Construction and Performance Evaluation of Nicandra physalodes (Linn.) Gaertn. Polysaccharide-Based Nanogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Nicandra Physalodes Polysaccharide
2.3. Structural Characterizations of NPGP
2.3.1. Chemical Compositions
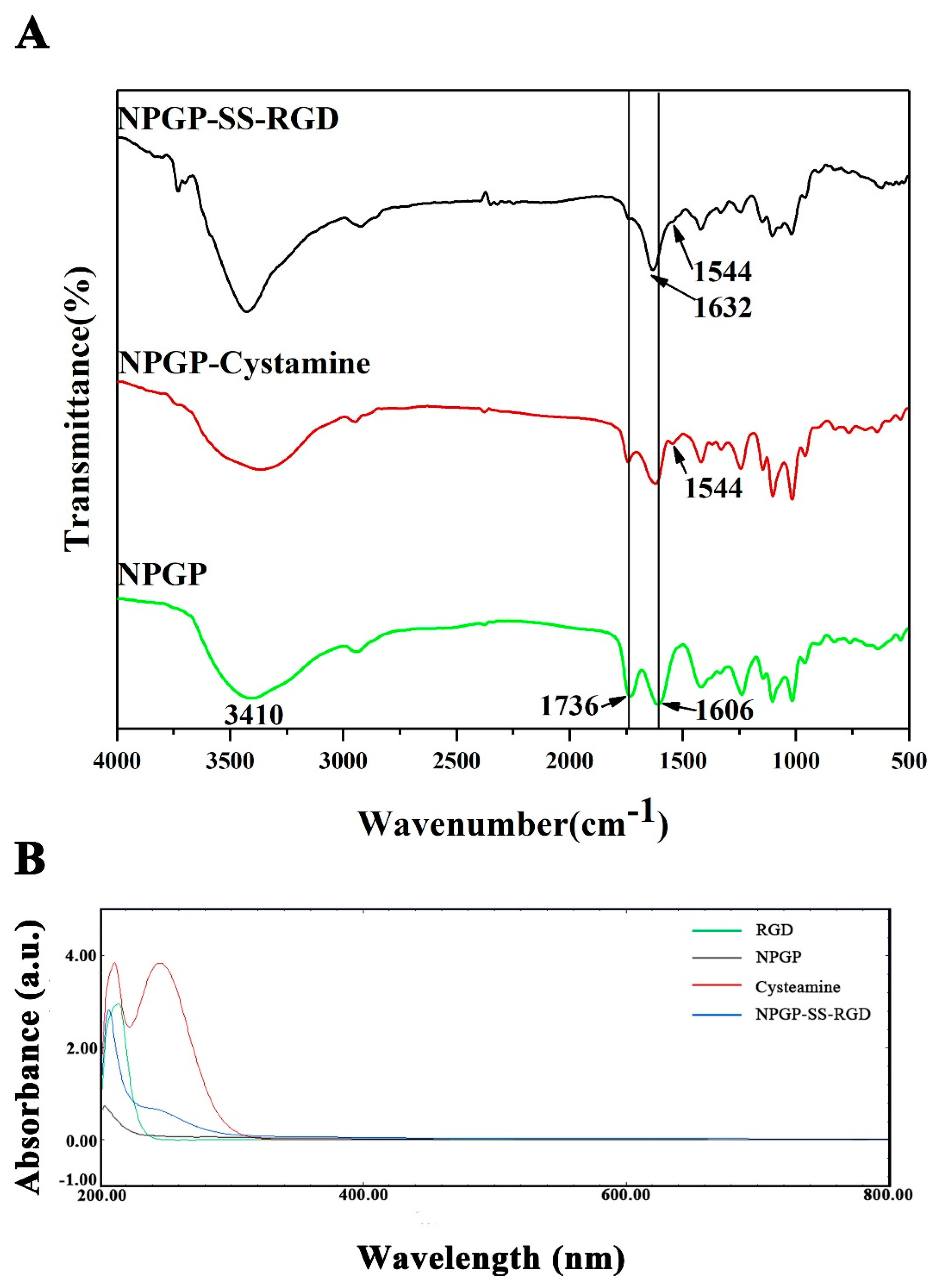
2.3.2. FT-IR Spectrum Analysis
2.3.3. Carboxyl-Reduction and Methylation Analysis
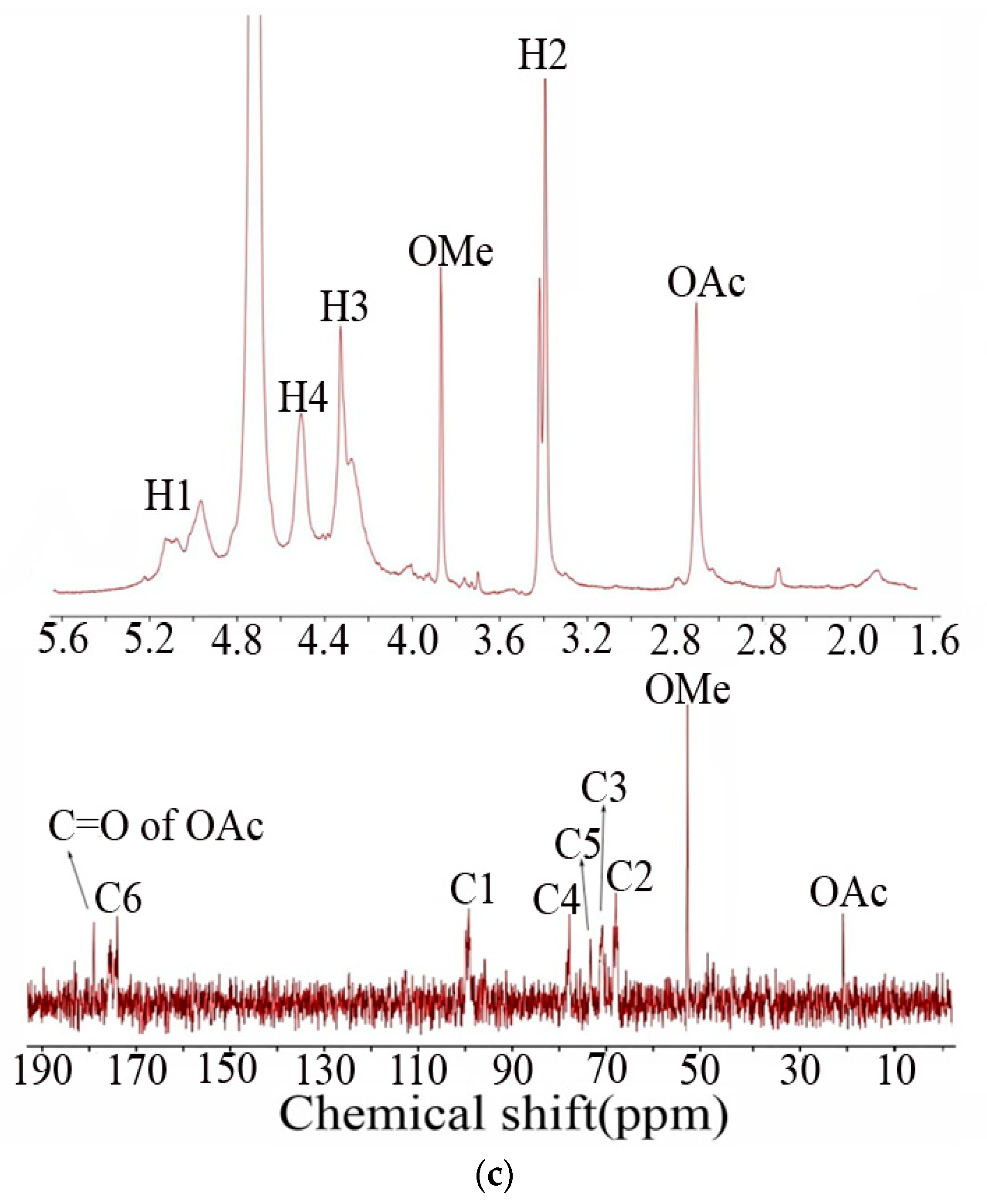
2.3.4. NMR Spectroscopy Analysis
2.4. The Preparation and Characterization of Nanogel
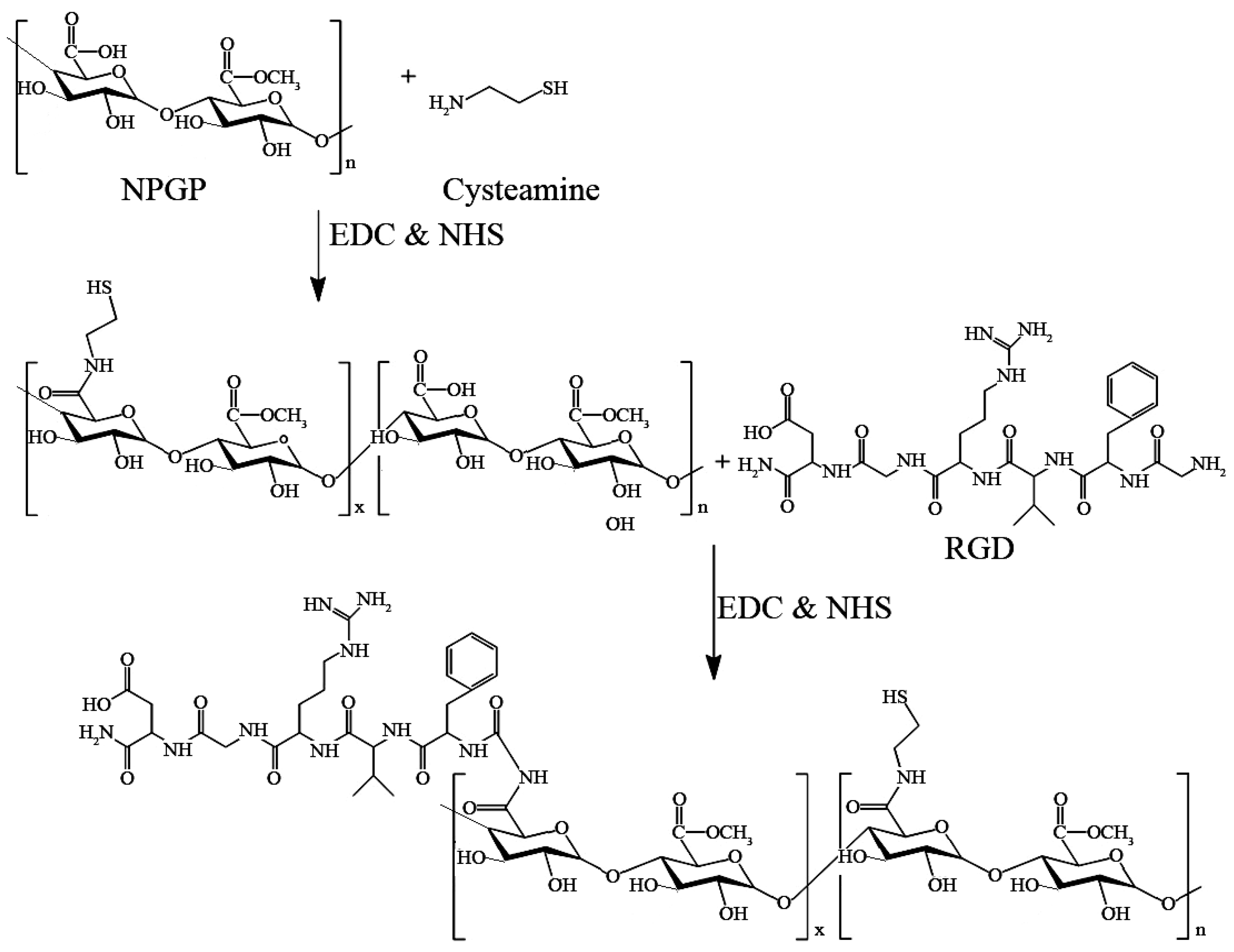
2.4.1. Synthesis of NPGP-Cysteamine-RGD (NPGP-SS-RGD) Conjugate
2.4.2. Preparation of Nanogels
2.5. Characterization of NGs
2.6. In Vitro Drug Release Studies
2.7. Cell Viability Assay
2.8. Cellular Uptake Assays
2.9. Cellular Uptake Pathways of NGs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Composition and Structural Characteristics of NPGP
3.2. Preparation and Characterization of NGs
3.3. Drug Loading and Drug Release Behaviors
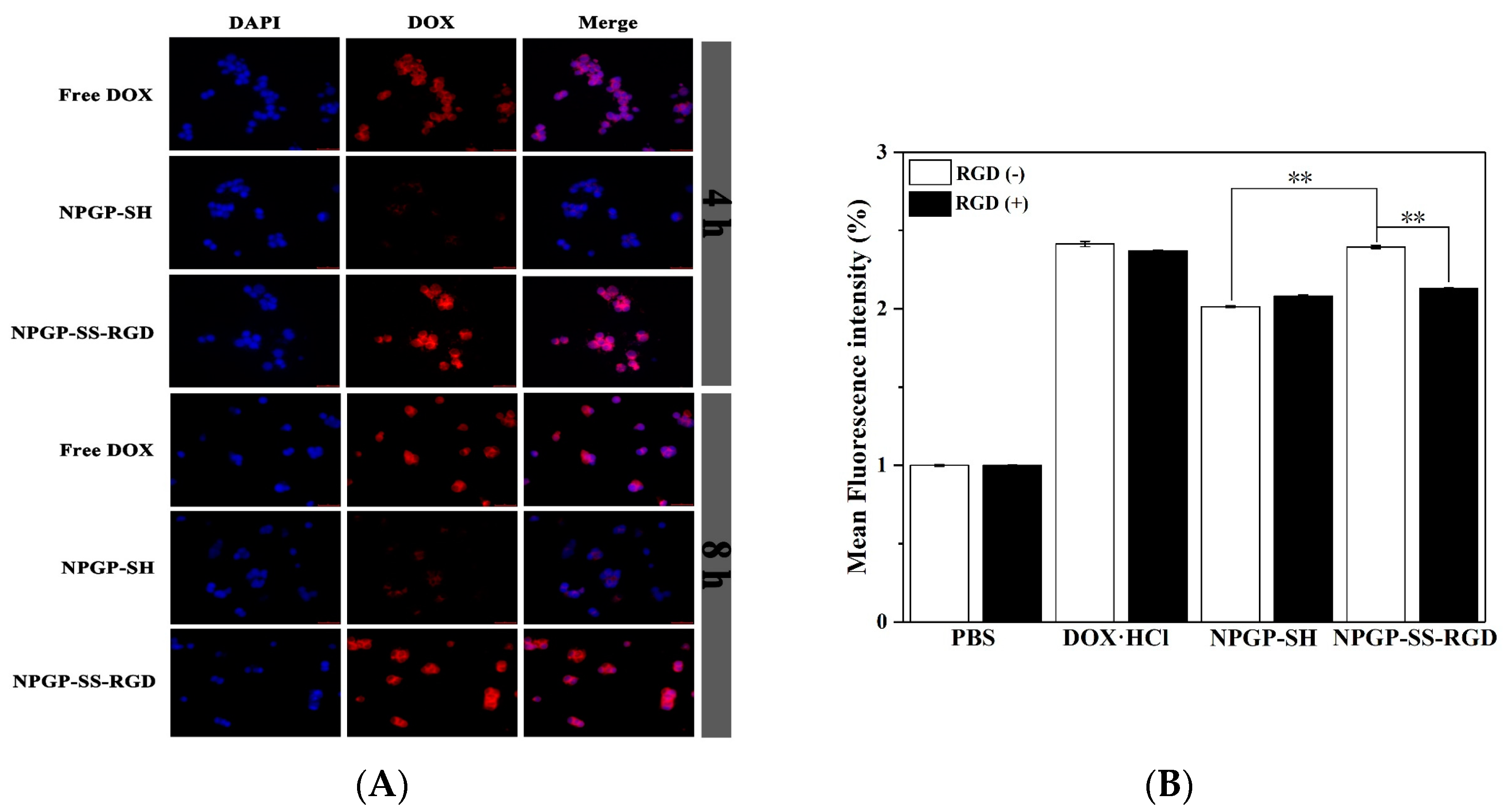
3.4. In Vitro Cytotoxicity and Cellular Uptake
3.5. Cellular Uptake of DOX-Loaded NGs
3.6. Cellular Uptake Pathways of NGs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.; Narkhede, J.; Naik, J.B. Nanogels as nanocarriers for drug delivery: A review. ADMET DMPK 2019, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. 3-Polysaccharides as biomaterials. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 37–70. [Google Scholar]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, S.; Zheng, H.; Dong, H.; Liu, J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015, 123, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, Q.; Ye, L.; Zhang, H.; Zhang, A.; Feng, Z. The preparation of pH and GSH dual responsive thiolated heparin/DOX complex and its application as drug carrier. Carbohydr. Polym. 