From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology
Abstract
1. Review Contents
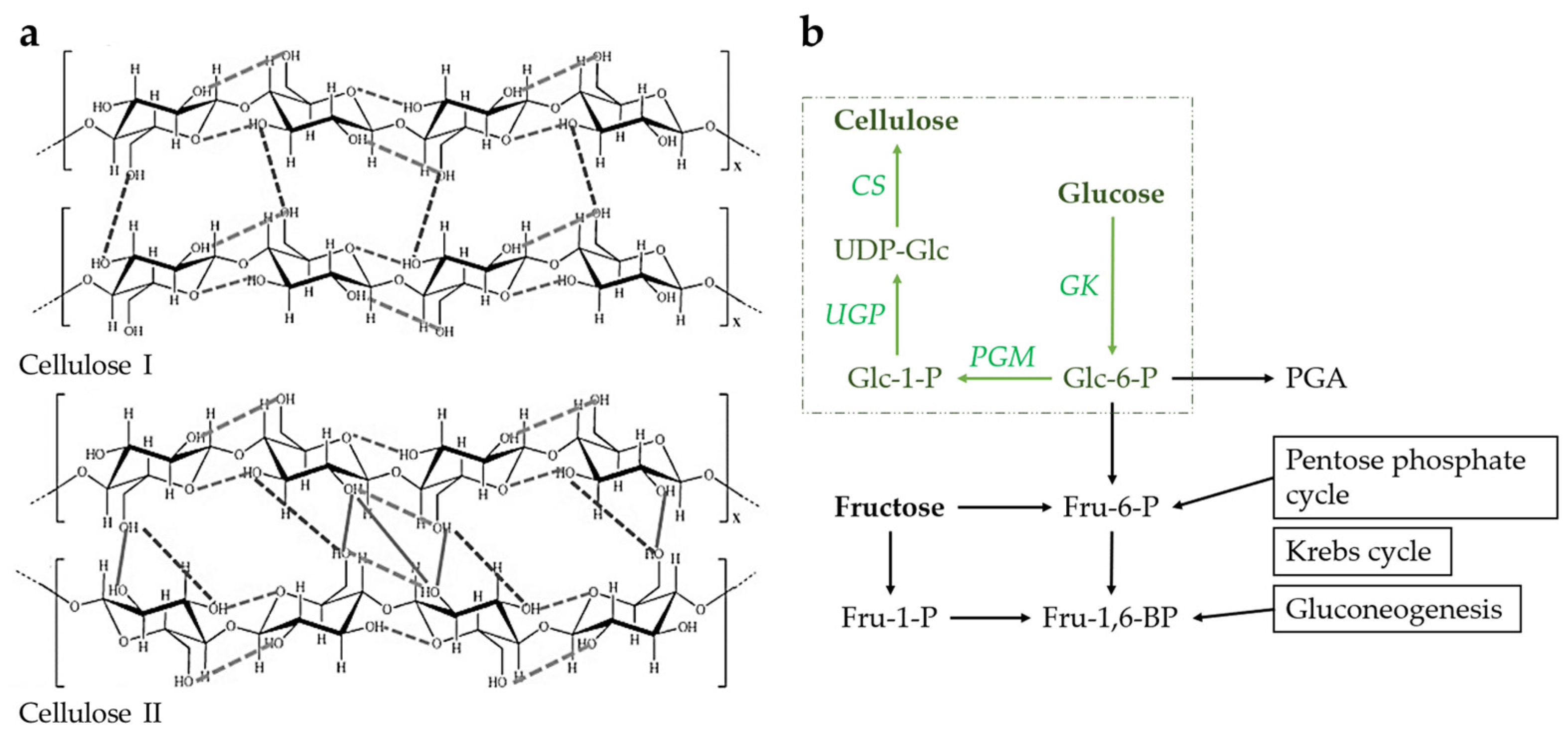
2. Bacterial Cellulose as Featured Nanomaterial
2.1. Introduction to Bacterial Cellulose
2.2. Growth Media for Bacterial Cellulose Production
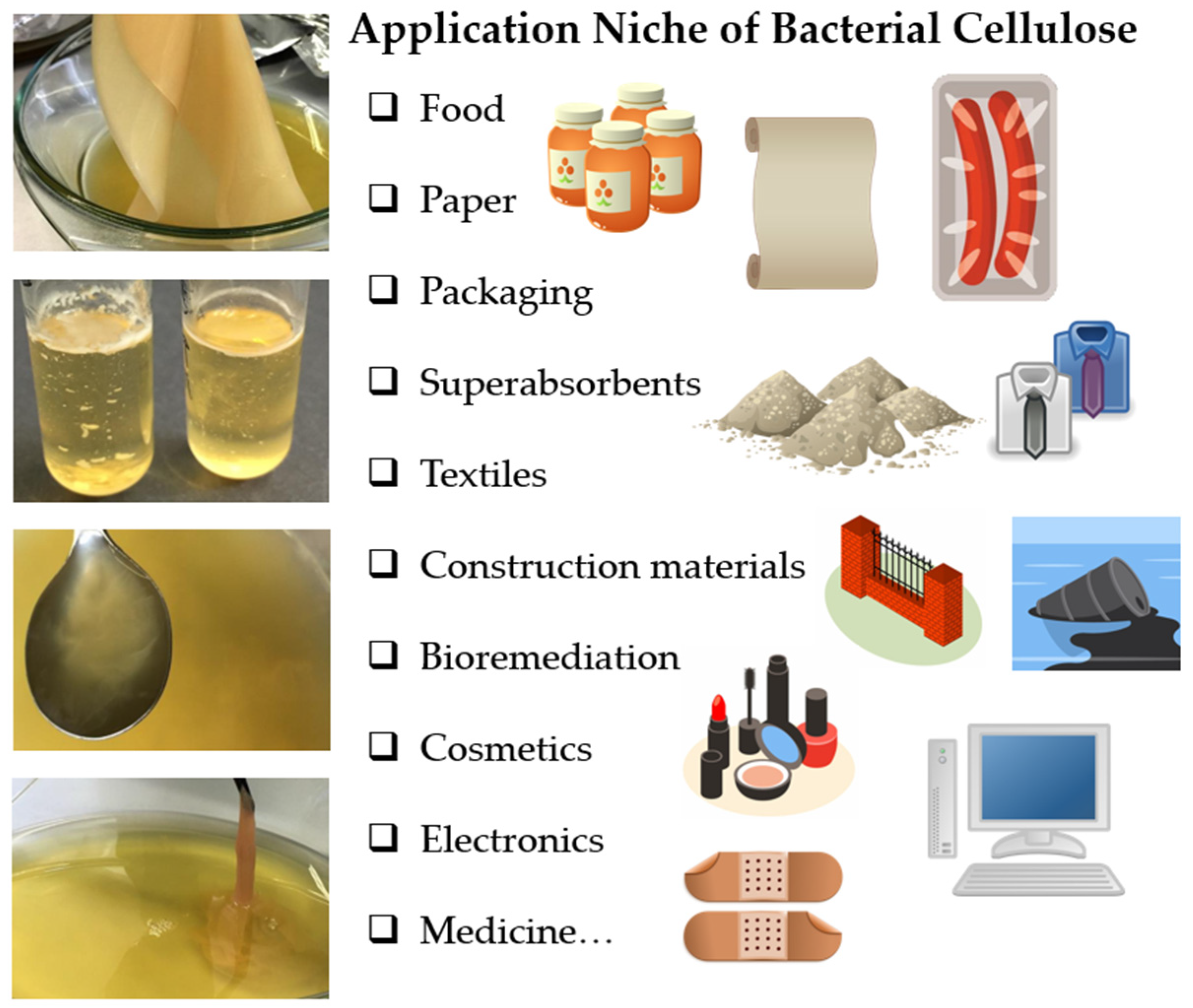
2.3. Application Niches of Bacterial Cellulose
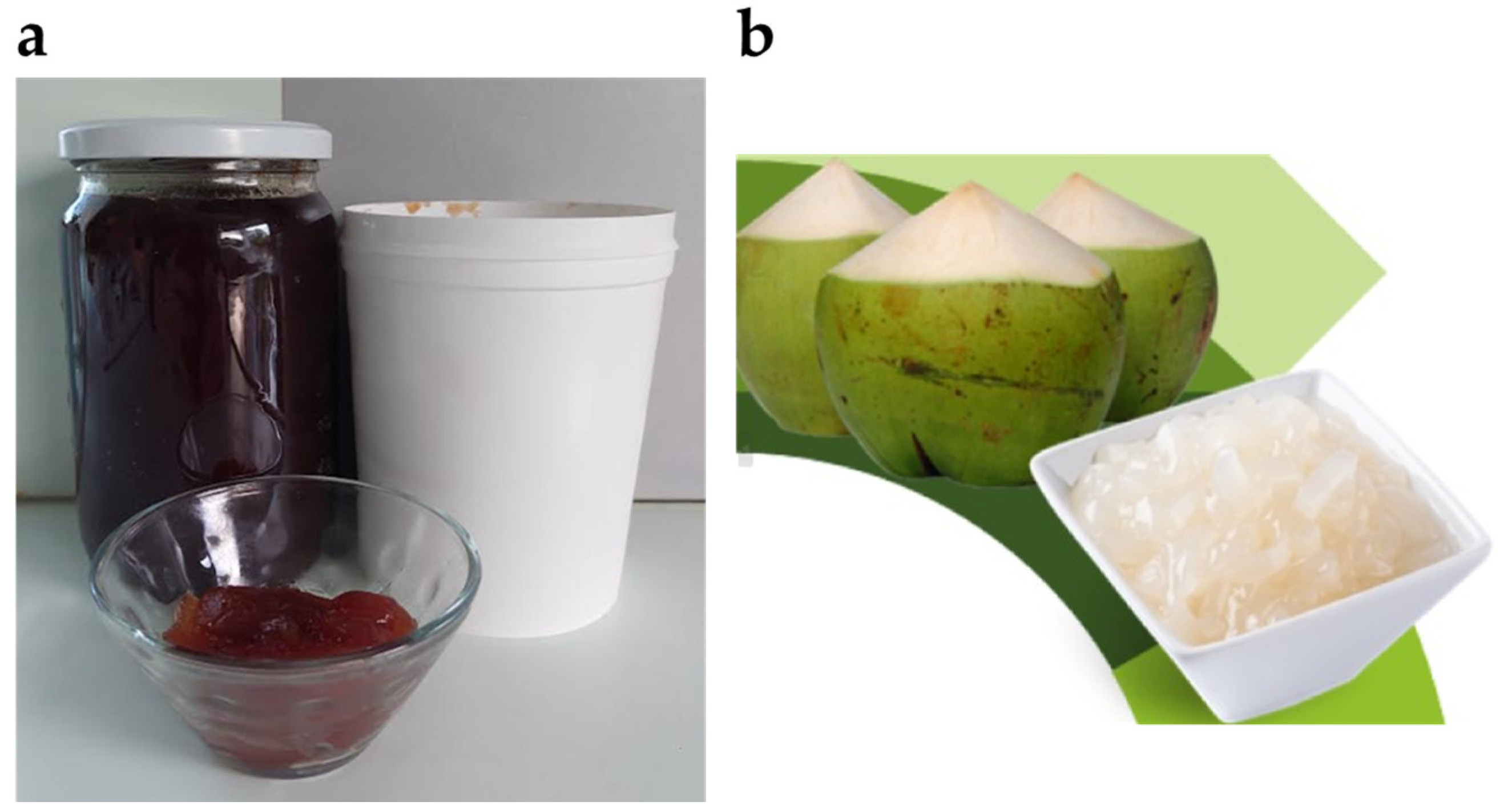
2.3.1. Application of Bacterial Cellulose in Food and Cosmetics
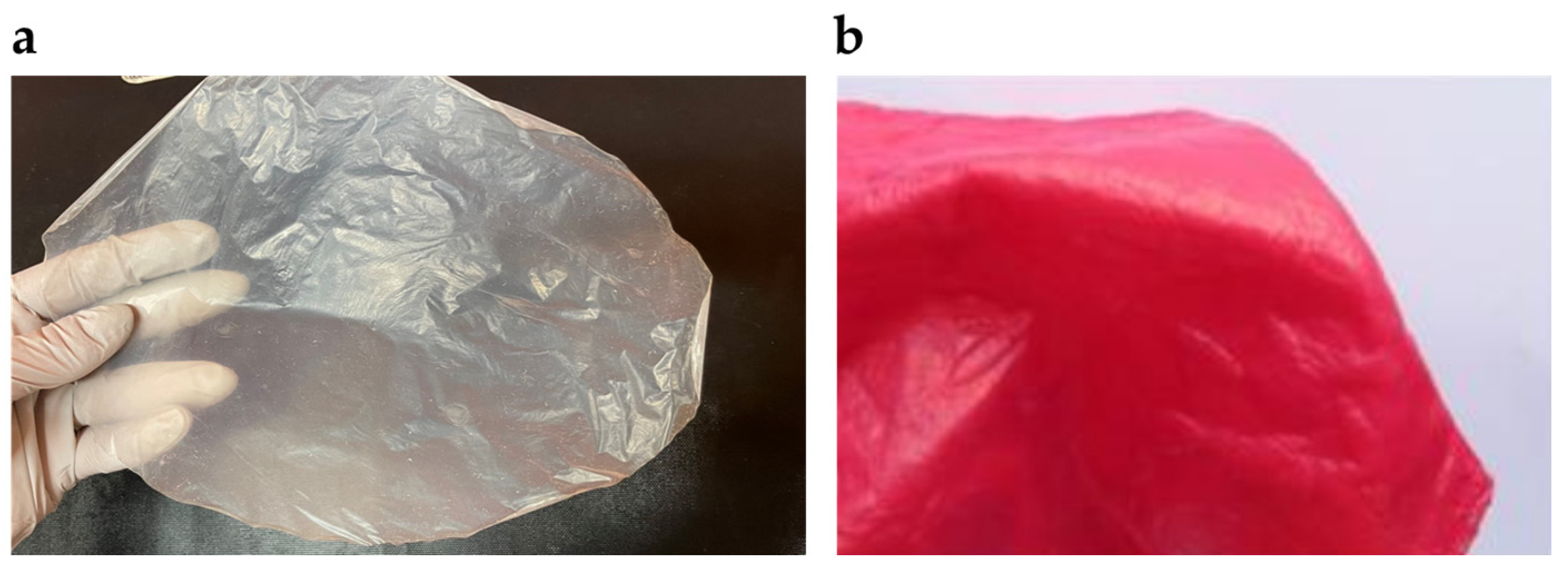
2.3.2. Application of Bacterial Cellulose as Advanced Material
2.3.3. Application of Bacterial Cellulose in Medicine
3. Strategies for Improvement of Bacterial Cellulose Production and Modification
3.1. Conventional Methods for Improvements of Bacterial Cellulose Production and Modification
3.2. Co-Culturing Bacteria for Improvements of Bacterial Cellulose Production and Functionality
3.3. Genetic Engineering of Bacterial Cellulose Producing Strains
3.3.1. Conventional Gene Targeted Approaches for Modification of Bacterial Cellulose Production
3.3.2. Standardized Genetic Tools for Bacterial Cellulose Production with Advanced Characteristics
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological production of cellulose by acetic acid bacteria: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef]
- Marič, L.; Cleenwerck, I.; Accetto, T.; Vandamme, P.; Trček, J. Description of Komagataeibacter melaceti sp. nov. and Komagataeibacter melomenusus sp. nov. Isolated from Apple Cider Vinegar. Microorganisms 2020, 8, 1178. [Google Scholar] [CrossRef]
- La China, S.; Bezzecchi, A.; Moya, F.; Petroni, G.; Di Gregorio, S.; Gullo, M. Genome sequencing and phylogenetic analysis of K1G4: A new Komagataeibacter strain producing bacterial cellulose from different carbon sources. Biotechnol. Lett. 2020, 42, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Adachi, O.; Moonmangmee, D.; Toyama, H.; Yamada, M.; Shinagawa, E.; Matsushita, K. New developments in oxidative fermentation. Appl. Microbiol. Biotechnol. 2003, 60, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Ryngajłło, M.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Jacek, P.; Bielecki, S. Comparative genomics of the Komagataeibacter strains—Efficient bionanocellulose producers. Microbiologyopen 2019, 8, e731. [Google Scholar] [CrossRef]
- Brandão, P.R.; Crespo, M.T.B.; Nascimento, F.X. Phylogenomic and comparative analyses support the reclassification of several Komagataeibacter species as novel members of the Novacetimonas gen. nov. and bring new insights into the evolution of cellulose synthase genes. Int. J. Syst. Evol. Microbiol. 2022, 72, 005252. [Google Scholar] [CrossRef]
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and tolerance of bacteria against acetic acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef]
- Bimmer, M.; Mientus, M.; Klingl, A.; Ehrenreich, A.; Liebl, W. The Roles of the Various Cellulose Biosynthesis Operons in Komagataeibacter hansenii ATCC 23769. Appl. Environ. Microbiol. 2022, 88, e02460-21. [Google Scholar] [CrossRef]
- Anguluri, K.; La China, S.; Brugnoli, M.; Cassanelli, S.; Gullo, M. Better under stress: Improving bacterial cellulose production by Komagataeibacter xylinus K2G30 (UMCC 2756) using adaptive laboratory evolution. Front. Microbiol. 2022, 13, 994097. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Walker, K.T.; Ledesma-Amaro, R.; Ellis, T. Engineering Bacterial Cellulose by Synthetic Biology. Int. J. Mol. Sci. 2020, 21, 9185. [Google Scholar] [CrossRef] [PubMed]
