Compressible Cellulose Wood Prepared with Deep Eutectic Solvents and Its Improved Technology
Abstract
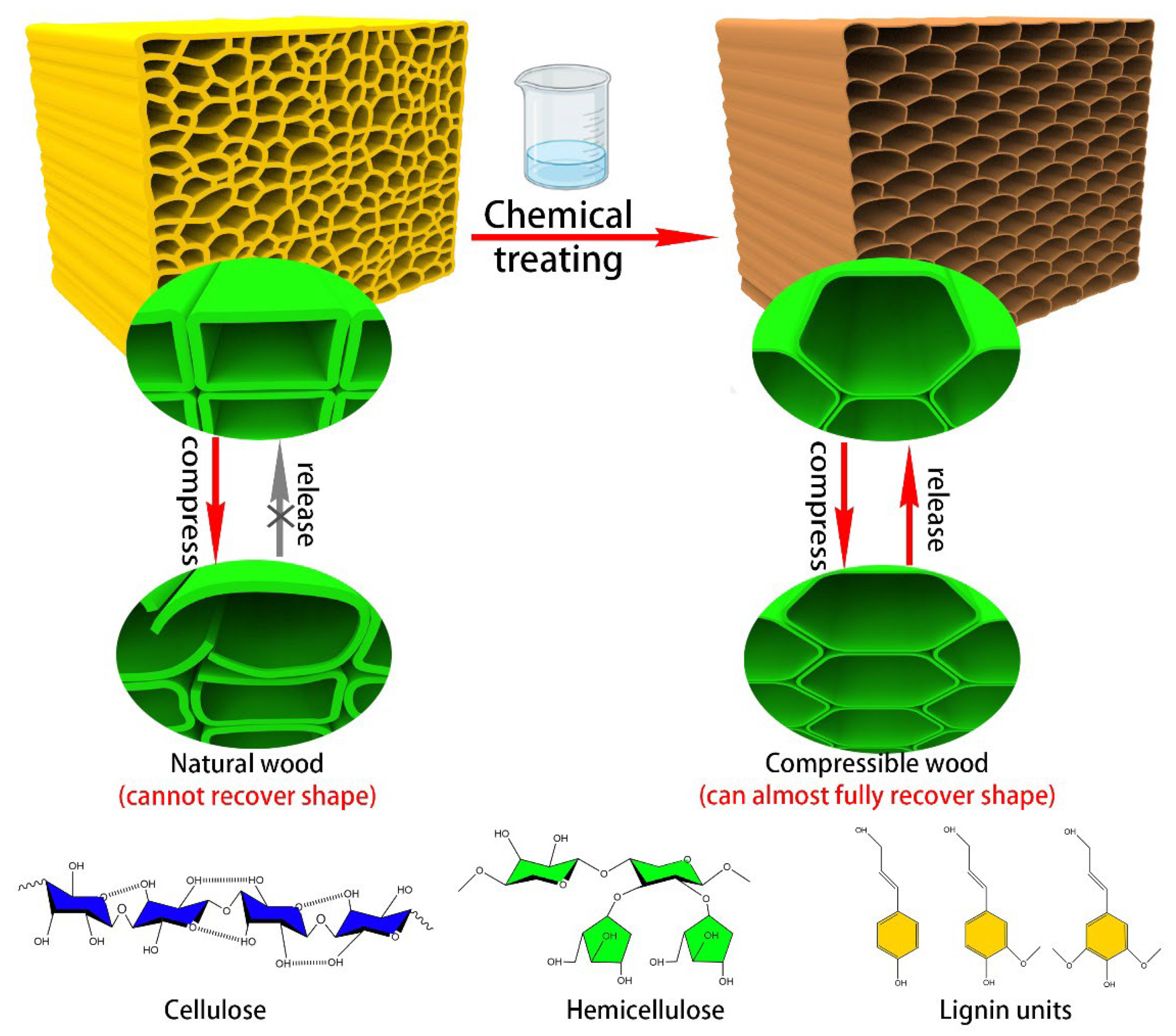
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure for Compressible Wood
2.2.1. Preprocessing
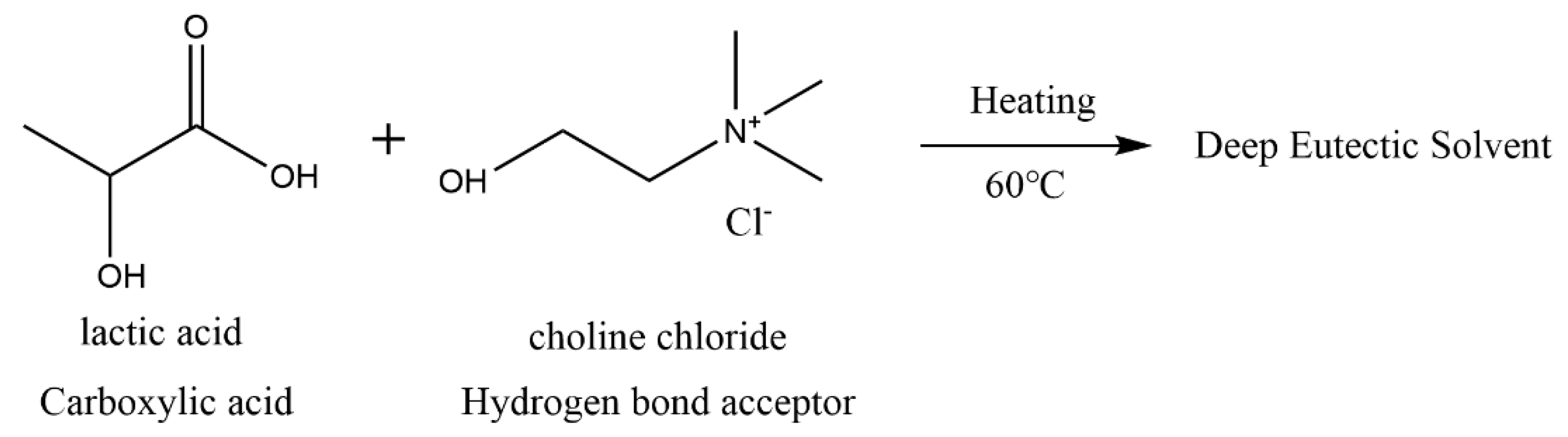
2.2.2. DES Solution Treatment
2.2.3. Alkaline Solution Treatment
2.2.4. Freeze Drying
2.3. Characterization
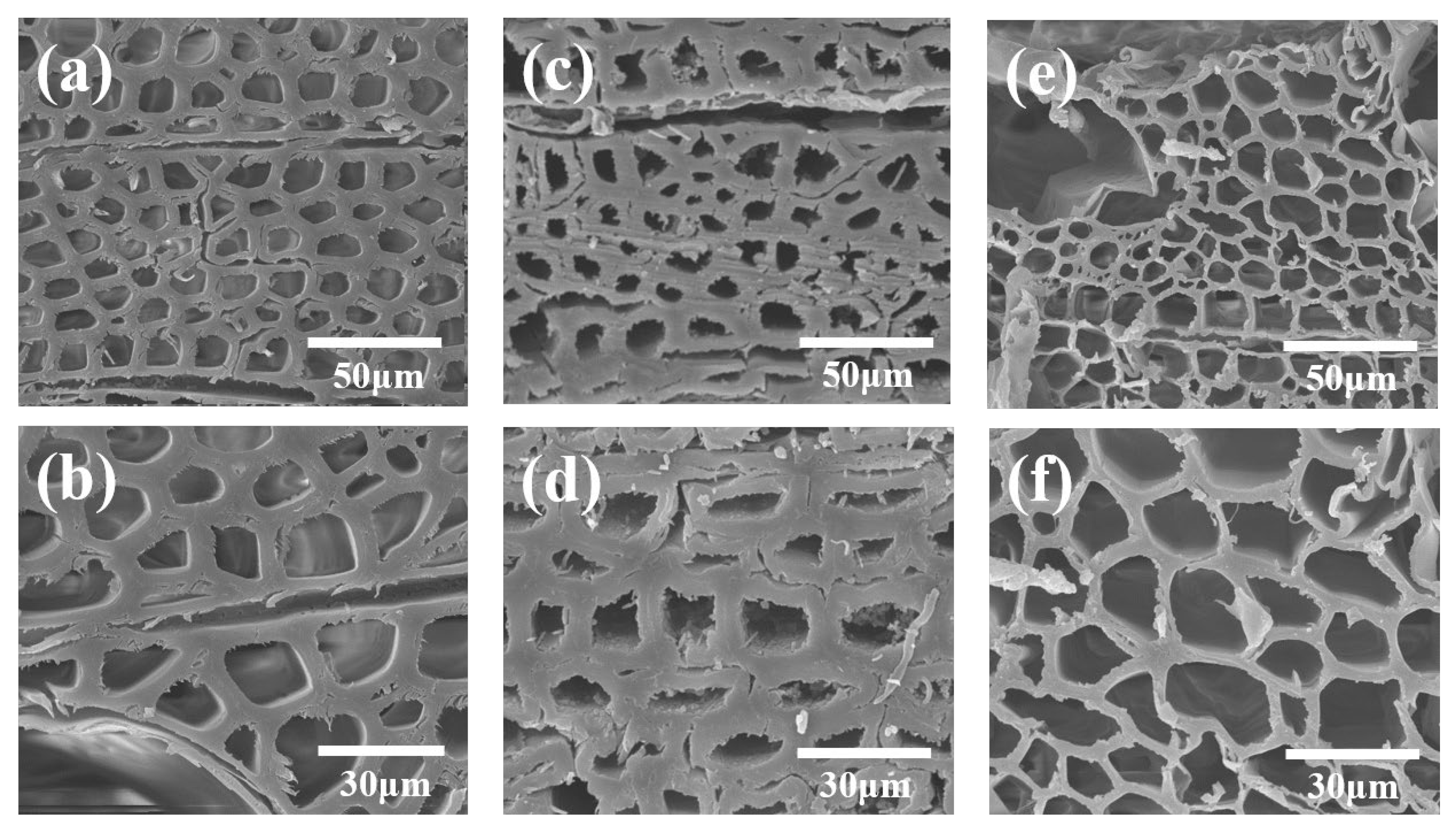
2.3.1. Scanning Electron Microscopy
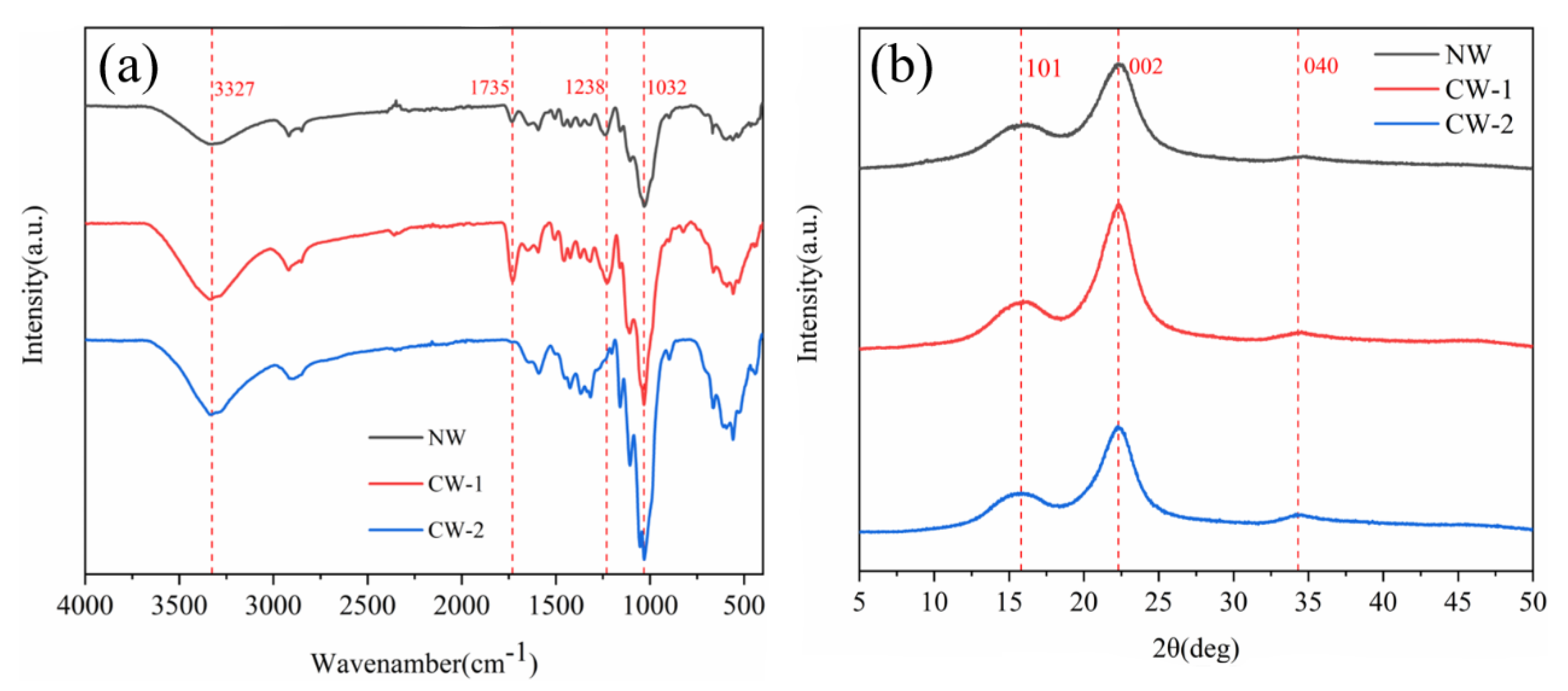
2.3.2. X-ray Diffraction and Fourier Infrared Spectroscopy
2.3.3. Chemical Composition Content Test
2.3.4. Mechanical Performance Test
2.3.5. Laser Confocal Test
3. Results and Discussion
3.1. Morphology Analysis
3.2. Chemical Structure Analysis
3.3. Mechanical Performance
3.4. Chemical Composition Content Analysis
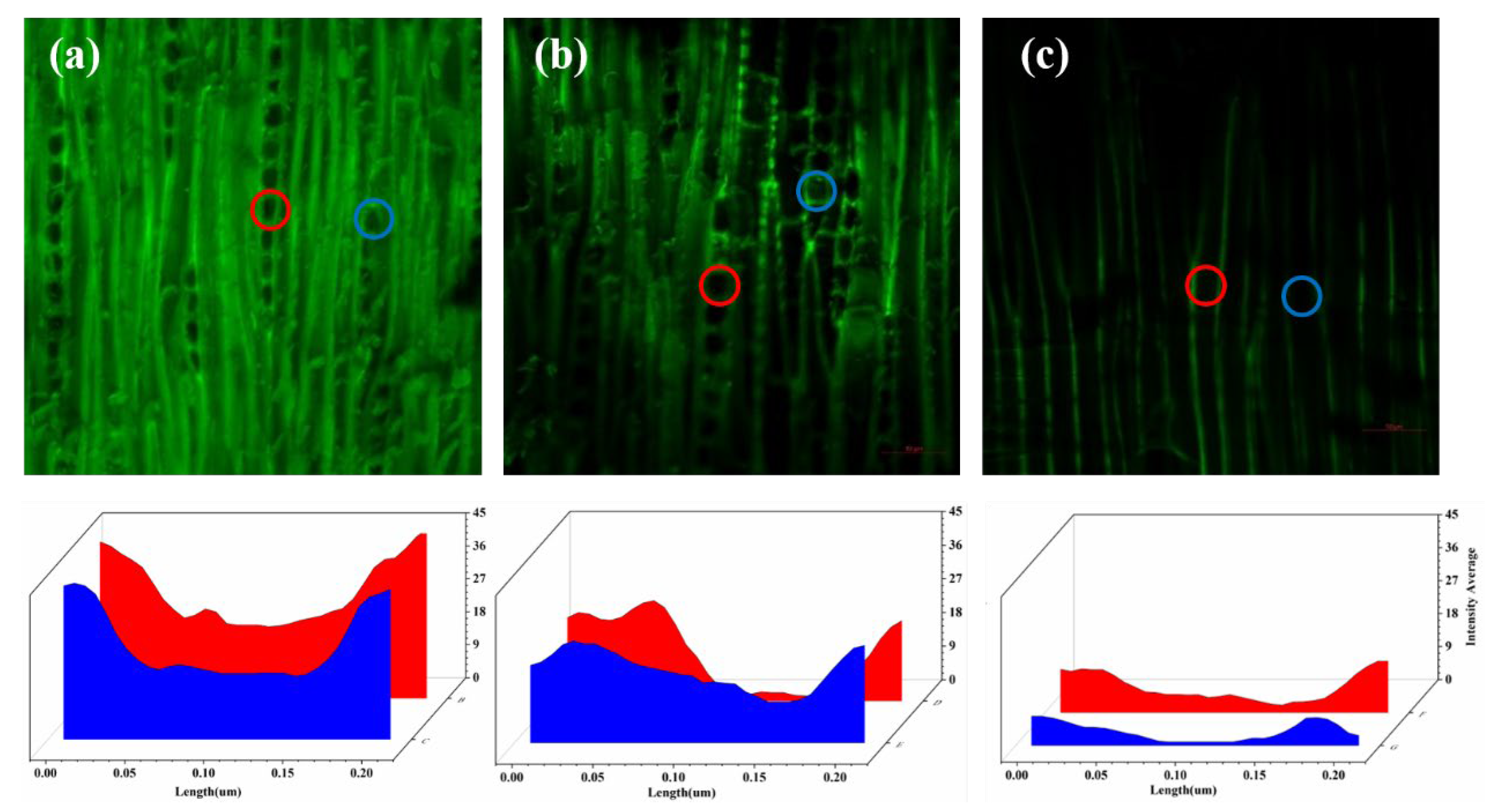
3.5. Confocal Fluorescence Figure
3.6. Thermal Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef] [PubMed]
- Emi, T.T.; Barnes, T.; Orton, E.; Reisch, A.; Tolouei, A.E.; Madani, S.Z.M.; Kennedy, S.M. Pulsatile Chemotherapeutic Delivery Profiles Using Magnetically Responsive Hydrogels. ACS Biomater. Sci. Eng. 2018, 4, 2412–2423. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-Dimensional MXene (Ti3C2)-Integrated Cellulose Hydrogels: Toward Smart Three-Dimensional Network Nanoplatforms Exhibiting Light-Induced Swelling and Bimodal Photothermal/Chemotherapy Anticancer Activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Z.; Huang, X.; Wang, Y.; Huang, Y.; Duan, X. Functionalized Graphene Hydrogel-Based High-Performance Supercapacitors. Adv. Mater. 2013, 25, 5779. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Lu, C.; Zhang, W. Cellulose hydrogels prepared from micron-sized bamboo cellulose fibers. Carbohyd. Polym. 2014, 114, 166–169. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Wang, C.; Kuang, Y.; Li, Y.; Jiang, F.; Li, Y.; Hitz, E.; Zhang, Y.; Liu, B.; et al. Superflexible Wood. ACS Appl. Mater. Interfaces 2017, 9, 23520–23527. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, L.; Nie, K.; Jin, C.; Sun, Q.; Xu, X. Green Construction of an Oil-Water Separator at Room Temperature and Its Promotion to an Adsorption Membrane. Langmuir 2019, 35, 11071–11079. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Tan, Y.; Zhang, W.; Zhang, S.; Li, J. Two-dimensional membrane and three-dimensional bulk aerogel materials via top-down wood nanotechnology for multibehavioral and reusable oil/water separation. Chem. Eng. J. 2019, 371, 769–780. [Google Scholar] [CrossRef]
- Chen, Z.; Ragauskas, A.; Wan, C. Lignin extraction and upgrading using deep eutectic solvents. Ind. Crops Prod. 2020, 147, 112241. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, X.; Sun, X.; Dai, S. New ionic liquids based on the complexation of dipropyl sulfide and AlCl3 for electrodeposition of aluminum. Chem. Commun. 2015, 51, 13286–13289. [Google Scholar] [CrossRef]
- Carriazo, D.; Gutierrez, M.C.; Luisa Ferrer, M.; Del Monte, F. Resorcinol-Based Deep Eutectic Solvents as Both Carbonaceous Precursors and Templating Agents in the Synthesis of Hierarchical Porous Carbon Monoliths. Chem. Mater. 2010, 22, 6146–6152. [Google Scholar] [CrossRef]
- Rogosic, M.; Kucan, K.Z. Deep eutectic solvent based on choline chloride and propylene glycol as a potential medium for extraction denitrification of hydrocarbon fuels. Chem. Eng. Res. Des. 2020, 161, 45–57. [Google Scholar] [CrossRef]
