2-Hydroxyethyl Methacrylate/Gelatin/Alginate Scaffolds Reinforced with Nano TiO2 as a Promising Curcumin Release Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
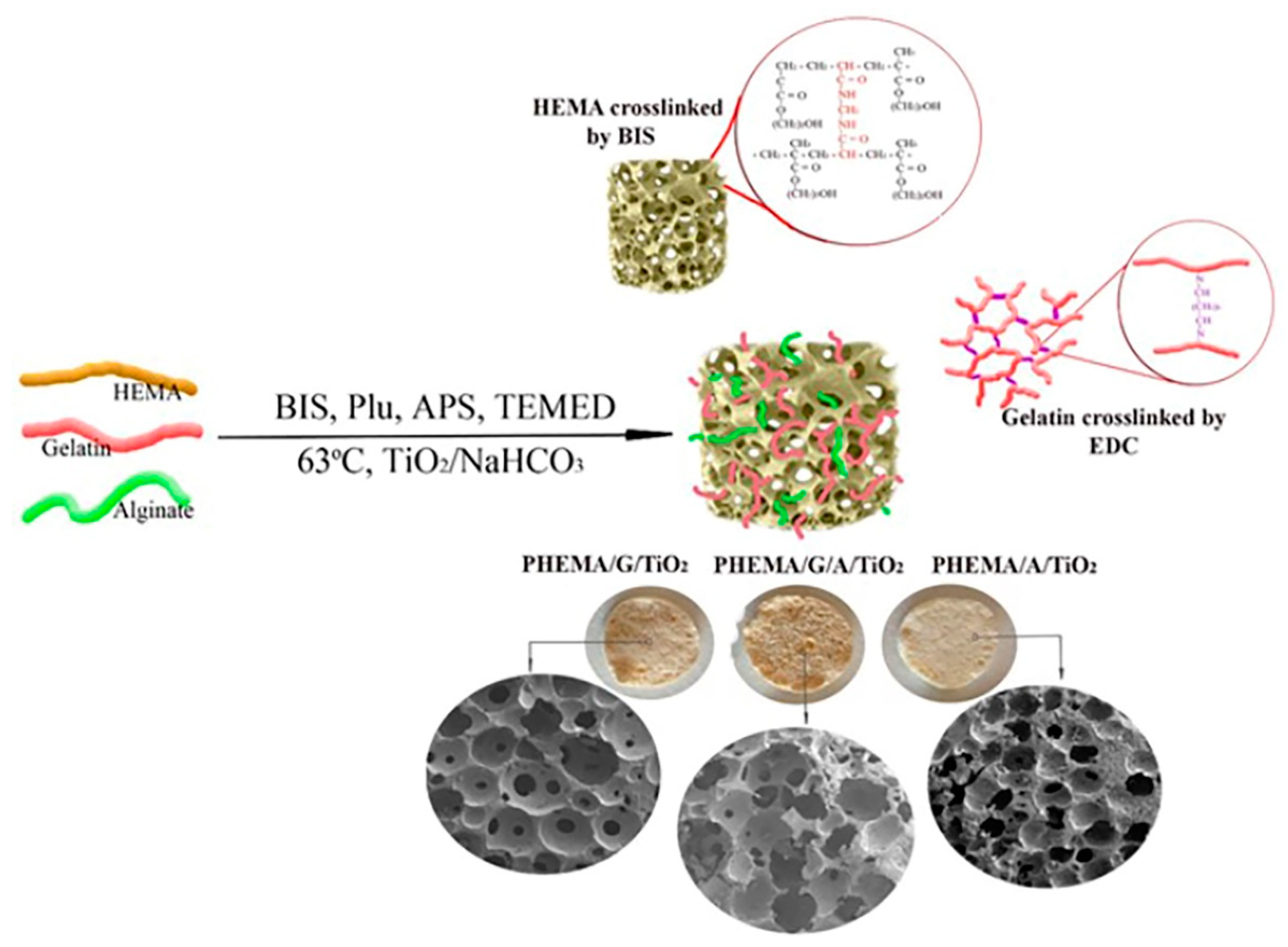
2.2. Hybrid Scaffolds Synthesis
2.3. Hybrid Scaffolds Characterization
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Porosity Measurements
2.3.4. Mechanical Properties Testing
2.3.5. In Vitro Swelling Study
2.3.6. Water Contact Angle Measurements
2.3.7. In Vitro Degradation Study
2.4. In Vitro Cytotoxicity Assay
2.5. In Vitro Controlled Curcumin Release Study
2.5.1. Drug Loading and Entrapment Efficiency of Hybrid Scaffolds
2.5.2. In vitro Curcumin Release Study
2.5.3. Analysis of Curcumin Transport Mechanism
2.6. Statistical Analyses
3. Results and Discussion
3.1. Structural Characteristics of Hybrid Scaffolds
3.2. Morphology of Hybrid Scaffolds
3.3. Porosity of Hybrid Scaffolds
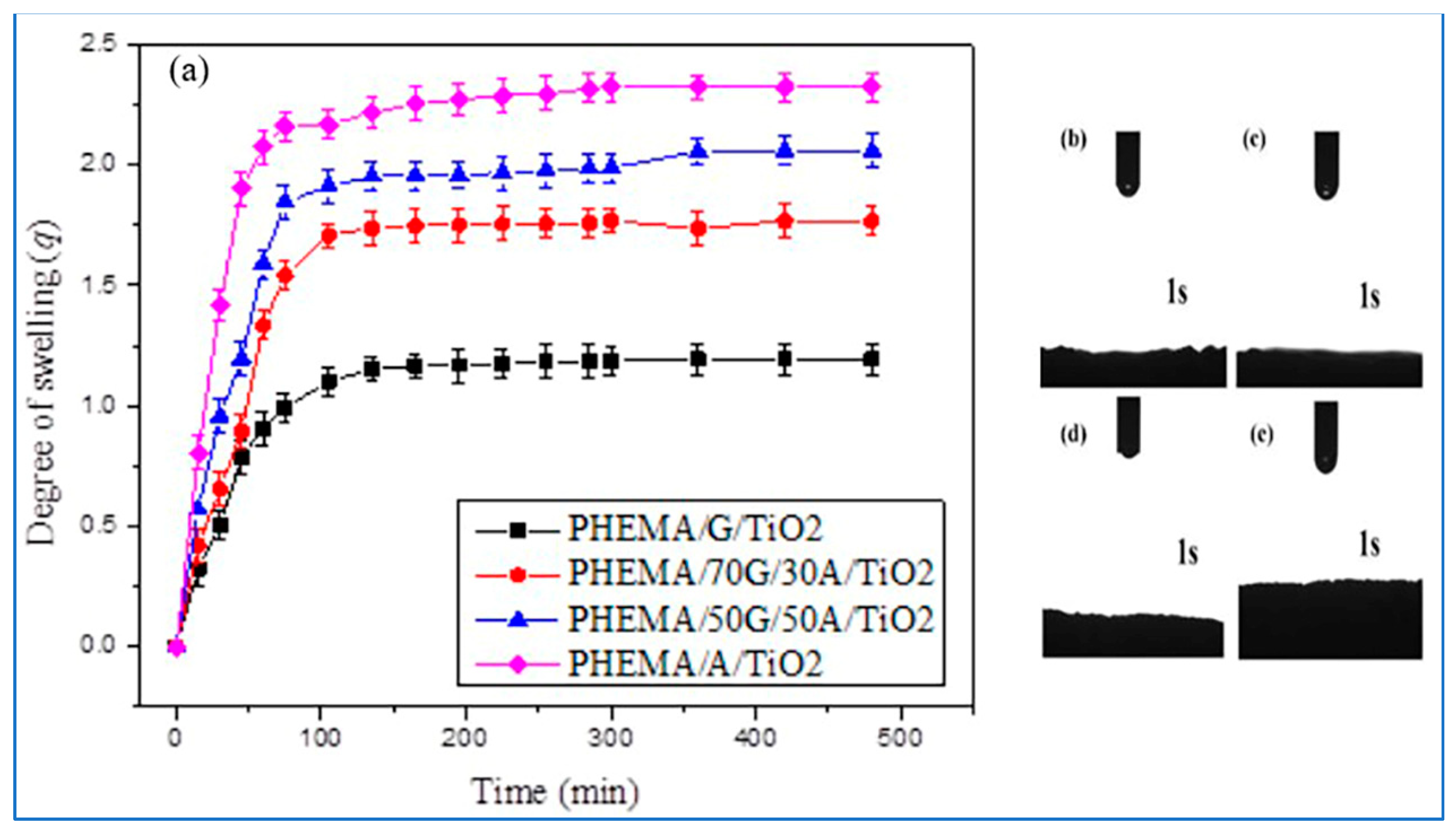
3.4. Swelling Properties of Hybrid Scaffolds
3.5. Hydrophilicity of Hybrid Scaffolds
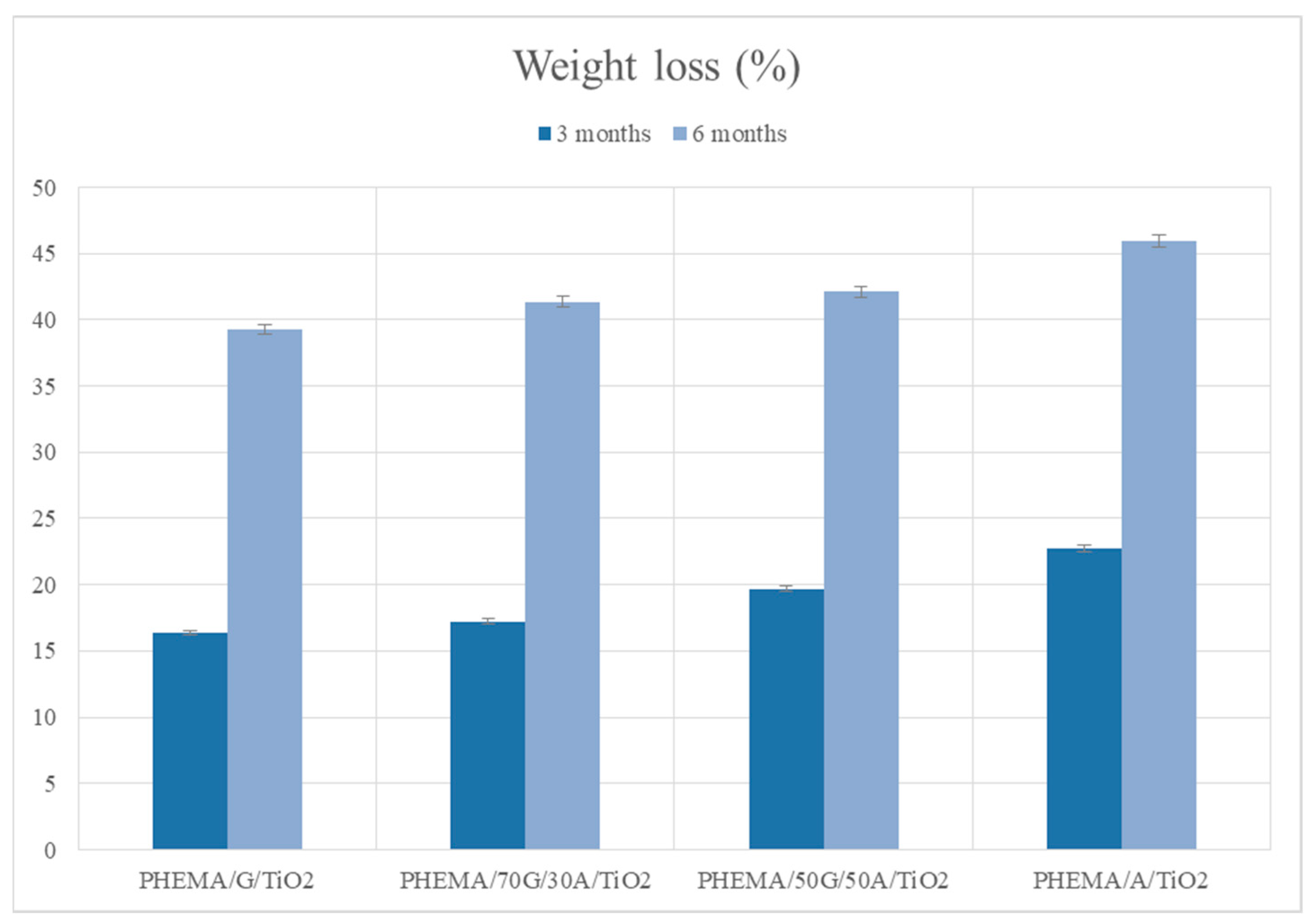
3.6. In Vitro Degradation Behavior of Hybrid Scaffolds
3.7. Mechanical Properties of Hybrid Scaffolds
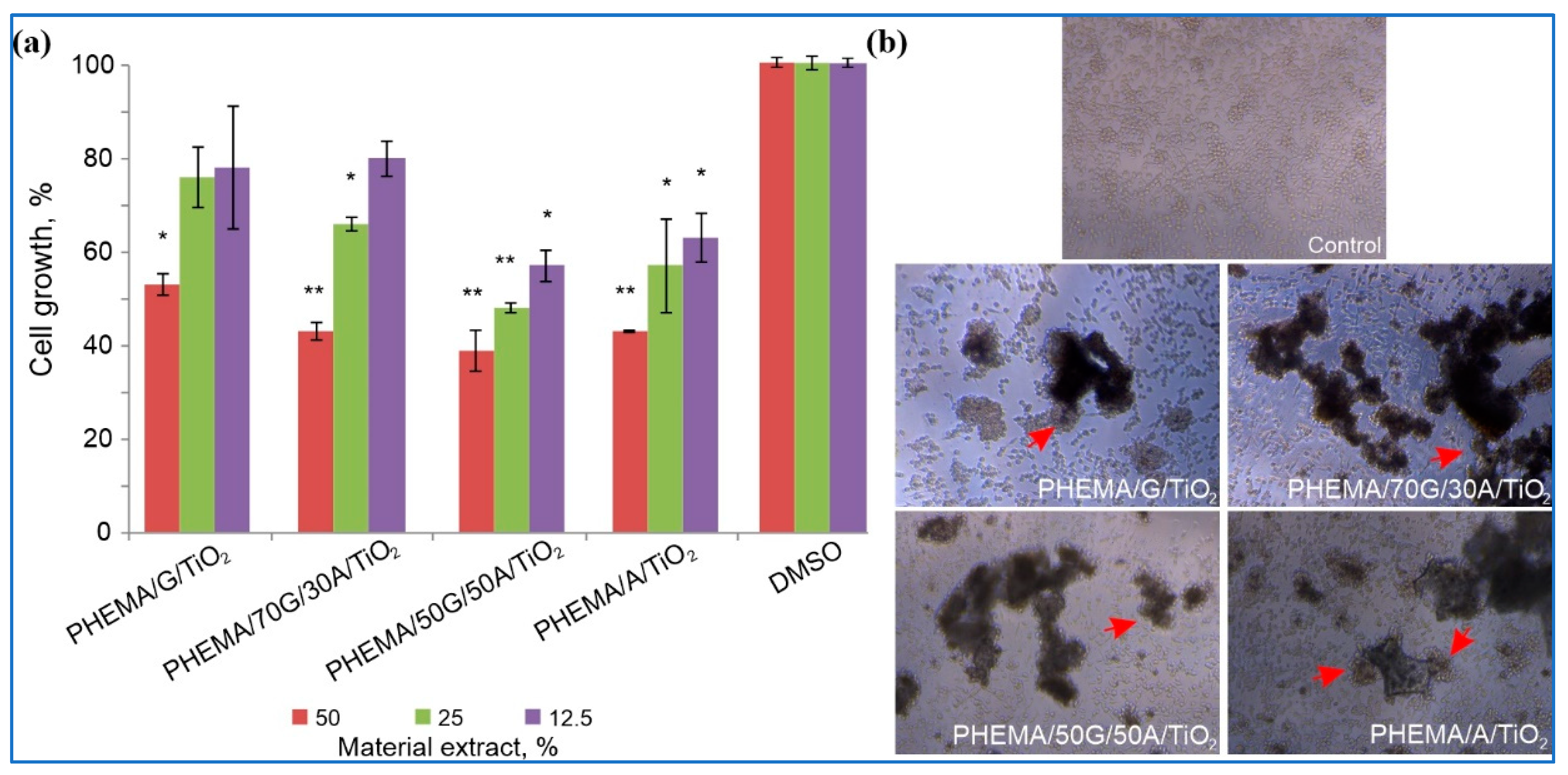
3.8. In Vitro Cytotoxicity of Hybrid Scaffolds
3.9. Drug Loading and Entrapment Efficiency for Curcumin
3.10. In Vitro Curcumin Release Study
Analysis of the Curcumin Transport Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremiao, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Kasinski, A.; Zielinska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels–synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Divyashri, G.; Badhe, R.V.; Sadanandan, B.; Vijayalakshmi, V.; Kumari, M.; Ashrit, P.; Bijukumar, D.; Mathew, M.T.; Shetty, K.; Raghu, A.V. Applications of hydrogel-based delivery systems in wound care and treatment: An up-to-date review. Polym. Adv. Technol. 2022, 33, 2025–2043. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Soleimani Rad, J. A review on the construction of hydrogel scaffolds by various chemically techniques for tissue engineering. Eur. Polym. J. 2019, 117, 64–76. [Google Scholar] [CrossRef]
