Polymer Screening for Efficient Water Cut Reduction in a Sandstone Oilfield in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymer Solutions
2.2. Rock Samples
2.3. Screening Methodology
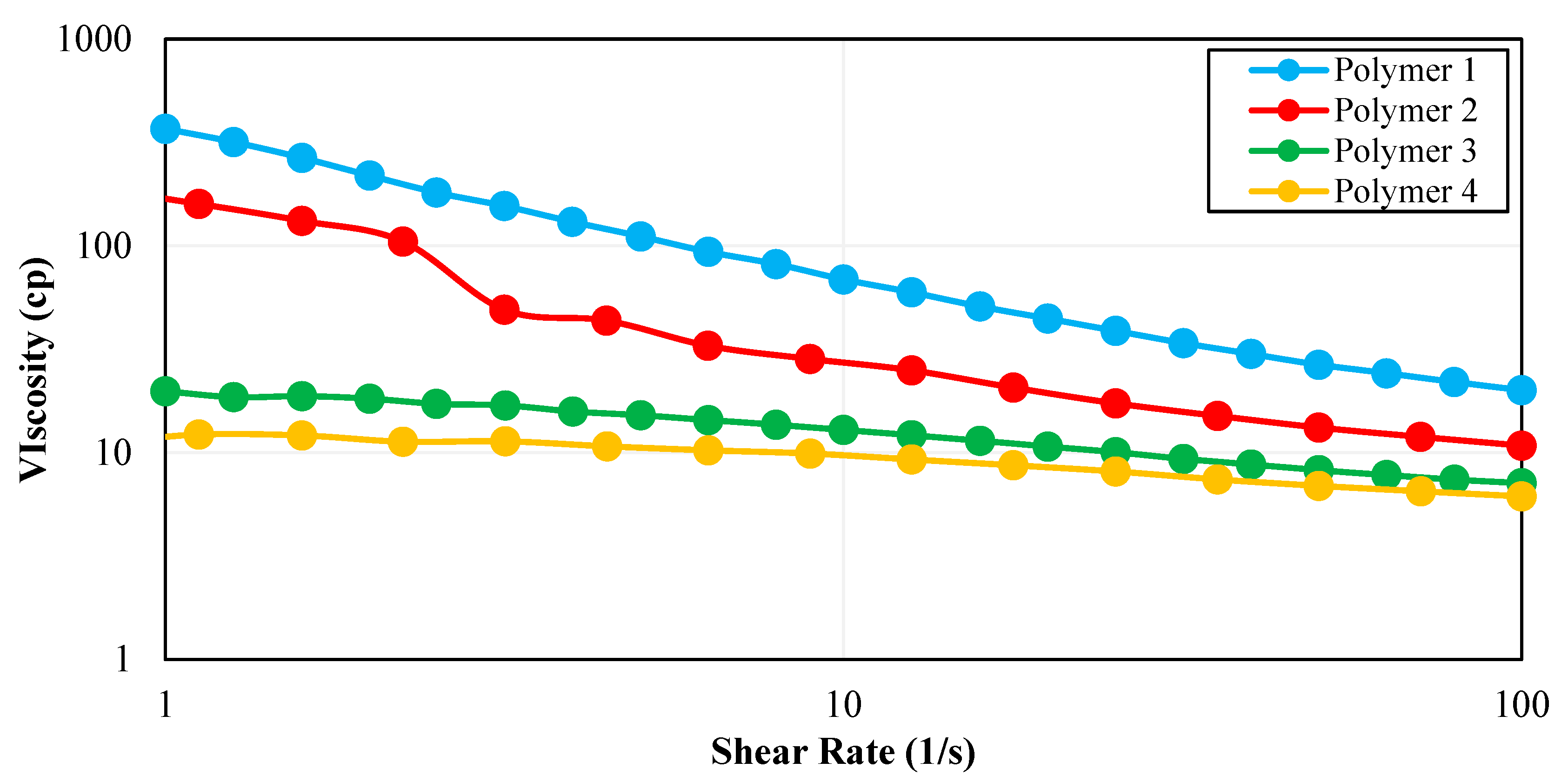
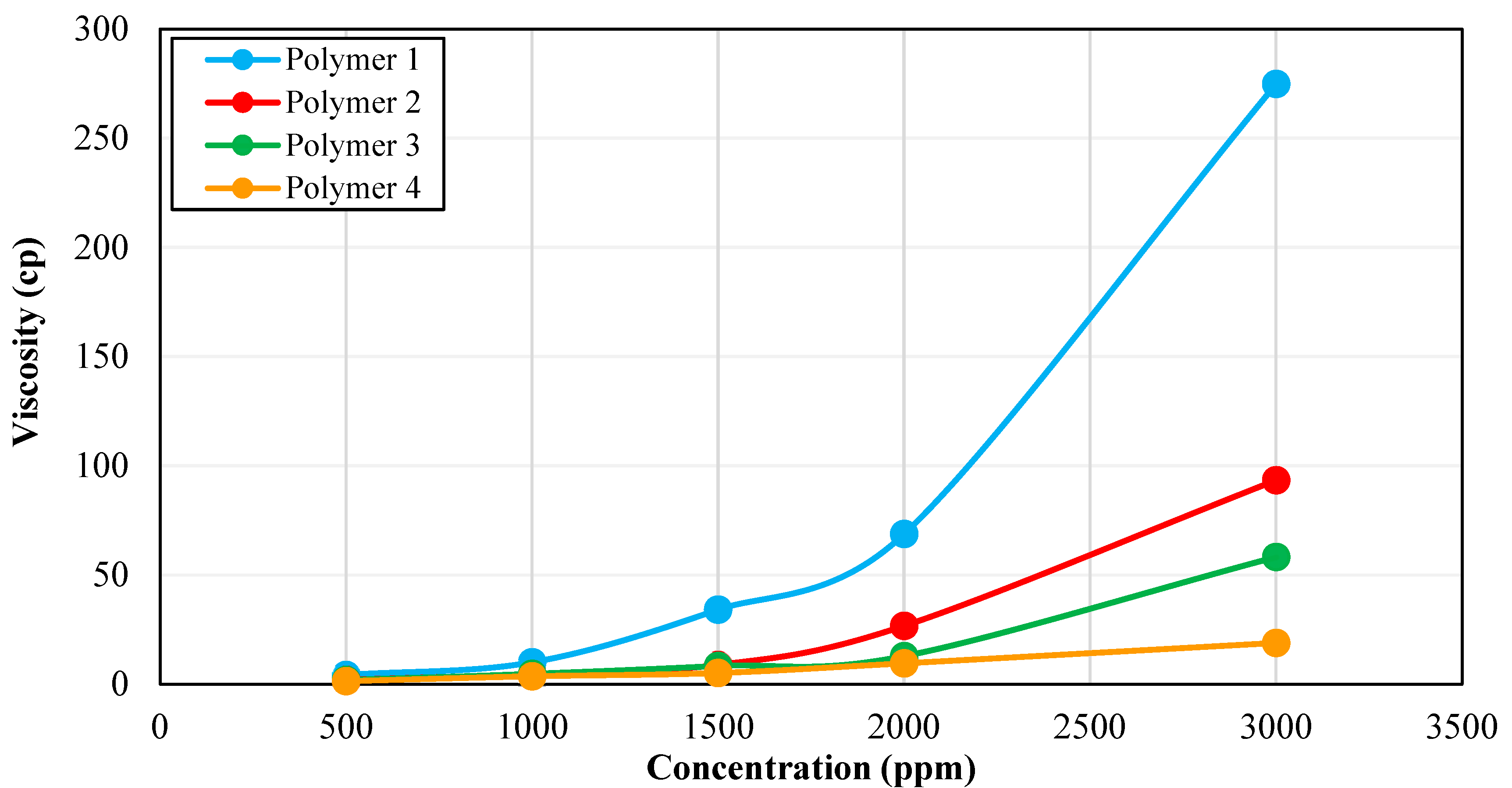
2.4. Rheology Analysis
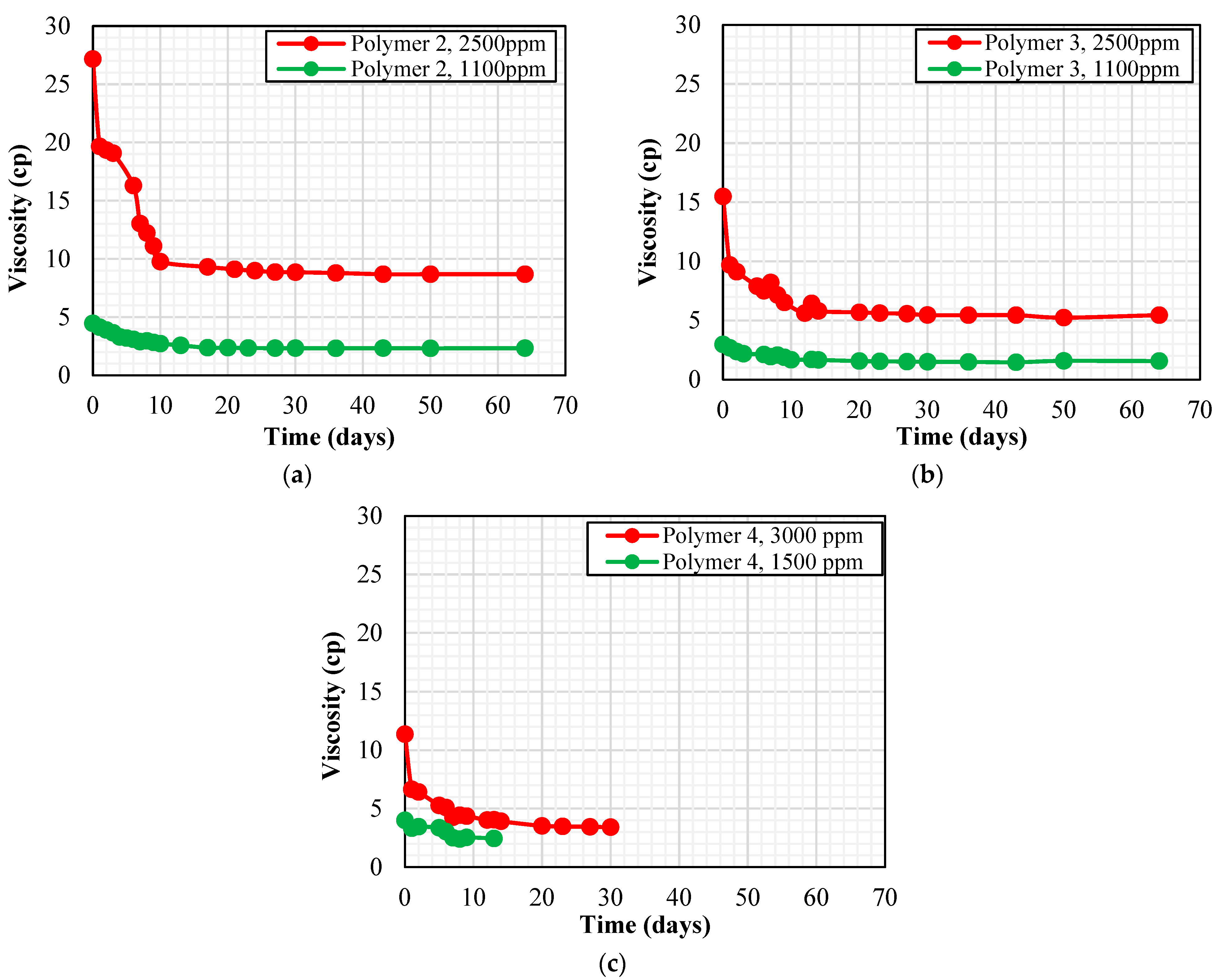
2.5. Thermal Stability Study
2.6. Static Adsorption Test
3. Results and Discussion
3.1. Rheological Characterization
3.2. Long-Term Thermal Stability
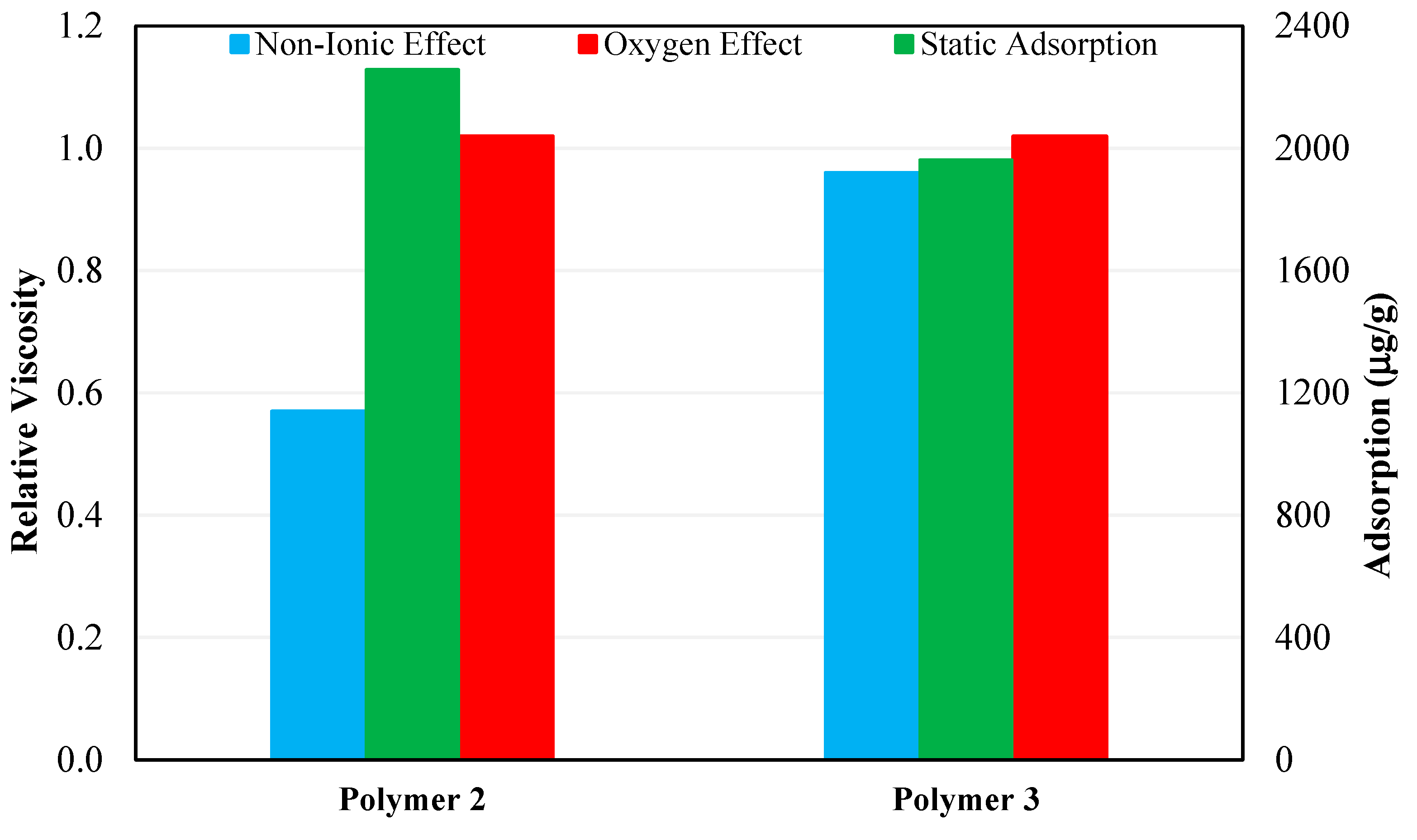
3.3. Effect of Non-Ionic Materials
3.4. Effect of Oxygen
3.5. Static Adsorption Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, N.; Mishra, L.; Ghosh, P.; Samal, S.; Priya, P. Application of Dead Oil Viscosity for Water Shut-Off Job Designs in Reservoirs with Biodegradation: A Case Study of Bhagyam Field. In Proceedings of the SPE Oil & Gas India Conference and Exhibition, Mumbai, India, 24–26 November 2015. [Google Scholar]
- Al-Hajri, S.; Mahmood, S.M.; Abdulrahman, A.; Abdulelah, H.; Akbari, S.; Saraih, N. An experimental study on hydrodynamic retention of low and high molecular weight sulfonated polyacrylamide polymer. Polymers 2019, 11, 1453. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajri, S.; Mahmood, S.M.; Akbari, S.; Abdulelah, H.; Yekeen, N.; Saraih, N. Experimental investigation and development of correlation for static and dynamic polymer adsorption in porous media. J. Pet. Sci. Eng. 2020, 189, 106864. [Google Scholar] [CrossRef]
- Al-Saadi, F.S.; Amri, B.A.; Nofli, S.; Van Wunnik, J.; Jaspers, H.F.; Harthi, S.; Shuaili, K.; Cherukupalli, P.K.; Chakravarthi, R. Polymer Flooding in a large field in South Oman-initial results and future plans. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16 April 2012. [Google Scholar]
