Study on the Difference of Superhydrophobic Characteristics of Different Wood Furniture Substrates
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
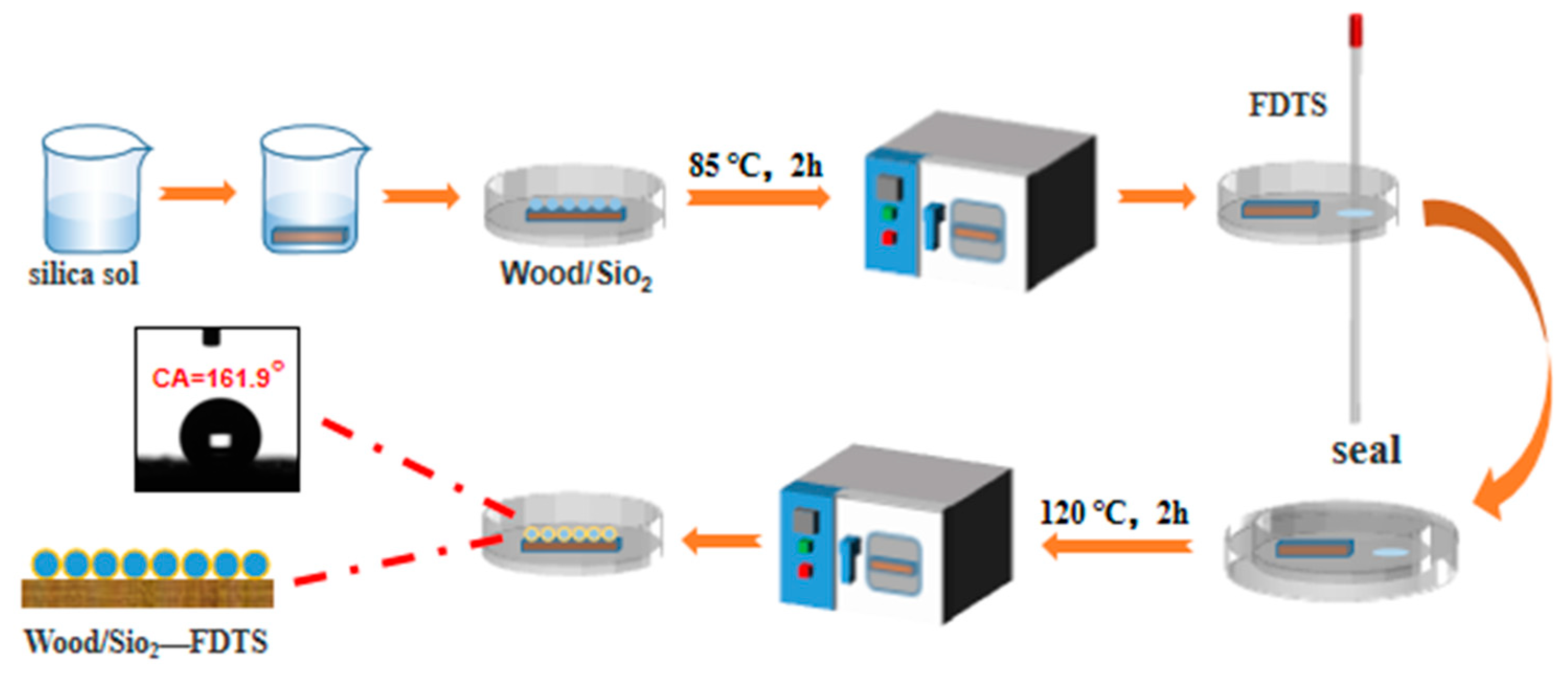
2.2. Preparation of Silica Micro–Nano Coating on Wood Surface
2.3. Modification of Silica Micro–Nano Coating on Wood Surface by FDTS
2.4. Wood Density, Porosity, and Surface Roughness Test
2.5. Surface Morphology and Wettability Analysis
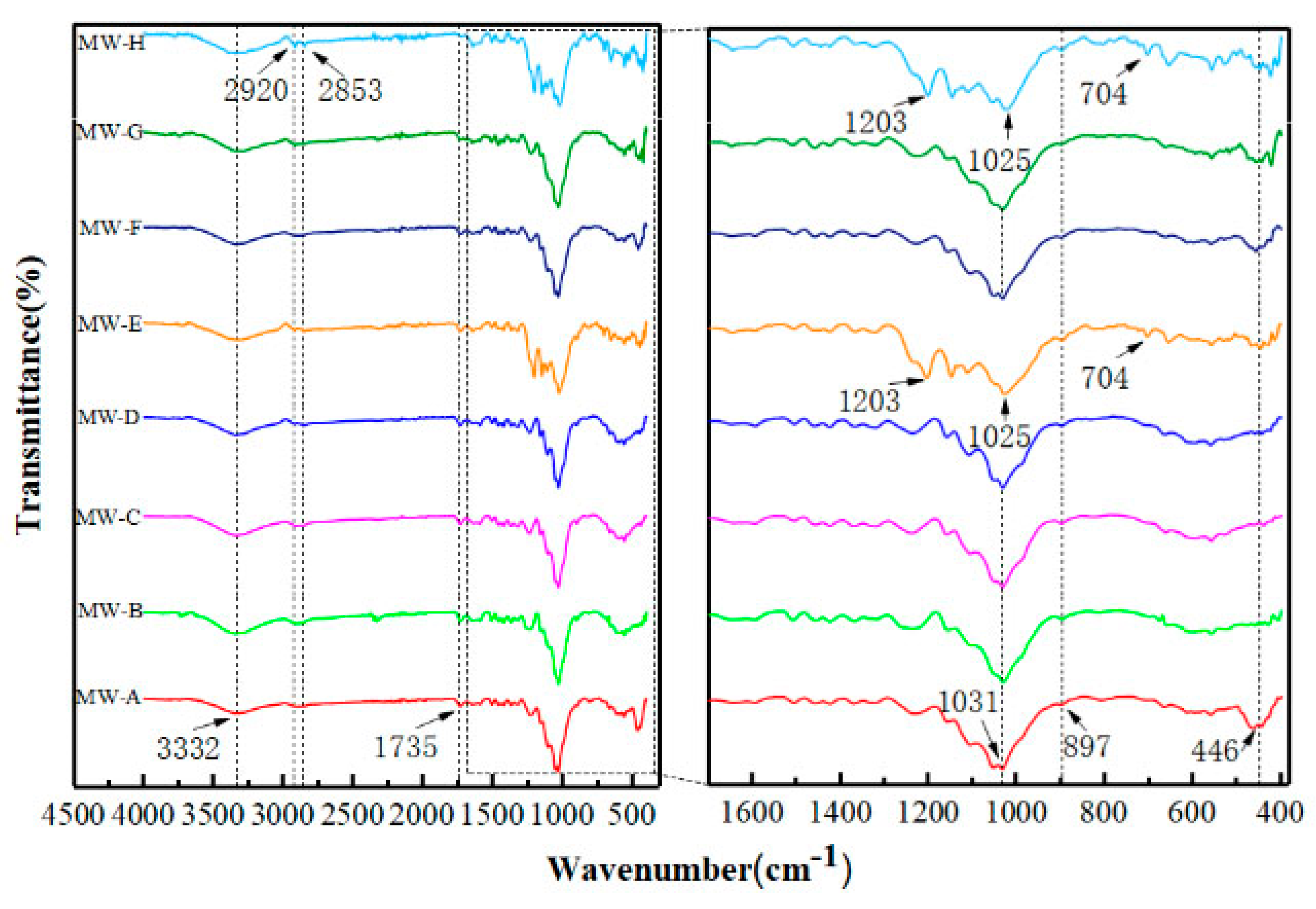
2.6. Surface Functional Group Test
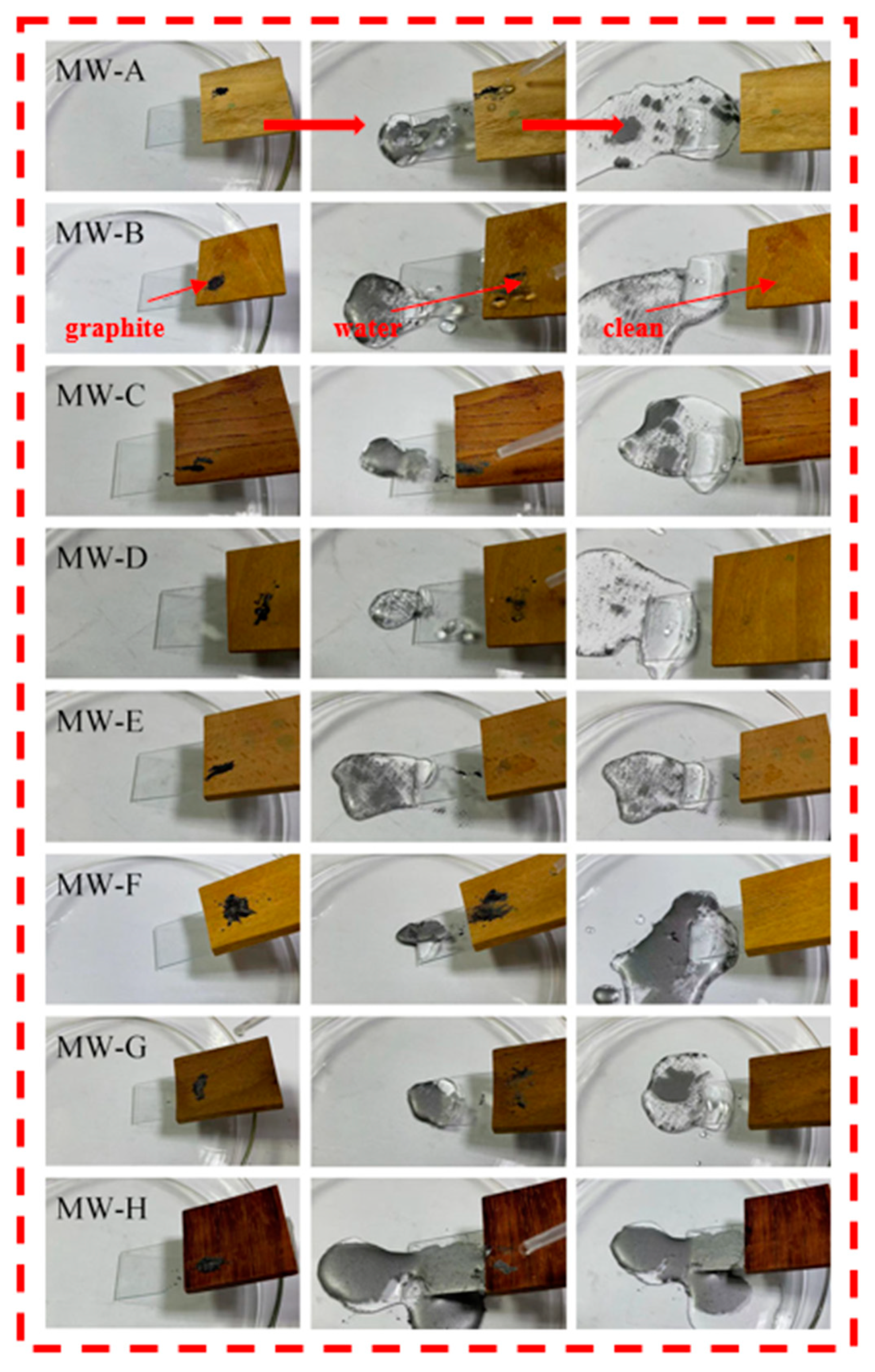
2.7. Pollution Resistance and Self-Cleaning Ability Test
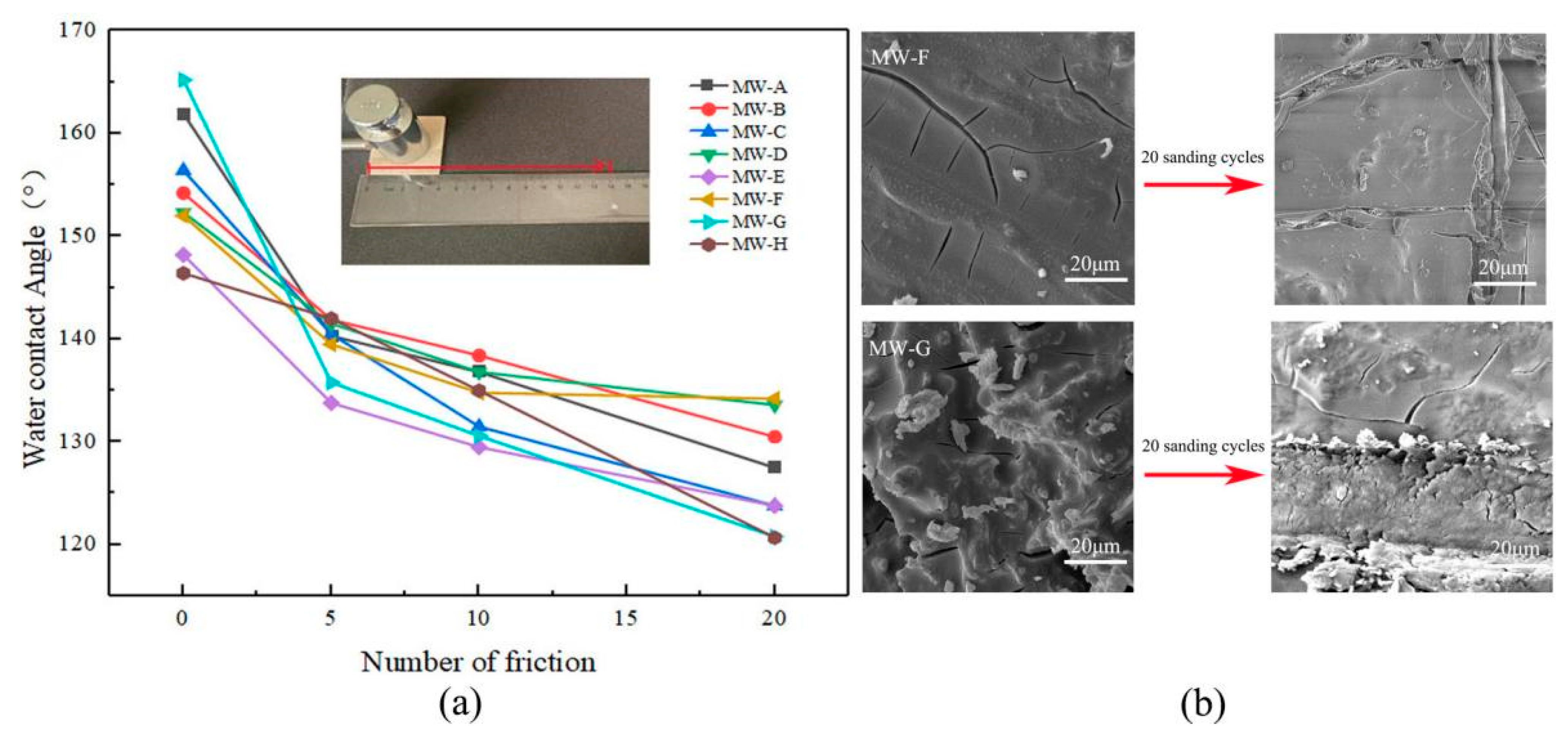
2.8. Mechanical Wear Resistance Test
2.9. Water Resistance and Acid-Base Resistance Test
3. Results and Discussion
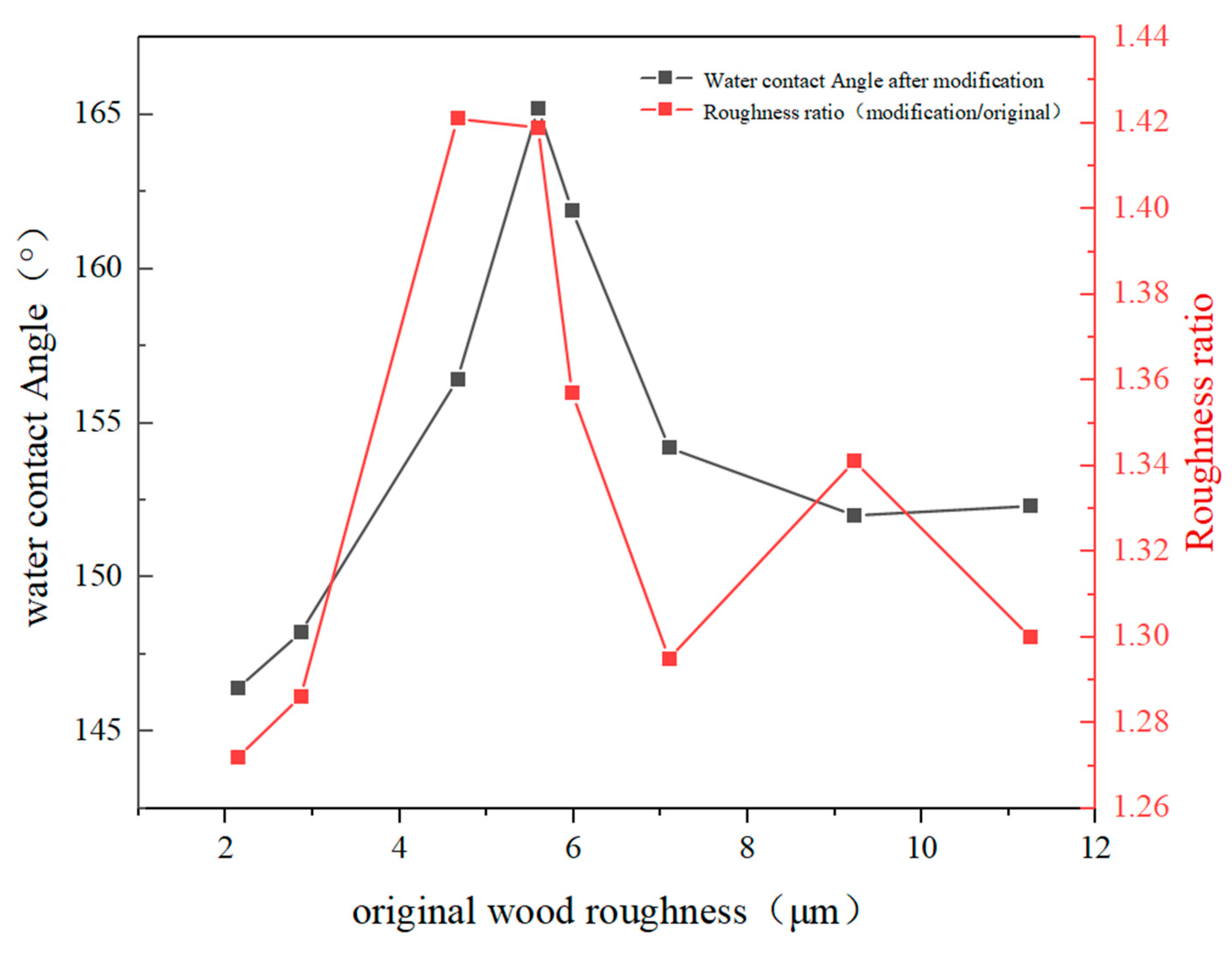
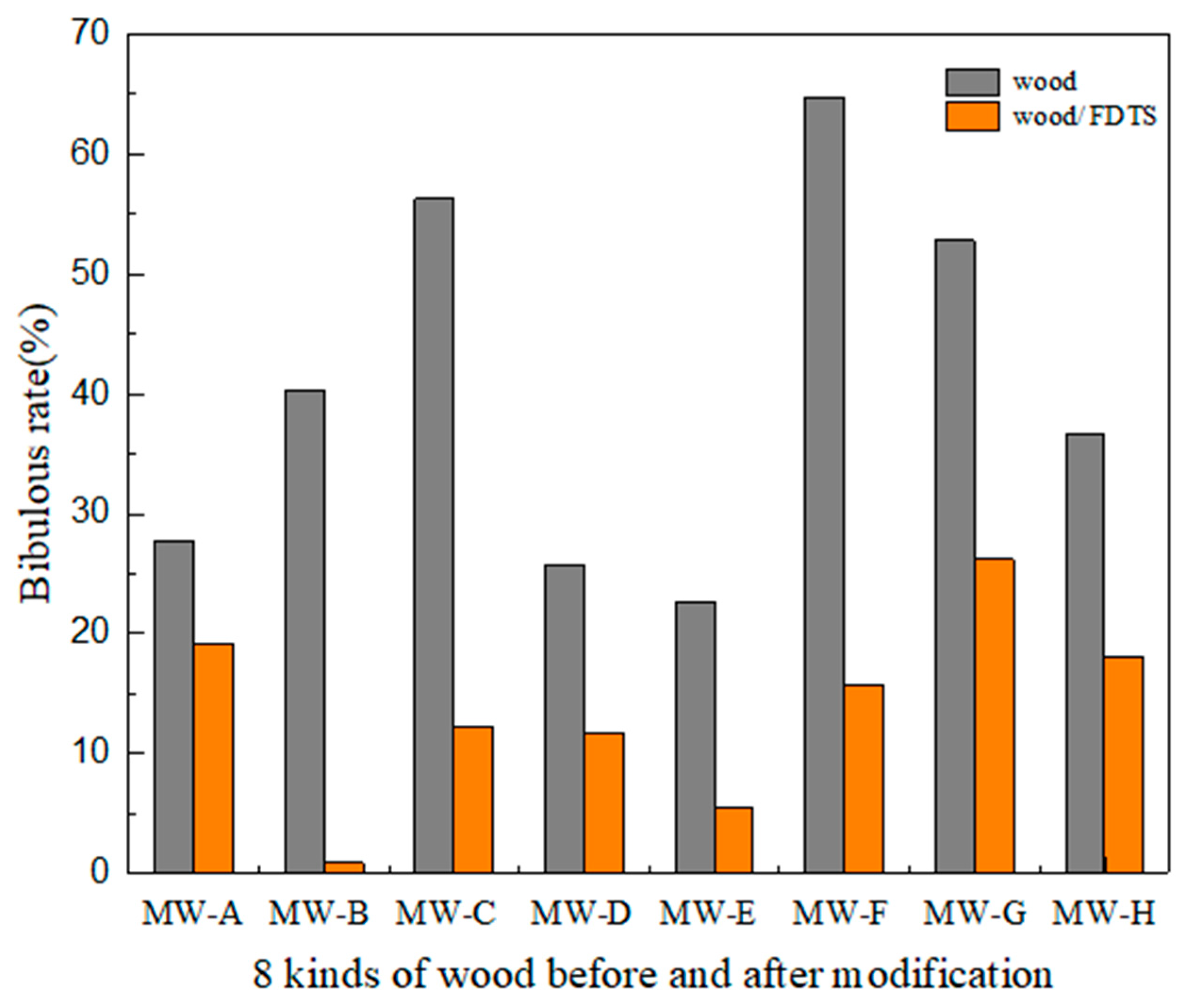
3.1. Analysis of Wood Density, Porosity, Roughness, and Wettability
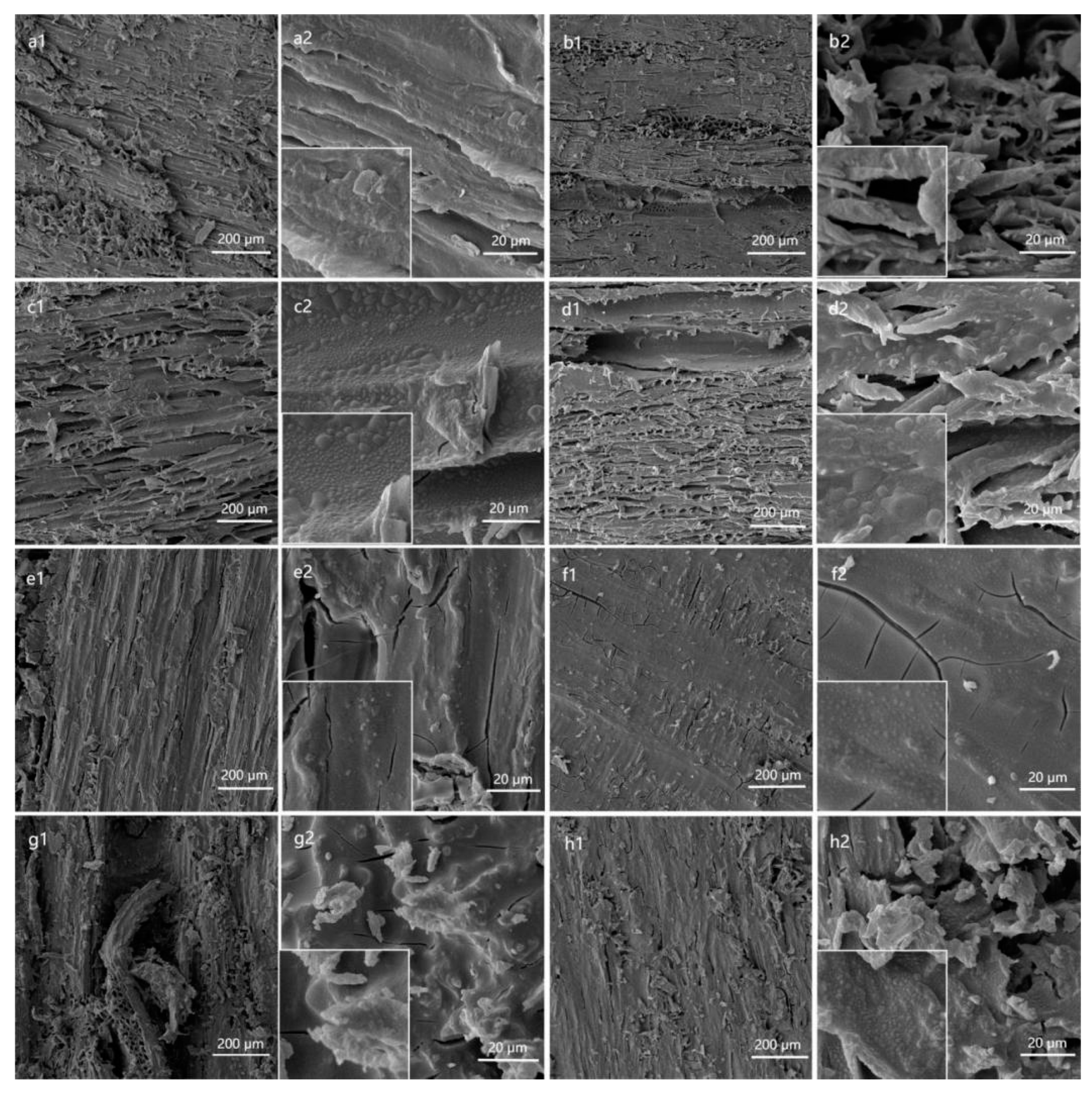
3.2. Surface Morphology and Wettability Analysis
3.3. Analysis of Reaction Mechanism
3.4. Analysis of Contaminant Resistance Test and Self-Cleaning Test
3.5. Mechanical Wear Resistance Analysis
3.6. Water Resistance Analysis
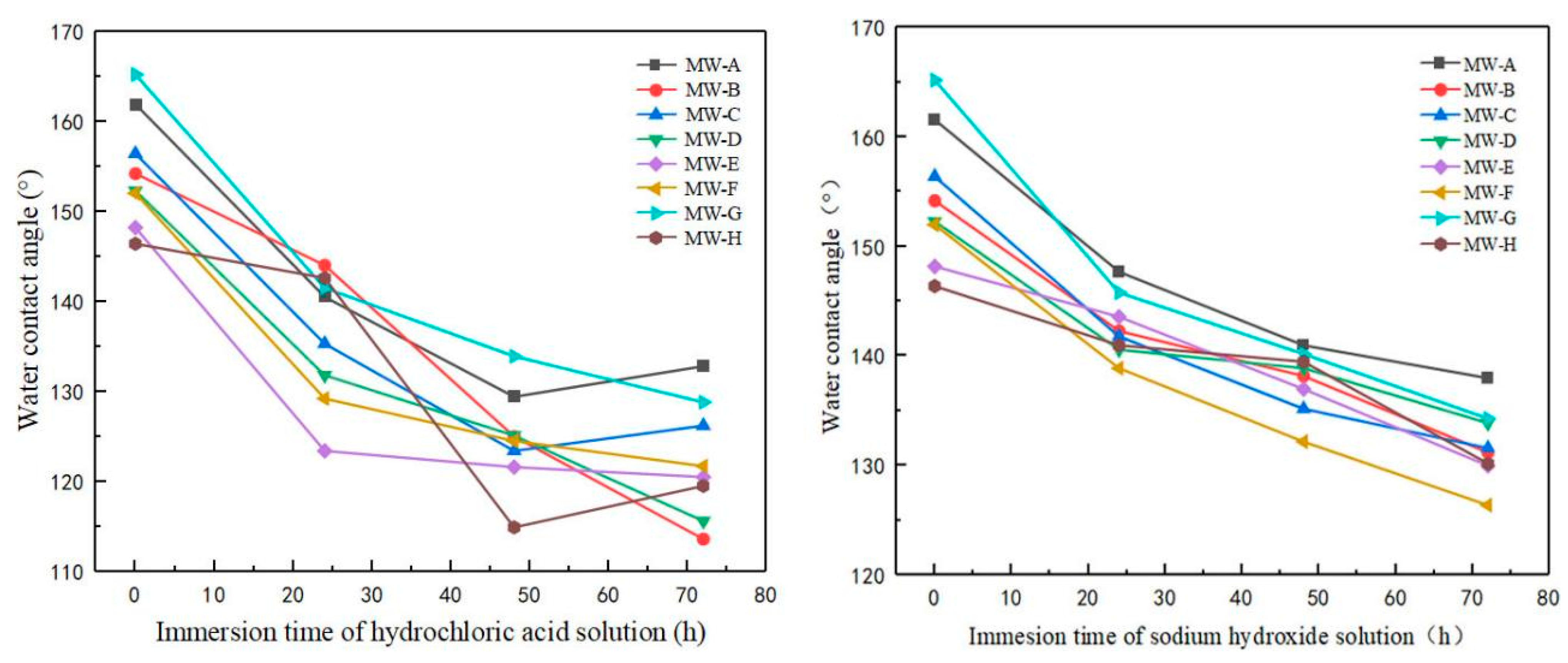
3.7. Acid and Alkali Resistance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spear, M.J.; Curling, S.F.; Dimitriou, A.; Ormondroyd, G.A. Review of Functional Treatments for Modified Wood. Coatings 2021, 11, 327. [Google Scholar] [CrossRef]