- Tiomnova, O.T.; Pellizaro, T.A.G.; Rodriguez Chanfrau, J.E.; de Oliveira Capote, T.S.; Basmaji, P.; Veranes Pantoja, Y.; Guastaldi, A.C. Preparation of scaffolds of amorphous calcium phosphate and bacterial cellulose for use in tissue regeneration by freeze-drying process. Biointerface Res. Appl. Chem. 2020, 11, 7357–7367. [Google Scholar]
- Francisco, E.M.; Zoccolotti, J.D.; Tiomnov, O.T.; Tolaba, A.G.; Chanfrau, J.E.R.; Jorge, J.H.; Basmaji, P.; Guastaldi, A.C. Sterilization of scaffolds of calcium phosphates and bacterial cellulose for their use in tissue regeneration. Biointerface Res. Appl. Chem. 2020, 11, 10089–10098. [Google Scholar]
- Simpson, L.W.; Good, T.A.; Leach, J.B. Protein folding and assembly in confined environments: Implications for protein aggregation in hydrogels and tissues. Biotechnol. Adv. 2020, 42, 107573. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-responsive hydrogels: Intelligent materials for biosensing and drug delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, Y.; Liu, P.; Luo, D. Bioresponsive DNA hydrogels: Beyond the conventional Stimuli responsiveness. Acc. Chem. Res. 2017, 50, 733–739. [Google Scholar] [CrossRef]
- Fanfan, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, 8580. [Google Scholar]
- Kaya, G.; Oytun, F. Rheological properties of injectable hyaluronic acid hydrogels for soft tissue engineering applications. Biointerface Res. Appl. Chem. 2020, 11, 8424–8430. [Google Scholar]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar]
- Divband, B.; Samiei, M.; Davaran, S.; Roshangar, L.; Shahi, S.; Aghazadeh, M. Synthesis and in vitro evaluation of thermosensitive PLA-g-P(HEM-co-NIPAAM) hydrogel used for delivery of VEGF. Biointerface Res. Appl. Chem. 2020, 11, 8043–8051. [Google Scholar]