2020, 230, 115592. [Google Scholar] [CrossRef]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.-K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. Bioimaging: In Vivo Photoacoustic Imaging of Livers Using Biodegradable Hyaluronic Acid-Conjugated Silica Nanoparticles (Adv. Funct. Mater. 22/2018). Adv. Funct. Mater. 2018, 28, 1870153. [Google Scholar] [CrossRef]
- Yang, S.; Tang, Z.; Zhang, D.; Deng, M.; Chen, X. pH and redox dual-sensitive polysaccharide nanoparticles for the efficient delivery of doxorubicin. Biomater. Sci. 2017, 5, 2169–2178. [Google Scholar] [CrossRef]
- Tie, S.; Tan, M. Current Advances in Multifunctional Nanocarriers Based on Marine Polysaccharides for Colon Delivery of Food Polyphenols. J. Agric. Food Chem. 2022, 70, 903–915. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomás, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef]
- Sun, Y.; Jing, X.; Ma, X.; Feng, Y.; Hu, H. Versatile Types of Polysaccharide-Based Drug Delivery Systems: From Strategic Design to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9159. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.P.; Ghilan, A.; Neamtu, I.; Nita, L.E.; Rusu, A.G.; Chiriac, V.M. Advancement in the Biomedical Applications of the (Nano)gel Structures Based on Particular Polysaccharides. Macromol. Biosci. 2019, 19, e1900187. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Dai, T.; Liang, L.; Deng, L.; Liu, C.; Li, Q.; Liang, R.; Chen, J. Physicochemical properties of pectin extracted from navel orange peel dried by vacuum microwave. LWT 2021, 151, 112100. [Google Scholar] [CrossRef]