- Alfazazi, U.; AlAmeri, W.; Hashmet, M.R. Experimental investigation of polymer flooding with low-salinity preconditioning of high temperature–high-salinity carbonate reservoir. J. Pet. Explor. Prod. Technol. 2019, 9, 1517–1530. [Google Scholar] [CrossRef]
- Borthakur, A.; Rahman, M.; Sarmah, A.; Subrahmanyam, B. Partially Hydrolyzed Polyacrylamide for Enhanced Oil-Recovery. Res. Ind. 1995, 40, 90–94. [Google Scholar]
- Chen, R.; Qi, M.; Zhang, G.; Yi, C. Comparative experiments on polymer degradation technique of produced water of polymer flooding oilfield. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 12208. [Google Scholar] [CrossRef]
- Cheraghian, G.; Khalili Nezhad, S.S.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Adsorption polymer on reservoir rock and role of the nanoparticles, clay and SiO2. Int. Nano Lett. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Ezeh, O.; Ikiensikimama, S.S.; Akaranta, O. Critical Review of Polymer Flooding in Daqing Field and Pelican Field: Case Studies of the World’s Largest Polymer Flooding in Light and Heavy Oil Reservoirs, Respectively. J. Eng. Res. Rep. 2021, 21, 25–40. [Google Scholar] [CrossRef]
- Firozjaii, A.M.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020, 6, 115–122. [Google Scholar] [CrossRef]
- Gaillard, N.; Giovannetti, B.; Favero, C.; Caritey, J.-P.; Dupuis, G.; Zaitoun, A. New water soluble anionic NVP acrylamide terpolymers for use in harsh EOR conditions. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Gaillard, N.; Giovannetti, B.; Leblanc, T.; Thomas, A.; Braun, O.; Favero, C. Selection of customized polymers to enhance oil recovery from high temperature reservoirs. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Quito, Ecuador, 18–20 November 2015. [Google Scholar]
