Controllable Production of Natural Silk Nanofibrils for Reinforcing Silk-Based Orthopedic Screws
Abstract
1. Introduction
2. Materials and Methods
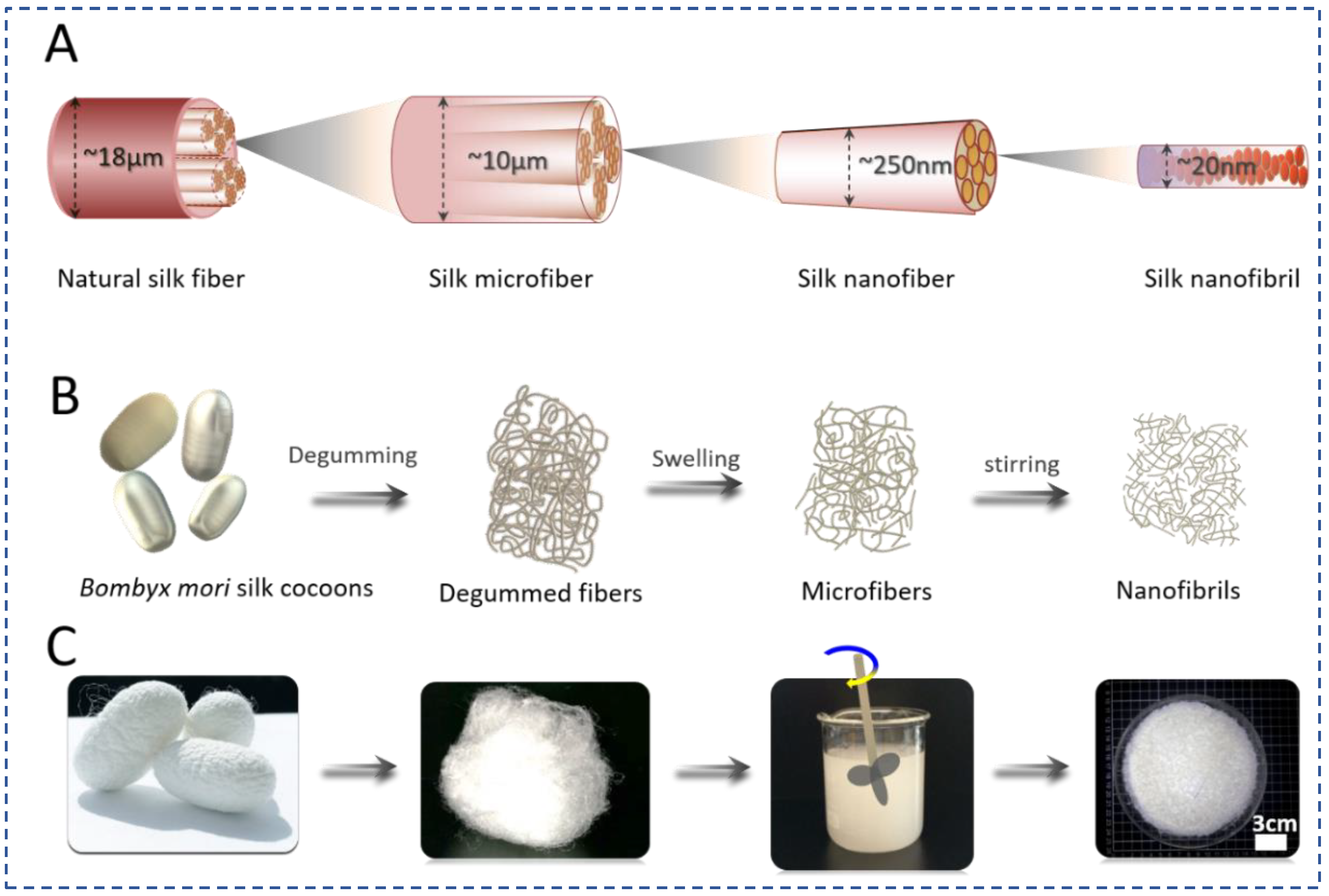
2.1. Exfoliation of Natural Silk Nanofibers (SNFs)
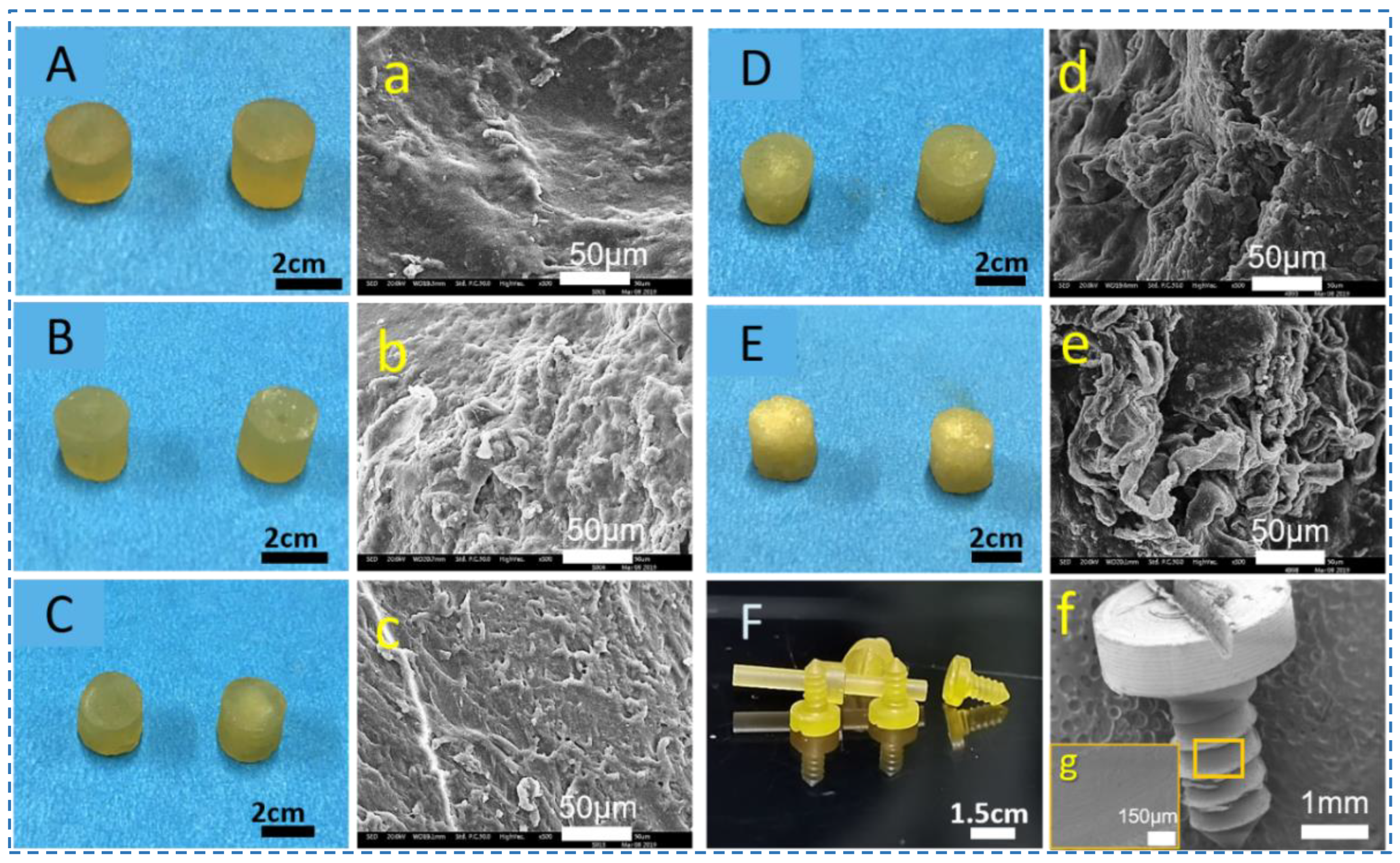
2.2. Preparation of Silk Screws
2.3. Morphology and Mechanical Properties of SNFs, Silk Screws
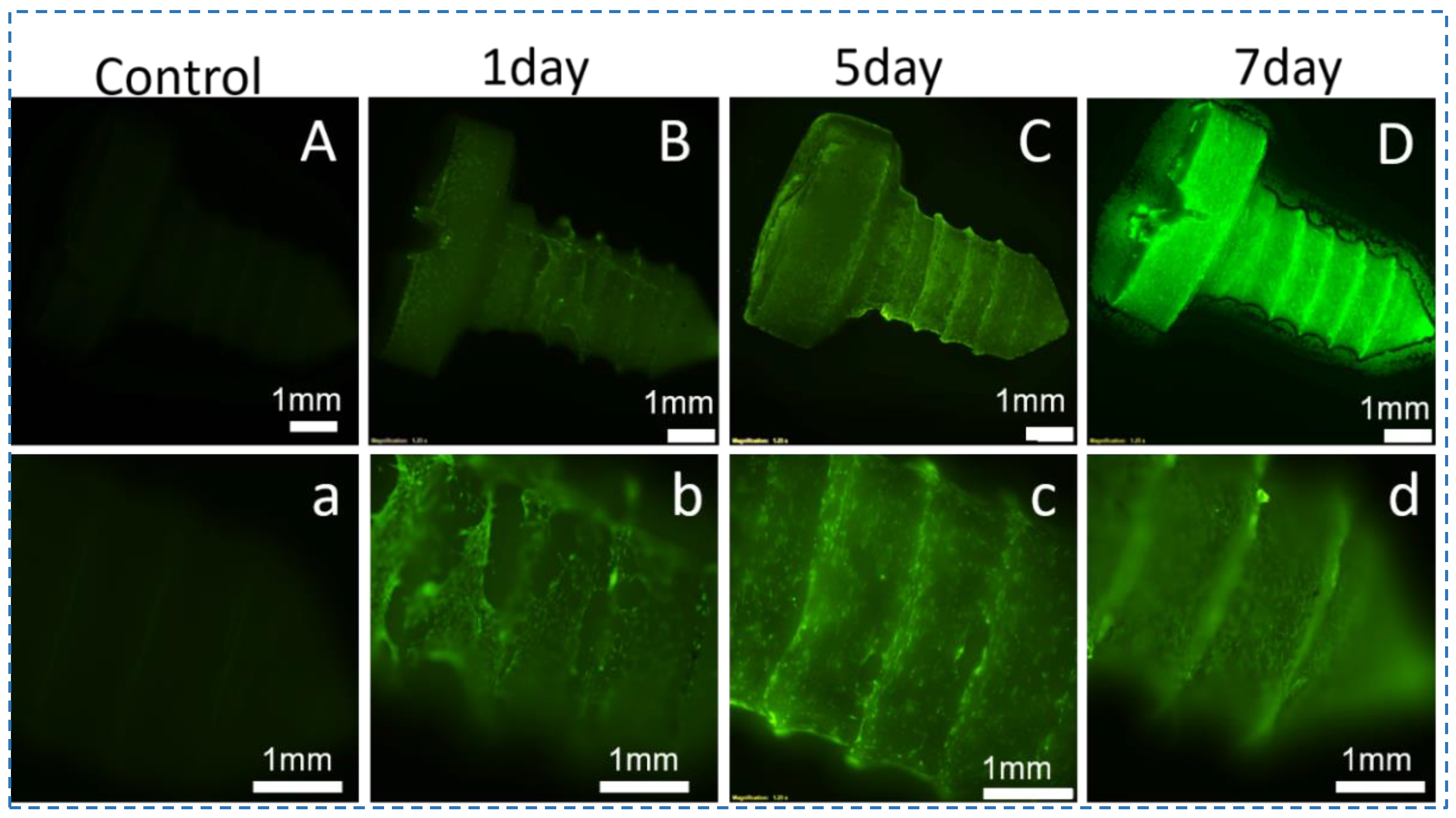
2.4. Biodegradation of the Silk Screws In Vitro
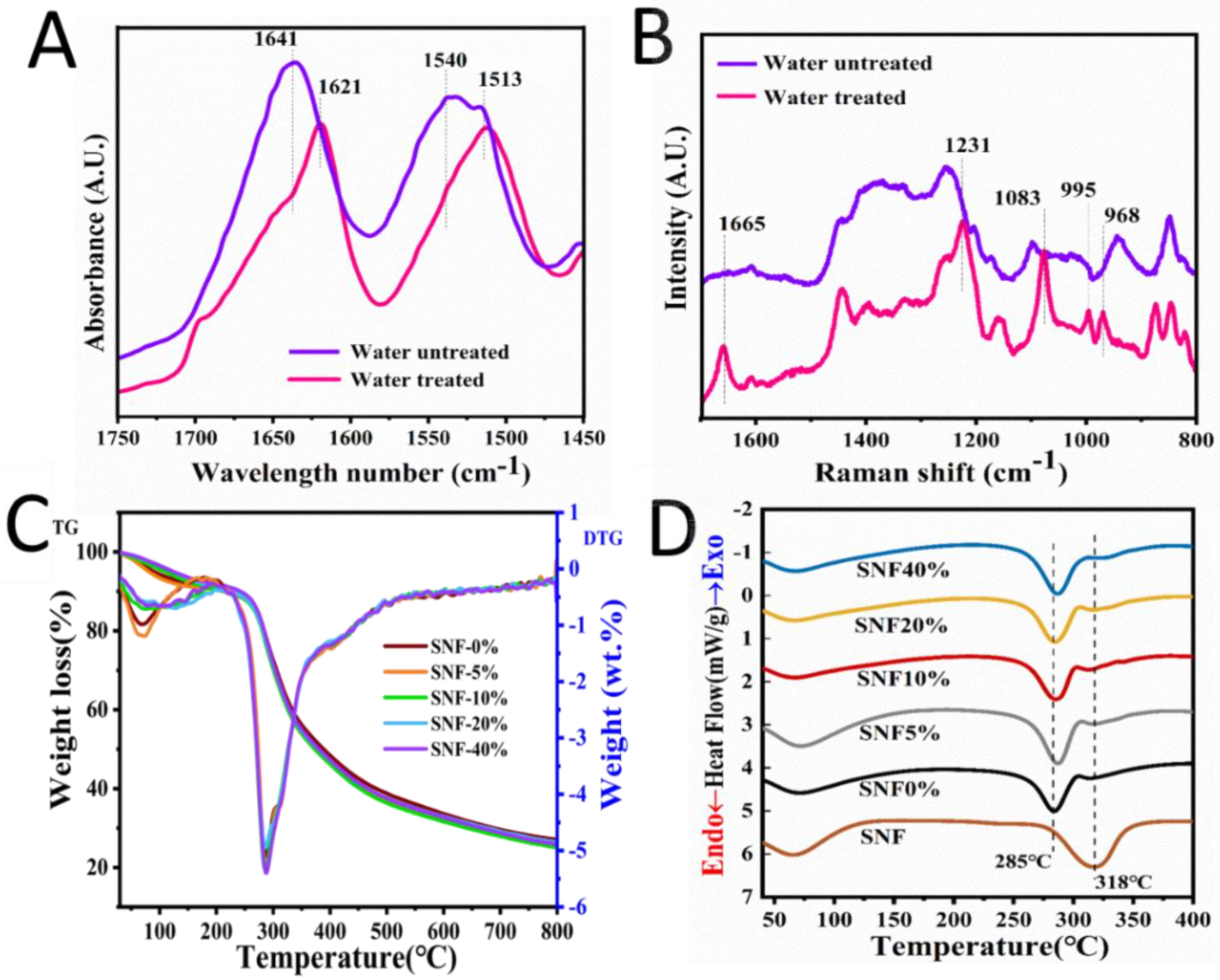
2.5. Structure and Stability Analysis of Silk Screws
2.6. Cell Culture
2.7. Statistical Analysis
3. Results and Discussion
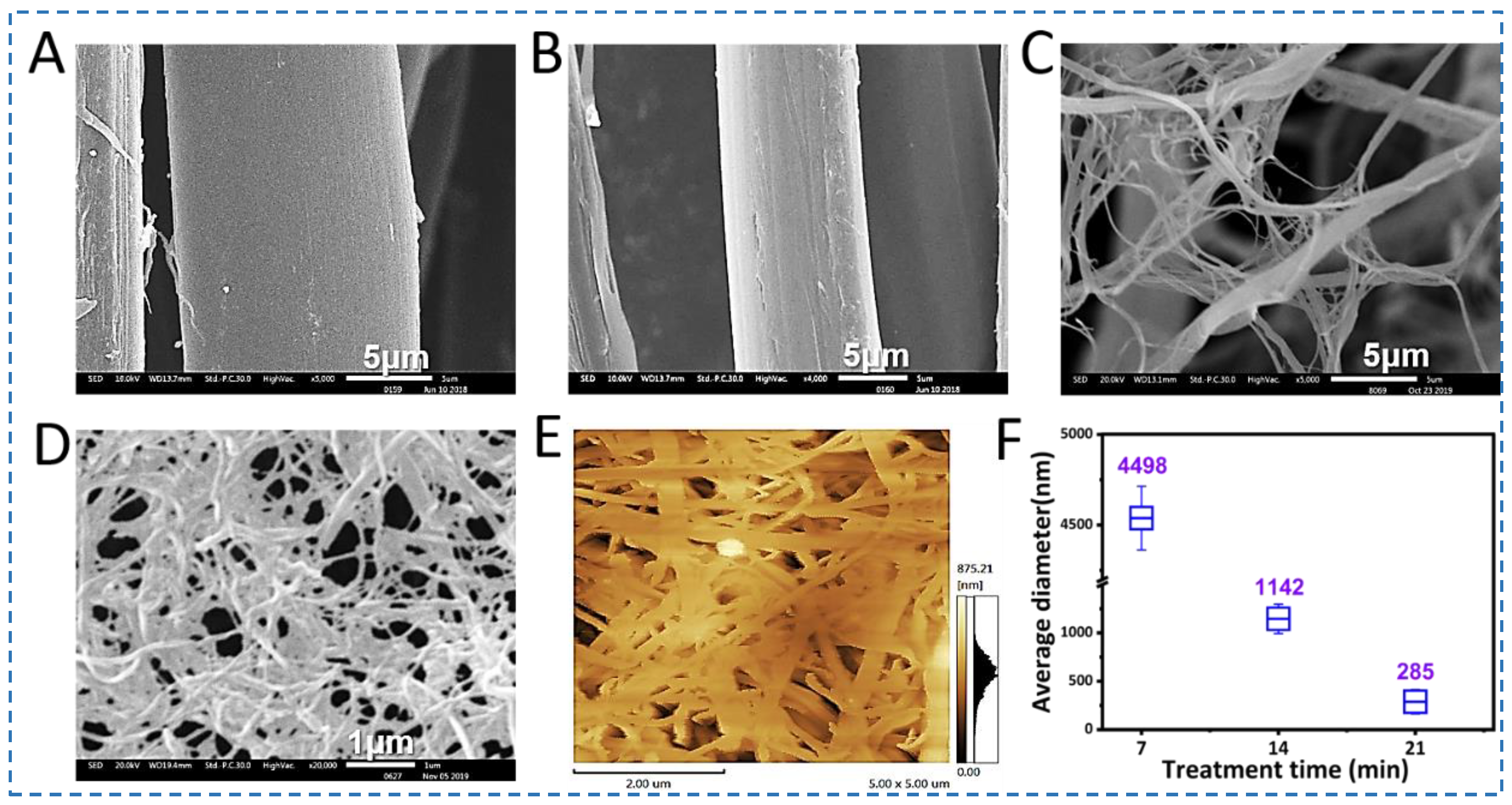
3.1. Exfoliation of SNFs
3.2. Preparation and Morphology of Silk Screws of Silk Screws
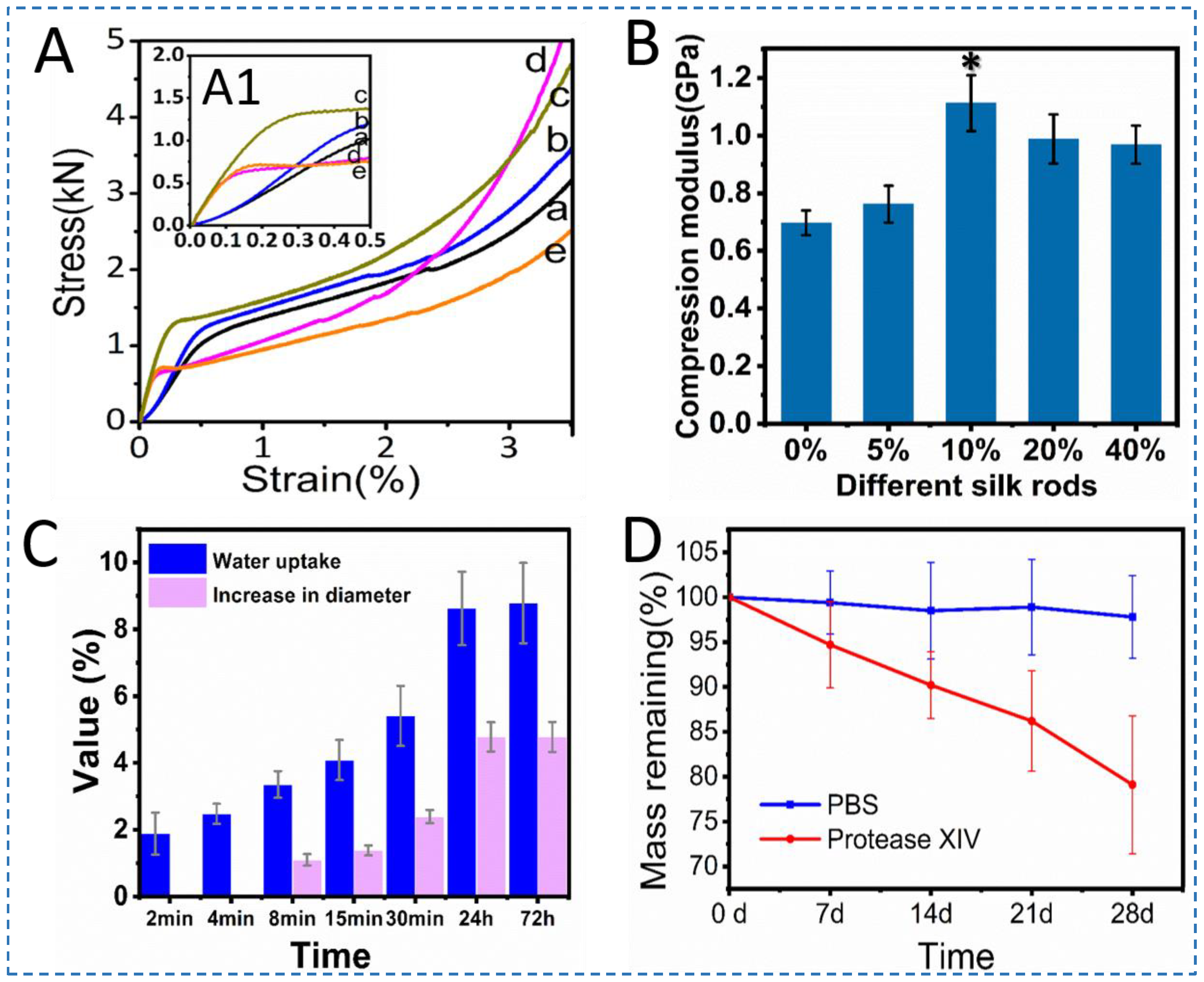
3.3. Mechanical Performance and Degradation of Silk Screws
3.4. Structural Analysis of Silk Rods
3.5. Cytocompatibility of the Silk Screws
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Ebraheim, N.; Lausé, G.E.; Xiao, B.; Xu, R. A Comparison of Absorbable Screws and Metallic Plates in Treating Calcaneal Fractures: A Prospective Randomized Trial. J. Trauma Acute Care Surg. 2012, 72, E106–E110. [Google Scholar] [CrossRef] [PubMed]