- Feng, X.H.; Chen, J.Y.; Yu, S.X.; Wu, Z.H.; Huang, Q.T. Mild Hydrothermal Modification of Beech Wood (Zelkova Schneideriana Hand-Mzt): Its Physical, Structural, and Mechanical Properties. Eur. J. Wood Wood Prod. 2022, 80, 933–945. [Google Scholar] [CrossRef]
- Deng, Y.B.; Mager, D.; Bai, Y.; Zhou, T.; Liu, Z.Y.; Wen, L.P.; Wu, Y.H.; Korvink, J.G. Inversely Designed Micro-Textures for Robust Cassie-Baxter Mode of Super-Hydrophobicity. Comput. Meth. Appl. Mech. Eng. 2018, 341, 113–132. [Google Scholar] [CrossRef]
- Xie, A.L.; Chen, X.P.; Ai, X.; Wang, Y.H.; Wang, Y.R.; Zhu, X.W.; Xing, T.L.; Chen, G.Q. Novel Fabrication of Robust Superhydrophobic Polyester Fabric with Eugenol Based on Thiol-Ene Click Chemistry for Self-Cleaning and Water-Oil Separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127947. [Google Scholar] [CrossRef]
- Zhang, W.G.; Jiang, S.N.; Lv, D.D. Fabrication and Characterization of a Pdms Modified Polyurethane/Al Composite Coating with Super-Hydrophobicity and Low Infrared Emissivity. Prog. Org. Coat. 2020, 143, 105622. [Google Scholar] [CrossRef]
- Chen, X.D.; Hu, L.A.; Du, Y.Z. Anti-Icing and Anti-Frost Properties of Structured Superhydrophobic Coatings Based on Aluminum Honeycombs. Mater. Chem. Phys. 2022, 291, 126683. [Google Scholar] [CrossRef]
- Zhang, M.M.; Zhao, W.J.; Zhang, X.; Li, Z.W.; Yu, L.G.; Li, X.H.; Zhang, Z.J. Nonfluoride-Modified Halloysite Nanotube-Based Hybrid: Potential for Acquiring Super-Hydrophobicity and Improving Flame Retardancy of Epoxy Resin. J. Nanostructure Chem. 2021, 11, 353–366. [Google Scholar] [CrossRef]
