New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Synthesis of ZnO Nanoparticles (ZnO–NPs)
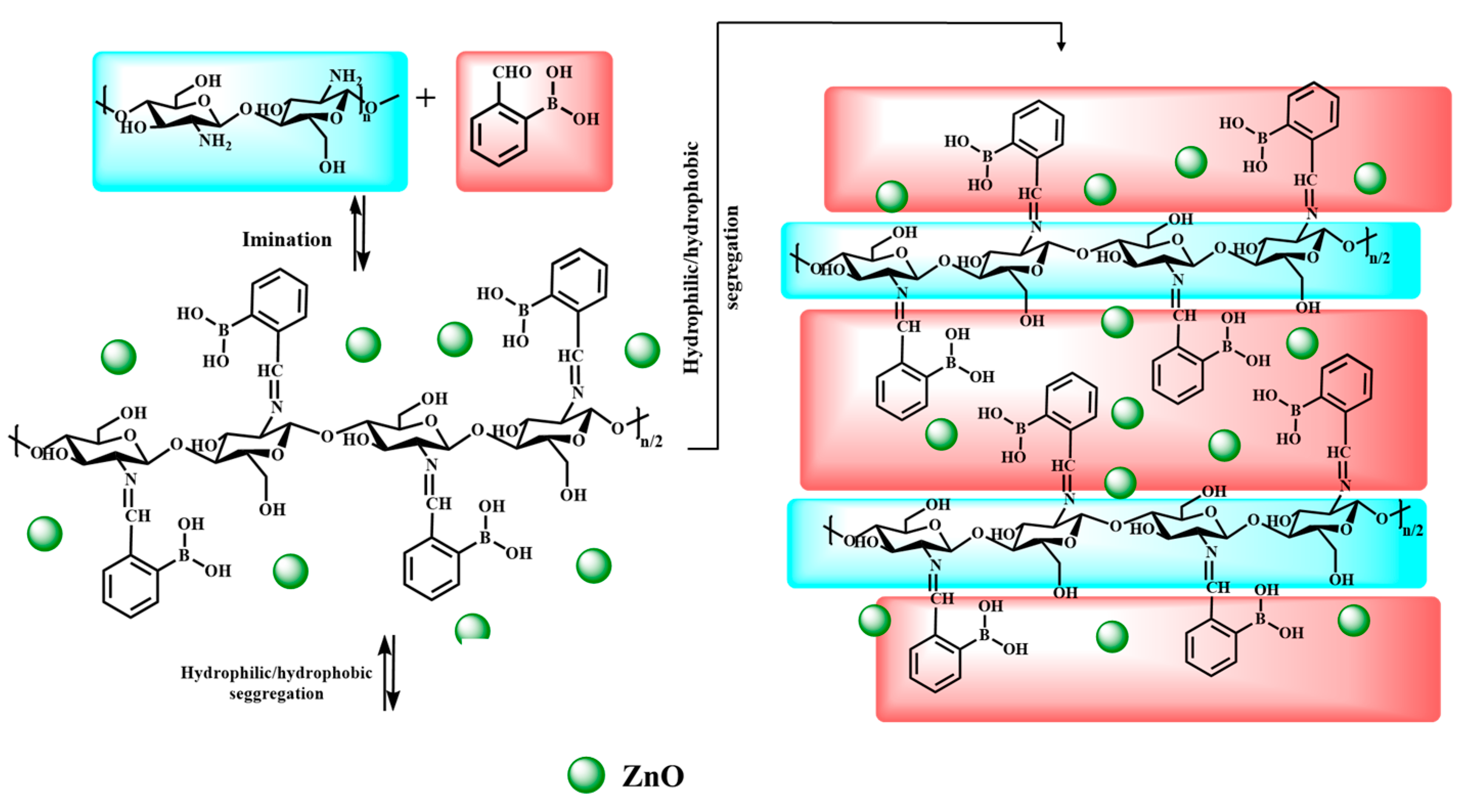
2.3. The Synthesis of the Hydrogels Containing ZnO–NPs
2.4. Characterisation
2.4.1. Hydrogels’ Lyophilisation
2.4.2. FTIR Spectroscopy
2.4.3. NMR Spectroscopy
2.4.4. Scanning Electron Microscopy
2.4.5. Wide-Angle X-ray Diffraction
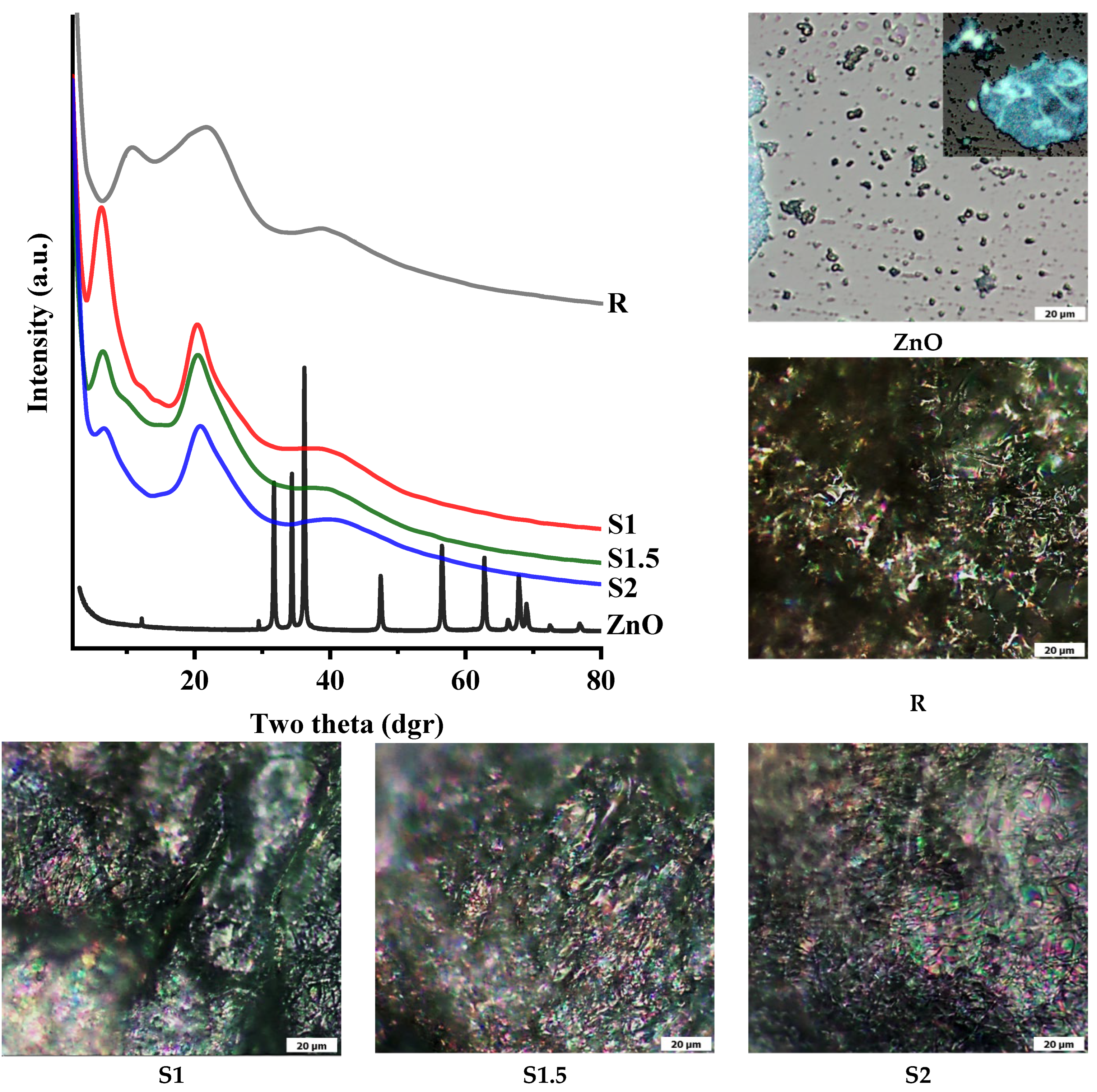
2.4.6. Polarised Optical Microscopy
2.4.7. Atomic Force Spectroscopy (AFM)
2.5. Antioxidant Activity
2.6. Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Structural Characterisation by 1H-NMR and FTIR Spectroscopy
3.2. Supramolecular Characterisation via Wide-Angle X-ray Diffraction
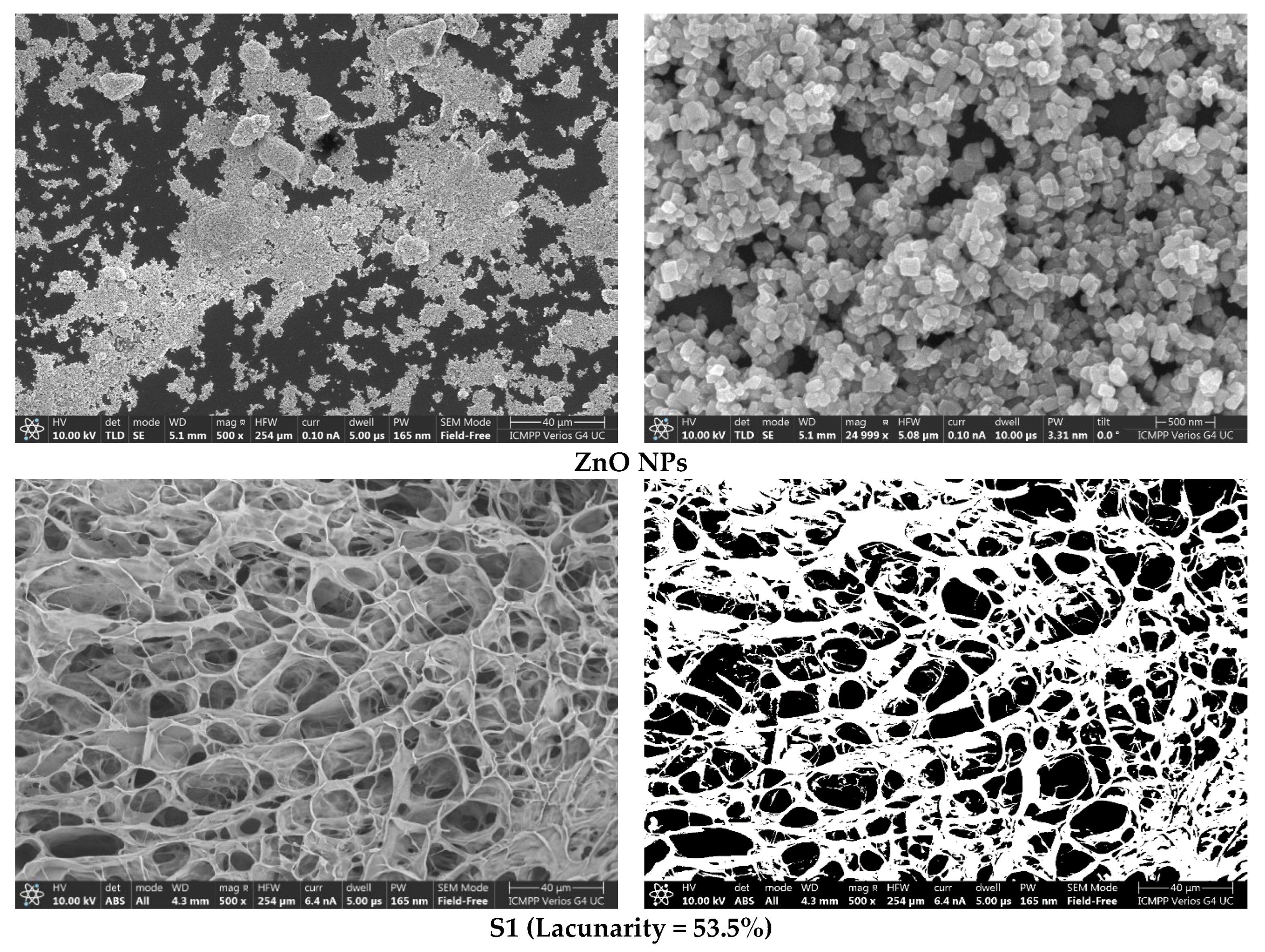
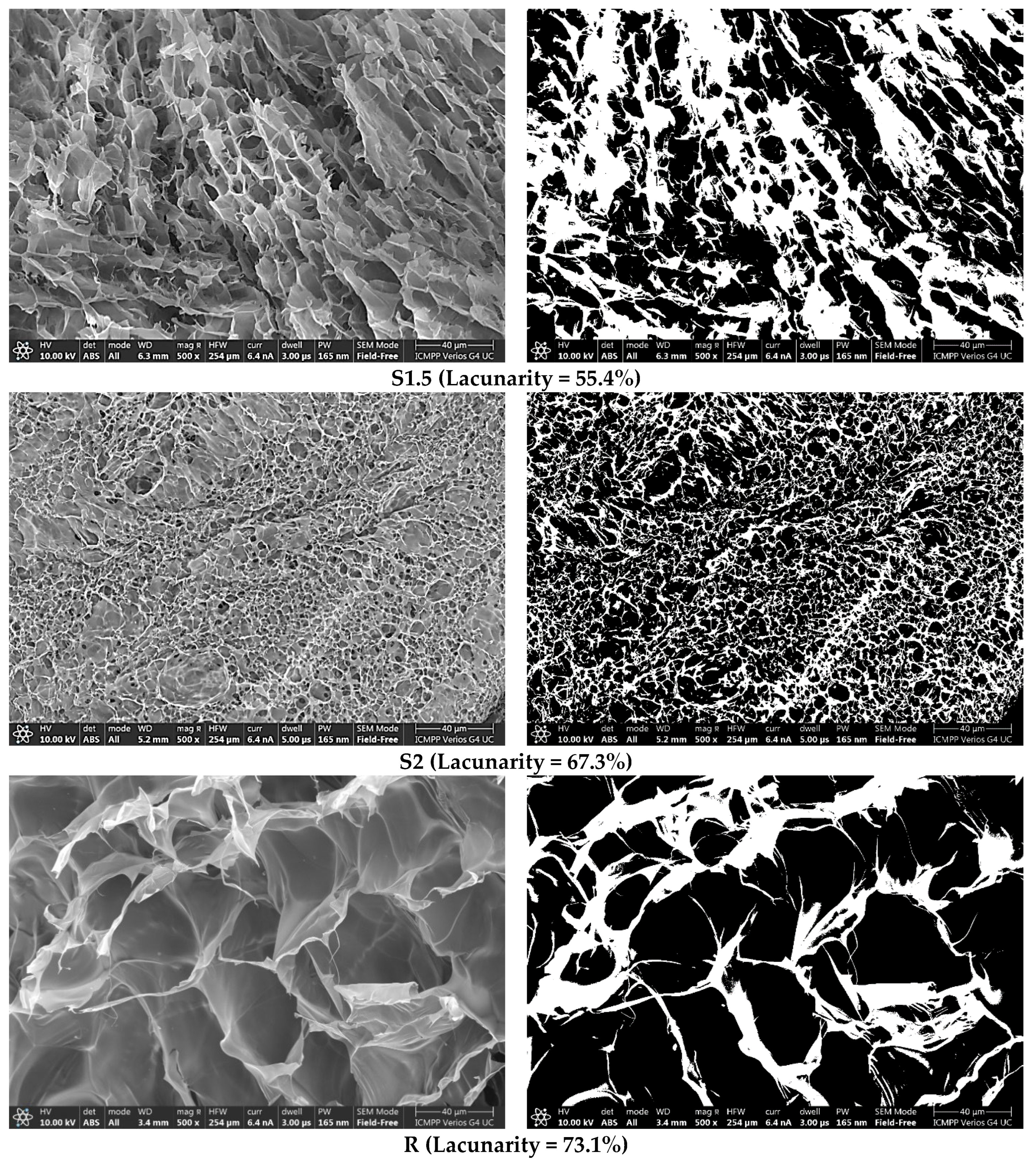
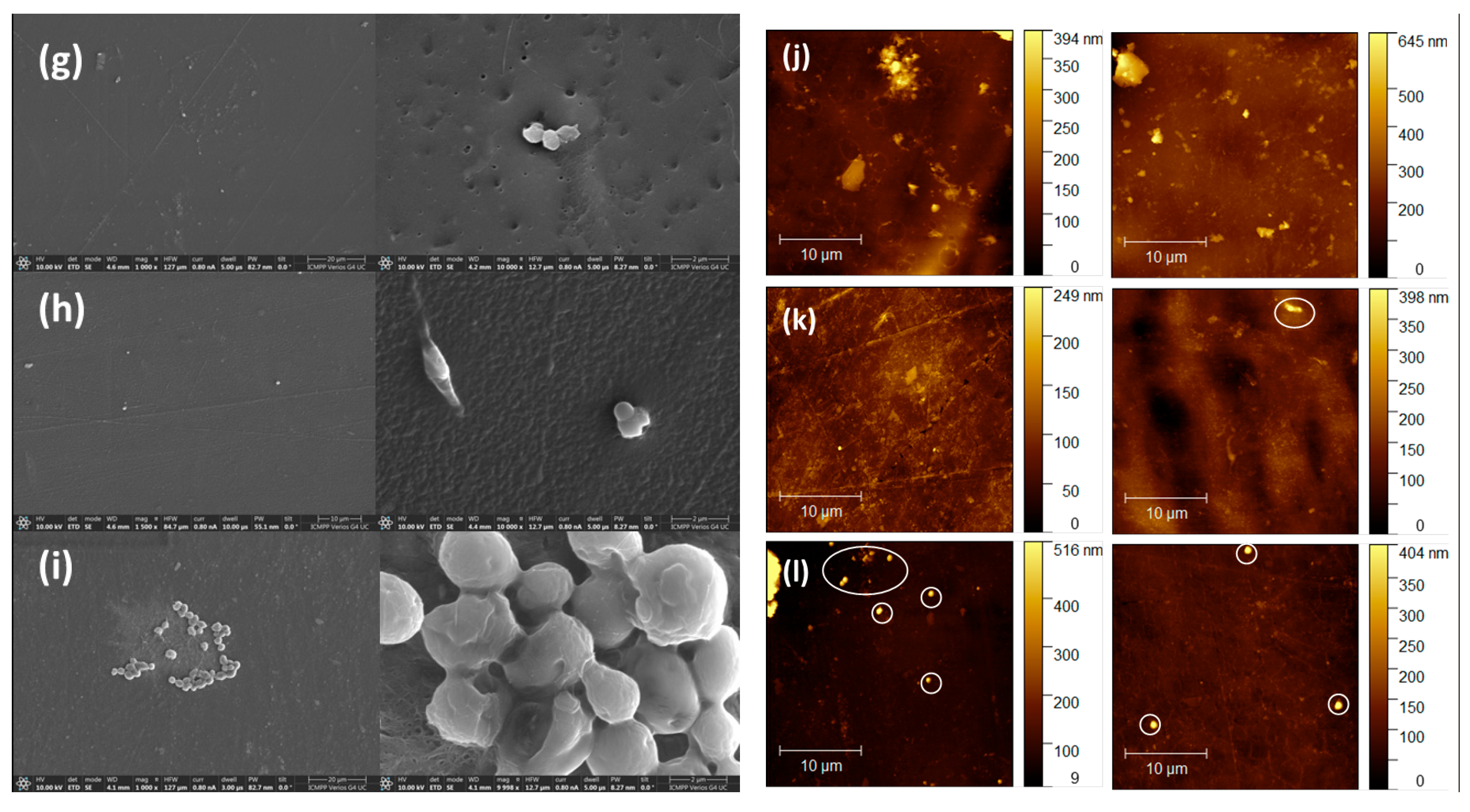
3.3. Morphological and Elementary Characterisation by SEM and EDAX
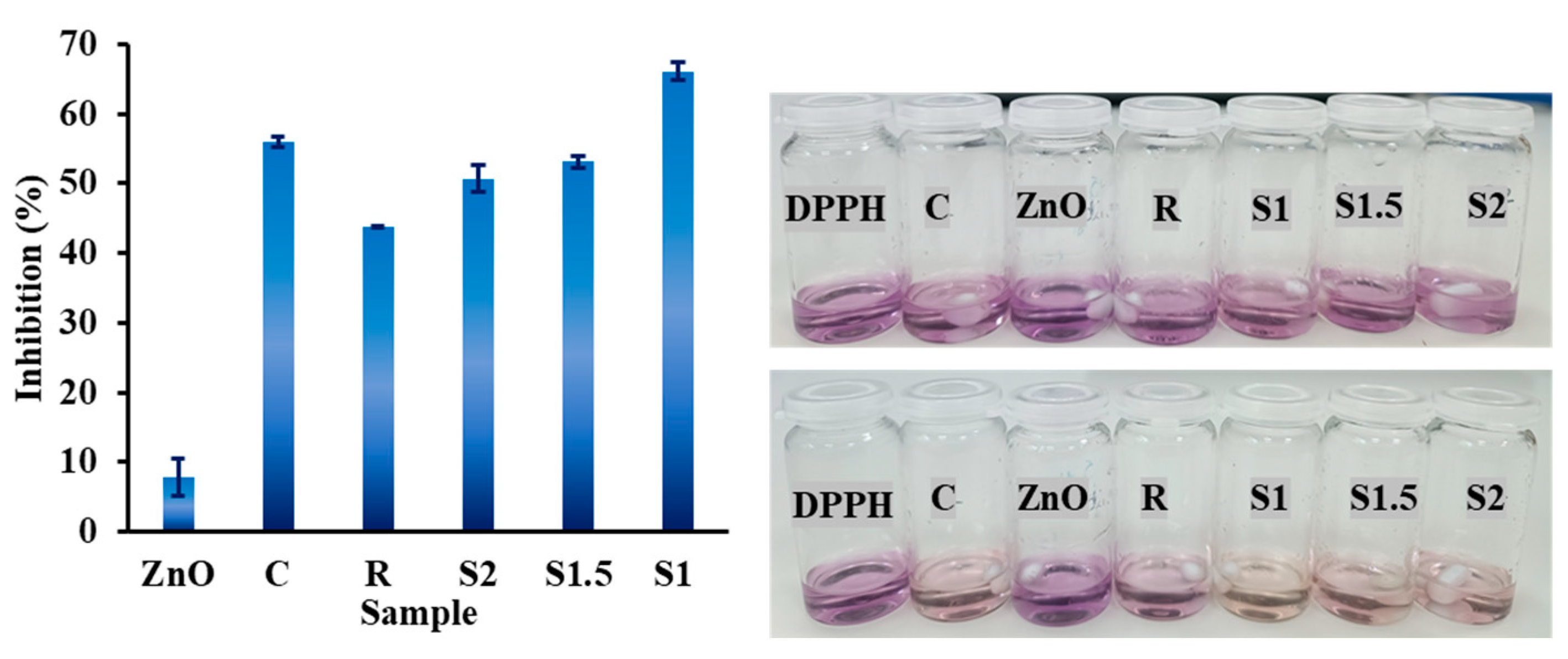
3.4. Antioxidant Activity
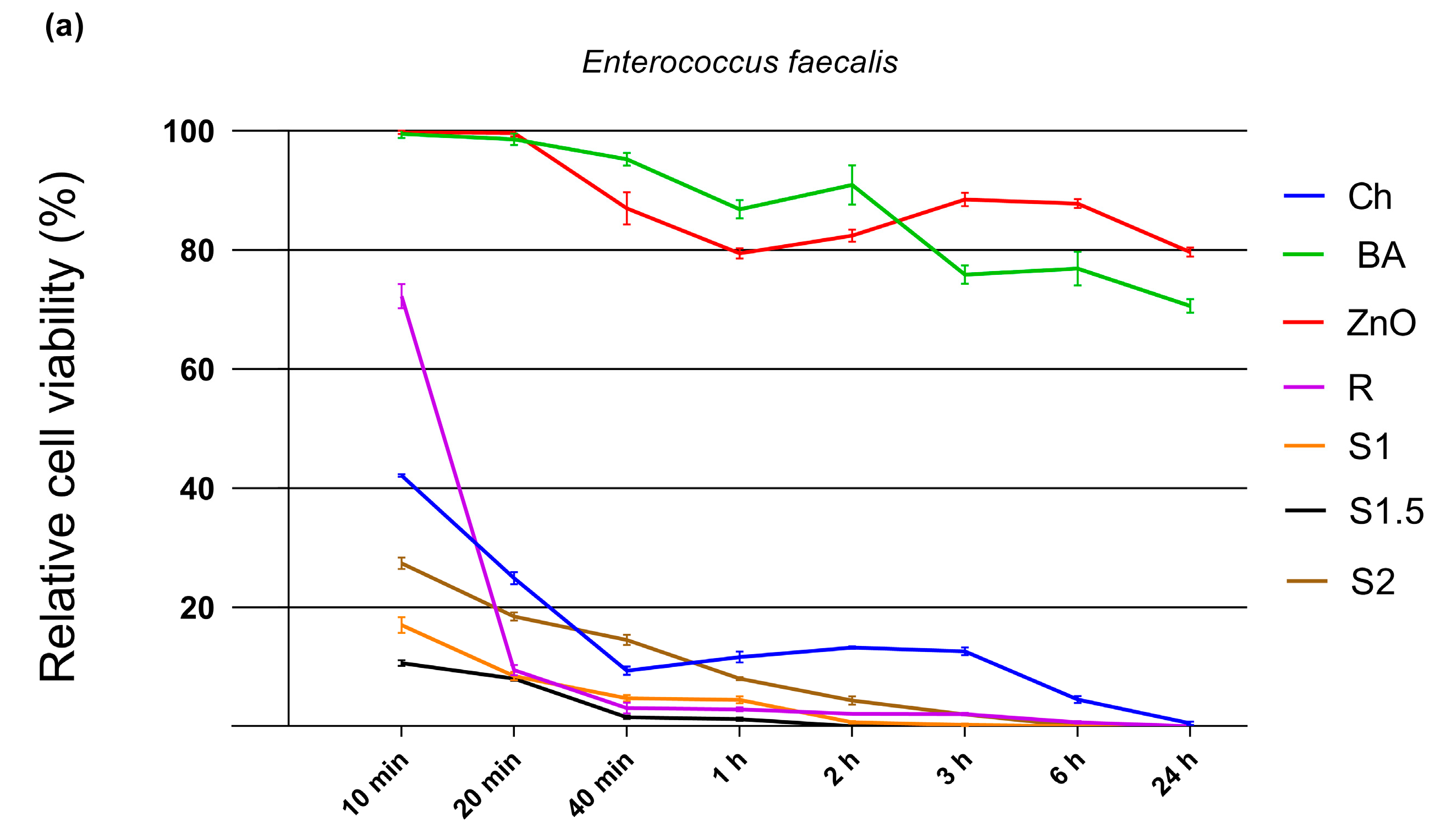
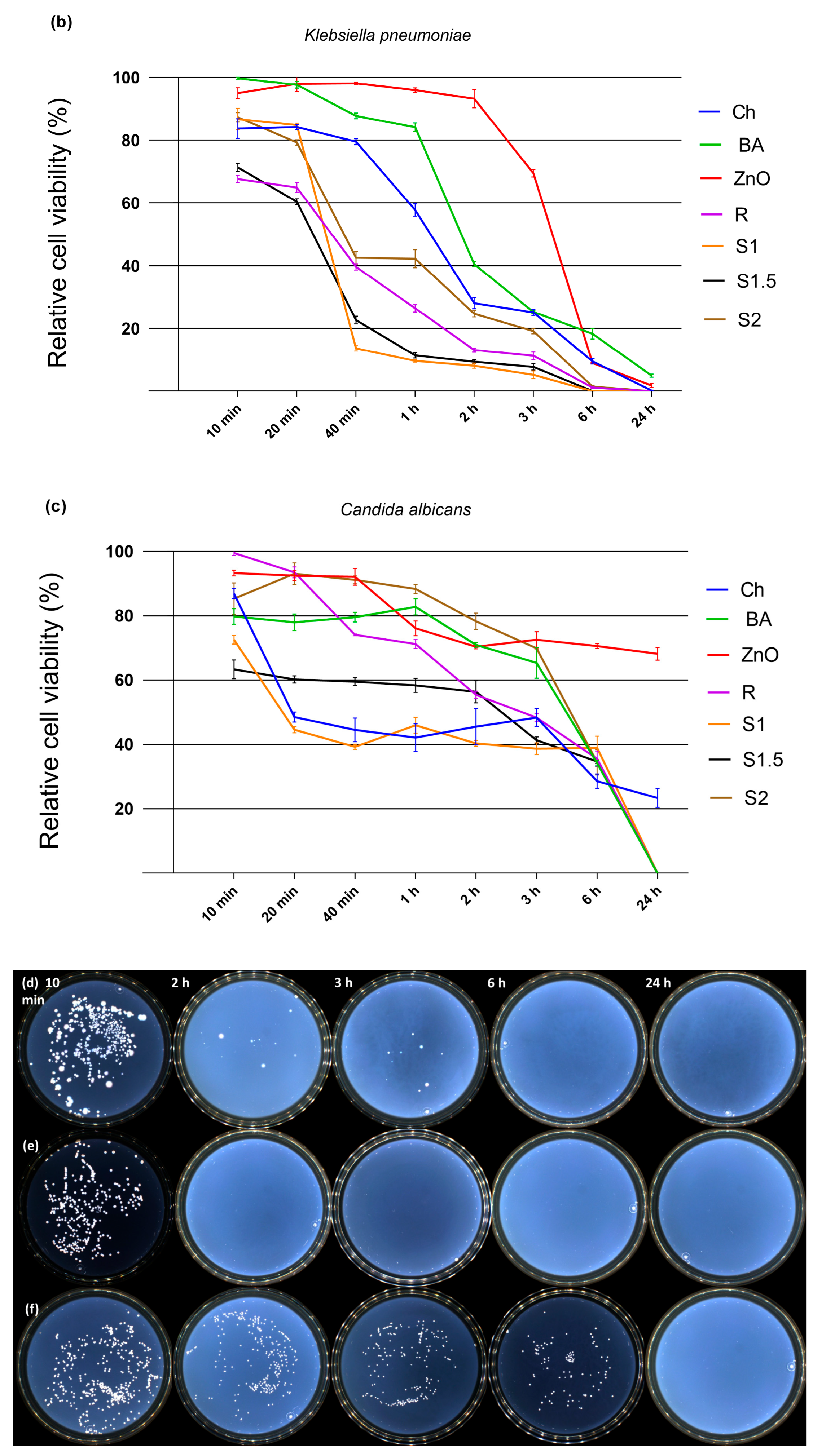
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Larsen, S.; Russell, R.V.; Ockert, L.K.; Spanos, S.; Travis, H.S.; Ehlers, L.H.; Mærkedahl, A. Rate and Impact of Duodenoscope Contamination: A Systematic Review and Meta-Analysis. EClinicalMedicine 2020, 25, 100451. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G.; Lieb, J.G.; Cohen, J.; Pike, I.M.; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; Scheiman, J.M.; Shaheen, N.J.; Sherman, S.; et al. Quality Indicators for ERCP. Gastrointest. Endosc. 2015, 81, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Sczaniecka, A.; Hirano, M.; Benedict, M.; Baba, S.; Horino, Y.; Takenouchi, M.; Morizane, Y.; Yana, I.; Segan, R.; et al. A Prospective, Multicenter, Clinical Study of Duodenoscope Contamination after Reprocessing. Infect. Control Hosp. Epidemiol 2022, 43, 1901–1909. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; de Oliveira, A.C.; Ribeiro, S.M.C.P.; Watanabe, E.; de Resende Stoianoff, M.A.; Ferreira, J.A.G. Effectiveness of Flexible Gastrointestinal Endoscope Reprocessing. Infect. Control Hosp. Epidemiol. 2013, 34, 309–312. [Google Scholar] [CrossRef]
- Kovaleva, J. Infectious Complications in Gastrointestinal Endoscopy and Their Prevention. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 689–704. [Google Scholar] [CrossRef]
