicago/Turabian StyleAragosa, Amina, Valeria Specchia, and Mariaenrica Frigione. 2023. "Valorization of Waste from Argan Seeds for Polyhydroxybutyrate Production Using Bacterial Strains Isolated from Argan Soils" Polymers 15, no. 8: 1972. https://doi.org/10.3390/polym15081972
APA StyleAragosa, A., Specchia, V., & Frigione, M. (2023). Valorization of Waste from Argan Seeds for Polyhydroxybutyrate Production Using Bacterial Strains Isolated from Argan Soils. Polymers, 15(8), 1972. https://doi.org/10.3390/polym15081972