- Gong, L.; Zhu, H.X.; Wu, W.H.; Lin, D.H.; Yang, K. A Durable Superhydrophobic Porous Polymer Coated Sponge for Efficient Separation of Immiscible Oil/Water Mixtures and Oil-in-Water Emulsions. J. Hazard. Mater. 2022, 425, 127980. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Wu, M.; Duan, G.G.; Gong, X. One-Step Fabrication of Eco-Friendly Superhydrophobic Fabrics for High-Efficiency Oil/Water Separation and Oil Spill Cleanup. Nanoscale 2022, 14, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, X.Z.; Huang, T.; Zhang, N.; Qi, X.D.; Yang, J.H.; Zhou, Z.W.; Wang, Y. Electrospun Fibrous Membranes with Dual-Scaled Porous Structure: Super Hydrophobicity, Super Lipophilicity, Excellent Water Adhesion, and Anti-Icing for Highly Efficient Oil Adsorption/Separation. ACS Appl. Mater. Interfaces 2019, 11, 5073–5083. [Google Scholar] [CrossRef]
- Kundu, D.; Banerjee, D.; Ghosh, S.; Tewari, R.C.; Das, N.S.; Das, B.; Chattopadhyay, K.K. Achievement of Nearly Super Hydrophobicity in Plasma Enhanced Chemical Vapour Deposited One and Two Dimensional Carbon Nanostructures. Physica E 2019, 108, 7–14. [Google Scholar] [CrossRef]
- Liu, S.H.; Han, Y.M.; Qie, J.Q.; Chen, S.Q.; Liu, D.; Duo, L.; Chen, H.; Lin, Q.K. Environment Friendly Superhydrophobic and Transparent Surface Coating Via Layer-by-Layer Self-Assembly for Antifogging of Optical Lenses. J. Biomater. Sci. Polym. Ed. 2022, 33, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.W.; Dong, X.L.; Huang, J.Y.; Li, S.H.; Li, Y.W.; Chen, Z.; Lai, Y.K. Rational Construction of Highly Transparent Superhydrophobic Coatings Based on a Non-Particle, Fluorine-Free and Water-Rich System for Versatile Oil-Water Separation. Chem. Eng. J. 2018, 333, 621–629. [Google Scholar] [CrossRef]