- Buldum, G.; Mantalaris, A. Systematic Understanding of Recent Developments in Bacterial Cellulose Biosynthesis at Genetic, Bioprocess and Product Levels. Int. J. Mol. Sci. 2021, 22, 7192. [Google Scholar] [CrossRef]
- Vadanan, S.V.; Basu, A.; Lim, S. Bacterial cellulose production, functionalization, and development of hybrid materials using synthetic biology. Polym. J. 2022, 54, 481–492. [Google Scholar] [CrossRef]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An integrated approach to optimizing cellulose mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef]
- Abidi, W.; Torres-Sánchez, L.; Siroy, A.; Krasteva, P.V. Weaving of bacterial cellulose by the Bcs secretion systems. FEMS Microbiol. Rev. 2022, 46, fuab051. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Deng, Y.; Wei, Q. Research progress of the biosynthetic strains and pathways of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab071. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul Islam, M.; Khan, S.; Shah, N.; Park, J.K. Recent advancements in bioreactions of cellular and cell-free systems: A study of bacterial cellulose as a model. Korean J. Chem. Eng. 2017, 34, 1591–1599. [Google Scholar] [CrossRef]
- Gorgieva, S. Bacterial Cellulose as a Versatile Platform for Research and Development of Biomedical Materials. Processes 2020, 8, 624. [Google Scholar] [CrossRef]
- Bacterial Cellulose Market by Product Type. Available online: https://www.profsharemarketresearch.com/bacterial-cellulose-market-report/ (accessed on 25 July 2023).
- Erbas Kiziltas, E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Brazilian J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Jančič, U.; Cepec, E.; Trček, J. Production efficiency and properties of bacterial cellulose membranes in a novel grape pomace hydrolysate by Komagataeibacter melomenusus AV436T and Komagataeibacter xylinus LMG 1518. Int. J. Biol. Macromol. 2023, 244, 125368. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Uzyol, H.K.; Saçan, M.T. Bacterial cellulose production by Komagataeibacter hansenii using algae-based glucose. Environ. Sci. Pollut. Res. 2017, 24, 11154–11162. [Google Scholar] [CrossRef]
- Jozala, A.F.; Pértile, R.A.N.; dos Santos, C.A.; de Carvalho Santos-Ebinuma, V.; Seckler, M.M.; Gama, F.M.; Pessoa, A. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2015, 99, 1181–1190. [Google Scholar] [CrossRef]
- Guimarães, D.T.; de Oliveira Barros, M.; de Araújo e Silva, R.; Silva, S.M.F.; de Almeida, J.S.; de Freitas Rosa, M.; Gonçalves, L.R.B.; Brígida, A.I.S. Superabsorbent bacterial cellulose film produced from industrial residue of cashew apple juice processing. Int. J. Biol. Macromol. 2023, 242, 124405. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef]
- Sar, T.; Yesilcimen Akbas, M. Potential use of olive oil mill wastewater for bacterial cellulose production. Bioengineered 2022, 13, 7659–7669. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Jin, K.; Jin, C.; Wu, Y. Synthetic biology-powered microbial co-culture strategy and application of bacterial cellulose-based composite materials. Carbohydr. Polym. 2022, 283, 119171. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial cellulose in food industry: Current research and future prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Dourado, F.; Gama, M.; Rodrigues, A.C. A Review on the toxicology and dietetic role of bacterial cellulose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Leksawasdi, N.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Effect of monochloroacetic acid on properties of carboxymethyl bacterial cellulose powder and film from nata de coco. Polymers 2021, 13, 488. [Google Scholar] [CrossRef]