- Zhang, W.; Mahuta, K.M.; Mikulski, B.A.; Harvestine, J.N.; Crouse, J.Z.; Lee, J.C.; Kaltchev, M.G.; Tritt, C.S. Novel pectin-based carriers for colonic drug delivery. Pharm. Dev. Technol. 2016, 21, 127–130. [Google Scholar] [CrossRef]
- An, H.; Yang, Y.; Zhou, Z.; Bo, Y.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Pectin-based injectable and biodegradable self-healing hydrogels for enhanced synergistic anticancer therapy. Acta Biomater. 2021, 131, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, T.; Yuan, P.; Tian, R.; Hu, W.; Tang, Y.; Jia, Y.; Zhang, L. Encapsulation of honokiol into self-assembled pectin nanoparticles for drug delivery to HepG2 cells. Carbohydr. Polym. 2015, 133, 31–38. [Google Scholar] [CrossRef]
- Park, S.-H.; Zheng, J.H.; Nguyen, V.H.; Jiang, S.-N.; Kim, D.-Y.; Szardenings, M.; Min, J.H.; Hong, Y.; Choy, H.E. RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy. Theranostics 2016, 6, 1672–1682. [Google Scholar] [CrossRef]
- Jin, M.-Y.; Li, M.-Y.; Huang, R.-M.; Wu, X.-Y.; Sun, Y.-M.; Xu, Z.-L. Structural features and anti-inflammatory properties of pectic polysaccharides: A review. Trends Food Sci. Technol. 2021, 107, 284–298. [Google Scholar] [CrossRef]
- Liu, H.; Dai, T.; Chen, J.; Liu, W.; Liu, C.; Deng, L.; Liang, R. Extraction, characterization and spontaneous gelation mechanism of pectin from Nicandra physaloides (Linn.) Gaertn seeds. Int. J. Biol. Macromol. 2021, 195, 523–529. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef]