- Hashjin, R.R.; Ranjbar, Z.; Yari, H.; Momen, G. Tuning Up Sol-Gel Process to Achieve Highly Durable Superhydrophobic Coating. Surf. Interfaces 2022, 33, 102282. [Google Scholar] [CrossRef]
- Xing, T.; Dong, C.Q.; Wang, X.D.; Hu, X.Y.; Liu, C.R.; Lv, H.Y. Biodegradable, Superhydrophobic Walnut Wood Membrane for the Separation of Oil/Water Mixtures. Front. Chem. Sci. Eng. 2022, 16, 1377–1386. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Lu, Y.Q.; Yu, H.; Jiang, G.J.; Zhao, X.T.; Gao, C.J.; Xue, L.X. Super-Hydrophobic F-Tio2@Pp Membranes with Nano-Scale Coral-Like Synapses for Waste Oil Recovery. Sep. Purif. Technol. 2021, 267, 118579. [Google Scholar] [CrossRef]
- Song, L.J.; Hu, J.; Huang, X.F.; Zhong, L.; Pei, Y.B.; Wu, L.B.; Zhang, X.Y. Superhydrophobic Self-Healing Coatings Comprised of Hemispherical Particles Arrays Decorated by Fluorocarbon-Coated Nanoscale Fe2O3 Rods and SiO2 Particles. ACS Appl. Nano Mater. 2020, 3, 10342–10348. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Q.; Xu, S.S.; Qu, L.; Shi, Z.Q. Robust Superhydrophobic Wood Surfaces with Mechanical Durability. Colloid Surf. A Physicochem. Eng. Asp. 2021, 608, 125624. [Google Scholar] [CrossRef]
- Jia, S.S.; Chen, H.B.; Luo, S.; Qing, Y.; Deng, S.L.; Yan, N.; Wu, Y.Q. One-Step Approach to Prepare Superhydrophobic Wood with Enhanced Mechanical and Chemical Durability: Driving of Alkali. Appl. Surf. Sci. 2018, 455, 115–122. [Google Scholar] [CrossRef]
