Moisture Sorption Isotherms and Thermodynamic Properties of Biodegradable Polymers for Application in Food Packaging Industry
Abstract
1. Introduction
2. Materials and Methods
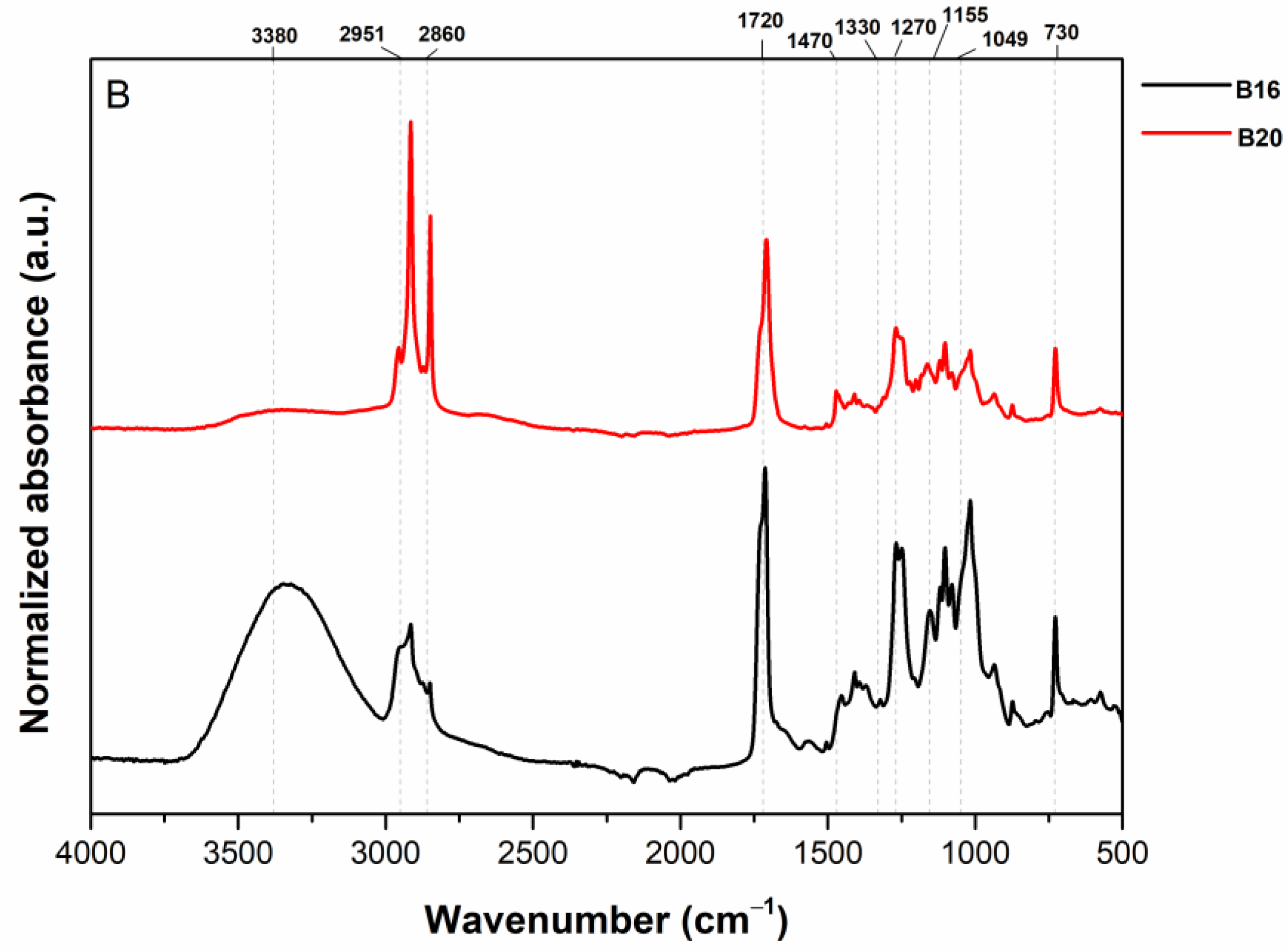
2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
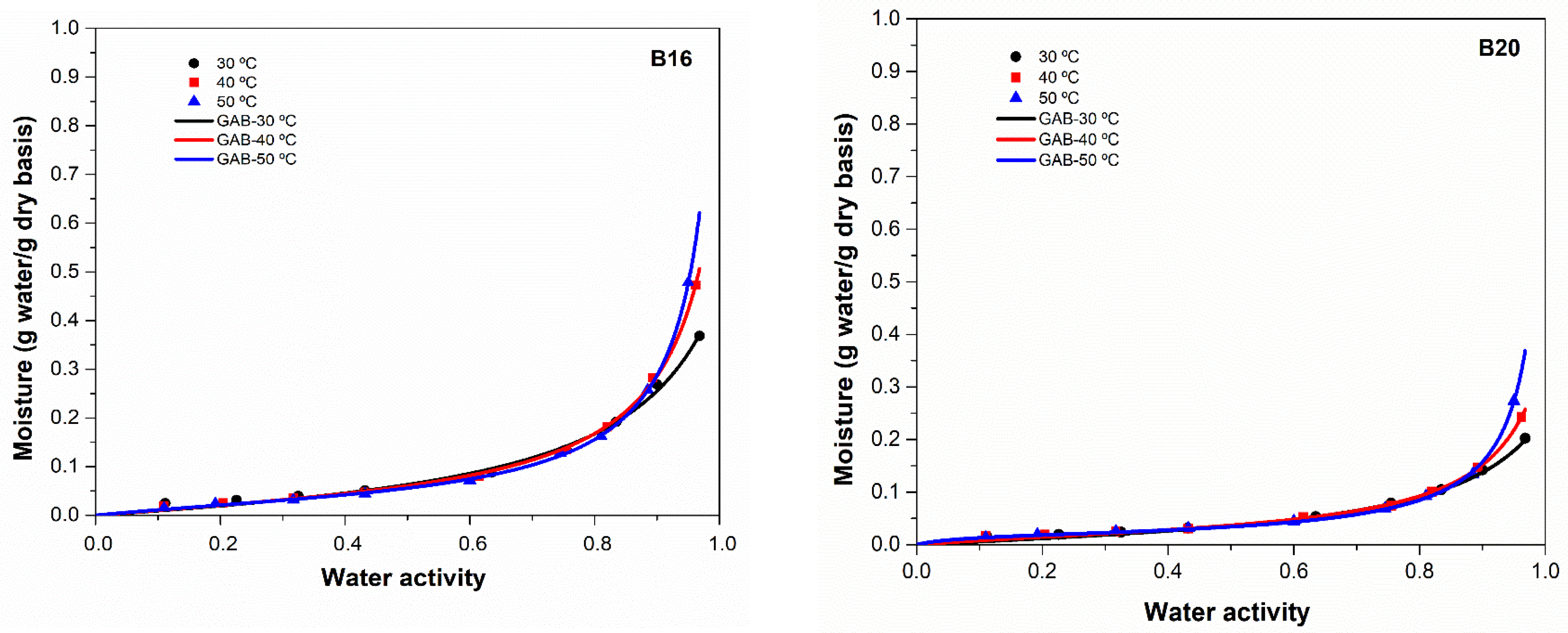
2.2. Water Adsorption Isotherms
2.3. Data Analysis of Thermodynamic Properties
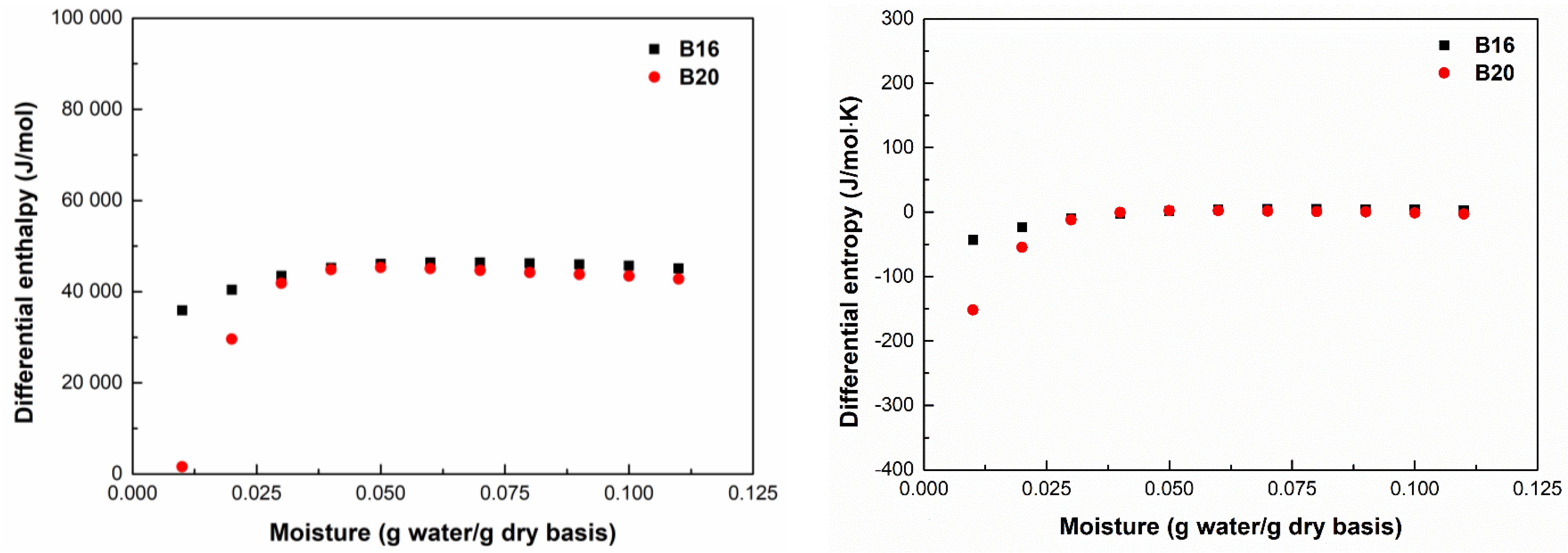
2.3.1. Net Isosteric Heat of Sorption
2.3.2. Enthalpy–Entropy Compensation Theory
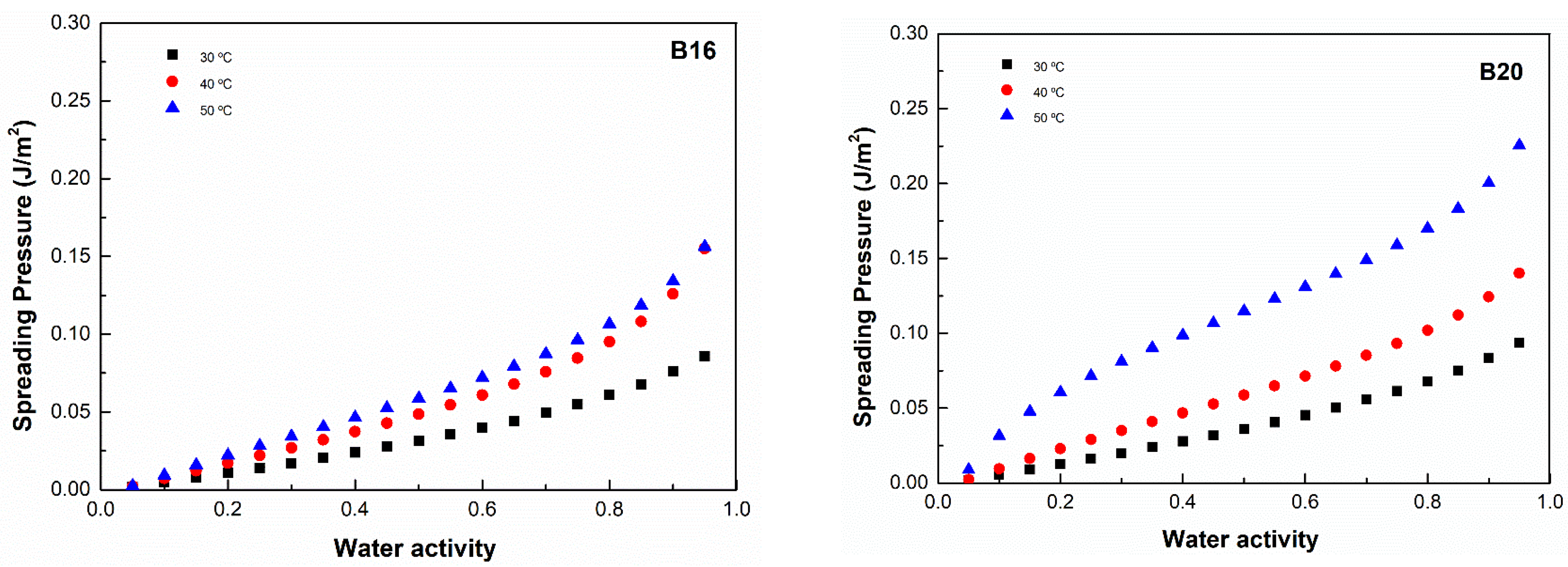
2.3.3. Spreading Pressure
2.3.4. Integral Enthalpy and Entropy
2.3.5. Specific Surface Area and Pore Size Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. FTIR Spectra
3.2. Water Sorption Isotherm
3.3. Differential Enthalpy and Entropy
3.4. Enthalpy–Entropy Compensation
3.5. Spreading Pressure
3.6. Integral Enthalpy and Entropy
3.7. Surface Area and Porosity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiess, W.; Wolf, W. Results of the COST 90 project on water activity. In Physical Properties of Foods; Jowitt, R., Ed.; Applied Science Publishers: London, UK, 1983. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Tsami, E. Net isosteric heat of sorption in dried fruits. J. Food Eng. 1991, 14, 327–335. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Water sorption isotherms of starch powders. Part 2: Thermodynamic characteristics. J. Food Eng. 2004, 62, 135–142. [Google Scholar] [CrossRef]
- Aguerre, R.J.; Suárez, C.; Viollaz, P.E. Enthalpy-Entropy Compensation in Sorption Phenomena: Application to the Prediction of the Effect of Temperature on Food Isotherms. J. Food Sci. 1986, 51, 1547–1549. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van’t Hoff and Arrhenius data. J. Phys. Chem. 1976, 80, 2335–2341. [Google Scholar] [CrossRef]