- Visrodia, K.H.; Ofstead, C.L.; Yellin, H.L.; Wetzler, H.P.; Tosh, P.K.; Baron, T.H. The Use of Rapid Indicators for the Detection of Organic Residues on Clinically Used Gastrointestinal Endoscopes with and without Visually Apparent Debris. Infect Control Hosp. Epidemiol. 2014, 35, 987–994. [Google Scholar] [CrossRef]
- Ross, A.S.; Baliga, C.; Verma, P.; Duchin, J.; Gluck, M. A Quarantine Process for the Resolution of Duodenoscope-Associated Transmission of Multidrug-Resistant Escherichia Coli. Gastrointest. Endosc. 2015, 82, 477–483. [Google Scholar] [CrossRef]
- Chapman, C.G.; Siddiqui, U.D.; Manzano, M.; Konda, V.J.; Murillo, C.; Landon, E.M.; Waxman, I. Risk of Infection Transmission in Curvilinear Array Echoendoscopes: Results of a Prospective Reprocessing and Culture Registry. Gastrointest. Endosc. 2017, 85, 390–397.e1. [Google Scholar] [CrossRef] [PubMed]
- Rauwers, A.W.; Voor In ’t Holt, A.F.; Buijs, J.G.; de Groot, W.; Hansen, B.E.; Bruno, M.J.; Vos, M.C. High Prevalence Rate of Digestive Tract Bacteria in Duodenoscopes: A Nationwide Study. Gut 2018, 67, 1637–1645. [Google Scholar] [CrossRef]
- Balan, G.G.; Sfarti, C.V.; Chiriac, S.A.; Stanciu, C.; Trifan, A. Duodenoscope-Associated Infections: A Review. Eur. J. Clin. Microbiol. Infect Dis. 2019, 38, 2205–2213. [Google Scholar] [CrossRef]
- Mark, J.A.; Underberg, K.; Kramer, R.E. Results of Duodenoscope Culture and Quarantine after Manufacturer-Recommended Cleaning Process. Gastrointest. Endosc. 2020, 91, 1328–1333. [Google Scholar] [CrossRef]
- Heuvelmans, M.; Wunderink, H.F.; van der Mei, H.C.; Monkelbaan, J.F. A Narrative Review on Current Duodenoscope Reprocessing Techniques and Novel Developments. Antimicrob. Resist. Infect. Control 2021, 10, 171. [Google Scholar] [CrossRef]
- Kwakman, J.A.; Bexkens, M.L.; Bruno, M.J.; Vos, M.C. Persistent Contamination of a Duodenoscope Working Channel in a Non-Clinical Simulated ERCP Setting. Endoscopy 2022, 54, 1085–1090. [Google Scholar] [CrossRef]
- Bajolet, O.; Ciocan, D.; Vallet, C.; de Champs, C.; Vernet-Garnier, V.; Guillard, T.; Brasme, L.; Thiefin, G.; Cadiot, G.; Bureau-Chalot, F. Gastroscopy-Associated Transmission of Extended-Spectrum Beta-Lactamase-Producing Pseudomonas Aeruginosa. J. Hosp. Infect. 