- Hadjmohammadi, M.R.; Fatemi, M.H.; Taneh, T. Coacervative extraction of phthalates from water and their determination by high performance liquid chromatography. J. Iran. Chem. Soc. 2011, 8, 100–106. [Google Scholar] [CrossRef]
- Hongen, D.O.U.; Zhang, H.; Sibo, S. Correct understanding and application of waterflooding characteristic curves. Pet. Explor. Dev. 2019, 46, 796–803. [Google Scholar]
- Imanbayev, B.; Kushekov, R.; Sagyndikov, M.; Shyrakbayev, D. Feasibility Study of a Polymer Flood for the Uzen Brownfield Conditions. In Proceedings of the SPE Annual Caspian Technical Conference, Nur-Sultan, Kazakhstan, 15–17 November 2022. [Google Scholar]
- Ivanov, M.V.; Savvichev, A.S.; Klyuvitkin, A.A.; Chul’tsova, A.L.; Zakharova, E.E.; Rusanov, I.I.; Lein, A.Y.; Lisitsyn, A.P. Resumption of hydrogen sulfide contamination of the water column of deep basins in the Caspian Sea. Dokl. Earth Sci. 2013, 453, 1094. [Google Scholar] [CrossRef]
- Jensen, T.; Kadhum, M.; Kozlowicz, B.; Sumner, E.S.; Malsam, J.; Muhammed, F.; Ravikiran, R. Chemical EOR under harsh conditions: Scleroglucan as a viable commercial solution. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018. [Google Scholar]
- Jouenne, S.; Klimenko, A.; Levitt, D. Polymer flooding: Establishing specifications for dissolved oxygen and iron in injection water. SPE J. 2017, 22, 438–446. [Google Scholar] [CrossRef]
- Jung, J.C.; Zhang, K.; Chon, B.H.; Choi, H.J. Rheology and polymer flooding characteristics of partially hydrolyzed polyacrylamide for enhanced heavy oil recovery. J. Appl. Polym. Sci. 2013, 127, 4833–4839. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Karbasi, A.R.; NOURI, J.A.; Ayaz, G.O. Flocculation of Cu, Zn, Pb, Ni and Mn during mixing of Talar river water with Caspian seawater. Int. J. Environ. Res. 2007, 1, 66–73. [Google Scholar]