- Imola, M.J.; Hamlar, D.D.; Shao, W.; Chowdhury, K.; Tatum, S. Resorbable Plate Fixation in Pediatric Craniofacial Surgery: Long-Term Outcome. Arch. Facial Plast. Surg. 2001, 3, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, R.; Krishnan, P.D.; Zennifer, A.; Kannan, V.; Manigandan, A.; Arul, M.R.; Jaiswal, D.; Subramanian, A.; Kumbar, S.G.; Sethuraman, S. Additive Manufacturing of Biodegradable Porous Orthopaedic Screw. Bioact. Mater. 2020, 5, 458–467. [Google Scholar] [CrossRef]
- Eppley, B.L. Use of Resorbable Plates and Screws in Pediatric Facial Fractures. J. Oral Maxillofac. Surg. 2005, 63, 385–391. [Google Scholar] [CrossRef]
- Ramos, D.M.; Dhandapani, R.; Subramanian, A.; Sethuraman, S.; Kumbar, S.G. Clinical Complications of Biodegradable Screws for Ligament Injuries. Mat. Sci. Eng. C 2020, 109, 110423. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.M.; Fung, S.; Wood, D.G. The Natural History of Biointerference Screw Cyst and New Bone Formation in Anterior Cruciate Ligament Reconstruction: 16-Year Follow-Up. Am. J. Sport Med. 2016, 44, 113–117. [Google Scholar] [CrossRef]
- Brito-Pereira, R.; Correia, D.M.; Ribeiro, C.; Francesko, A.; Etxebarria, I.; Pérez-Álvarez, L.; Vilas, J.L.; Martins, P.; Lanceros-Mendez, S. Silk Fibroin-Magnetic Hybrid Composite Electrospun Fibers for Tissue Engineering Applications. Compos. Part B Eng. 2018, 141, 70–75. [Google Scholar] [CrossRef]
- Ghanaati, S.; Unger, R.E.; Webber, M.J.; Barbeck, M.; Orth, C.; Kirkpatrick, J.A.; Booms, P.; Motta, A.; Migliaresi, C.; Sader, R.A.; et al. Scaffold Vascularization in vivo Driven by Primary Human Osteoblasts in Concert with Host Inflammatory Cells. Biomaterials 2011, 32, 8150–8160. [Google Scholar] [CrossRef]
