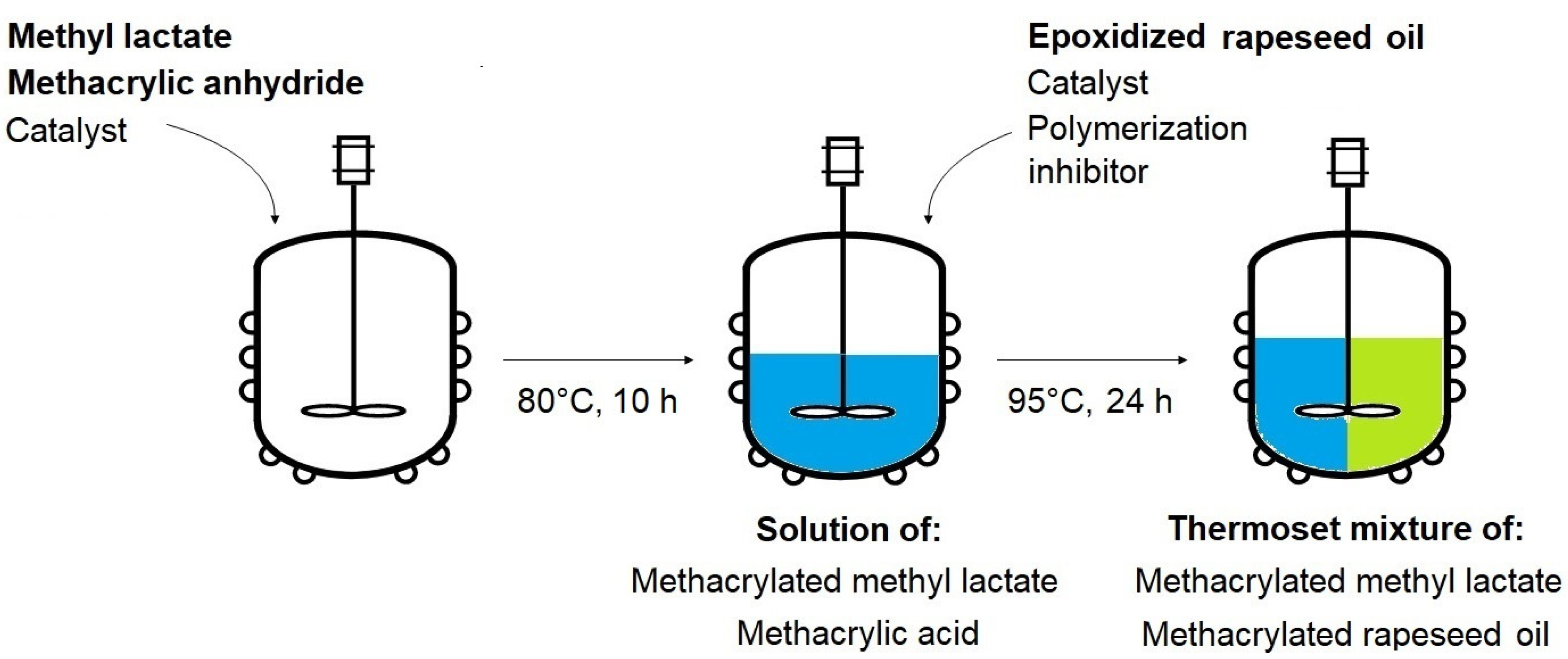
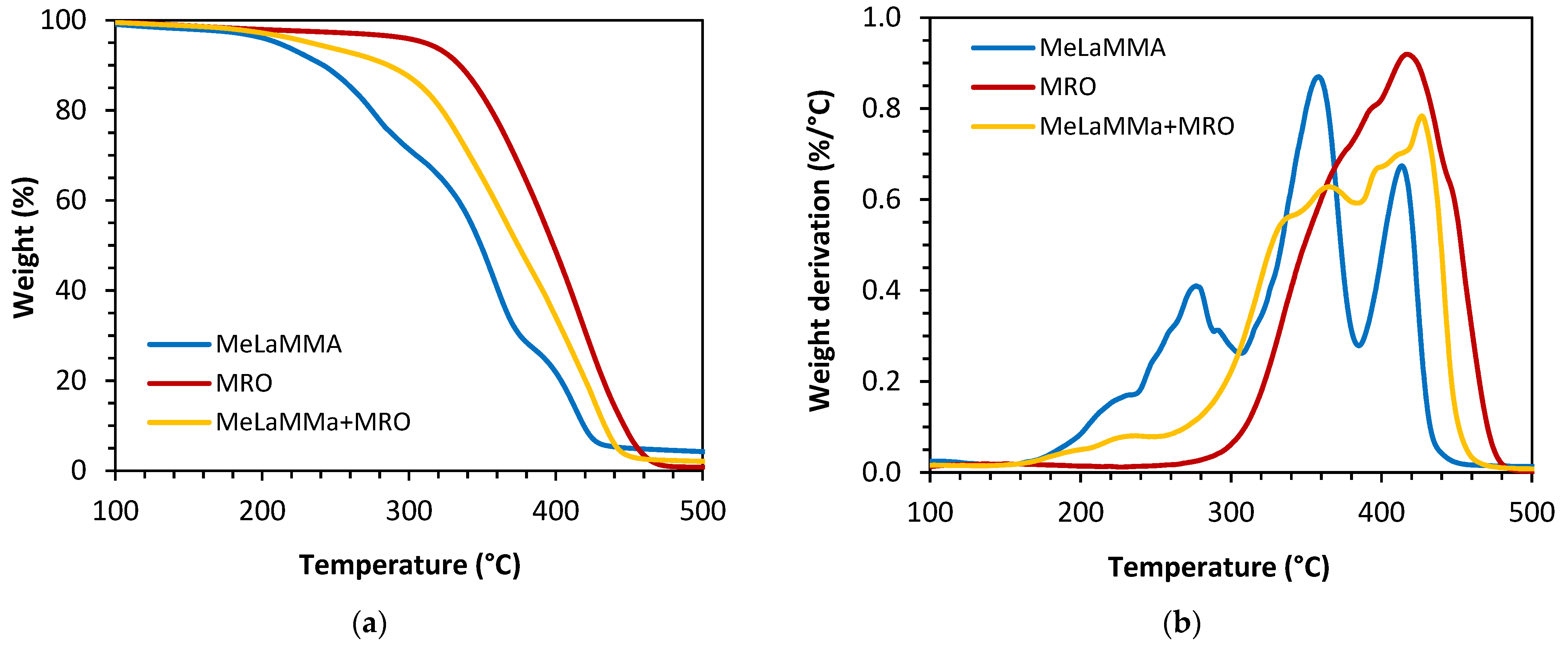
3.1. Polymerizable Thermoset Mixture Synthesis
The analysis of used rapeseed oil was performed before it was epoxidized due to the fact that the information on iodine value is especially necessary for the preparation of the reaction mixture. Reactants that undergo the epoxidation reaction (hydrogen peroxide and formic acid) are calculated according to the iodine value as a parameter determining the amount of modifiable double bonds. The results of all measured volumetric parameters are shown in
Table 1.
The used rapeseed oil had a particular composition of fatty acid within its structure. This factor might be important for comparison with other vegetable oils modified the same way. However, only the oil with the highest ratio of mono-unsaturated fatty acids has been studied in this work. The particular composition of occurring fatty acids is displayed in
Table 2. The saturated fatty acid share is an important parameter for the used vegetable oil since this type of structure cannot be modified for further polymerizable purposes.