- Koduru, N.; Choudhury, N.N.; Kumar, V.; Prasad, D.; Raj, R.; Barua, D.; Singh, A.K.; Jain, S.; Gupta, A.K.; Pandey, A. Bhagyam Full Field Polymer Flood: Implementation and Initial Production Response. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 15–18 November 2021. [Google Scholar]
- Liu, J.-F.; Feng, J.-Y.; Hu, H.; Li, C.-Y.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Decrease in viscosity of partially hydrolyzed polyacrylamide solution caused by the interaction between sulfide ion and amide group. J. Pet. Sci. Eng. 2018, 170, 738–743. [Google Scholar] [CrossRef]
- Ma, F.; Wei, L.; Wang, L.; Chang, C.-C. Isolation and identification of the sulphate-reducing bacteria strain H1 and its function for hydrolysed polyacrylamide degradation. Int. J. Biotechnol. 2008, 10, 55–63. [Google Scholar] [CrossRef]
- Moradi-Araghi, A.; Doe, P.H. Hydrolysis and precipitation of polyacrylamides in hard brines at elevated temperatures. SPE Reserv. Eng. 1987, 2, 189–198. [Google Scholar] [CrossRef]
- Nazina, T.N.; Ivanova, A.E.; Borzenkov, I.A.; Belyaev, S.S.; Ivanov, M.V. Occurrence and geochemical activity of microorganisms in high-temperature, water-flooded oil fields of Kazakhstan and Western Siberia. Geomicrobiol. J. 1995, 13, 181–192. [Google Scholar] [CrossRef]
- Needham, R.B.; Doe, P.H. Polymer flooding review. J. Pet. Technol. 1987, 39, 1503–1507. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Costa, J.A.; Oliveira, L.F.S.; Mota, L.S.; de Oliveira, L.A.; Mansur, C.R.E. Hydrolysis and thermal stability of partially hydrolyzed polyacrylamide in high-salinity environments. J. Appl. Polym. Sci. 2019, 136, 47793. [Google Scholar] [CrossRef]
