Mechanical and Gas Barrier Properties of Poly(Lactic Acid) Modified by Blending with Poly(Butylene 2,5-Furandicarboxylate): Based on Molecular Dynamics
Abstract
1. Introduction
2. Simulation Details
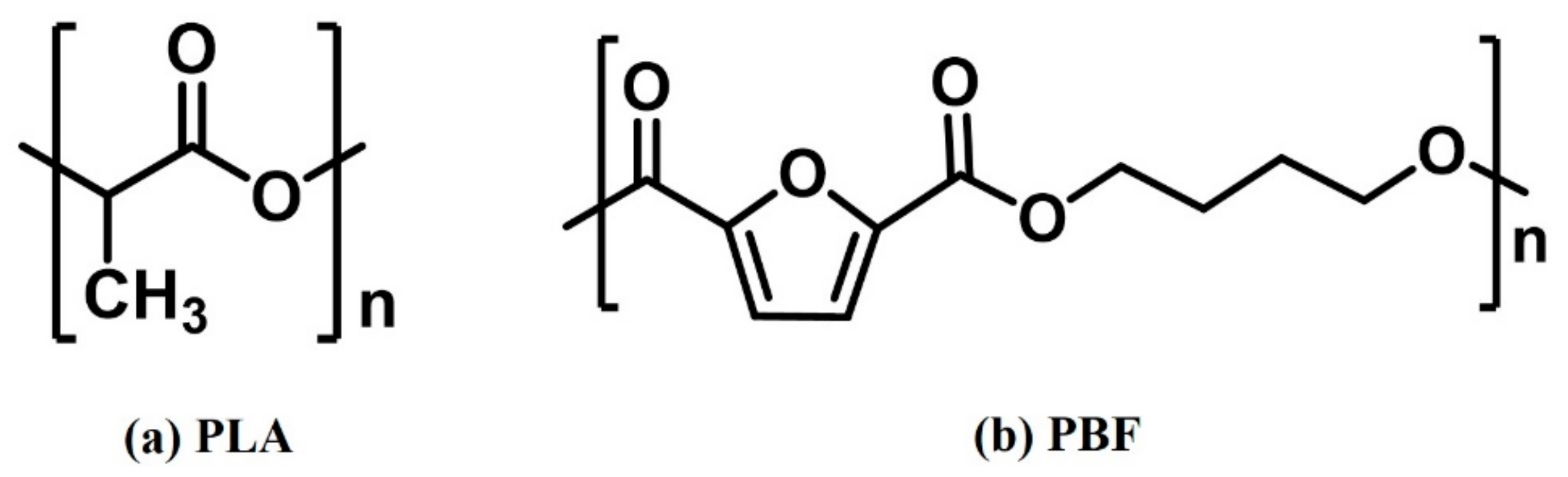
2.1. Construction of Polymer Chain Model
2.2. Construction of Polymer Model
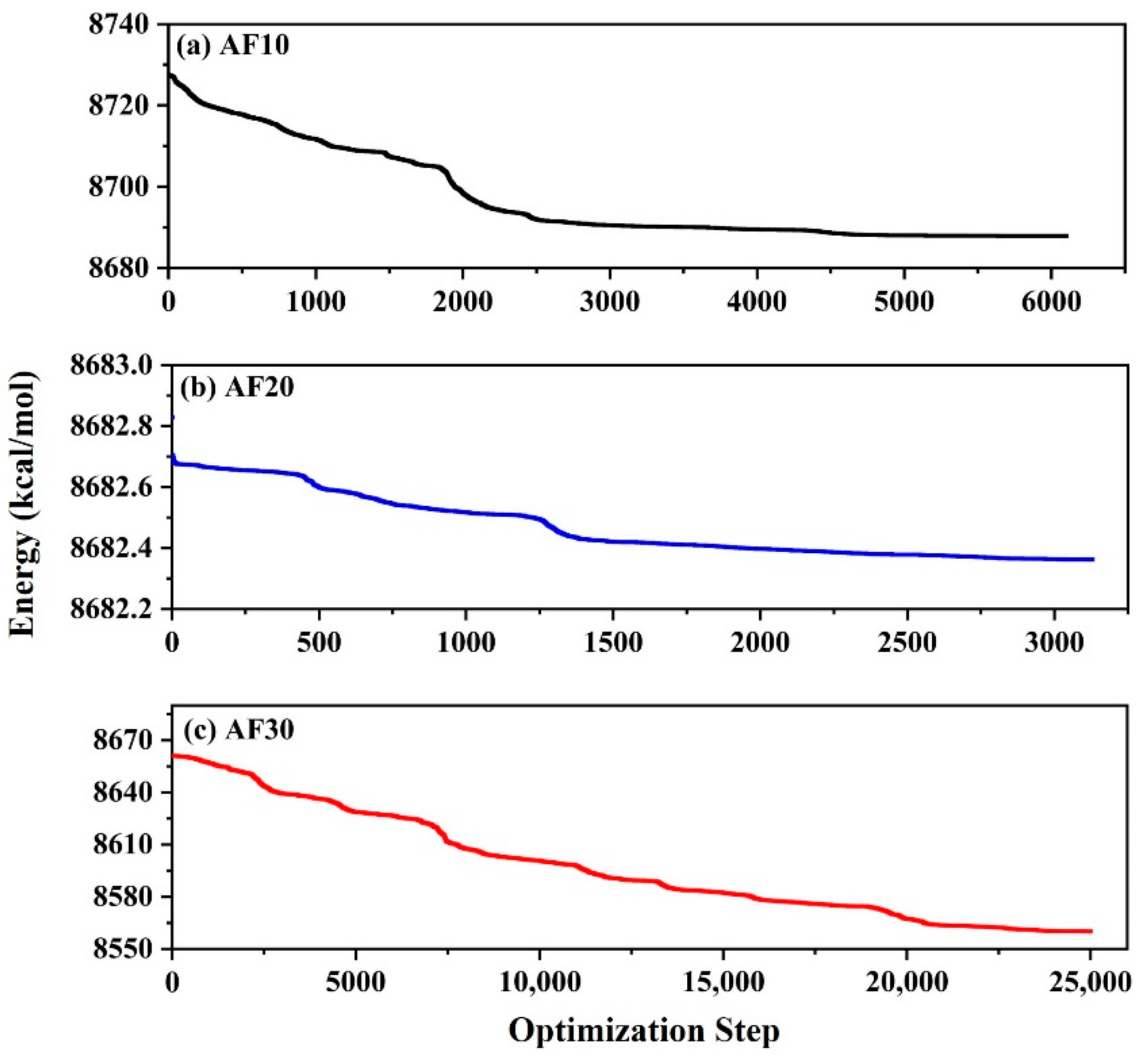
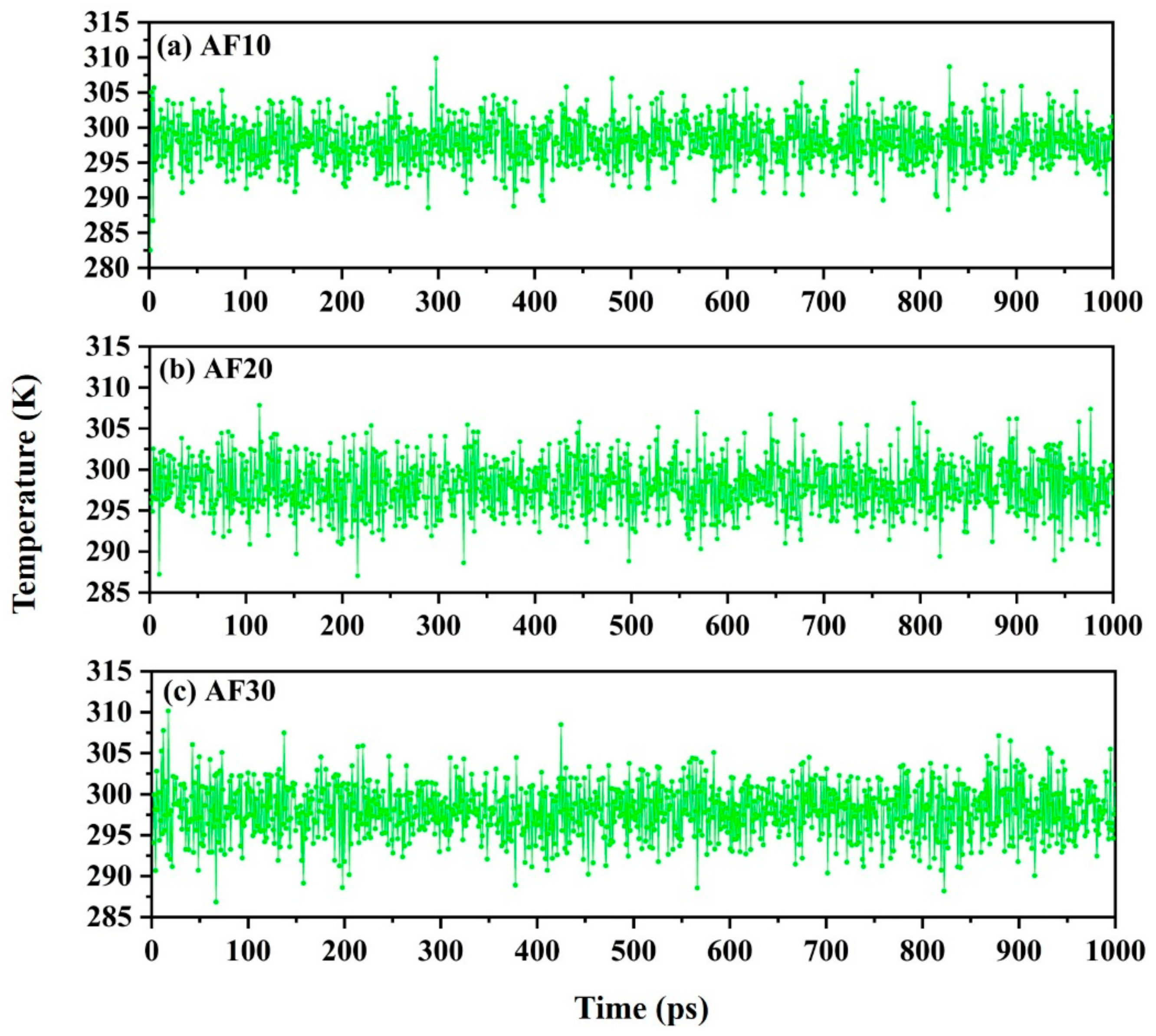
2.3. Model Optimization
3. Results and Discussion
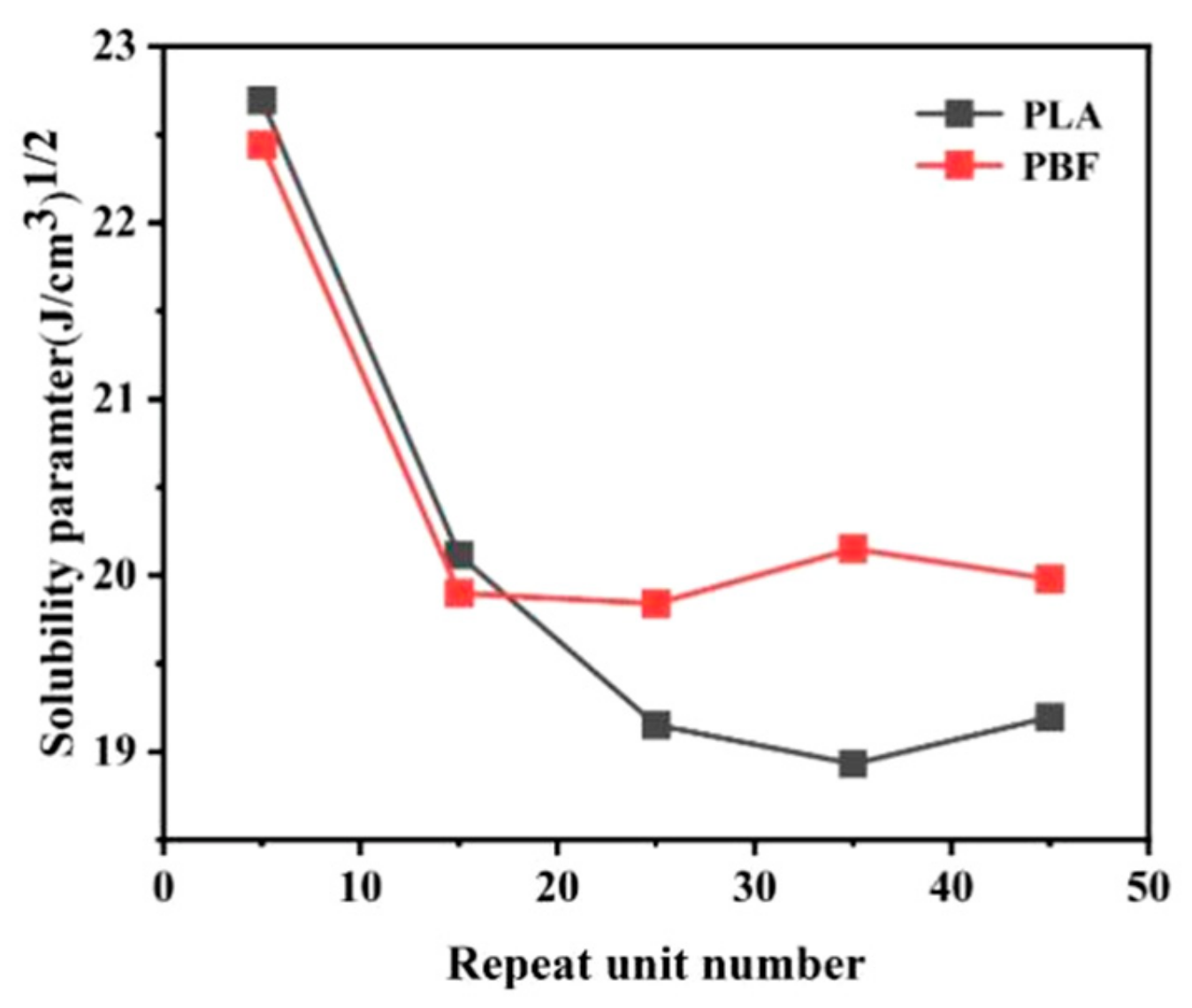
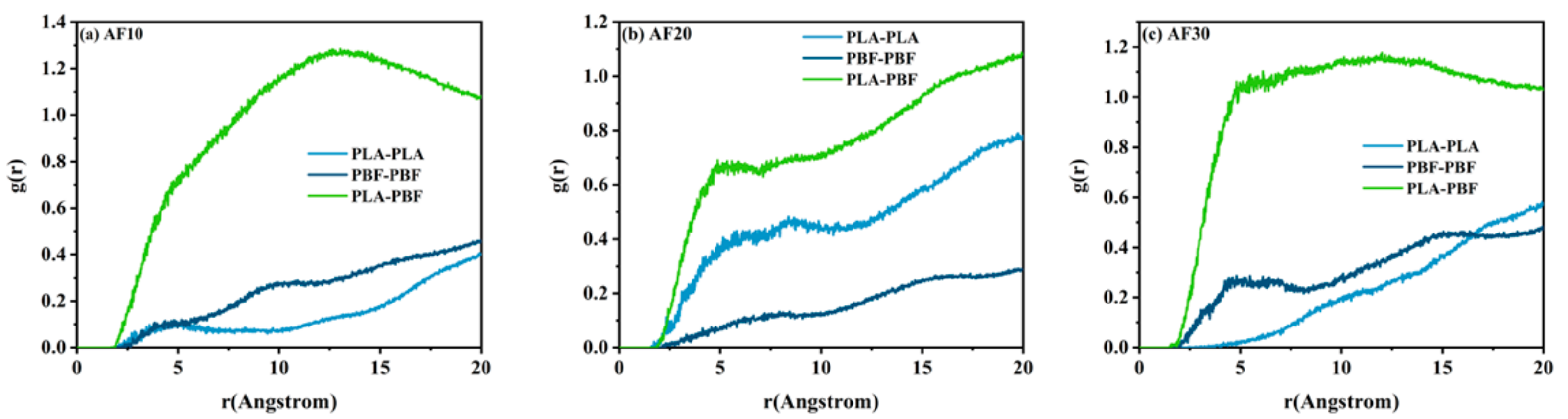
3.1. Compatibility of PLA and PBF
3.2. Mechanical Properties
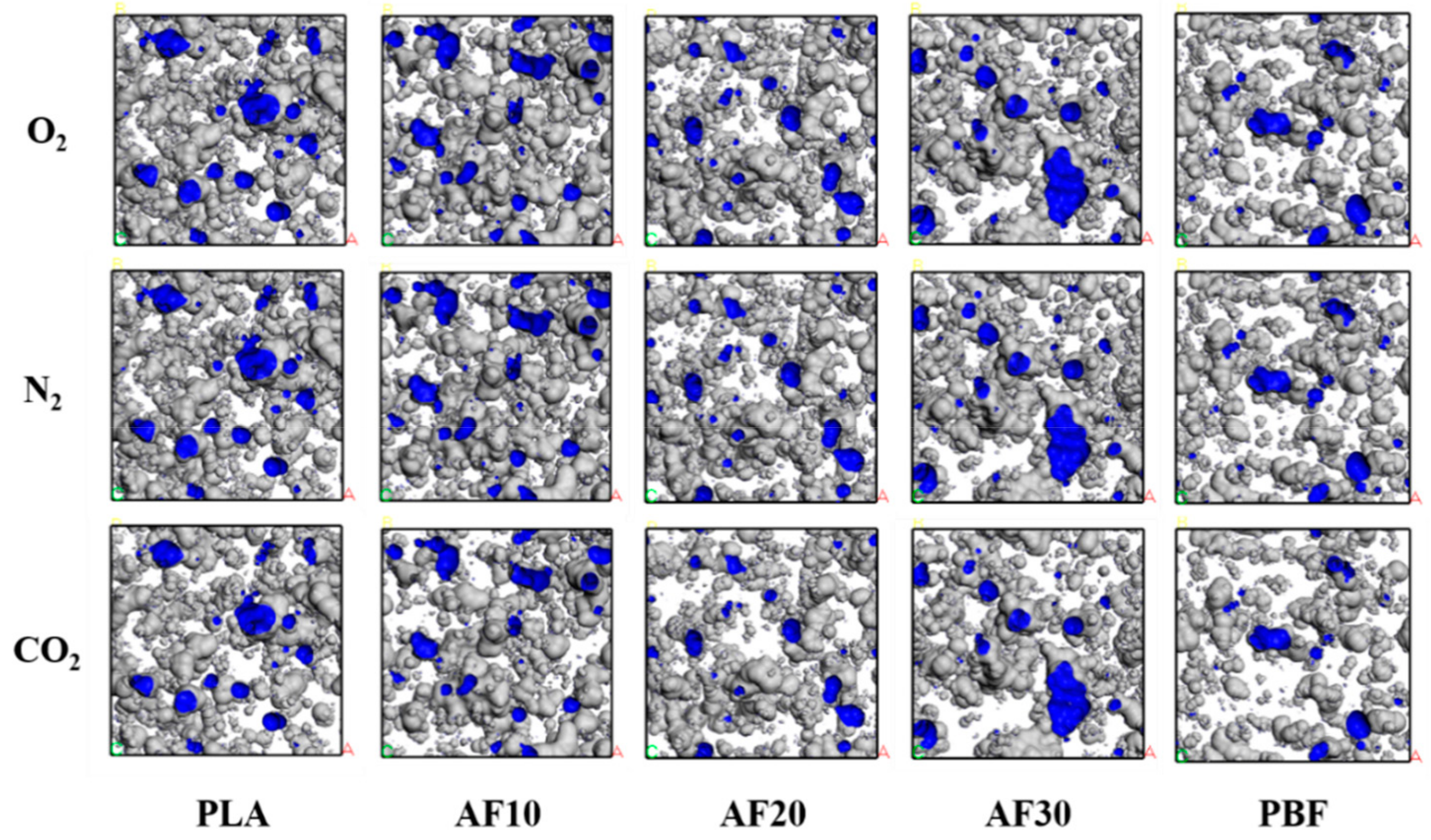
3.3. Free Volume
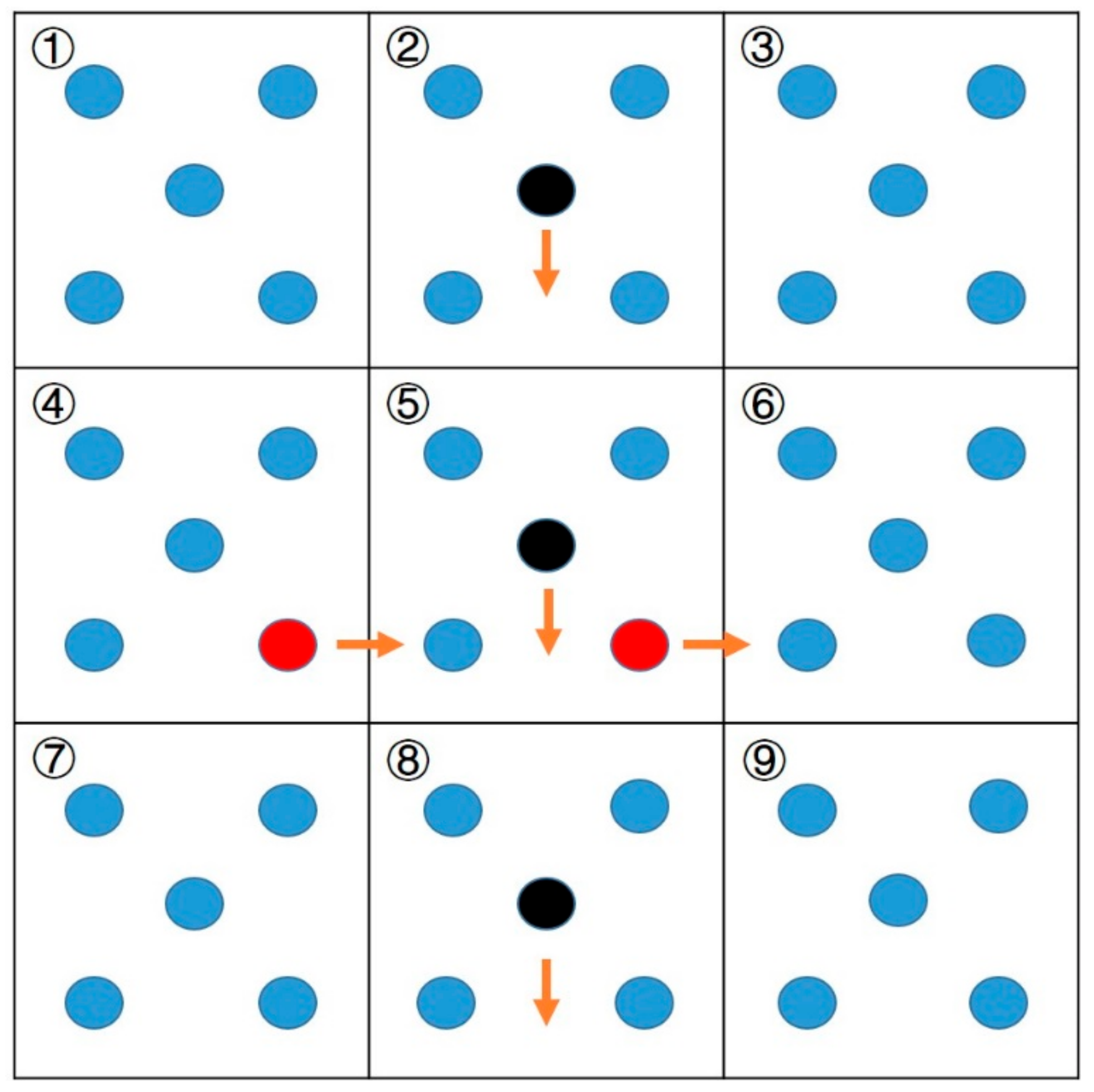
3.4. Diffusion Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.N.; Song, M.Z.; Xu, Y.N.; Wang, W. Bio-based polyesters: Recent progress and future prospects. Prog. Polym. Sci. 2021, 120, 42. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, P.J. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Lalanne, L.; Nyanhongo, G.S.; Guebitz, G.M.; Pellis, A. Biotechnological production and high potential of furan-based renewable monomers and polymers. Biotechnol. Adv. 2021, 48, 107707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (Bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef]