2013, 83, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.; Hunter, J.C.; Arwady, M.A.; Tsai, V.; Stein, L.; Gribogiannis, M.; Frias, M.; Guh, A.Y.; Laufer, A.S.; Black, S.; et al. New Delhi Metallo-β-Lactamase–Producing Carbapenem-Resistant Escherichia Coli Associated with Exposure to Duodenoscopes. JAMA 2014, 312, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.L.; Oh, Y.S.; Saeian, K.; Edmiston, C.E.; Khan, A.H.; Massey, B.T.; Dua, K.S. Transmission of Carbapenem-Resistant Enterobacteriaceae during ERCP: Time to Revisit the Current Reprocessing Guidelines. Gastrointest. Endosc. 2015, 81, 1041–1045. [Google Scholar] [CrossRef]
- Yang, S.; Hemarajata, P.; Hindler, J.; Li, F.; Adisetiyo, H.; Aldrovandi, G.; Sebra, R.; Kasarskis, A.; MacCannell, D.; Didelot, X.; et al. Evolution and Transmission of Carbapenem-Resistant Klebsiella Pneumoniae Expressing the BlaOXA-232 Gene During an Institutional Outbreak Associated With Endoscopic Retrograde Cholangiopancreatography. Clin. Infect. Dis. 2017, 64, 894–901. [Google Scholar] [CrossRef]
- Bourigault, C.; Le Gallou, F.; Bodet, N.; Musquer, N.; Juvin, M.-E.; Corvec, S.; Ferronnière, N.; Wiesel, S.; Gournay, J.; Birgand, G.; et al. Duodenoscopy: An Amplifier of Cross-Transmission during a Carbapenemase-Producing Enterobacteriaceae Outbreak in a Gastroenterology Pathway. J. Hosp. Infect. 2018, 99, 422–426. [Google Scholar] [CrossRef]
- Bălan, G.G.; Roșca, I.; Ursu, E.-L.; Doroftei, F.; Bostănaru, A.-C.; Hnatiuc, E.; Năstasă, V.; Șandru, V.; Ștefănescu, G.; Trifan, A.; et al. Plasma-Activated Water: A New and Effective Alternative for Duodenoscope Reprocessing. IDR 2018, 11, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Balan, G.G.; Rosca, I.; Ursu, E.-L.; Fifere, A.; Varganici, C.-D.; Doroftei, F.; Turin-Moleavin, I.-A.; Sandru, V.; Constantinescu, G.; Timofte, D.; et al. Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing. Molecules 2019, 24, 2343. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.q.; Lin, M. Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia Coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Narayanan, P.M.; Wilson, W.S.; Abraham, A.T.; Sevanan, M. Synthesis, Characterization, and Antimicrobial Activity of Zinc Oxide Nanoparticles Against Human Pathogens. BioNanoScience 2012, 2, 329–335. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Soares, F.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Watson, C.Y.; Molina, R.M.; Louzada, A.; Murdaugh, K.M.; Donaghey, T.C.; Brain, J.D. Effects of Zinc Oxide Nanoparticles on Kupffer Cell Phagosomal Motility, Bacterial Clearance, and Liver Function. IJN 2015, 10, 4173–4184. [Google Scholar] [CrossRef]