- Cao, Y.; Shen, X.; Chen, Y.; Guo, J.; Chen, Q.; Jiang, X. pH-Induced self-assembly and capsules of sodium alginate. Biomacromolecules 2005, 6, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Hulmes, D.J. Building collagen molecules, fibril, and suprafibrillar structures. J. Struct. Biol. 2002, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Xiong, Z.; Liu, H.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Direct construction of a three-dimensional structure with cells and hydrogel. J. Bioact. Compat. Polym. 2005, 20, 259–269. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Barnard, Z.Z.; Keen, I.; Hill, D.J.T.; Chirila, T.V.; Harkin, D.G. PHEMA hydrogels modified through the grafting of phosphate groups by ATRP support the attachment and growth of human corneal epithelial cells. J. Biomater. Appl. 2008, 23, 147–168. [Google Scholar]
- González-Henriquez, C.M.; Pizarro, G.d.C.; Sarabia-Vallejos, M.A.; Terraza, C.A.; López-Cabaña, Z.E. In situ-preparation and characterization of silver-HEMA/PEGDA hydrogel matrix nanocomposites: Silver inclusion studies into hydrogel matrix. Arabian J. Chem. 2019, 12, 1413–1423. [Google Scholar] [CrossRef]
- Praveen Suzuki, S.; Carson, C.F.; Saunders, M.; Clode, P.L.; Myers, M.; Chirila, T.V.; Baker, M.V. Poly(2-hydroxyethyl methacrylate) sponges doped with Ag nanoparticles as antibacterial agents. ACS Appl. Nano Mater. 2020, 3, 1630–1639. [Google Scholar] [CrossRef]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. pHEMA: An overview for biomedical applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Hemraj, M.Y.; Sachin, V.O.; Valmiki, B.K.; Sawanta, S.M.; Chang, K.H.; Shivaji, H.P.; Sagar, D.D. Preparation and characterisation of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A Chem. 2014, 280, 32–38. [Google Scholar]
- Wu, S.; Weng, Z.; Liu, X.; Young, K.W.K.; Chu, P.K. Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Kang, W.; Cui, Y.; Qin, L.; Yang, Y.; Zhao, Z.; Wang, X.; Liu, X. A novel robust adsorbent for efficient oil/water separation: Magnetic carbon nanospheres/graphene composite aerogel. J. Hazard. Mater. 2020, 392, 122499. [Google Scholar] [CrossRef]