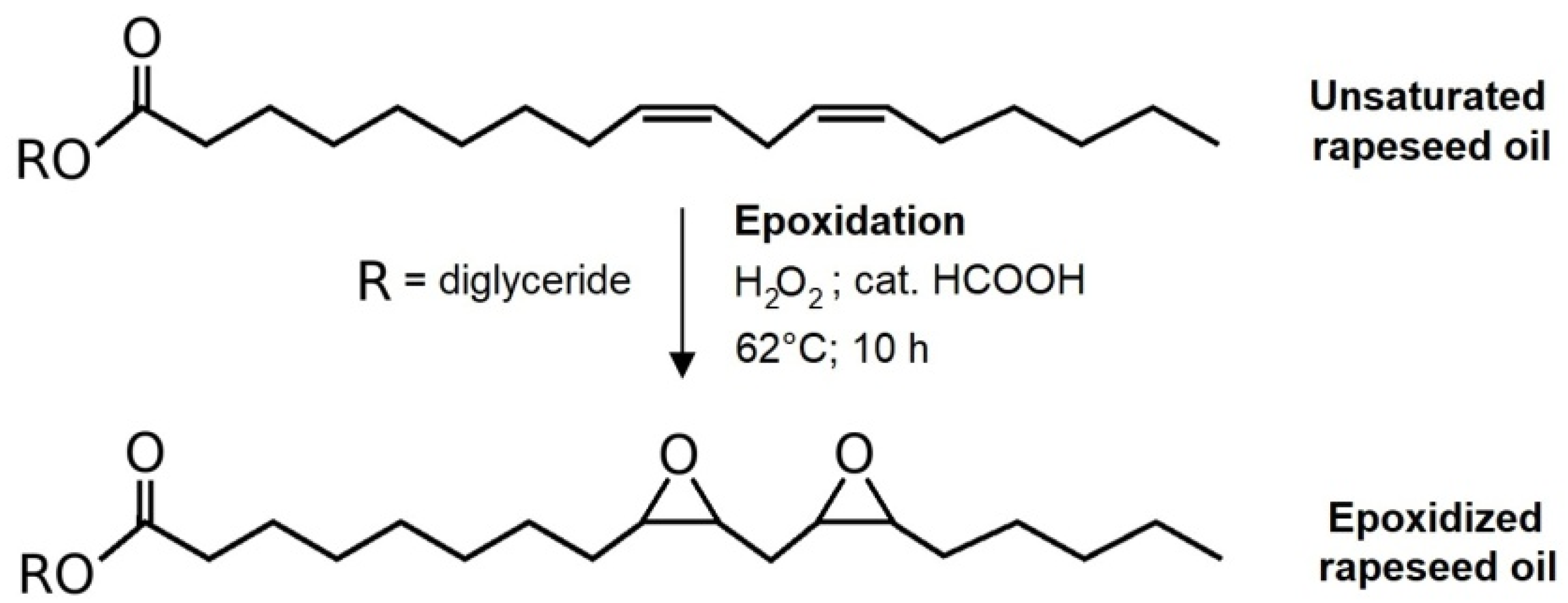
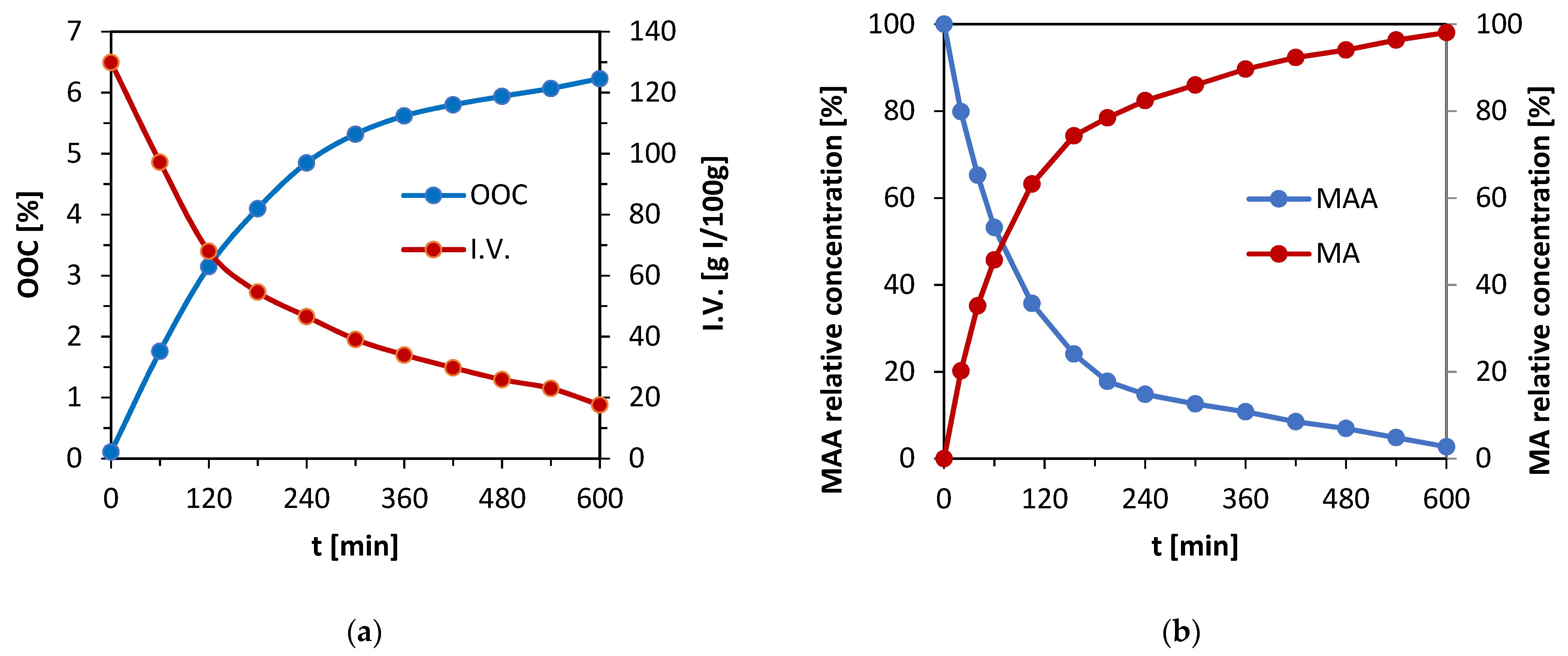
The epoxidation mixture prepared from reactants according to the determined iodine value of rapeseed oil underwent a reaction for 10 h. Both iodine value and OOC parameters were monitored during the reaction for the confirmation of forming epoxy functional groups within the structure of the used oil. The OOC parameter increases over time, as is shown in
Figure 2a, as a result of forming of oxirane cycles. On the other hand, double bonds disappear from the carbon backbone of used oil as an iodine value decreases. The mixture was treated with potassium iodide and sodium thiosulfate after the reaction to reduce the number of peroxide functional groups occurring in the oil’s structure after the epoxidation. The solution was eventually washed with distilled water to remove all formed structures and the remaining hydrogen peroxide and formic acid. Finally, the epoxidized oil was distilled to separate residual water from the emulsion.
There is evidence of positive progress of the epoxidation from
Figure 2a. The OOC parameter raised to the value of 6.23%. Since the maximal OOC which can be reached with the initial iodine value of 120.5 g I
2/100 g is 7.22%, the conversion of double bonds into epoxy groups is 86.3% according to OOC. The iodine value decreased in time during the reaction and reached a value of 17.6 g I
2/100 g. If the conversion of double bonds is calculated via the iodine value, the result is 86.5%. The value calculated from the decrease in iodine value is slightly higher than the one from OOC. The reason for this outcome is the fact that a part of the double bonds participates in the formation of peroxide functional groups within the oil’s structure. However, both parameters correlate. It was observed that when the oil undergoes the reaction for a longer time, the OOC parameter tends to decrease. Therefore, the time of 10 h was determined as optimal. Published epoxidation processes involving oils such as camelina oil, flax oil, or canola oil reached similar or lower OOC parameter values in comparison with presented results, except for the reaction time, which was longer [
28]. It was found that 10 h is an optimal reaction time, particularly for rapeseed oil.
The epoxidation process appeared to be exothermic after the addition of the catalyst. The temperature increase is an important factor for the potential scale-up and production technology suggestions. The heating jacket of the reactor can temper the temperature and cool down the system. However, the amount of released energy during this process is non-negligible. The temperature increase after the catalyst’s addition and the following cool-down is shown in
Figure S3.