- Feghali, E.; Tauk, L.; Ortiz, P.; Vanbroekhoven, K.; Eevers, W. Catalytic chemical recycling of biodegradable polyesters. Polym. Degrad. Stab. 2020, 179, 109241. [Google Scholar] [CrossRef]
- Billiet, S.; Trenor, S.R. 100th Anniversary of macromolecular science viewpoint: Needs for plastics packaging circularity. ACS Macro Lett. 2020, 9, 1376–1390. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Blasing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides-methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef]
- Tessanan, W.; Chanthateyanonth, R.; Yamaguchi, M.; Phinyocheep, P. Improvement of mechanical and impact performance of poly(lactic acid) by renewable modified natural rubber. J. Clean. Prod. 2020, 276, 123800. [Google Scholar] [CrossRef]
- Goh, K.; Heising, J.K.; Yuan, Y.; Karahan, H.E.; Wei, L.; Zhai, S.; Koh, J.X.; Htin, N.M.; Zhang, F.; Wang, R.; et al. Sandwich-architectured poly(lactic acid)-graphene composite food packaging films. ACS Appl. Mater. Interfaces 2016, 8, 9994–10004. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Nam, D.H.; Taitt, B.J.; Choi, K.S. Copper-based catalytic anodes to produce 2,5-furandicarboxylic acid, a biomass-derived alternative to terephthalic acid. ACS Catal. 2018, 8, 1197–1206. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Su, S.; Yin, D. Advances in catalytic conversion of biomass carbohydrates into 2,5-furandicarboxylic acid. Petrochem. Technol. 2016, 45, 872–879. [Google Scholar]
- Fei, X.; Wang, J.; Zhang, X.; Jia, Z.; Jiang, Y.; Liu, X. Recent progress on bio-based polyesters derived from 2,5-furandicarbonxylic acid (FDCA). Polymers 2022, 14, 625. [Google Scholar] [CrossRef]
- Banella, M.B.; Bonucci, J.; Vannini, M.; Marchese, P.; Lorenzetti, C.; Celli, A. Insights into the synthesis of poly(ethylene 2,5-Furandicarboxylate) from 2,5-furandicarboxylic acid: Steps toward environmental and food safety excellence in packaging applications. Ind. Eng. Chem. Res. 2019, 58, 8955–8962. [Google Scholar] [CrossRef]
- Zhou, G.N.; Li, L.; Jiang, M.; Wang, G.; Wang, R.; Wu, G.; Zhou, G. Renewable poly(butene 2, 5-furan dicarboxylate) nanocomposites constructed by TiO2 nanocubes: Synthesis, crystallization, and properties. Polym. Degrad. Stab. 2021, 189, 109591. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Terzopoulou, Z.; Vlachopoulos, A.; Klonos, P.A.; Kyritsis, A.; Tzetzis, D.; Papageorgiou, G.Z.; Bikiaris, D. Synthesis and characterization of novel polymer/clay nanocomposites based on poly (butylene 2,5-furan dicarboxylate). Appl. Clay Sci. 2020, 190, 105588. [Google Scholar] [CrossRef]
- Fojtikova, J.; Kalvoda, L.; Sedlak, P. Molecular Dynamics Simulations of Poly(dimethylsiloxane) Properties. Acta Phys. Pol. A 2015, 128, 637–639. [Google Scholar] [CrossRef]
- Rastegar, S.; Montazeri, A. Atomistic insights into the toughening role of surface-treated boron nitride nanosheets in PLA-based nanocomposites. Eur. Polym. J. 2022, 168, 111071. [Google Scholar] [CrossRef]
- Song, J.F.; Zhao, G.; Ding, Q.J.; Yang, Y. Molecular dynamics study on the thermal, mechanical and tribological properties of PBI/PI composites. Mater. Today Commun. 2022, 30, 103077. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Nonelectrolytes, 3rd ed.; Reinhold: New York, NY, USA, 1950. [Google Scholar]
- Wei, Q.H.; Wang, Y.N.; Chai, W.H.; Wang, T.; Zhang, Y.F. Effects of composition ratio on the properties of poly(vinyl alcohol)/poly(acrylic acid) blend membrane: A molecular dynamics simulation study. Mater. Des. 2016, 89, 848–855. [Google Scholar] [CrossRef]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient; Danish Technical Press: Copenhagen, Denmark, 1967. [Google Scholar]
- Gupta, J.; Nunes, C.; Vyas, S.; Jonnalagadda, S. Prediction of solubility parameters and miscibility of pharmaceutical compounds by molecular dynamics simulations. J. Phys. Chem. B 2011, 115, 2014–2023. [Google Scholar] [CrossRef]
- Luo, Y.L.; Wang, R.G.; Wang, W.; Zhang, L.; Wu, S. Molecular dynamics simulation insight into two-component solubility parameters of graphene and thermodynamic compatibility of graphene and styrene butadiene rubber. J. Phys. Chem. C 2017, 121, 10163–10173. [Google Scholar] [CrossRef]
- Agrawal, A.; Saran, A.D.; Rath, S.S.; Khanna, A. Constrained nonlinear optimization for solubility parameters of poly(lactic acid) and poly(glycolic acid)—Validation and comparison. Polymer 2004, 45, 8603–8612. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Lu, Z.Y.; Li, Z.S. Phase separation in blmodal molecular weight high density polyethylene with differing branch contents by molecular dynamics and mesodyn simulation. Chin. J. Polym. Sci. 2009, 27, 493–500. [Google Scholar] [CrossRef]