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial Cellulose: A Sustainable Source to Develop Value-Added Products—A Review. BioResources 2016, 11, 5641–5655. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Zywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT—Food Sci. Technol. 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Oliveira-Alcântara, A.V.; Abreu, A.A.S.; Gonçalves, C.; Fuciños, P.; Cerqueira, M.A.; Gama, F.M.P.; Pastrana, L.M.; Rodrigues, S.; Azeredo, H.M.C. Bacterial cellulose/cashew gum films as probiotic carriers. LWT—Food Sci. Technol. 2020, 130, 109699. [Google Scholar] [CrossRef]
- Sabio, L.; González, A.; Ramírez-Rodríguez, G.B.; Gutiérrez-Fernández, J.; Bañuelo, O.; Olivares, M.; Gálvez, N.; Delgado-López, J.M.; Dominguez-Vera, J.M. Probiotic cellulose: Antibiotic-free biomaterials with enhanced antibacterial activity. Acta Biomater. 2021, 124, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bianchet, R.T.; Vieira Cubas, A.L.; Machado, M.M.; Siegel Moecke, E.H. Applicability of bacterial cellulose in cosmetics—Bibliometric review. Biotechnol. Rep. 2020, 27, e00502. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncu, E.E.; Pesman, E. Bacterial Cellulose as Reinforcement in Paper Made from Recycled Office Waste Pulp. BioResource 2020, 15, 8496–8514. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Basta, A.H.; El-Saied, H. Performance of Improved Bacterial Cellulose Application in the Production of Functional Paper. J. Appl. Microbiol. 2009, 107, 2098–2107. [Google Scholar] [CrossRef]
- Janbade, A.; Zaidi, S.; Vats, M.; Kumar, N.; Dhiman, J.; Gupta, M.K. A Mini Review on Current Advancement in Application of Bacterial Cellulose in Pulp and Paper Industry. In Proceedings of the International Conference on Innovative Technologies for Clean and Sustainable Development (ICITCSD—2021); Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Dikshit, P.K. Production of Low-Cost Nano-Functionalized Bacterial Cellulose Films for Smart/Intelligent Packaging. Mater. Proc. 2023, 14, 58. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Mohite, B.V.; Koli, S.H.; Patil, S.V. Bacterial Cellulose-Based Hydrogels: Synthesis, Properties, and Applications. In Cellulose-Based Superabsorbent Hydrogels; Polymers and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 1255–1276. [Google Scholar]
- Walker, K.T.; Keane, J.; Goosens, V.J.; Song, W.; Lee, K.-Y.; Ellis, T. Self-dyeing textiles grown from cellulose-producing bacteria with engineered tyrosinase expression. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fernandes, M.; Souto, A.P.; Dourado, F.; Gama, M. Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites. Polysaccharides 2021, 2, 566–581. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Silvestre, J.; Silvestre, N.; De Brito, J. Review on concrete nanotechnology. Eur. J. Environ. Civ. Eng. 2016, 20, 455–485. [Google Scholar] [CrossRef]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Galdino, C.J.S.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Shen, H.; Liao, S.; Jiang, C.; Zhang, J.; Wei, Q.; Ghiladi, R.A.; Wang, Q. In situ grown bacterial cellulose/MoS2 composites for multi-contaminant wastewater treatment and bacteria inactivation. Carbohydr. Polym. 2022, 277, 118853. [Google Scholar] [CrossRef]