- Cao, M.T.; Tang, M.W.; Lin, W.S.; Ding, Z.H.; Cai, S.; Chen, H.X.; Zhan, X.X. Facile Fabrication of Fluorine-Free, Anti-Icing, and Multifunctional Superhydrophobic Surface on Wood Substrates. Polymers 2022, 14, 1953. [Google Scholar] [CrossRef]
- Wu, X.Y.; Yang, F.; Gan, J.; Kong, Z.Q.; Wu, Y. A Superhydrophobic, Antibacterial, and Durable Surface of Poplar Wood. Nanomaterials 2021, 11, 1885. [Google Scholar] [CrossRef]
- Yan, X.X.; Peng, W.W.; Qian, X.Y. Effect of Water-Based Acrylic Acid Microcapsules on the Properties of Paint Film for Furniture Surface. Appl. Sci. 2021, 11, 7586. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Niu, Y.T.; Zhou, Z.R.; Ren, J. Development and Application of a New Flame-Retardant Adhesive. Polymers 2020, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Samyn, P.; Stanssens, D.; Paredes, A.; Becker, G. Performance of Organic Nanoparticle Coatings for Hydrophobization of Hardwood Surfaces. J. Coat. Technol. Res. 2014, 11, 461–471. [Google Scholar] [CrossRef]
- Gurau, L. Testing the Processing-Induced Roughness of Sanded Wood Surfaces Separated from Wood Anatomical Structure. Forests 2022, 13, 331. [Google Scholar] [CrossRef]
- Zhang, D.W.; Huang, Y. Influence of Surface Roughness and Bondline Thickness on the Bonding Performance of Epoxy Adhesive Joints on Mild Steel Substrates. Prog. Org. Coat. 2021, 153, 106135. [Google Scholar] [CrossRef]
- Soyturk, E.E.; Kartal, S.N.; Onses, M.S.; Celik, N. Preliminary Evaluation of Polydimethylsiloxane and Hydrophobic Silica Nanoparticles to Improve Water Repellency and Boron Leachability in Wood. Eur. J. Wood Wood Prod. 2022, 81, 89–98. [Google Scholar] [CrossRef]