- Abou-Rachid, H.; Lussier, L.-S.; Ringuette, S.; Lafleur-Lambert, X.; Jaidann, M.; Brisson, J. On the correlation between miscibility and solubility properties of energetic plasticizers/polymer blends: Modeling and simulation studies. Propellants Explos. Pyrotech. 2008, 33, 301–310. [Google Scholar] [CrossRef]
- Glova, A.D.; Falkovich, S.G.; Dmitrienko, D.I.; Lyulin, A.V.; Larin, S.V.; Nazarychev, V.M.; Karttunen, M.; Lyulin, S.V. Scale-dependent miscibility of polylactide and polyhydroxybutyrate: Molecular dynamics simulations. Macromolecules 2018, 51, 552–563. [Google Scholar] [CrossRef]
- Takhulee, A.; Takahashi, Y.; Vao-soongnern, V. Molecular simulation and experimental studies of the miscibility of polylactic acid/polyethylene glycol blends. J. Polym. Res. 2016, 24, 8. [Google Scholar] [CrossRef]
- Akten, E.D.; Mattice, W.L. Monte Carlo simulation of head-to-head, tail-to-tail polypropylene and its mixing with polyethylene in the melt. Macromolecules 2001, 34, 3389–3395. [Google Scholar] [CrossRef]