- Prilepskii, A.; Nikolaev, V.; Klaving, A. Conductive bacterial cellulose: From drug delivery to flexible electronics. Carbohydr. Polym. 2023, 313, 120850. [Google Scholar] [CrossRef] [PubMed]
- Anjan, A.; Bharti, V.K.; Sharma, C.S.; Khandelwal, M. Carbonized Bacterial Cellulose-Derived Binder-Free, Flexible, and Free-Standing Cathode Host for High-Performance Stable Potassium-Sulfur Batteries. ACS Appl. Energy Mater. 2023, 6, 3042–3051. [Google Scholar] [CrossRef]
- Gilbert, C.; Tang, T.-C.; Ott, W.; Dorr, B.A.; Shaw, W.M.; Sun, G.L.; Lu, T.K.; Ellis, T. Living materials with programmable functionalities grown from engineered microbial co-cultures. Nat. Mater. 2021, 20, 691–700. [Google Scholar] [CrossRef]
- Gilbert, C.; Ellis, T. Biological Engineered Living Materials: Growing Functional Materials with Genetically Programmable Properties. ACS Synth. Biol. 2019, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, M.; Wang, M.; Hu, Q.; Liu, J.; Duan, Y.; Liu, B. Direct Synthesis of Photosensitizable Bacterial Cellulose as Engineered Living Material for Skin Wound Repair. Adv. Mater. 2022, 34, 2109010. [Google Scholar] [CrossRef]
- Lin, S.P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.R.; Demirci, A.; Cheng, K.C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Navas-Martos, F.J.; Soriano-Cuadrado, B.; Paniza, J.M.L.; Simchi, A. Development of chitosan/bacterial cellulose composite films containing nanodiamonds as a potential flexible platform for wound dressing. Materials 2015, 8, 6401–6418. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.H.; Chou, S.C.; Chen, C.L.; Wang, Y.W.; Chang, S.J.; Fan, G.Y.; Leung, F.S.; Meng, E. Bacterial cellulose as a potential bio-scaffold for effective re-epithelialization therapy. Pharmaceutics 2021, 13, 1592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Urbina, L.; Eceiza, A.; Gabilondo, N.; Corcuera, M.Á.; Retegi, A. Tailoring the in situ conformation of bacterial cellulose-graphene oxide spherical nanocarriers. Int. J. Biol. Macromol. 2020, 163, 1249–1260. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Islan, G.A.; León, I.E.; Álvarez, V.A.; Chourpa, I.; Allard-Vannier, E.; García-Aranda, N.; Díaz-Riascos, Z.V.; Fernández, Y.; Schwartz, S.; et al. Bacterial cellulose hydrogel loaded with lipid nanoparticles for localized cancer treatment. Colloids Surf. B Biointerfaces 2018, 170, 596–608. [Google Scholar] [CrossRef]
- Islam, S.U.; Ul-Islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential applications of bacterial cellulose and its composites for cancer treatment. Int. J. Biol. Macromol. 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Autier, L.; Clavreul, A.; Cacicedo, M.L.; Franconi, F.; Sindji, L.; Rousseau, A.; Perrot, R.; Montero-Menei, C.N.; Castro, G.R.; Menei, P. A new glioblastoma cell trap for implantation after surgical resection. Acta Biomater. 2019, 84, 268–279. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.C.; Li, X.; Zhang, L.; Jiang, F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef] [PubMed]
- Niamsap, T.; Lam, N.T.; Sukyai, P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019, 205, 159–166. [Google Scholar] [CrossRef]