- Bell, C.L.; Peppas, N.A. Measurement of swelling force in ionic polymer networks. III. Swelling force of interpolymer complexes. J. Control. Release 1995, 37, 77–280. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymer. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Babić, M.M.; Antić, K.M.; Vuković, J.S.; Božić, B.Đ.; Davidović, S.Z.; Filipović, J.M.; Tomić, S.Lj. Oxaprozin/poly(2-hydroxyethyl acrylate/itaconic acid hydrogels: Morphological, thermal, swelling, drug release and antibacterial properties. J. Mater. Sci. 2015, 50, 906–922. [Google Scholar] [CrossRef]
- Jeong, C.G.; Hollister, S.J. Mechanical, permeability, and degradation properties of 3D designed poly(1,8 octane diol-co-citrate) scaffolds for soft tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 141–149. [Google Scholar]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Babić, M.M.; Vukomanović, M.; Stefanič, M.; Nikodinović Runić, J.; Tomić, S.Lj. Controlled Curcumin release from hydrogel scaffold platform based on 2-hydroxyethyl methacrylate/gelatin/alginate/iron(III) oxide. Macromol. Chem. Phys. 2020, 221, 2000186–2000198. [Google Scholar] [CrossRef]
- Babić Radić, M.M.; Filipović, V.V.; Vukomanović, M.; Nikodinović-Runić, J.; Tomić, S.Lj. Degradable 2-hydroxyethyl methacrylate/gelatin/alginate hydrogels infused by nanocolloidal graphene oxide as promising drug delivery and scaffolding biomaterials. Gels 2022, 8, 22. [Google Scholar] [CrossRef]
- Yamoaka, K.; Nakagawa, T.; Uno, T. Application of the Akaike information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhang, C.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Cestari, A.R.; Airoldi, C.; Loh, W. Polysaccharide-based hydrogels: Preparation, characterization, and drug interaction behavior. Biomacromolecules 2009, 9, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef] [PubMed]
- Abazovic, N.D.; Čomor, M.I.; Comor, M.D.; Dramicanin, D.J.; Jovanovic, S.P.; Nedeljković, J.M. Photoluminescence of anatase and rutile TiO2 particles. J. Phys. Chem. B. 2006, 110, 25366–25370. [Google Scholar] [CrossRef]