- Yazdanshenas, M.E.; Shateri-Khalilabad, M. In Situ Synthesis of Silver Nanoparticles on Alkali-Treated Cotton Fabrics. J. Ind. Text. 2013, 42, 459–474. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial Activity of Zinc and Titanium Dioxide Nanoparticles against Biofilm-Producing Methicillin-Resistant Staphylococcus Aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial Activity of Zinc Oxide (ZnO) Nanoparticle against Klebsiella Pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- Quek, J.-A.; Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. Visible Light Responsive Flower-like ZnO in Photocatalytic Antibacterial Mechanism towards Enterococcus Faecalis and Micrococcus Luteus. J. Photochem. Photobiol. B Biol. 2018, 187, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial Effect of Different Sizes of Nano Zinc Oxide on Oral Microorganisms. Front. Dent. 2019, 16, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.J.; Chi, L.Z.; Wong, L.S.; Rajamani, R.; Djearamane, S. Antibacterial Efficacy of Zinc Oxide Nanoparticles against Serratia Marcescens (ATCC 43862) and Enterococcus Faecalis (ATCC 29121). J. Exp. Biol. Agric. Sci. 2022, 10, 1069–1075. [Google Scholar] [CrossRef]
- Djearamane, S.; Loh, Z.C.; Lee, J.J.; Wong, L.S.; Rajamani, R.; Luque, P.A.; Gupta, P.K.; Liang, S.X.T. Remedial Aspect of Zinc Oxide Nanoparticles Against Serratia Marcescens and Enterococcus Faecalis. Front. Pharmacol. 2022, 13, 891304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Ghose, R. Synthesis of Zinc Oxide Nanoparticles by Homogeneous Precipitation Method and Its Application in Antifungal Activity against Candida Albicans. Ceram. Int. 2015, 41, 967–975. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual Crosslinked Iminoboronate-Chitosan Hydrogels with Strong Antifungal Activity against Candida Planktonic Yeasts and Biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Ailincai, D.; Rosca, I.; Morariu, S.; Mititelu-Tartau, L.; Marin, L. Iminoboronate-Chitooligosaccharides Hydrogels with Strong Antimicrobial Activity for Biomedical Applications. Carbohydr. Polym. 2022, 276, 118727. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Nemecek, T.; Komanec, M.; Nelsen, B.; Martan, T.; Suslov, D.; Hartmann, P.; Zvanovec, S. Experimentally and Analytically Derived Generalized Model for the Detection of Liquids with Suspended-Core Optical Fibers. Opt. Fiber Technol. 