- Fasina, O.; Ajibola, O.; Tyler, R. Thermodynamics of moisture sorption in winged bean seed and gari. J. Food Process Eng. 1999, 22, 405–418. [Google Scholar] [CrossRef]
- Iglesias, H.A.; Chirife, J.; Viollaz, P. Thermodynamics of water vapour sorption by sugar beet root. Int. J. Food Sci. Technol. 1976, 11, 91–101. [Google Scholar] [CrossRef]
- Fasina, O.; Sokhansanj, S.; Tyler, R. Thermodynamics of moisture sorption in alfalfa pellets. Drying Technol. 1997, 15, 1553–1570. [Google Scholar] [CrossRef]
- Labuza, T.P. Sorption phenomena in foods. Food Technol. 1968, 22, 15–19. [Google Scholar]
- Gregg, S. Adsorption of gases on porous solids. Surf. Colloid Sci. 1976, 6, 183–186. [Google Scholar]
- Gouveia, T.I.A.; Biernacki, K.; Castro, M.C.R.; Gonçalves, M.P.; Souza, H.K.S. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Thermoplastic starch blown films with improved mechanical and barrier properties. Int. J. Biol. Macromol. 2021, 188, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Barros, H.L.; Tavares, L.; Stefani, V. Dye-doped starch microparticles as a novel fluorescent agent for the visualization of latent fingermarks on porous and non-porous substrates. Forensic Chem. 2020, 20, 100264. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Moisture Sorption Isotherm Characteristics of Food Products: A Review. Food Bioprod. Process. 2002, 80, 118–128. [Google Scholar] [CrossRef]
- Aouaini, F.; Knani, S.; Ben Yahia, M.; Bahloul, N.; Kechaou, N.; Ben Lamine, A. Application of Statistical Physics on the Modeling of Water Vapor Desorption Isotherms. Dry. Technol. 2014, 32, 1905–1922. [Google Scholar] [CrossRef]
- Lomauro, C.; Bakshi, A.; Labuza, T. Evaluation of food moisture sorption isotherm equations part II: Milk, coffee, tea, nuts, oilseeds, spices and starchy foods. LWT—Food Sci. Technol. 1985, 18, 118–124. [Google Scholar]