- Shen, C.; Wang, T.; Guo, F.; Sun, K.; Wang, B.; Wang, J.; Zhang, Z.; Zhang, X.; Zhao, Y.; Chen, Y. Structural characterization and intestinal protection activity of polysaccharides from Sea buckthorn (Hippophae rhamnoides L.) berries. Carbohydr. Polym. 2021, 274, 118648. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.; Shan, X.; Zhang, X.; Zhao, X.; Li, Q.; Wang, X.; Cai, C.; Li, G.; Yu, G. Antithrombotic activities of fucosylated chondroitin sulfates and their depolymerized fragments from two sea cucumbers. Carbohydr. Polym. 2016, 152, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Matute, A.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC-MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Hsu, M.-W.; Su, Y.-C.; Chang, H.-M.; Chang, C.-H.; Jan, J.-S. Naturally derived DNA nanogels as pH- and glutathione-triggered anticancer drug carriers. Mater. Sci. Eng. C 2020, 114, 111025. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Zhou, L.; Dai, H. Drug-loaded chondroitin sulfate-based nanogels: Preparation and characterization. Colloids Surf. B Biointerfaces 2012, 100, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; Mügge, C.; Kroh, L.W. NMR analyses of complex d-glucose anomerization. Food Chem. 2018, 265, 222–226. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Caligiani, A.; Tedeschi, T.; Elst, K.; Sforza, S. Simple and validated quantitative ¹H NMR method for the determination of methylation, acetylation, and feruloylation degree of pectin. J. Agric. Food Chem. 2014, 62, 9081–9087. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Lu, T.; Nong, Z.; Li, G.; Pan, X.; Wei, Y.; Yang, Y.; Wu, N.; Huang, J.; Pan, M.; et al. Reductive response and RGD targeting nano-graphene oxide drug delivery system. J. Drug Deliv. Sci. Technol. 2019, 53, 101202. [Google Scholar] [CrossRef]