- Olkowska, E.; Ruman, M.; Kowalska, A.; Polkowska, Ż. Determination of surfactants in environmental samples. Part III. Non-ionic compounds. Ecol. Chem. Eng. S 2013, 20, 449–461. [Google Scholar] [CrossRef]
- Peck, L.S.; Chapelle, G. Reduced oxygen at high altitude limits maximum size. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S166–S167. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Blokhus, A.M.; Skauge, A. Viscosity and retention of sulfonated polyacrylamide polymers at high temperature. J. Appl. Polym. Sci. 2011, 119, 3623–3629. [Google Scholar] [CrossRef]
- Sandengen, K.; Meldahl, M.M.; Gjersvold, B.; Molesworth, P.; Gaillard, N.; Braun, O.; Antignard, S. Long term stability of ATBS type polymers for enhanced oil recovery. J. Pet. Sci. Eng. 2018, 169, 532–545. [Google Scholar] [CrossRef]
- Seright, R.S.S.; Skjevrak, I. Effect of dissolved iron and oxygen on stability of hydrolyzed polyacrylamide polymers. SPE J. 2015, 20, 433–441. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Shuhong, J.; Changbing, T.; Chengfang, S.H.I.; Jigen, Y.E.; Zhang, Z.; Xiujuan, F.U. New understanding on water-oil displacement efficiency in a high water-cut stage. Pet. Explor. Dev. 2012, 39, 362–370. [Google Scholar]
- Singh, A.K.; Vegesna, P.R.; Prasad, D.; Kachodi, S.C.; Lohiya, S.; Srivastava, D.; Raj, R.; Koduru, N.; Mishra, S.; Barua, D. Successful Implementation of Polymer Flood in Aishwariya Field, Rajasthan, India-Concept to Full Field. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 15–18 November 2021. [Google Scholar]
- Sokolova, D.S.; Semenova, E.M.; Grouzdev, D.S.; Bidzhieva, S.K.; Babich, T.L.; Loiko, N.G.; Ershov, A.P.; Kadnikov, V.V.; Beletsky, A.V.; Mardanov, A. V Sulfidogenic microbial communities of the uzen high-temperature oil field in Kazakhstan. Microorganisms 2021, 9, 1818. [Google Scholar] [CrossRef]