2018, 45, 295–299. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, D.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Biomaterials Based on N,N,N-Trimethyl Chitosan Fibers in Wound Dressing Applications. Int. J. Biol. Macromol. 2016, 89, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Bercea, M.; Mititelu Tartau, L.; Marin, L. Biocompatible Drug Delivery Systems Able to Co-Deliver Antifungal and Antiviral Agents. Carbohydr. Polym. 2022, 298, 120071. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Rosca, I. New Hydrogels and Formulations Based on Piperonyl-Imino-Chitosan Derivatives. Polymers 2023, 15, 753. [Google Scholar] [CrossRef]
- Rajendran, S.P.; Sengodan, K. Synthesis and Characterization of Zinc Oxide and Iron Oxide Nanoparticles Using Sesbania Grandiflora Leaf Extract as Reducing Agent. J. Nanosci. 2017, 2017, e8348507. [Google Scholar] [CrossRef]
- Marin, L.; Moraru, S.; Popescu, M.-C.; Nicolescu, A.; Zgardan, C.; Simionescu, B.C.; Barboiu, M. Out-of-Water Constitutional Self-Organization of Chitosan–Cinnamaldehyde Dynagels. Chem. A Eur. J. 2014, 20, 4814–4821. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. Int. Sch. Res. Not. 2012, 2012, e372505. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Razzaghi, D.; Arshadi Pirlar, M. ZnO Nanocrystals with Narrow-Band Blue Emission. J. Lumin. 2019, 205, 508–518. [Google Scholar] [CrossRef]
- Balan, G.G.; Pavel, L.; Sandu, A.V.; Stefanescu, G.; Trifan, A.V. Preliminary Study on Erosion of Polymer Coatings of Duodenoscopes. Mater. Plast. 2016, 53, 791–795. [Google Scholar]
- Verfaillie, C.J.; Bruno, M.J.; Voor in ’t Holt, A.F.; Buijs, J.G.; Poley, J.-W.; Loeve, A.J.; Severin, J.A.; Abel, L.F.; Smit, B.J.; de Goeij, I.; et al. Withdrawal of a Novel-Design Duodenoscope Ends Outbreak of a VIM-2-Producing Pseudomonas Aeruginosa. Endoscopy 2015, 47, 493–502. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Gozdalik, J.T.; Wieczorek, D.; Madura, I.D.; Kaczorowska, E.; Brzezińska, E.; Sporzyński, A.; Lipok, J. Synthesis, Properties and Antimicrobial Activity of 5-Trifluoromethyl-2-Formylphenylboronic Acid. Molecules 2020, 25, 799. [Google Scholar] [CrossRef]