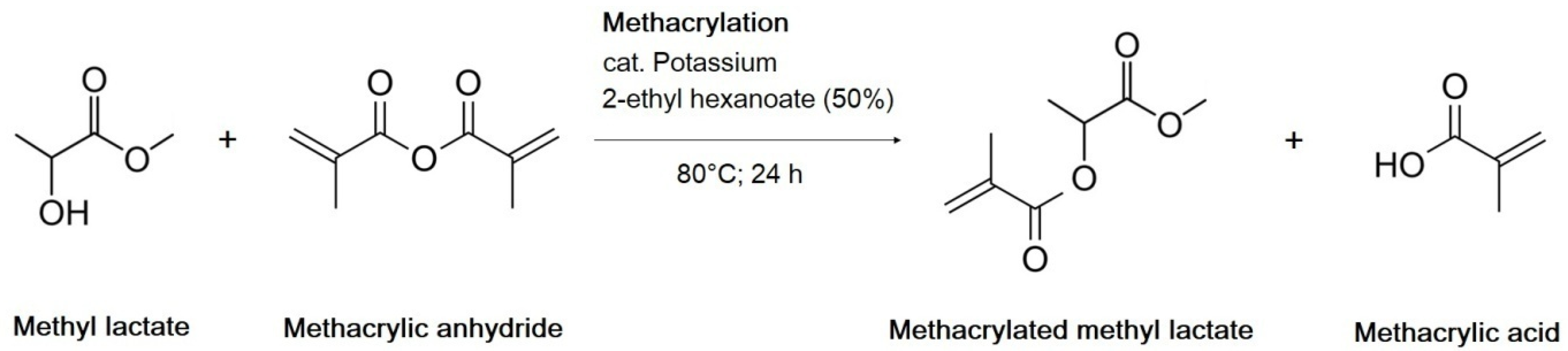
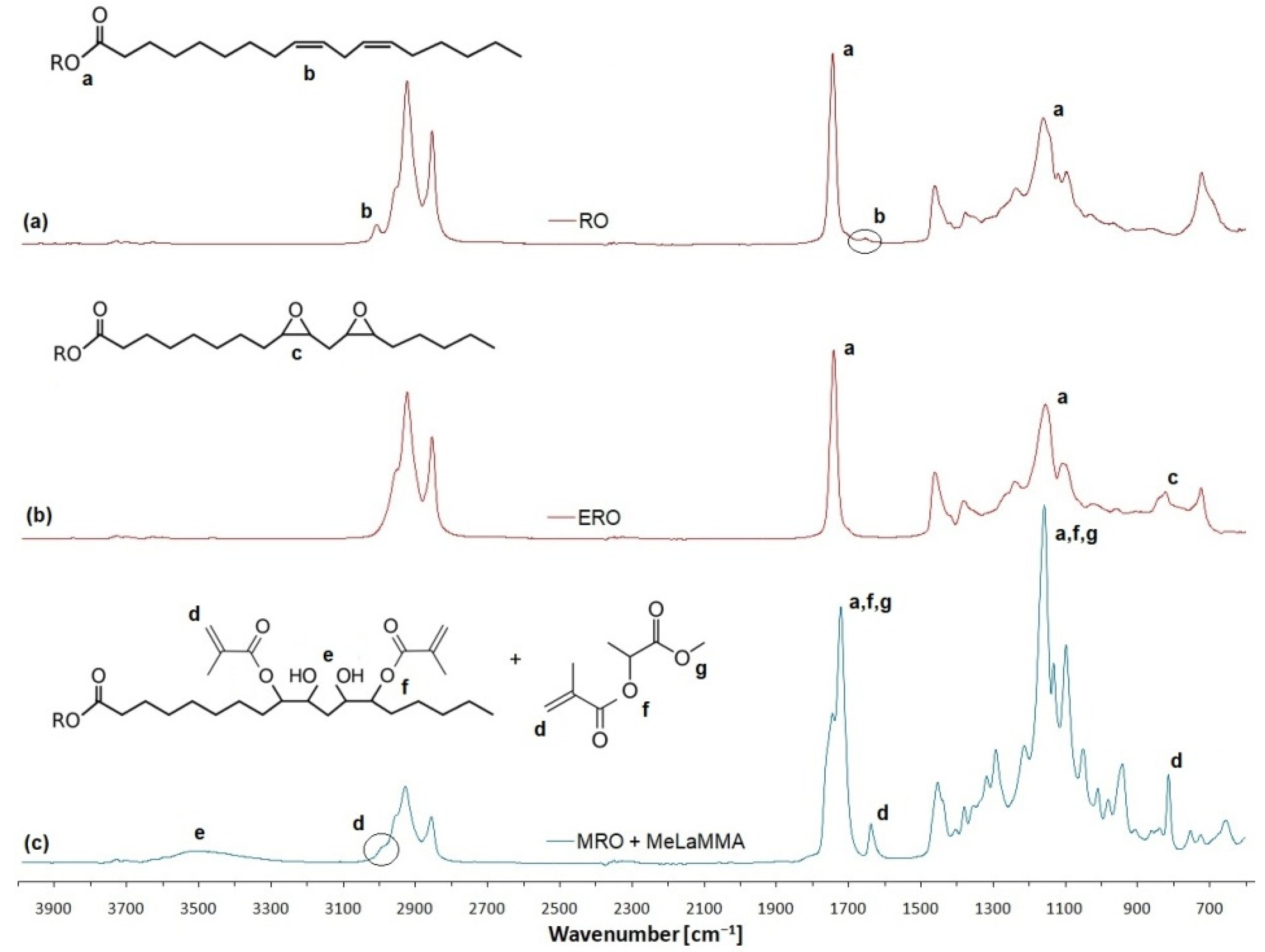
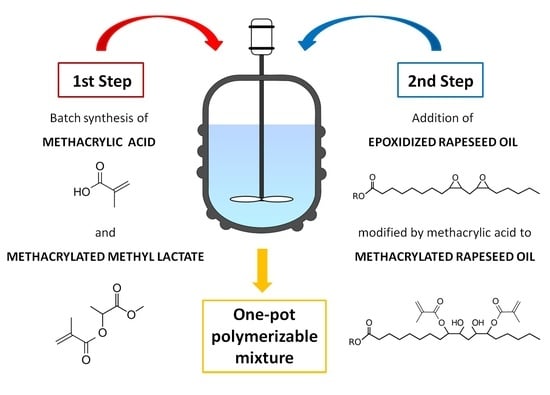
Separately, the synthesis of methacrylated methyl lactate (MeLaMMA) was performed to generate both polymerizable monomer (MeLaMMA) and reaction methacrylic acid (MA) for a further methacrylated oil synthesis (illustrated in
Figure 1). The polymerizable monomer forms a continuum for an additional reaction step as well as methacrylic acid. The reaction’s progress in time is shown in
Figure 2, which is a progress of the MAA concentration’s decrease and MA concentration’s increase in time monitored via the GC-FID method.
There is evidence of a reaction’s progressing trend from the chromatography results in
Figure 2b. Methacrylic anhydride reacts with methyl lactate (MeLa), so its concentration in the reaction mixture decreases over time. At the same time, methacrylic acid is formed as one of the products during the reaction, which illustrates the dependence above as well. Since the methacrylation reaction of MeLa and MAA does not require the withdrawing of the forming by-product