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020, 149, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Hribernik, S. Microstructured and degradable bacterial cellulose–gelatin composite membranes: Mineralization aspects and biomedical relevance. Nanomaterials 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Bae, S.; Shoda, M. Statistical Optimization of Culture Conditions for Bacterial Cellulose Production Using Box-Behnken Design. Biotechnol. Bioeng. 2005, 90, 20–28. [Google Scholar] [CrossRef]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr. Polym. 2012, 89, 613–622. [Google Scholar] [CrossRef]
- Son, H.-J.; Heo, M.-S.; Kim, Y.-G.; Lee, S.-J. Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter sp. A9 in shaking cultures. Biotechnol. Appl. Biochem. 2001, 33, 1–5. [Google Scholar] [CrossRef]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Jagannath, A.; Kalaiselvan, A.; Manjunatha, S.S.; Raju, P.S.; Bawa, A.S. The effect of pH, sucrose and ammonium sulphate concentrations on the production of bacterial cellulose (Nata-de-coco) by Acetobacter xylinum. World J. Microbiol. Biotechnol. 2008, 24, 2593–2599. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.d.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Kim, H.G.; Kim, K.K.; Kim, H.S.; Kim, Y.G.; Lee, S.J. Increased production of bacterial cellulose by Acetobacter sp. V6 in synthetic media under shaking culture conditions. Bioresour. Technol. 2003, 86, 215–219. [Google Scholar] [CrossRef]
- Zhou, L.L.; Sun, D.P.; Hu, L.Y.; Li, Y.W.; Yang, J.Z. Effect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. J. Ind. Microbiol. Biotechnol. 2007, 34, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Flanagan, B.; Gidley, M.J.; Dykes, G.A. Characterization of cellulose production by a Gluconacetobacter xylinus strain from Kombucha. Curr. Microbiol. 2008, 57, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Shoda, M. Bacterial cellulose production by fed-batch fermentation in molasses medium. Biotechnol. Prog. 2004, 20, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors affecting the yield and properties of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef]
- Ishihara, M.; Matsunaga, M.; Hayashi, N.; Tišler, V. Utilization of D-xylose as carbon source for production of bacterial cellulose. Enzyme Microb. Technol. 2002, 31, 986–991. [Google Scholar] [CrossRef]
- Keshk, S.; Sameshima, K. The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Appl. Microbiol. Biotechnol. 2006, 72, 291–296. [Google Scholar] [CrossRef]
- Pourramezan, G.Z.; Roayaei, A.M.; Qezelbash, Q.R. Optimization of Culture Conditions for Bacterial Cellulose Production by Acetobacter sp. 4B-2. Biotechnology 2009, 8, 150–154. [Google Scholar] [CrossRef]
- Fang, L.; Catchmark, J.M. Characterization of cellulose and other exopolysaccharides produced from Gluconacetobacter strains. Carbohydr. Polym. 2015, 115, 663–669. [Google Scholar] [CrossRef]
- Basu, A.; Vadanan, S.V.; Lim, S. Rational design of a scalable bioprocess platform for bacterial cellulose production. Carbohydr. Polym. 2019, 207, 684–693. [Google Scholar] [CrossRef]
- Toyosaki, H.; Naritomi, T.; Seto, A.; Matsuoka, M.; Tsuchida, T.; Yoshinaga, F. Screening of Bacterial Cellulose-producing Acetobacter Strains Suitable for Agitated Culture. Biosci. Biotechnol. Biochem. 1995, 59, 1498–1502. [Google Scholar] [CrossRef]