- Wolf, W.; Spiess, W.E.L.; Jung, G. Standardization of Isotherm Measurements (Cost-Project 90 and 90 BIS). In Properties of Water in Foods: In Relation to Quality and Stability; Simatos, D., Multon, J.L., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 661–679. [Google Scholar]
- Lara, B.R.B.; Dias, M.V.; Guimarães Junior, M.; de Andrade, P.S.; de Souza Nascimento, B.; Ferreira, L.F.; Yoshida, M.I. Water sorption thermodynamic behavior of whey protein isolate/ polyvinyl alcohol blends for food packaging. Food Hydrocoll. 2020, 103, 105710. [Google Scholar] [CrossRef]
- McMinn, W.A.M.; Magee, T.R.A. Thermodynamic properties of moisture sorption of potato. J. Food Eng. 2003, 60, 157–165. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, Y.; Yang, J.; Jiang, N.; Wang, Q.; Chu, D.-T.; Han, Y.; Zhou, J. Thermodynamic sorption properties, water plasticizing effect and particle characteristics of blueberry powders produced from juices, fruits and pomaces. Powder Technol. 2018, 323, 208–218. [Google Scholar] [CrossRef]
- Arthur, E.; Tuller, M.; Moldrup, P.; Greve, M.H.; Knadel, M.; de Jonge, L.W. Applicability of the Guggenheim–Anderson–Boer water vapour sorption model for estimation of soil specific surface area. Eur. J. Soil Sci. 2018, 69, 245–255. [Google Scholar] [CrossRef]
- Monte, M.L.; Moreno, M.L.; Senna, J.; Arrieche, L.S.; Pinto, L.A.A. Moisture sorption isotherms of chitosan-glycerol films: Thermodynamic properties and microstructure. Food Biosci. 2018, 22, 170–177. [Google Scholar] [CrossRef]
- Mutungi, C.; Schuldt, S.; Onyango, C.; Schneider, Y.; Jaros, D.; Rohm, H. Dynamic Moisture Sorption Characteristics of Enzyme-Resistant Recrystallized Cassava Starch. Biomacromolecules 2011, 12, 660–671. [Google Scholar] [CrossRef]