- Sorbie, K.S. Polymer-Improved Oil Recovery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Standnes, D.C.; Skjevrak, I. Literature review of implemented polymer field projects. J. Pet. Sci. Eng. 2014, 122, 761–775. [Google Scholar] [CrossRef]
- Thakuria, C.; Al-Amri, M.S.; Al-Saqri, K.A.; Jaspers, H.F.; Al-Hashmi, K.H.; Zuhaimi, K. Performance review of polymer flooding in a major brown oil field of Sultanate of Oman. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar]
- Thomas, A. Polymer flooding. In Chemical Enhanced Oil Recovery (CEOR)—A Practical Overview; InTech: Rijeka, Croatia, 2016; pp. 55–99. [Google Scholar]
- Ulasbek, K.; Hashmet, M.R.; Pourafshary, P.; Muneer, R. Laboratory Investigation of Nanofluid-Assisted Polymer Flooding in Carbonate Reservoirs. Nanomaterials 2022, 12, 4258. [Google Scholar] [CrossRef]
- Vermolen, E.C.; Van Haasterecht, M.J.; Masalmeh, S.K.; Faber, M.J.; Boersma, D.M.; Gruenenfelder, M. Pushing the envelope for polymer flooding towards high-temperature and high-salinity reservoirs with polyacrylamide based ter-polymers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. [Google Scholar]
- Wei, B. Flow characteristics of three enhanced oil recovery polymers in porous media. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Zhou, X.; Wang, L.; Fu, P.; Yakushev, V.S.; Khaidina, M.P.; Zhang, D.; Shi, X.; Zhou, R. Experimental studies of surfactant-polymer flooding: An application case investigation. Int. J. Hydrogen Energy 2022, 47, 32876–32892. [Google Scholar] [CrossRef]
- Xu, J.; Guo, C.; Wei, M.; Jiang, R. Impact of paramete’ time variation on waterflooding reservoir performance. J. Pet. Sci. Eng. 2015, 126, 181–189. [Google Scholar] [CrossRef]