- Seto, A.; Kojima, Y.; Tonouchi, N.; Tsuchida, T.; Yoshinaga, F. Screening of Bacterial Cellulose-producing Acetobacter Strains Suitable for Sucrose as a Carbon Source. Biosci. Biotechnol. Biochem. 1997, 61, 735–736. [Google Scholar] [CrossRef][Green Version]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of cellulose in aqueous NaOH/urea solution: Role of urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Shanshan, G.; Jianqing, W.; Zhengwei, J. Preparation of cellulose films from solution of bacterial cellulose in NMMO. Carbohydr. Polym. 2012, 87, 1020–1025. [Google Scholar] [CrossRef]
- Jin, H.; Zha, C.; Gu, L. Direct dissolution of cellulose in NaOH/thiourea/urea aqueous solution. Carbohydr. Res. 2007, 342, 851–858. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Vadanan, S.V.; Lim, S. Enhanced rheological properties and conductivity of bacterial cellulose hydrogels and aerogels through complexation with metal ions and PEDOT/PSS. Cellulose 2020, 27, 8075–8086. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining Cellulose Nanofibers with a Uniform Width of 15 nm from Wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, Q. A Novel Process to Isolate Fibrils from Cellulose Fibers by High-Intensity Ultrasonication, Part 1: Process Optimization. J. Appl. Polym. Sci. 2009, 113, 1270–1275. [Google Scholar] [CrossRef]
- Saibuatong, O.; Phisalaphong, M. Novo aloe vera-bacterial cellulose composite film from biosynthesis. Carbohydr. Polym. 2010, 79, 455–460. [Google Scholar] [CrossRef]
- De Lima Fontes, M.; Meneguin, A.B.; Tercjak, A.; Gutierrez, J.; Cury, B.S.F.; dos Santos, A.M.; Ribeiro, S.J.L.; Barud, H.S. Effect of in situ modification of bacterial cellulose with carboxymethylcellulose on its nano/microstructure and methotrexate release properties. Carbohydr. Polym. 2018, 179, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Mandelli, J.S.; Marins, J.A.; Soares, B.G.; Porto, L.M.; Rambo, C.R.; Barra, G.M.O. Electrically conducting nanocomposites: Preparation and properties of polyaniline (PAni)-coated bacterial cellulose nanofibers (BC). Cellulose 2012, 19, 1645–1654. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; He, Y.; Duan, T. In-situ biopreparation of biocompatible bacterial cellulose/graphene oxide composites pellets. Appl. Surf. Sci. 2015, 338, 22–26. [Google Scholar] [CrossRef]
- Arias, S.L.; Shetty, A.R.; Senpan, A.; Echeverry-Rendón, M.; Reece, L.M.; Allain, J.P. Fabrication of a functionalized magnetic bacterial nanocellulose with iron oxide nanoparticles. J. Vis. Exp. 2016, 2016, e52951. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, S.; Wang, H.; Wang, B.; Jiang, J. Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr. Polym. 2008, 74, 659–665. [Google Scholar] [CrossRef]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. In situ modifications to bacterial cellulose with the water insoluble polymer poly-3-hydroxybutyrate. Carbohydr. Polym. 2013, 92, 1717–1723. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, G.; Zhou, Y.; Liu, Y.; Feng, Y.; Li, J. Effects of Sodium Alginate on Microstructural and Properties of Bacterial Cellulose Nanocrystal Stabilized Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125474. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Shah, N.; Ha, J.H.; Park, J.K. Effect of chitosan penetration on physico-chemical and mechanical properties of bacterial cellulose. Korean J. Chem. Eng. 2011, 28, 1736–1743. [Google Scholar] [CrossRef]
- Zhijiang, C.; Guang, Y. Bacterial cellulose/collagen composite: Characterization and first evaluation of cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar] [CrossRef]