- Abid, H.M.U.; Hanif, M.; Mahmood, K.; Aziz, M.; Abbas, G.; Latif, H. Wound-Healing and Antibacterial Activity of the Quercetin–4-Formyl Phenyl Boronic Acid Complex against Bacterial Pathogens of Diabetic Foot Ulcer. ACS Omega 2022, 7, 24415–24422. [Google Scholar] [CrossRef]
- Borys, K.M.; Wieczorek, D.; Pecura, K.; Lipok, J.; Adamczyk-Woźniak, A. Antifungal Activity and Tautomeric Cyclization Equilibria of Formylphenylboronic Acids. Bioorganic Chem. 2019, 91, 103081. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymer 2021, 13, 904. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Fernandes, M.M.; Mendoza, E.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Sonochemical Coating of Textiles with Hybrid ZnO/Chitosan Antimicrobial Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 1164–1172. [Google Scholar] [CrossRef]
- Sathiya, S.M.; Okram, G.S.; Maria Dhivya, S.; Manivannan, G.; Jothi Rajan, M.A. Interaction of Chitosan/Zinc Oxide Nanocomposites and Their Antibacterial Activities with Escherichia Coli. Mater. Today Proc. 2016, 3, 3855–3860. [Google Scholar] [CrossRef]
- Barreto, M.S.; Andrade, C.T.; Azero, E.G.; Paschoalin, V.M.; Del Aguila, E.M. Production of Chitosan/Zinc Oxide Complex by Ultrasonic Treatment with Antibacterial Activity. J. Bacteriol. Parasitol. 2017, 08, 1000330. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-Shaped Gold Nanoparticles with Nanogram Killing Efficiency as Potential Antimicrobial Surface Coatings for the Medical Devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef]
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Xin, L. Synthesis and Characterization of Chitosan/ZnO Nanoparticle Composite Membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Facile Fabrication and Characterization of Chitosan-Based Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Antibiofilm Activity. Int. Nano Lett. 2014, 4, 107. [Google Scholar] [CrossRef]
- Mohammed, A.N.; Abdel Aziz, S.A.A. The Prevalence of Campylobacter Species in Broiler Flocks and Their Environment: Assessing the Efficiency of Chitosan/Zinc Oxide Nanocomposite for Adopting Control Strategy. Environ. Sci. Pollut. Res. 2019, 26, 30177–30187. [Google Scholar] [CrossRef] [PubMed]


| Sample Code | mchitosan (mg) | Vwater (mL) | Vac.acid (μL) | maldehyde (mg) | Vethanol (mL) | NH2/CHO Molar Ratio | mZnO (mg) |
|---|---|---|---|---|---|---|---|
| S1 | 60 | 6 | 42 | 44.8 | 4.48 | 1 | 18 |
| S1.5 | 60 | 6 | 42 | 33.3 | 3.33 | 1.5 | 18 |
| S2 | 60 | 6 | 42 | 22.4 | 2.24 | 2 | 18 |