- Qiu, W.; Patil, A.; Hu, F.; Liu, X.Y. Hierarchical Structure of Silk Materials Versus Mechanical Performance and Mesoscopic Engineering Principles. Small 2019, 15, 1903948. [Google Scholar] [CrossRef]
- Jin, H.; Kaplan, D.L. Mechanism of Silk Processing in Insects and Spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef]
- Patel, M.; Dubey, D.K.; Singh, S.P. Insights into Nanomechanical Behavior and Molecular Mechanisms in Bombyx mori Silk Fibroin in Saline Environment Using Molecular Dynamics Analysis. Macromol. Res. 2021, 29, 694–712. [Google Scholar]
- Yamane, T.; Umemura, K.; Nakazawa, Y.; Asakura, T. Molecular Dynamics Simulation of Conformational Change of Poly(Ala–Gly) from Silk I to Silk II in Relation to Fiber Formation Mechanism of Bombyx mori Silk Fibroin. Macromolecules 2003, 36, 6766–6772. [Google Scholar] [CrossRef]
- Patel, M.; Dubey, D.K.; Singh, S.P. Phenomenological Models of Bombyx mori Silk Fibroin and their Mechanical Behavior Using Molecular Dynamics Simulations. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110414. [Google Scholar] [CrossRef]
- Grant, A.M.; Kim, H.S.; Dupnock, T.L.; Hu, K.; Yingling, Y.G.; Tsukruk, V.V. Silk Fibroin–Substrate Interactions at Heterogeneous Nanocomposite Interfaces. Adv. Funct. Mater. 2016, 26, 6380–6392. [Google Scholar] [CrossRef]