- Wang, F.; Kim, H.J.; Park, S.; Kee, C.D.; Kim, S.J.; Oh, I.K. Bendable and flexible supercapacitor based on polypyrrole-coated bacterial cellulose core-shell composite network. Compos. Sci. Technol. 2016, 128, 33–40. [Google Scholar] [CrossRef]
- Lopes, T.D.; Riegel-Vidotti, I.C.; Grein, A.; Tischer, C.A.; Faria-Tischer, P.C. de S. Bacterial cellulose and hyaluronic acid hybrid membranes: Production and characterization. Int. J. Biol. Macromol. 2014, 67, 401–408. [Google Scholar] [CrossRef]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; O’Neill, H.M.; Rawn, C.J. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Golmohammadi, H.; Naghdi, T.; Yousefi, H. Green in-situ synthesized silver nanoparticles embedded in bacterial cellulose nanopaper as a bionanocomposite plasmonic sensor. Biosens. Bioelectron. 2015, 74, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; Barrios, C.; Regiani, T.; Marques, R.F.C.; Verelst, M.; Dexpert-Ghys, J.; Messaddeq, Y.; Ribeiro, S.J.L. Self-supported silver nanoparticles containing bacterial cellulose membranes. Mater. Sci. Eng. C 2008, 28, 515–518. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Nanoreinforced bacterial cellulose-montmorillonite composites for biomedical applications. Carbohydr. Polym. 2012, 89, 1189–1197. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef]
- Seto, A.; Saito, Y.; Matsushige, M.; Kobayashi, H.; Sasaki, Y.; Tonouchi, N.; Tsuchida, T.; Yoshinaga, F.; Ueda, K.; Beppu, T. Effective cellulose production by a coculture of Gluconacetobacter xylinus and Lactobacillus mali. Appl. Microbiol. Biotechnol. 2006, 73, 915–921. [Google Scholar] [CrossRef]
- Liu, K.; Catchmark, J.M. Enhanced mechanical properties of bacterial cellulose nanocomposites produced by co-culturing Gluconacetobacter hansenii and Escherichia coli under static conditions. Carbohydr. Polym. 2019, 219, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Catchmark, J.M. Bacterial cellulose/hyaluronic acid nanocomposites production through co-culturing Gluconacetobacter hansenii and Lactococcus lactis in a two-vessel circulating system. Bioresour. Technol. 2019, 290, 121715. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Hu, S.; Xu, M.; Hu, Q.; Jiang, S.; Xu, K.; Tremblay, P.L.; Zhang, T. The facile and controllable synthesis of a bacterial cellulose/polyhydroxybutyrate composite by co-culturing Gluconacetobacter xylinus and Ralstonia eutropha. Carbohydr. Polym. 2021, 252, 117137. [Google Scholar] [CrossRef]
- Gunduz, G.; Erbas Kiziltas, E.; Kiziltas, A.; Gencer, A.; Aydemir, D.; Asik, N. Production of bacterial cellulose fibers in the presence of effective microorganism. J. Nat. Fibers 2019, 16, 567–575. [Google Scholar] [CrossRef]
- Brugnoli, M.; Mazzini, I.; La China, S.; De Vero, L.; Gullo, M. A Microbial Co-Culturing System for Producing Cellulose-Hyaluronic Acid Composites. Microorganisms 2023, 11, 1504. [Google Scholar] [CrossRef]
- Nakai, T.; Tonouchi, N.; Konishi, T.; Kojima, Y.; Tsuchida, T.; Yoshinaga, F.; Sakai, F.; Hayashi, T. Enhancement of cellulose production by expression of sucrose synthase in Acetobacter xylinum. Proc. Natl. Acad. Sci. USA 1999, 96, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Battad-Bernardo, E.; McCrindle, S.L.; Couperwhite, I.; Neilan, B.A. Insertion of an E. coli lacZ gene in Acetobacter xylinus for the production of cellulose in whey. FEMS Microbiol. Lett. 2004, 231, 253–260. [Google Scholar] [CrossRef]