- Wang, S.L.; Liu, C.Y.; Liu, G.C.; Zhang, M.; Li, J.; Wang, C.Y. Fabrication of Superhydrophobic Wood Surface by a Sol-Gel Process. Appl. Surf. Sci. 2011, 258, 806–810. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Wejrzanowski, T.; Przybyszewski, B.; Kozera, R.; Garcia-Casas, X.; Barranco, A. Role of Surface Topography in the Superhydrophobic Effect-Experimental and Numerical Studies. Materials 2022, 15, 3112. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chen, W.Y.; Wu, F.L.; Hung, W.M. Superhydrophobicity of a Three-Tier Roughened Texture of Microscale Carbon Fabrics Decorated with Silica Spheres and Carbon Nanotubes. Diam. Relat. Mat. 2010, 19, 26–30. [Google Scholar] [CrossRef]
- Vinogradova, E.; Estrada, M.; Moreno, A. Colloidal Aggregation Phenomena: Spatial Structuring of Teos-Derived Silica Aerogels. J. Colloid Interface Sci. 2006, 298, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhang, M.; Xu, Y.; Wang, S.L.; Liu, F.; Ma, M.L.; Zang, D.L.; Gao, Z.X. One-Step Synthesis of Unique Silica Particles for the Fabrication of Bionic and Stably Superhydrophobic Coatings on Wood Surface. Adv. Powder Technol. 2014, 25, 530–535. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.F.; Jia, X.H.; Chen, B.B.; Li, Y.; Wang, S.Z.; Shao, D.; Feng, L.; Song, H.J. A Mechanically Robust Slippery Coating for Anti-Corrosion, Photothermal Deicing, and Anti-Sticking Applications. Surf. Coat. Technol. 2022, 438, 128395. [Google Scholar] [CrossRef]