| R | 60 | 6 | 42 | - | 2.24 | - | 18 |
| (a) Incubation Time | Relative Cell Viability (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| Ch | BA | ZnO | R | S1 | S1.5 | S2 | |
| 10 min | 42.10 ± 0.22 *** | 99.48 ± 0.68 *** | 99.75 ± 0.28 *** | 72.25 ± 2.01 *** | 16.98 ± 1.37 *** | 10.60 ± 0.50 *** | 27.36 ± 0.99 *** |
| 20 min | 24.89 ± 1.01 *** | 98.58 ± 0.94 *** | 99.66 ± 0.58 *** | 9.45 ± 0.86 *** | 8.43 ± 0.70 *** | 7.99 ± 0.40 *** | 18.43 ± 0.70 *** |
| 40 min | 9.32 ± 0.71 *** | 95.23 ± 1.06 *** | 86.96 ± 2.76 *** | 3.08 ± 0.95 *** | 4.70 ± 0.55 *** | 1.52 ± 0.26 *** | 14.52 ± 0.86 *** |
| 1 h | 11.61 ± 0.91 *** | 86.82 ± 1.52 *** | 79.45 ± 0.87 *** | 2.82 ± 0.34 *** | 4.46 ± 0.60 *** | 1.17 ± 0.26 *** | 8.02 ± 0.27 *** |
| 2 h | 13.23 ± 0.22 *** | 90.94 ± 3.29 *** | 82.42 ± 1.04 *** | 2.11 ± 0.10 *** | 0.66 ± 0.20 *** | 0.00 ± 0.00 *** | 4.34 ± 0.67 *** |
| 3 h | 12.58 ± 0.64 *** | 75.84 ± 1.58 *** | 88.48 ± 1.12 *** | 2.04 ± 0.22 *** | 0.23 ± 0.24 *** | 0.00 ± 0.00 *** | 2.00 ± 0.13 *** |
| 6 h | 4.49 ± 0.60 *** | 76.87 ± 2.83 *** | 87.78 ± 0.75 *** | 0.66 ± 0.21 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** |
| 24 h | 0.49 ± 0.29 *** | 70.59 ± 1.12 *** | 79.63 ± 0.74 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** |
| (b) Incubation Time | Relative Cell Viability (Mean ± SD) | ||||||
| Ch | BA | ZnO | R | S1 | S1.5 | S2 | |
| 10 min | 86.66 ± 3.16 *** | 99.75 ± 0.30 *** | 94.95 ± 1.72 *** | 67.70 ± 1.13 *** | 86.71 ± 3.39 *** | 71.28 ± 1.26 *** | 87.30 ± 1.47 *** |
| 20 min | 84.19 ± 0.86 *** | 97.63 ± 1.06 *** | 97.96 ± 2.54 *** | 64.89 ± 1.56 *** | 84.79 ± 0.58 *** | 60.36 ± 0.97 *** | 79.27 ± 0.88 *** |
| 40 min | 79.51 ± 0.97 *** | 87.73 ± 0.90 *** | 98.09 ± 0.35 *** | 39.62 ± 1.01 *** | 13.62 ± 0.85 *** | 22.62 ± 1.26 *** | 42.56 ± 2.06 *** |
| 1 h | 57.73 ± 1.99 *** | 84.13 ± 1.35 *** | 95.93 ± 0.70 *** | 26.42 ± 1.18 *** | 9.66 ± 0.41 *** | 11.43 ± 0.84 *** | 42.23 ± 2.86 *** |
| 2 h | 28.08 ± 1.77 *** | 40.48 ± 0.80 *** | 93.23 ± 2.89 *** | 13.09 ± 0.65 *** | 8.12 ± 0.79 *** | 9.40 ± 0.67 *** | 24.64 ± 1.01 *** |
| 3 h | 25.10 ± 0.97 *** | 25.26 ± 0.46 *** | 69.44 ± 1.19 *** | 11.30 ± 1.21 *** | 5.23 ± 1.21 *** | 7.73 ± 1.02 *** | 19.11 ± 0.87 *** |
| 6 h | 9.49 ± 0.88 *** | 18.28 ± 1.70 *** | 8.96 ± 0.22 *** | 1.18 ± 0.29 *** | 0.00 ± 0.00 *** | 0.07 ± 0.12 *** | 1.50 ± 0.45 *** |