- Watt, J.P.; Davies, G.F.; Oconnell, R.J. Elastic properties of composite-materials. Rev. Geophys. 1976, 14, 541–563. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, R.; Huang, J.; Wang, J.; Jiang, Y.; Hu, G.-H.; Yang, J.; Zhu, J. Tensile property balanced and gas barrier improved poly(lactic acid) by blending with biobased poly(butylene 2,5-furan dicarboxylate). ACS Sustain. Chem. Eng. 2017, 5, 9244–9253. [Google Scholar] [CrossRef]
- Wells, B.A.; Chaffee, A.L. Ewald summation for molecular simulations. J. Chem. Theory Comput. 2015, 11, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.L.; Zhu, A.M.; Zhang, Q.G.; Wu, J.Y. Molecular simulation of CO2/CH4 permeabilities in polyamide-imide isomers. J. Membr. Sci. 2010, 348, 204–212. [Google Scholar] [CrossRef]
- Yang, J.Z.; Liu, Q.L.; Wang, H.T. Analyzing adsorption and diffusion behaviors of ethanol/water through silicalite membranes by molecular simulation. J. Membr. Sci. 2007, 291, 1–9. [Google Scholar] [CrossRef]
- Keil, F.J.; Krishna, R.; Coppens, M.O. Modeling of diffusion in zeolites. Rev. Chem. Eng. 2000, 16, 71–197. [Google Scholar] [CrossRef]




| PLA | PBF | |||
|---|---|---|---|---|
| Simulation | Exp. [10] | Simulation | Exp. [17] | |