MA to modify the equilibrium, this reaction was performed until the maximum conversion of both reactants (MA and MeLaMMA) was acquired. The reached yield values of both products are shown in
Table 3.
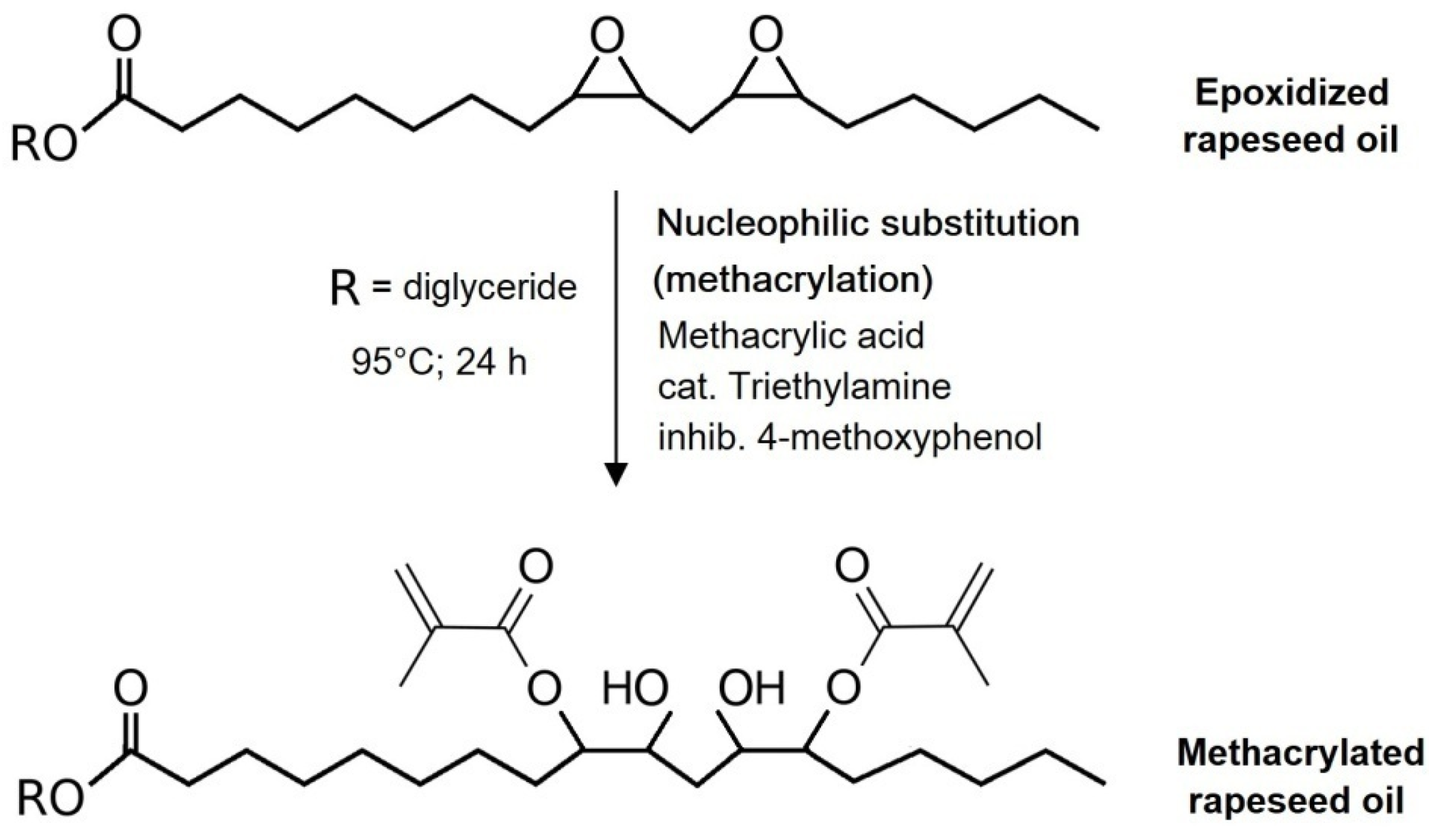
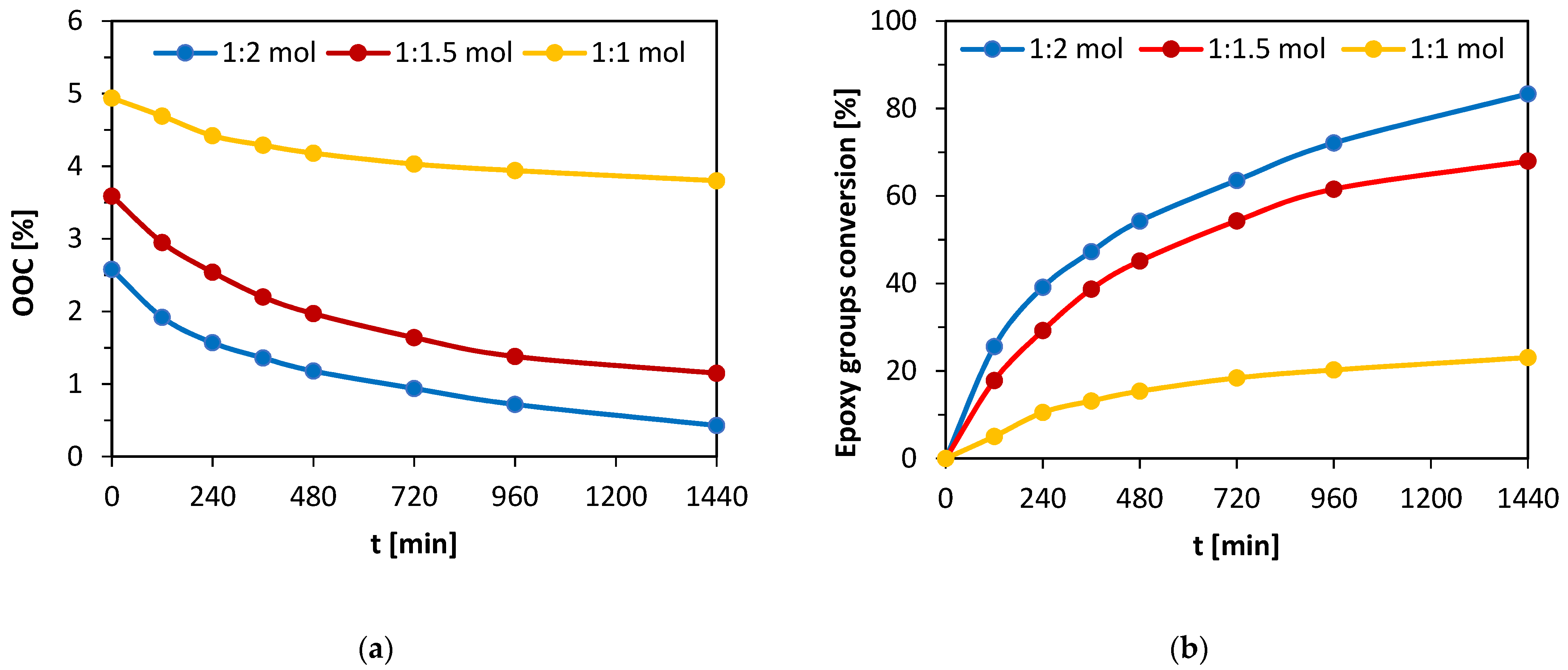
The post-reaction mixture containing both formed MeLaMMA and MA was heated from 80 °C to 95 °C before the addition of prepared epoxidized oil and appropriate catalyst (TEA). An additional inhibitor (4-methoxyphenol) was dissolved in the solution to prevent the mixture from spontaneous polymerization. This additive is also present in used methacrylic anhydride at 2000 ppm. However, since the thermoset mixture preparation process took two steps both involving increased temperature conditions, the amount of inhibitor increased. All three chosen molar ratios of reactants were monitored during the 24 h long reaction and the decreases of OOC parameters were measured for all of them regularly. The molar ratios contain an either equimolar amount of MA (1:1 mol) or an excess of MA (1:1.5 mol; 1:2 mol). The dependences of OOC parameter changes in time and calculated epoxy functional groups conversions from the OOC parameters’ theoretical and real values are shown in