- Tavares, L.; Noreña, C.P.Z. Characterization of the physicochemical, structural and thermodynamic properties of encapsulated garlic extract in multilayer wall materials. Powder Technol. 2021, 378, 388–399. [Google Scholar] [CrossRef]
- Gichau, A.W.; Okoth, J.K.; Makokha, A. Moisture sorption isotherm and shelf life prediction of complementary food based on amaranth-sorghum grains. J. Food Sci. Technol. 2020, 57, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Bennaceur, S.; Draoui, B.; Touati, B.; Benseddik, A.; Saad, A.; Bennamoun, L. Determination of the Moisture-Sorption Isotherms and Isosteric Heat of Henna Leaves. J. Eng. Phys. Thermophys. 2015, 88, 52–62. [Google Scholar] [CrossRef]
- Viollaz, P.E.; Rovedo, C.O. Equilibrium sorption isotherms and thermodynamic properties of starch and gluten. J. Food Eng. 1999, 40, 287–292. [Google Scholar] [CrossRef]
- Moghaddam, M.R.A.; Razavi, S.M.A.; Jahani, Y. Effects of Compatibilizer and Thermoplastic Starch (TPS) Concentration on Morphological, Rheological, Tensile, Thermal and Moisture Sorption Properties of Plasticized Polylactic Acid/TPS Blends. J. Polym. Environ. 2018, 26, 3202–3215. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Van den Berg, C.; Bruin, S. Water activity and its estimation in food systems. In Proceedings of the International Symposium Properties of Water in Relation to Food Quality and Stability, Osaka, Japan, 10–16 September 1978. [Google Scholar]
- Halsey, G. Physical adsorption on non-uniform surfaces. J. Chem. Phys. 1948, 16, 931–937. [Google Scholar] [CrossRef]
- Smith, S.E. The sorption of water vapor by high polymers. J. Am. Chem. Soc. 1947, 69, 646–651. [Google Scholar] [CrossRef]
- Oswin, C. The kinetics of package life. III. The isotherm. J. Soc. Chem. Ind. 1946, 65, 419–421. [Google Scholar] [CrossRef]
- Peleg, M. Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms 1. J. Food Process Eng. 1993, 16, 21–37. [Google Scholar] [CrossRef]