| Density(g·cm−3) | 1.17 | 1.20 | 1.29 | 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jiang, G.; Shao, X.; Pu, S.; Jiang, D.; Lan, Y. Mechanical and Gas Barrier Properties of Poly(Lactic Acid) Modified by Blending with Poly(Butylene 2,5-Furandicarboxylate): Based on Molecular Dynamics. Polymers 2023, 15, 1657. https://doi.org/10.3390/polym15071657
Wang Y, Jiang G, Shao X, Pu S, Jiang D, Lan Y. Mechanical and Gas Barrier Properties of Poly(Lactic Acid) Modified by Blending with Poly(Butylene 2,5-Furandicarboxylate): Based on Molecular Dynamics. Polymers. 2023; 15(7):1657. https://doi.org/10.3390/polym15071657
Chicago/Turabian StyleWang, Ye, Gongliang Jiang, Xiancheng Shao, Shikun Pu, Dengbang Jiang, and Yaozhong Lan. 2023. "Mechanical and Gas Barrier Properties of Poly(Lactic Acid) Modified by Blending with Poly(Butylene 2,5-Furandicarboxylate): Based on Molecular Dynamics" Polymers 15, no. 7: 1657. https://doi.org/10.3390/polym15071657
APA StyleWang, Y., Jiang, G., Shao, X., Pu, S., Jiang, D., & Lan, Y. (2023). Mechanical and Gas Barrier Properties of Poly(Lactic Acid) Modified by Blending with Poly(Butylene 2,5-Furandicarboxylate): Based on Molecular Dynamics. Polymers, 15(7), 1657. https://doi.org/10.3390/polym15071657