Figure 3a,b.
A big influence on the methacrylation reaction rate (of epoxidized oil) is caused by particular excess of methacrylic acid.
Figure 3a shows the OOC parameter values decrease in time for all performed mixtures. The initial OOC parameter differs in the graph due to the unequal mass participation of oil in studied mixtures (the equimolar mixture has twice the amount of epoxidized oil than the solution with double MA excess). Therefore, the results comparing OOC values do not show an accurate reaction conversion. On the other hand,
Figure 3b is composed of the particular dependences of epoxy functional groups conversions in time during the reaction. The conversion values were calculated as follows:
where OOC
t is the value of epoxy groups percentage in particular time t (%), OOC
0 is the value of epoxy groups percentage in time t= 0 min (%). The conversions reached after 24 h of reaction for each mixture containing different amounts of MA excess are shown in
Table 4.
The resulting mixture containing both methacrylated rapeseed oil and methacrylated methyl lactate exhibited particular oil numbers as a confirmation of performed modifications and reactions. The values of measured parameters are written in
Table 5. There is an evident presence of hydroxyl functional groups from the hydroxyl value confirming the bonded methacrylic functional groups in the oil’s structure. This bonding is also evident from the raised iodine value. The remaining OOC parameter value is a measurement of the reaction conversion and gives information about possible further modification performance possibilities. However, the mixture was set at 95 °C to modify the epoxidized oil for only 24 h so the solution does not spontaneously polymerize. When the calculated conversions are compared to the reported results using epodixized soybean oil and acrylic acid the excess of (1:10) (oil:acid), the final products reached a similar conversion as the reported ones. Therefore, the used excesses of the acid investigated in the work should increase the profitability of the process [
29].