- Wikene, K.O.; Bruzell, E.; Tonnesen, H.H. Improved antibacterial phototoxicity of a neutral porphyrin in natural deep eutectic solvents. J. Photochem. Photobiol. B 2015, 148, 188–196. [Google Scholar] [CrossRef]
- Jablonsky, M.; Skulcova, A.; Kamenska, L.; Vrska, M.; Sima, J. Deep Eutectic Solvents: Fractionation of Wheat Straw. Bioresources 2015, 10, 8039–8047. [Google Scholar] [CrossRef]
- Bi, Z.; Li, T.; Su, H.; Ni, Y.; Yan, L. Transparent Wood Film Incorporating Carbon Dots as Encapsulating Material for White Light-Emitting Diodes. ACS Sustain. Chem. Eng. 2018, 6, 9314–9323. [Google Scholar] [CrossRef]
- Chen, Z.; Dang, B.; Luo, X.; Li, W.; Li, J.; Yu, H.; Liu, S.; Li, S. Deep Eutectic Solvent-Assisted In Situ Wood Delignification: A Promising Strategy To Enhance the Efficiency of Wood-Based Solar Steam Generation Devices. ACS Appl. Mater. Interfaces 2019, 11, 26032–26037. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin. Bioresour. Technol. 2019, 281, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohyd. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Ma, H.; Zhang, S.; Li, J.; Xia, C.; Shi, S.Q.; Cai, L. In-Situ Chemosynthesis of ZnO Nanoparticles to EndowWood with Antibacterial and UV-Resistance Properties. J. Mater. Sci. Technol. 2017, 33, 266–270. [Google Scholar] [CrossRef]