- Mugundan, S.; Rajamannan, G.; Viruthagiri, N.; Shanmugam, R.; Gobi, P. Synthesis and characterization of undoped and cobalt-doped TiO2 nanoparticles via sol-gel technique. Appl. Nanosci. 2015, 5, 449–456. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New insights of scaffolds based on hydrogels in tissue engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Vadav, P.; Beniwal, G.; Saxena, K.K. A review on pore and porosity in tissue engineering. Mater. Today Proc. 2021, 44, 2623. [Google Scholar] [CrossRef]
- Staruch, R.M.; Glass, G.E.; Rickard, R.; Hettiaratchy, S.P.; Butler, P.E.M. Injectable pore-forming hydrogel scaffolds for complex wound tissue engineering: Designing and controlling their porosity and mechanical properties. Tissue Eng. Part B Rev. 2017, 23, 183–198. [Google Scholar] [CrossRef]
- Zhang, J.C.; Wu, L.B.; Jing, D.Y.; Ding, J.D. A comparative study of porous scaffolds with cubic and spherical macropores. Polymer 2005, 46, 4979–4985. [Google Scholar] [CrossRef]
- Chong, E.; Phan, T.; Lim, I.; Zhang, Y.; Bay, B.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef]
- Chamkouri, H.; Chamkouri, M. A Review of hydrogels, their properties and applications in medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Yildirim, E.D.; Besunder, R.; Pappas, D.; Allen, F.; Gu¨c¸eri, S.; Sun, W. Accelerated differentiation of osteoblast cells on polycaprolactone scaffolds driven by a combined effect of protein coating and plasma modification. Biofabrication 2010, 2, 014109. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N. Mechanical and biological properties of scaffold materials. In Functional 3D Tissue Engineering Scaffolds; Woodhead Publishing: Sawston, UK, 2018; pp. 1–21. [Google Scholar]
- Kirchmajer, D.; Watson, C.; Ranson, M.; Panhuis, M. Gelatin, a degradable genipin cross-linked gelatin hydrogel. RSC Adv. 2012, 3, 1073–1081. [Google Scholar] [CrossRef]
- Elsner, J.J.; Zilberman, M. Novel antibiotic-eluting wound dressings: An in vitro study and engineering aspects in the dressing’s design. J. Tissue Viability 2010, 19, 54–66. [Google Scholar] [CrossRef]