- Zeinijahromi, A.; Lemon, P.; Bedrikovetsky, P. Effects of induced fines migration on water cut during waterflooding. J. Pet. Sci. Eng. 2011, 78, 609–617. [Google Scholar] [CrossRef]
- Zhangaliyev, M.M.; Hashmet, M.R.; Pourafshary, P. Laboratory Investigation of Hybrid Nano-Assisted-Polymer Method for EOR Applications in Carbonate Reservoirs. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 22–25 March 2022. [Google Scholar]
- Zhao, F.-L.; Wang, Y.-F.; Dai, C.-L.; Ren, S.; Jiao, C. Techniques of enhanced oil recovery after polymer flooding. Zhongguo Shi You Daxue Xuebao. J. China Univ. Pet. Ed. Nat. Sci. 2006, 30, 86–89. [Google Scholar]
- Zhong, H.; Zhang, W.; Fu, J.; Lu, J.; Yin, H. The performance of polymer flooding in heterogeneous type II reservoirs—An experimental and field investigation. Energies 2017, 10, 454. [Google Scholar] [CrossRef]
- Zudakina, Y.A.; Yefremova, L.N.; Vorontsova, G.I.; Rmyantsev, A.A. Effect of conditions of sedimentation and of post-sedimentary alterations on reservoir properties of polymict sandstones of the Uzen Field. Pet. Geol. A Dig. Russ. Lit. Pet. Geol. 1979, 17, 286–290. [Google Scholar]





| Ion | Concentration (ppm) |
|---|---|
| Na+ | 3513.1 |
| Ca2+ | 400.8 |
| Mg2+ | 790.4 |
| Cl− | 6026 |
| SO42− | 3138 |
| HCO3− | 256.2 |
| K+ | 87.6 |
| CO32− | 36 |
| Parameters | Units | Polymer 1 | Polymer 2 | Polymer 3 | Polymer 4 |
|---|---|---|---|---|---|
| Molecular weight | ×106, Dalton | 12.2 | 11.1 | 7.6 | 8.4 |
| Intrinsic viscosity | dL/g (deciliter/g) | 18.8 | 17.3 | 12.9 | 14 |
| Degree of hydrolysis | % | 19.7 | 1.2 | 6.4 | 7.4 |
| Test | Criterion | Polymer 1 | Polymer 2 | Polymer 3 | Polymer 4 | Remarks |
|---|---|---|---|---|---|---|
| Rheology | ≥5 cp viscosity, No gelling |  |  |  |  | Polymer 1 appeared more like a gel and was discarded. |