- Collins, A.M.; Skaer, N.J.V.; Gheysens, T.; Knight, D.; Bertram, C.; Roach, H.I.; Oreffo, R.O.C.; Von–Aulock, S.; Baris, T.; Skinner, J.; et al. Bone-Like Resorbable Silk-Based Scaffolds for Load-Bearing Osteoregenerative Applications. Adv. Mater. 2009, 21, 75–78. [Google Scholar] [CrossRef]
- Ling, S.; Li, C.; Jin, K.; Kaplan, D.L.; Buehler, M.J. Liquid Exfoliated Natural Silk Nanofibrils: Applications in Optical and Electrical Devices. Adv. Mater. 2016, 28, 7783–7790. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Q.; Ming, J.; Dou, H.; Liu, Z.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Silk Dissolution and Regeneration at the Nanofibril Scale. J. Mater. Chem. B 2014, 2, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Peng, Q.; Lu, L.; Fan, S.; Shao, H.; Zhang, H.; Wu, R.; Hsiao, B.S.; Zhang, Y. Single Molecular Layer of Silk Nanoribbon as Potential Basic Building Block af Silk Materials. ACS Nano 2018, 12, 11860–11870. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhong, J.; Qi, Z.; Ling, S.; Kaplan, D.L. Isolation of Silk Mesostructures for Electronic and Environmental Applications. Adv. Funct. Mater. 2018, 28, 1806380. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, W.; Mu, T. Controllable Exfoliation of Natural Silk Fibers into Nanofibrils by Protein Denaturant Deep Eutectic Solvent, Nanofibrous Strategy for Multifunctional Membranes. Green Chem. 2018, 20, 3625–3633. [Google Scholar] [CrossRef]