- Khoee, S.; Soleymani, M. Janus arrangement of smart polymer on magnetite nanoparticles through solvent evaporation from emulsion droplets. Appl. Surf. Sci. 2019, 494, 805–816. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, L.; Zhang, D.; Wang, F.; Sharma, V.; Wang, C. Amido-functionalized carboxymethyl chitosan/montmorillonite composite for highly efficient and cost-effective mercury removal from aqueous solution. J. Colloid Interface Sci. 2019, 554, 479–487. [Google Scholar] [CrossRef]
- Chang, D.; Lei, J.; Cui, H.; Lu, N.; Sun, Y.; Zhang, X.; Gao, C.; Zheng, H.; Yin, Y. Disulfide cross-linked nanospheres from sodium alginate derivative for inflammatory bowel disease: Preparation, characterization, and in vitro drug release behavior. Carbohydr. Polym. 2012, 88, 663–669. [Google Scholar] [CrossRef]
- Uhljar, L.; Kan, S.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In Vitro Drug Release, Permeability, and Structural Test of Ciprofloxacin-Loaded Nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Deepak, K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagina, S.; Rao, D.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]
- Hou, J.; Diao, Y.; Li, W.; Yang, Z.; Zhang, L.; Chen, Z.; Wu, Y. RGD peptide conjugation results in enhanced antitumor activity of PD0325901 against glioblastoma by both tumor-targeting delivery and combination therapy. Int. J. Pharm. 2016, 505, 329–340. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]