- Abd-Khorsand, S.; Saber-Samandari, S.; Saber-Samandari, S. Development of nanocomposite scaffolds based on TiO2 doped in grafted chitosan/hydroxyapatite by freeze drying method and evaluation of biocompatibility. Int. J. Biol. Macromol. 2017, 101, 51–58. [Google Scholar] [CrossRef]
- Naik, K.; Girish Chandran, V.; Rajashekaran, R.; Waigaonkar, S.; Kowshik, M. Mechanical properties, biological behaviour and drug release capability of nano TiO2-HAp-alginate composite scaffolds for potential application as bone implant material. J. Biomater. Appl. 2016, 31, 387–399. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Yekta, H.; Ahmadi, S.; Alamara, K. The role of titanium dioxide on the morphology, microstructure, and bioactivity of grafted cellulose/hydroxyapatite nanocomposites for a potential application in bone repair. Int. J. Biol. Macromol. 2018, 106, 481–488. [Google Scholar] [CrossRef]
- Ruan, G.; Feng, S.S. Preparation and characterization of poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid)(PLA-PEG-PLA) microspheres for controlled release of paclitaxel. Biomaterials 2003, 24, 5037–5044. [Google Scholar] [CrossRef]
- Filipović, V.V.; Božić Nedeljković, B.Đ.; Vukomanović, M.; Tomić, S.Lj. Biocompatible and degradable scaffolds based on 2-hydroxyethyl methacrylate, gelatin, and poly(beta amino ester) crosslinkers. Polym. Test. 2018, 68, 270–278. [Google Scholar] [CrossRef]
- Nikolić, L.; Urošević, M.; Nikolić, V.; Gajić, I.; Dinić, A.; Miljković, V.; Rakić, S.; Đokić, S.; Kesić, J.; Ilić-Stojanović, S.; et al. The formulation of curcumin: 2-hydroxypropyl-β-cyclodextrin complex with smart hydrogel for prolonged release of curcumin. Pharmaceutics 2023, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Madeo, L.F.; Curcio, M.; Iemma, F.; Nicoletta, F.P.; Hampel, S.; Cirillo, G. Release of bioactive molecules from graphene oxide-alginate hybrid hydrogels: Effect of crosslinking method. C 2023, 9, 8. [Google Scholar] [CrossRef]
- Udeni Gunathilake, T.M.S.; Ching, Y.C.; Chuah, C.H. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef]