- Yang, H.; Wang, P.; Yang, Q.; Wang, D.; Wang, Y.; Kuai, L.; Wang, Z. Superelastic and Multifunctional Fibroin Aerogels from Multiscale Silk Micro-Nanofibrils Exfoliated via Deep Eutectic Solvent. Int. J. Biol. Macromol. 2023, 224, 1412–1422. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, S.; Han, G.; Li, X.; You, R.; Zhang, Q.; Li, M.; Kaplan, D.L. Facile Production of Natural Silk Nanofibers for Electronic Device Applications. Compos. Sci. Technol. 2020, 187, 107950. [Google Scholar] [CrossRef]
- Hu, Z.; Yan, S.; Li, X.; You, R.; Zhang, Q.; Kaplan, D.L. Natural Silk Nanofibril Aerogels with Distinctive Filtration Capacity and Heat-Retention Performance. ACS Nano 2021, 15, 8171–8183. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.Q.; Han, G.C.; Wang, Q.S.; Zhang, S.Y.; You, R.C.; Luo, Z.W.; Xu, A.C.; Li, X.F.; Zhang, Q.; Kaplan, D.L. Directed Assembly of Robust and Biocompatible Silk Fibroin/Hyaluronic Acid Composite Hydrogels. Compos. Part B Eng. 2019, 167, 107204. [Google Scholar] [CrossRef]
- Yan, S.; Wang, L.; Fan, H.; Li, X.; You, H.; You, R.; Zhang, Q.; Xu, W.; Zhang, Y. Biomimetic Natural Silk Nanofibrous Microspheres for Multifunctional Biomedical Applications. ACS Nano 2022, 16, 15115–15123. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, C.; Vu, H.V.; Hanna, P.; Lechtig, A.; Qiu, Y.; Mu, X.; Ling, S.; Nazarian, A.; Lin, S.J.; et al. Thermoplastic Moulding of Regenerated Silk. Nat. Mater. 