- Pinkert, A.; Goeke, D.F.; Marsh, K.N.; Pang, S. Extracting wood lignin without dissolving or degrading cellulose: Investigations on the use of food additive-derived ionic liquids. Green Chem. 2011, 13, 3124–3136. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Zhang, N.; Li, S.; Xiong, L.; Hong, Y.; Chen, Y. Cellulose-hemicellulose interaction in wood secondary cell-wall. Model. Simul. Mater. Sci. Eng. 2015, 23, 085010. [Google Scholar] [CrossRef]
- Decou, R.; Serk, H.; Menard, D.; Pesquet, E. Analysis of Lignin Composition and Distribution Using Fluorescence Laser Confocal Microspectroscopy. Methods Mol. Biol. 2017, 1544, 233–247. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Kim, H.J.; Yang, H.S. Thermal properties of bio-flour-filled polyolefin composites with different compatibilizing agent type and content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Shebani, A.N.; van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood species. Thermochim. Acta 2008, 471, 43–50. [Google Scholar] [CrossRef]
- Shebani, A.N.; Van Reenen, A.J.; Meincken, M. The Effect of Wood Species on the Mechanical and Thermal Properties of Wood-LLDPE Composites. J. Compos. Mater. 2009, 43, 1305–1318. [Google Scholar] [CrossRef]



| Sample | T0 (°C) | Tf (°C) | Tmax (°C) | Residue at 600 °C(%) |
|---|---|---|---|---|
| NW | 249.51 | 377.38 | 368.54 | 21.95 |
| CW-1 | 212.20 | 367.24 | 355.72 | 18.19 |
| CW-2 | 207.13 | 332.01 | 297.15 | 31.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Chen, M.; Wu, Y. Compressible Cellulose Wood Prepared with Deep Eutectic Solvents and Its Improved Technology. Polymers 2023, 15, 1593. https://doi.org/10.3390/polym15071593
Wang W, Chen M, Wu Y. Compressible Cellulose Wood Prepared with Deep Eutectic Solvents and Its Improved Technology. Polymers. 2023; 15(7):1593. https://doi.org/10.3390/polym15071593
Chicago/Turabian StyleWang, Wenhao, Mengyao Chen, and Yan Wu. 2023. "Compressible Cellulose Wood Prepared with Deep Eutectic Solvents and Its Improved Technology" Polymers 15, no. 7: 1593. https://doi.org/10.3390/polym15071593
APA StyleWang, W., Chen, M., & Wu, Y. (2023). Compressible Cellulose Wood Prepared with Deep Eutectic Solvents and Its Improved Technology. Polymers, 15(7), 1593. https://doi.org/10.3390/polym15071593