- Hamilton, A.E.; Gilbert, R.J. Curcumin release from biomaterials for enhanced tissue regeneration following injury or disease. Bioengineering 2023, 10, 262. [Google Scholar] [CrossRef]
- Amirazad, H.; Baradar Khoshfetrat, A.; Zarghami, N. A dual synergistic effect of titanium and curcumin co-embedded on extracellular matrix hydrogels of decellularized bone: Potential application in osteoblastic differentiation of adipose-derived mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2023, 34, 372–397. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to modify the drug release from pharmaceutical systems; Woodhead Publishing: Cambridge, UK, 2015. Caccavo, D. An overview on the mathematical modeling of hydrogels’ behavior for drug delivery systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar]
- Gouda, R.; Baishya, H.; Qing, Z. Application of mathematical models in drug release kinetics of Carbidopa and Levodopa ER tablets. J. Develop. Drugs 2017, 6, 171. [Google Scholar]
- Liu, S.; Li, W.; Xu, Z.; Hu, J.; Wu, F.; Zheng, Y. Preparation and synergistic effect of biomimetic poly(lactic acid)/graphene oxide composite scaffolds loaded with dual drugs. Polymers 2022, 14, 5348. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, M.; Bizari, D.; Soltani, M.; Khshmohabat, H.; Raahemifar, K.; Akbarzade Amirdehi, M. The effects of curcumin nanoparticles incorporated into collagen-alginate scaffold on wound healing of skin tissue in trauma patients. Polymers 2021, 13, 4291. [Google Scholar] [CrossRef] [PubMed]






| Sample | HEMA (µL) | 10% Gelatin (µL) | 10% Alginate (µL) | TiO2 (mg) |
|---|---|---|---|---|
| PHEMA/G/TiO2 | 2250 | 2250 | 0 | 2.50 |
| PHEMA/70G/30A/TiO2 | 2250 | 1575 | 675 | 2.50 |
| PHEMA/50G/50A/TiO2 | 2250 | 1125 | 1125 | 2.50 |
| PHEMA/A/TiO2 | 2250 | 0 | 2250 | 2.50 |
| Release Model | Parameters | PHEMA/A/TiO2 | PHEMA/50G/50A/TiO2 | PHEMA/70G/30A/TiO2 | PHEMA/G/TiO2 |
|---|---|---|---|---|---|
| (a) Higuchi model | kH | 0.086 | 0.039 | 0.026 | 0.019 |
| SSR | 0.007 | 0.001 | 0.014 | 0.964 | |
| AIC | −19.21 | −34.19 | −55.26 | −49.88 | |
| R2 | 0.976 | 0.965 | 0.985 | 0.974 | |
| (b) Ritger–Peppas model | K1 | 0.103 | 0.063 | 0.030 | 0.030 |