2020, 19, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hotz, B.; Ling, S.; Guo, J.; Haas, D.S.; Marelli, B.; Omenetto, F.; Lin, S.J.; Kaplan, D.L. Regenerated Silk Materials for Functionalized Silk Orthopedic Devices by Mimicking Natural Processing. Biomaterials 2016, 110, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Birman, V.; Chandrashekhara, K.; Hopkins, M.S.; Volz, J.S. Strength Analysis of Particulate Polymers. Compos. Part B Eng. 2013, 54, 278–288. [Google Scholar] [CrossRef]
- Phillips, D.; Drummy, L.; Naik, R.; Long, H.; Fox, D.; Trulove, P.; Mantz, R. Regenerated Silk Fiber Wet Spinning from an Ionic Liquid Solution. J. Mater. Chem. 2005, 15, 4206–4208. [Google Scholar] [CrossRef]
- Lao, U.L.; Sun, M.; Matsumoto, M.; Mulchandani, A.; Chen, W. Genetic Engineering of Self–Assembled Protein Hydrogel Based on Elastin-Like Sequences with Metal Binding Functionality. Biomacromolecules 2007, 8, 3736–3739. [Google Scholar] [CrossRef]
- Cheng, Y.; Koh, L.D.; Li, D.; Ji, B.; Han, M.Y.; Zhang, Y.W. On the Strength of Β-Sheet Crystallites of Bombyx mori Silk Fibroin. J. R. Soc. Interface 2014, 11, 20140305. [Google Scholar] [CrossRef]
- Askarieh, G.; Hedhammar, M.; Nordling, K.; Saenz, A.; Casals, C.; Rising, A.; Johansson, J.; Knight, S.D. Self-Assembly of Spider Silk Proteins is Controlled by a Ph-Sensitive Relay. Nature 2010, 465, 236–238. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Li, X.; Yan, S.; Xu, A.; You, R.; Zhang, Q. Natural Silk Nanofibrils as Reinforcements for the Preparation of Chitosan-Based Bionanocomposites. Carbohydr. Polym. 2020, 253, 117214. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Kaplan, D.L.; Buehler, M.J. Nanofibrils in Nature and Materials Engineering. Nat. Rev. Mater. 2018, 3, 18016. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.Y.; Lau, K.T.; Pow, Y.F.; Zhao, Y.-Q.; Hui, D. Biodegradation of a Silkworm Silk/PLA Composite. Compos. Part B Eng. 2010, 41, 223–228. [Google Scholar] [CrossRef]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.; et al. The Use of Silk-Based Devices for Fracture Fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, M.M.; Gortemaker, A.; Van’t Hof, M.A.; Jansen, J.A.; Creugers, N.H. Implant Surface Roughness and Bone Healing: A Systematic Review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Zilkens, C.; Zanger, K.; Krauspe, R. Significance of Nano- and Microtopography for Cell-Surface Interactions in Orthopaedic Implants. J. Biomed. Biotechnol. 2007, 2007, 69036. [Google Scholar] [CrossRef]
- Guo, C.; Li, C.; Kaplan, D.L. Enzymatic Degradation of Bombyx mori Silk Materials: A Review. Biomacromolecules 2020, 21, 1678–1686. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Li, G.; Wang, P.; Yang, Y. Tailoring Degradation Rates of Silk Fibroin Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. A 2019, 107, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.; Kaplan, D.L. Design of Biodegradable Implantable Devices towards Clinical Translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]