| 24 h | 0.21 ± 0.11 *** | 4.96 ± 0.48 *** | 1.84 ± 0.65 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** |
| (c) Incubation Time | Relative Cell Viability (Mean ± SD) | ||||||
| Ch | BA | ZnO | R | S1 | S1.5 | S2 | |
| 10 min | 86.92 ± 1.60 *** | 79.82 ± 2.49 *** | 93.34 ± 0.95 *** | 99.52 ± 0.70 *** | 72.57 ± 1.34 *** | 63.34 ± 2.95 *** | 85.31 ± 4.89 *** |
| 20 min | 48.53 ± 1.52 *** | 77.98 ± 2.54 *** | 92.54 ± 1.58 *** | 93.54 ± 1.70 *** | 44.56 ± 0.97 *** | 60.20 ± 1.13 *** | 93.13 ± 3.36 *** |
| 40 min | 44.51 ± 3.67 *** | 79.57 ± 1.48 *** | 92.13 ± 2.63 *** | 74.13 ± 0.31 *** | 39.27 ± 0.83 *** | 59.54 ± 1.25 *** | 91.20 ± 1.24 *** |
| 1 h | 42.12 ± 4.29 *** | 82.80 ± 2.51 *** | 76.14 ± 2.30 *** | 71.24 ± 1.39 *** | 45.93 ± 2.45 *** | 58.33 ± 2.17 *** | 88.38 ± 1.41 *** |
| 2 h | 45.54 ± 5.62 *** | 70.99 ± 0.74 *** | 70.36 ± 0.68 *** | 55.44 ± 1.14 *** | 40.30 ± 0.91 *** | 56.38 ± 3.48 *** | 78.29 ± 2.55 *** |
| 3 h | 48.35 ± 2.79 *** | 65.36 ± 4.83 *** | 72.54 ± 2.50 *** | 48.34 ± 1.18 *** | 38.67 ± 1.82 *** | 41.35 ± 1.06 *** | 69.90 ± 0.20 *** |
| 6 h | 28.53 ± 2.25 *** | 34.21 ± 0.93 *** | 70.57 ± 0.76 *** | 35.52 ± 2.42 *** | 38.90 ± 3.65 *** | 34.66 ± 0.65 *** | 34.47 ± 3.95 *** |
| 24 h | 23.36 ± 2.93 *** | 0.00 ± 0.00 *** | 68.22 ± 2.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** | 0.00 ± 0.00 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailincai, D.; Turin Moleavin, I.-A.; Sarghi, A.; Fifere, A.; Dumbrava, O.; Pinteala, M.; Balan, G.G.; Rosca, I. New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing. Polymers 2023, 15, 2669. https://doi.org/10.3390/polym15122669
Ailincai D, Turin Moleavin I-A, Sarghi A, Fifere A, Dumbrava O, Pinteala M, Balan GG, Rosca I. New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing. Polymers. 2023; 15(12):2669. https://doi.org/10.3390/polym15122669
Chicago/Turabian StyleAilincai, Daniela, Ioana-Andreea Turin Moleavin, Alexandra Sarghi, Adrian Fifere, Oana Dumbrava, Mariana Pinteala, Gheorghe G. Balan, and Irina Rosca. 2023. "New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing" Polymers 15, no. 12: 2669. https://doi.org/10.3390/polym15122669
APA StyleAilincai, D., Turin Moleavin, I.-A., Sarghi, A., Fifere, A., Dumbrava, O., Pinteala, M., Balan, G. G., & Rosca, I. (2023). New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing. Polymers, 15(12), 2669. https://doi.org/10.3390/polym15122669