| Thermal Stability | ≥5 cp viscosity for at least two weeks aging at 63 °C | - |  |  |  | Polymer 4 was eliminated as it did not meet the set criterion. |
| Non-Ionic Material (Bacteria) | Little to negligible effect on thermal stability over a month | - |  |  | - | Polymer 2 showed higher thermal degradation in presence of bacteria. |
| Oxygen Content | Little to negligible effect on thermal stability over a month | - |  |  | - | Both polymers showed a negligible effect of oxygen present in CSW. |
| Static Adsorption | Ap ≤ 2000 μg/g | - |  |  | - | Static adsorption for Polymer 2 was higher. |
 Meets the criterion;
Meets the criterion;  Does not meet the criterion.
Does not meet the criterion.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerniyazov, D.; Yesmukhambet, M.; Kenes, R.; Bukayev, A.; Shakeel, M.; Pourafshary, P.; Musharova, D. Polymer Screening for Efficient Water Cut Reduction in a Sandstone Oilfield in Kazakhstan. Polymers 2023, 15, 1969. https://doi.org/10.3390/polym15081969
Yerniyazov D, Yesmukhambet M, Kenes R, Bukayev A, Shakeel M, Pourafshary P, Musharova D. Polymer Screening for Efficient Water Cut Reduction in a Sandstone Oilfield in Kazakhstan. Polymers. 2023; 15(8):1969. https://doi.org/10.3390/polym15081969
Chicago/Turabian StyleYerniyazov, Daniyar, Madi Yesmukhambet, Razida Kenes, Azamat Bukayev, Mariam Shakeel, Peyman Pourafshary, and Darya Musharova. 2023. "Polymer Screening for Efficient Water Cut Reduction in a Sandstone Oilfield in Kazakhstan" Polymers 15, no. 8: 1969. https://doi.org/10.3390/polym15081969
APA StyleYerniyazov, D., Yesmukhambet, M., Kenes, R., Bukayev, A., Shakeel, M., Pourafshary, P., & Musharova, D. (2023). Polymer Screening for Efficient Water Cut Reduction in a Sandstone Oilfield in Kazakhstan. Polymers, 15(8), 1969. https://doi.org/10.3390/polym15081969