| SAMPLES | SNF0% | SNF5% | SNF10% | SNF20% | SNF40% |
|---|---|---|---|---|---|
| Stress (kN) | 1.02 | 1.20 | 1.37 | 0.79 | 0.75 |
| Compression modulus (Gpa) | 0.697 ± 0.043 | 0.762 ± 0.064 | 1.112 ± 0.097 | 0.988 ± 0.085 | 0.968 ± 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; He, L.; Hai, A.M.; Hu, Z.; You, R.; Zhang, Q.; Kaplan, D.L. Controllable Production of Natural Silk Nanofibrils for Reinforcing Silk-Based Orthopedic Screws. Polymers 2023, 15, 1645. https://doi.org/10.3390/polym15071645
Yan S, He L, Hai AM, Hu Z, You R, Zhang Q, Kaplan DL. Controllable Production of Natural Silk Nanofibrils for Reinforcing Silk-Based Orthopedic Screws. Polymers. 2023; 15(7):1645. https://doi.org/10.3390/polym15071645
Chicago/Turabian StyleYan, Shuqin, Li He, Abdul Moqeet Hai, Zhanao Hu, Renchuan You, Qiang Zhang, and David L. Kaplan. 2023. "Controllable Production of Natural Silk Nanofibrils for Reinforcing Silk-Based Orthopedic Screws" Polymers 15, no. 7: 1645. https://doi.org/10.3390/polym15071645
APA StyleYan, S., He, L., Hai, A. M., Hu, Z., You, R., Zhang, Q., & Kaplan, D. L. (2023). Controllable Production of Natural Silk Nanofibrils for Reinforcing Silk-Based Orthopedic Screws. Polymers, 15(7), 1645. https://doi.org/10.3390/polym15071645