- Song, P.; Wang, C.; Sun, Y.; Bousquet, A.; Tomasella, E. Broadband and Wide-Temperature-Range Thermal Emitter with Super-Hydrophobicity Based on Oxidized High-Entropy Film. ACS Appl. Mater. Interfaces 2020, 12, 4123–4128. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Proverbio, E. Anticorrosion Superhydrophobic Surfaces on Aa6082 Aluminum Alloy by Hf/Hcl Texturing and Self-Assembling of Silane Monolayer. Materials 2022, 15, 8549. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Zhou, H.; Liu, S.; Shao, H.; Fu, S.D.; Rutledge, G.C.; Lin, T. Durable, Self-Healing, Superhydrophobic Fabrics from Fluorine-Free, Waterborne, Polydopamine/Alkyl Silane Coatings. Rsc Adv. 2017, 7, 33986–33993. [Google Scholar] [CrossRef]
- Huo, H.F.; Song, F.F.; Yang, Y.; Zhang, L.; Zhang, X.; Zhang, J.J.; Yue, K.; Zhang, Z.F. Preparation of Environmentally Friendly Glueless Boxwood Timber by Acidic Environmental Treatment and High-Temperature Pressing. Polymers 2023, 15, 11. [Google Scholar] [CrossRef] [PubMed]



| Wood | Poplar Wood | Elm Wood | Chinese Toon Wood | Paulownia Wood | Beech Wood | Ashtree | Black Walnut Wood | Rosewood |
|---|---|---|---|---|---|---|---|---|
| Original wood | OW-A | OW-B | OW-C | OW-D | OW-E | OW-F | OW-G | OW-H |
| Modified wood | MW-A | MW-B | MW-C | MW-D | MW-E | MW-F | MW-G | MW-H |
| Sample | Density (g/cm−3) | Porosity (%) | Roughness (um) |
|---|---|---|---|
| OW-A | 0.386 | 29.81 | 5.978 |
| OW-B | 0.680 | 13.66 | 7.093 |
| OW-C | 0.540 | 27.36 | 4.660 |
| OW-D | 0.312 | 42.36 | 11.244 |
| OW-E | 0.693 | 14.54 | 2.856 |
| OW-F | 0.794 | 30.27 | 9.222 |
| OW-G | 0.730 | 23.33 | 5.586 |