| Methylation Product | Linkage Type | Main MS (m/z) | Molar Ratio (%) |
|---|---|---|---|
| 1,5-Ac2-2,3,4,6-Me4-D-Glc | Glcp-(→ | 101,117,129,145,161,205 | 5.9 |
| 1,4,5-Ac3-2,3,6-Me3-D-Man | →4)-Manp-(→ | 101,113,117,131,161,173,233 | 5.2 |
| 1,4,5-Ac3-2,3,6-Me3-D-Glc | →4)-Glcp-(→ | 101,113,117,131,161,173,233 | 15.0 |
| 1,4,5-Ac3-2,3,6-Me3-D-Gal | →4)-Galp-(→ | 101,113,117,131,161,173,233 | 68.4 |
| 1,3,4,5-Ac4-2,6-Me2-D-Gal | →3,4)-Galp-(→ | 87,101,118,129,180,234,305 | 5.5 |
| Element Content (%) | C | O | N | S |
|---|---|---|---|---|
| NPGP | 61.23 | 38.77 | 0 | 0 |
| NPGP-SH | 60.19 | 31.12 | 5.85 | 2.83 |
| NPGP-SS-RGD | 60.25 | 30.29 | 6.86 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Shen, C.; Chen, X.; Gao, F.; Chen, Y. Construction and Performance Evaluation of Nicandra physalodes (Linn.) Gaertn. Polysaccharide-Based Nanogel. Polymers 2023, 15, 1933. https://doi.org/10.3390/polym15081933
Liu F, Shen C, Chen X, Gao F, Chen Y. Construction and Performance Evaluation of Nicandra physalodes (Linn.) Gaertn. Polysaccharide-Based Nanogel. Polymers. 2023; 15(8):1933. https://doi.org/10.3390/polym15081933
Chicago/Turabian StyleLiu, Fangyan, Chen Shen, Xuelian Chen, Fei Gao, and Yin Chen. 2023. "Construction and Performance Evaluation of Nicandra physalodes (Linn.) Gaertn. Polysaccharide-Based Nanogel" Polymers 15, no. 8: 1933. https://doi.org/10.3390/polym15081933
APA StyleLiu, F., Shen, C., Chen, X., Gao, F., & Chen, Y. (2023). Construction and Performance Evaluation of Nicandra physalodes (Linn.) Gaertn. Polysaccharide-Based Nanogel. Polymers, 15(8), 1933. https://doi.org/10.3390/polym15081933