- Aviara, N.A.; Ajibola, O.O. Thermodynamics of moisture sorption in melon seed and cassava. J. Food Eng. 2002, 55, 107–113. [Google Scholar] [CrossRef]
- Kaya, S.; Kahyaoglu, T. Influence of dehulling and roasting process on the thermodynamics of moisture adsorption in sesame seed. J. Food Eng. 2006, 76, 139–147. [Google Scholar] [CrossRef]
- Madamba, P.S.; Driscoll, R.H.; Buckle, K.A. Enthalpy-entropy compensation models for sorption and browning of garlic. J. Food Eng. 1996, 28, 109–119. [Google Scholar] [CrossRef]
- Leffler, J.E. The enthalpy-entropy relationship and its implications for organic chemistry. J. Org. Chem. 1955, 20, 1202–1231. [Google Scholar] [CrossRef]
- Lago, C.C.; Liendo-Cárdenas, M.; Noreña, C.P.Z. Thermodynamic sorption properties of potato and sweet potato flakes. Food Bioprod. Process. 2013, 91, 389–395. [Google Scholar] [CrossRef]
- Kaya, S.; Kahyaoglu, T. Thermodynamic properties and sorption equilibrium of pestil (grape leather). J. Food Eng. 2005, 71, 200–207. [Google Scholar] [CrossRef]
- Silva, E.K.; Fernandes, R.V.d.B.; Borges, S.V.; Botrel, D.A.; Queiroz, F. Water adsorption in rosemary essential oil microparticles: Kinetics, thermodynamics and storage conditions. J. Food Eng. 2014, 140, 39–45. [Google Scholar] [CrossRef]
- Singh, R.R.B.; Rao, K.H.; Anjaneyulu, A.S.R.; Patil, G.R. Moisture sorption properties of smoked chicken sausages from spent hen meat. Food Res. Int. 2001, 34, 143–148. [Google Scholar] [CrossRef]
- Kapsalis, J.G. Moisture sorption hysteresis. In Water Activity: Influences on Food Quality; Elsevier: Amsterdam, The Netherlands, 1981; pp. 143–177. [Google Scholar]
- Rosa, G.S.; Moraes, M.A.; Pinto, L.A.A. Moisture sorption properties of chitosan. LWT—Food Sci. Technol. 2010, 43, 415–420. [Google Scholar] [CrossRef]