| OW-H | 0.722 | 22.23 | 2.141 |
| Original Wood | Modified Wood | ||||
|---|---|---|---|---|---|
| Serial Number | Average Water Contact ANGLE | Standard Deviation | Serial Number | Average Water Contact ANGLE | Standard Deviation |
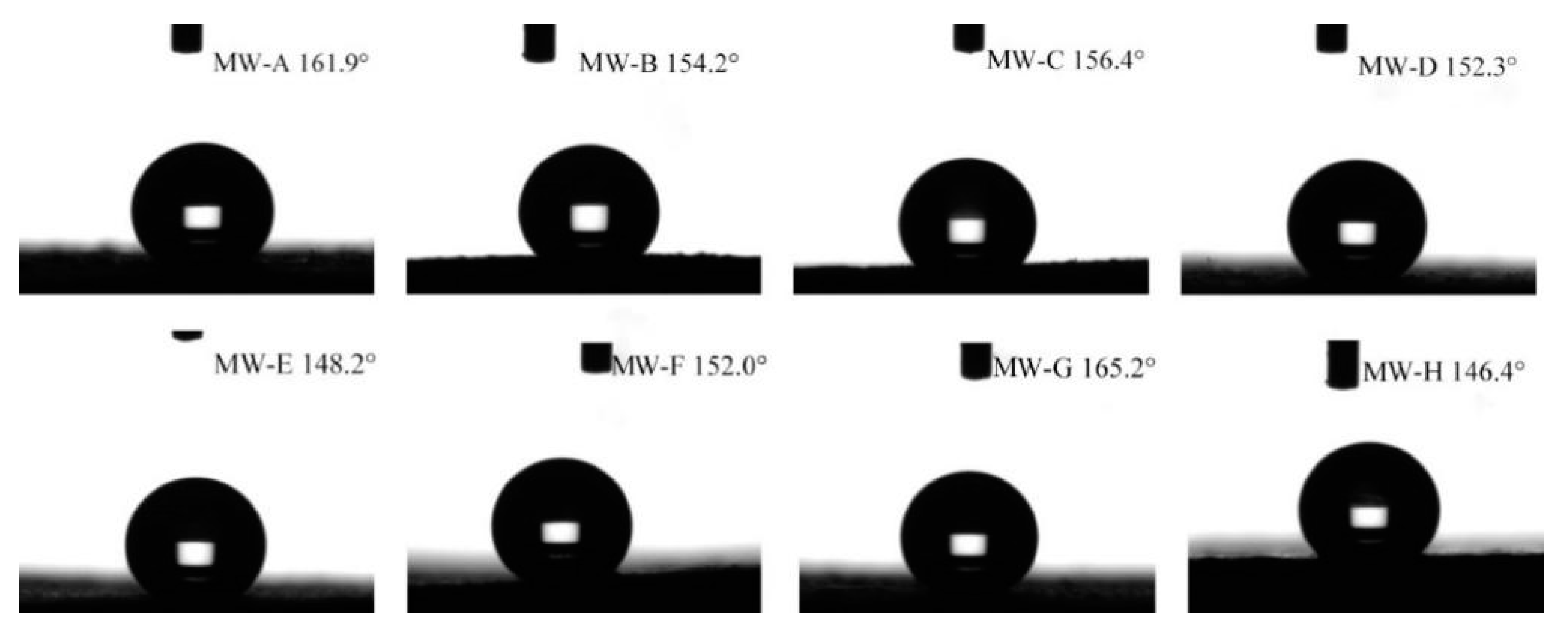
| OW-A | 3.2° | 1.08 | MW-A | 161.9° | 3.08 |
| OW-B | 12.6° | 2.36 | MW-B | 154.2° | 1.15 |
| OW-C | 15.4° | 2.21 | MW-C | 156.4° | 3.91 |
| OW-D | 7.5° | 1.68 | MW-D | 152.3° | 3.79 |
| OW-E | 10.6° | 2.58 | MW-E | 148.2° | 4.33 |
| OW-F | 2.1° | 3.14 | MW-F | 152.0° | 5.34 |
| OW-G | 4.5° | 2.16 | MW-G | 165.2° | 2.34 |
| OW-H | 5.2° | 1.45 | MW-H | 146.4° | 3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Kong, Z.; Yang, F.; Wu, X.; Wu, Y. Study on the Difference of Superhydrophobic Characteristics of Different Wood Furniture Substrates. Polymers 2023, 15, 1644. https://doi.org/10.3390/polym15071644
Yao X, Kong Z, Yang F, Wu X, Wu Y. Study on the Difference of Superhydrophobic Characteristics of Different Wood Furniture Substrates. Polymers. 2023; 15(7):1644. https://doi.org/10.3390/polym15071644
Chicago/Turabian StyleYao, Xingzhou, Zhangqian Kong, Feng Yang, Xinyu Wu, and Yan Wu. 2023. "Study on the Difference of Superhydrophobic Characteristics of Different Wood Furniture Substrates" Polymers 15, no. 7: 1644. https://doi.org/10.3390/polym15071644
APA StyleYao, X., Kong, Z., Yang, F., Wu, X., & Wu, Y. (2023). Study on the Difference of Superhydrophobic Characteristics of Different Wood Furniture Substrates. Polymers, 15(7), 1644. https://doi.org/10.3390/polym15071644