| n | 0.449 | 0.406 | 0.477 | 0.431 | |
| SSR | 0.006 | 0.006 | 0.006 | 0.005 | |
| AIC | −25.41 | −36.45 | −58.41 | −49.94 | |
| R2 | 0.974 | 0.984 | 0.985 | 0.988 | |
| (c) Peppas–Sahlin model | K1 | 0.095 | 0.039 | 0.019 | 0.022 |
| K2 | 0.0170 | −0.0006 | −0.0001 | −0.0002 | |
| m | 0.338 | 0.577 | 0.620 | 0.514 | |
| SSR | 0.005 | 0.003 | 0.004 | 0.004 | |
| AIC | −26.21 | −48.14 | −62.14 | −56.11 | |
| R2 | 0.971 | 0.990 | 0.989 | 0.990 | |
| (d) Peppas–Sahlin model when m = 0.5 | K1 | 0.090 | 0.051 | 0.029 | 0.023 |
| K2 | −0.0007 | 0.0008 | 0.0001 | 0.0001 | |
| SSR | 0.00333 | 0.00188 | 0.00203 | 0.00183 | |
| AIC | −25.54 | −49.46 | −59.24 | −52.12 | |
| R2 | 0.971 | 0.989 | 0.986 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babić Radić, M.M.; Filipović, V.V.; Vuković, J.S.; Vukomanović, M.; Ilic-Tomic, T.; Nikodinovic-Runic, J.; Tomić, S.L. 2-Hydroxyethyl Methacrylate/Gelatin/Alginate Scaffolds Reinforced with Nano TiO2 as a Promising Curcumin Release Platform. Polymers 2023, 15, 1643. https://doi.org/10.3390/polym15071643
Babić Radić MM, Filipović VV, Vuković JS, Vukomanović M, Ilic-Tomic T, Nikodinovic-Runic J, Tomić SL. 2-Hydroxyethyl Methacrylate/Gelatin/Alginate Scaffolds Reinforced with Nano TiO2 as a Promising Curcumin Release Platform. Polymers. 2023; 15(7):1643. https://doi.org/10.3390/polym15071643
Chicago/Turabian StyleBabić Radić, Marija M., Vuk V. Filipović, Jovana S. Vuković, Marija Vukomanović, Tatjana Ilic-Tomic, Jasmina Nikodinovic-Runic, and Simonida Lj. Tomić. 2023. "2-Hydroxyethyl Methacrylate/Gelatin/Alginate Scaffolds Reinforced with Nano TiO2 as a Promising Curcumin Release Platform" Polymers 15, no. 7: 1643. https://doi.org/10.3390/polym15071643
APA StyleBabić Radić, M. M., Filipović, V. V., Vuković, J. S., Vukomanović, M., Ilic-Tomic, T., Nikodinovic-Runic, J., & Tomić, S. L. (2023). 2-Hydroxyethyl Methacrylate/Gelatin/Alginate Scaffolds Reinforced with Nano TiO2 as a Promising Curcumin Release Platform. Polymers, 15(7), 1643. https://doi.org/10.3390/polym15071643