- Singh, R.R.B.; Rao, K.H.; Anjaneyulu, A.S.R.; Patil, G.R. Water desorption characteristics of raw goat meat: Effect of temperature. J. Food Eng. 2006, 75, 228–236. [Google Scholar] [CrossRef]



| Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 40 | 50 | ||||||
| Model | Equation | Constant | B16 | B20 | B16 | B20 | B16 | B20 |
| GAB | (Equation (1)) [32] | Xm | 0.064 | 0.033 | 0.047 | 0.025 | 0.038 | 0.019 |
| C | 1.602 | 1.986 | 2.359 | 3.839 | 3.358 | 15.506 | ||
| k | 0.873 | 0.875 | 0.940 | 0.934 | 0.972 | 0.981 | ||
| R2 | 0.997 | 0.997 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| E (%) | 9.153 | 9.037 | 9.249 | 9.998 | 5.624 | 3.604 | ||
| Halsey | (Equation (2)) [33] | A | 0.005 | 0.002 | 0.013 | 0.004 | 0.021 | 0.009 |
| B | 1.937 | 1.994 | 1.525 | 1.652 | 1.263 | 1.274 | ||
| R2 | 0.971 | 0.978 | 0.991 | 0.993 | 0.998 | 0.999 | ||
| E (%) | 35.878 | 29.697 | 30.192 | 17.553 | 18.696 | 3.104 | ||
| Smith | (Equation (3)) [34] | A | −0.003 | 0.002 | −0.028 | −0.007 | −0.037 | −0.015 |
| B | −0.251 | −0.134 | −0.325 | −0.162 | −0.344 | −0.186 | ||
| R2 | 0.994 | 0.997 | 0.983 | 0.986 | 0.966 | 0.952 | ||
| E | 126.29 | 116.51 | 161.75 | 132.96 | 179.363 | 155.013 | ||
| Oswin | (Equation (4)) [35] | A | 0.081 | 0.046 | 0.071 | 0.043 | 0.058 | 0.033 |
| B | 0.463 | 0.448 | 0.593 | 0.544 | 0.716 | 0.712 | ||
| R2 | 0.981 | 0.987 | 0.995 | 0.997 | 0.999 | 0.998 | ||
| E (%) | 23.306 | 18.285 | 13.618 | 8.887 | 6.011 | 14.304 | ||
| Peleg | (Equation (5)) [36] | k1 | 0.074 | 0.057 | 0.507 | 0.250 | 0.647 | 0.085 |
| k2 | 0.356 | 0.179 | 0.123 | 0.070 | 0.165 | 0.424 | ||
| n1 | 0.542 | 0.703 | 9.322 | 9.470 | 13.591 | 1.048 | ||
| n2 | 5.695 | 6.490 | 1.022 | 0.840 | 1.422 | 15.514 | ||
| R2 | 0.999 | 0.999 | 0.998 | 0.999 | 0.999 | 0.997 | ||
| E (%) | 3.14 | 5.44 | 7.04 | 7.47 | 13.71 | 11.38 | ||
| Moisture Content (g Water/g Dry Basis) | B16 | B20 | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | |||||
| 30 | 40 | 50 | 30 | 40 | 50 | |
| 0.01 | 0.92 | 0.87 | 0.83 | 1.08 | 0.96 | 0.76 |
| 0.02 | 1.16 | 1.11 | 1.06 | 1.48 | 1.34 | 1.15 |
| 0.03 | 1.39 | 1.34 | 1.31 | 1.90 | 1.79 | 1.72 |
| 0.04 | 1.61 | 1.58 | 1.57 | 2.37 | 2.28 | 2.31 |
| 0.05 | 1.84 | 1.83 | 1.84 | 2.88 | 2.81 | 2.90 |
| 0.06 | 2.09 | 2.08 | 2.12 | 3.44 | 3.37 | 3.50 |
| 0.07 | 2.34 | 2.35 | 2.41 | 4.06 | 3.97 | 4.10 |
| 0.08 | 2.60 | 2.62 | 2.70 | 4.76 | 4.60 | 4.71 |
| 0.09 | 2.88 | 2.90 | 3.00 | 5.54 | 5.28 | 5.33 |
| 0.1 | 3.16 | 3.19 | 3.30 | 6.42 | 6.01 | 5.95 |
| 0.2 | 7.18 | 6.62 | 6.57 | 35.27 | 17.62 | 12.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, L.; Sousa, L.R.; Magalhães da Silva, S.; Lima, P.S.; Oliveira, J.M. Moisture Sorption Isotherms and Thermodynamic Properties of Biodegradable Polymers for Application in Food Packaging Industry. Polymers 2023, 15, 1634. https://doi.org/10.3390/polym15071634
Tavares L, Sousa LR, Magalhães da Silva S, Lima PS, Oliveira JM. Moisture Sorption Isotherms and Thermodynamic Properties of Biodegradable Polymers for Application in Food Packaging Industry. Polymers. 2023; 15(7):1634. https://doi.org/10.3390/polym15071634
Chicago/Turabian StyleTavares, Loleny, Liliana R. Sousa, Sara Magalhães da Silva, Paulo S. Lima, and J. M. Oliveira. 2023. "Moisture Sorption Isotherms and Thermodynamic Properties of Biodegradable Polymers for Application in Food Packaging Industry" Polymers 15, no. 7: 1634. https://doi.org/10.3390/polym15071634
APA StyleTavares, L., Sousa, L. R., Magalhães da Silva, S., Lima, P. S., & Oliveira, J. M. (2023). Moisture Sorption Isotherms and Thermodynamic Properties of Biodegradable Polymers for Application in Food Packaging Industry. Polymers, 15(7), 1634. https://doi.org/10.3390/polym15071634