- Shigematsu, T.; Takamine, K.; Kitazato, M.; Morita, T.; Naritomi, T.; Morimura, S.; Kida, K. Cellulose production from glucose using a glucose dehydrogenase gene (gdh)-deficient mutant of Gluconacetobacter xylinus and its use for bioconversion of sweet potato pulp. J. Biosci. Bioeng. 2005, 99, 415–422. [Google Scholar] [CrossRef]
- Chien, L.J.; Chen, H.T.; Yang, P.F.; Lee, C.K. Enhancement of cellulose pellicle production by constitutively expressing Vitreoscilla hemoglobin in Acetobacter xylinum. Biotechnol. Prog. 2006, 22, 1598–1603. [Google Scholar] [CrossRef]
- Yadav, V.; Paniliatis, B.J.; Shi, H.; Lee, K.; Cebe, P.; Kaplan, D.L. Novel in vivo-degradable cellulose-chitin copolymer from metabolically engineered Gluconacetobacter xylinus. Appl. Environ. Microbiol. 2010, 76, 6257–6265. [Google Scholar] [CrossRef]
- Imai, T.; Sun, S.J.; Horikawa, Y.; Wada, M.; Sugiyama, J. Functional reconstitution of cellulose synthase in Escherichia coli. Biomacromolecules 2014, 15, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Kawano, S.; Tajima, K.; Kondo, T. In Vivo Curdlan/Cellulose Bionanocomposite Synthesis by Genetically Modified Gluconacetobacter xylinus. Biomacromolecules 2015, 16, 3154–3160. [Google Scholar] [CrossRef]
- Florea, M.; Hagemann, H.; Santosa, G.; Abbott, J.; Micklem, C.N.; Spencer-Milnes, X.; De Arroyo Garcia, L.; Paschou, D.; Lazenbatt, C.; Kong, D.; et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-prod ucing strain. Proc. Natl. Acad. Sci. USA 2016, 113, E3431–E3440. [Google Scholar] [CrossRef] [PubMed]
- Buldum, G.; Bismarck, A.; Mantalaris, A. Recombinant biosynthesis of bacterial cellulose in genetically modified Escherichia coli. Bioprocess Biosyst. Eng. 2018, 41, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Kim, T.Y.; Kim, H.U.; Shim, W.Y.; Ryu, J.Y.; Park, J.H.; Lee, S.Y. Genomic and metabolic analysis of Komagataeibacter xylinus DSM 2325 producing bacterial cellulose nanofiber. Biotechnol. Bioeng. 2019, 116, 3372–3381. [Google Scholar] [CrossRef]
- Gwon, H.; Park, K.; Chung, S.C.; Kim, R.H.; Kang, J.K.; Ji, S.M.; Kim, N.J.; Lee, S.; Ku, J.H.; Do, E.C.; et al. A safe and sustainable bacterial cellulose nanofiber separator for lithium rechargeable batteries. Proc. Natl. Acad. Sci. USA 2019, 116, 19288–19293. [Google Scholar] [CrossRef]
- Walker, K.T.; Goosens, V.J.; Das, A.; Graham, A.E.; Ellis, T. Engineered cell-to-cell signalling within growing bacterial cellulose pellicles. Microb. Biotechnol. 2019, 12, 611–619. [Google Scholar] [CrossRef]
- Teh, M.Y.; Ooi, K.H.; Danny Teo, S.X.; Bin Mansoor, M.E.; Shaun Lim, W.Z.; Tan, M.H. An Expanded Synthetic Biology Toolkit for Gene Expression Control in Acetobacteraceae. ACS Synth. Biol. 2019, 8, 708–723. [Google Scholar] [CrossRef]
- Jacek, P.; Ryngajłło, M.; Bielecki, S. Structural changes of bacterial nanocellulose pellicles induced by genetic modification of Komagataeibacter hansenii ATCC 23769. Appl. Microbiol. Biotechnol. 2019, 103, 5339–5353. [Google Scholar] [CrossRef]
- Liu, D.; Cao, Y.; Qu, R.; Gao, G.; Chen, S.; Zhang, Y.; Wu, M.; Ma, T.; Li, G. Production of bacterial cellulose hydrogels with tailored crystallinity from Enterobacter sp. FY-07 by the controlled expression of colanic acid synthetic genes. Carbohydr. Polym. 2019, 207, 563–570. [Google Scholar] [CrossRef]
- Huang, L.H.; Liu, Q.J.; Sun, X.W.; Li, X.J.; Liu, M.; Jia, S.R.; Xie, Y.Y.; Zhong, C. Tailoring bacterial cellulose structure through CRISPR interference-mediated downregulation of galU in Komagataeibacter xylinus CGMCC 2955. Biotechnol. Bioeng. 2020, 117, 2165–2176. [Google Scholar] [CrossRef] [PubMed]



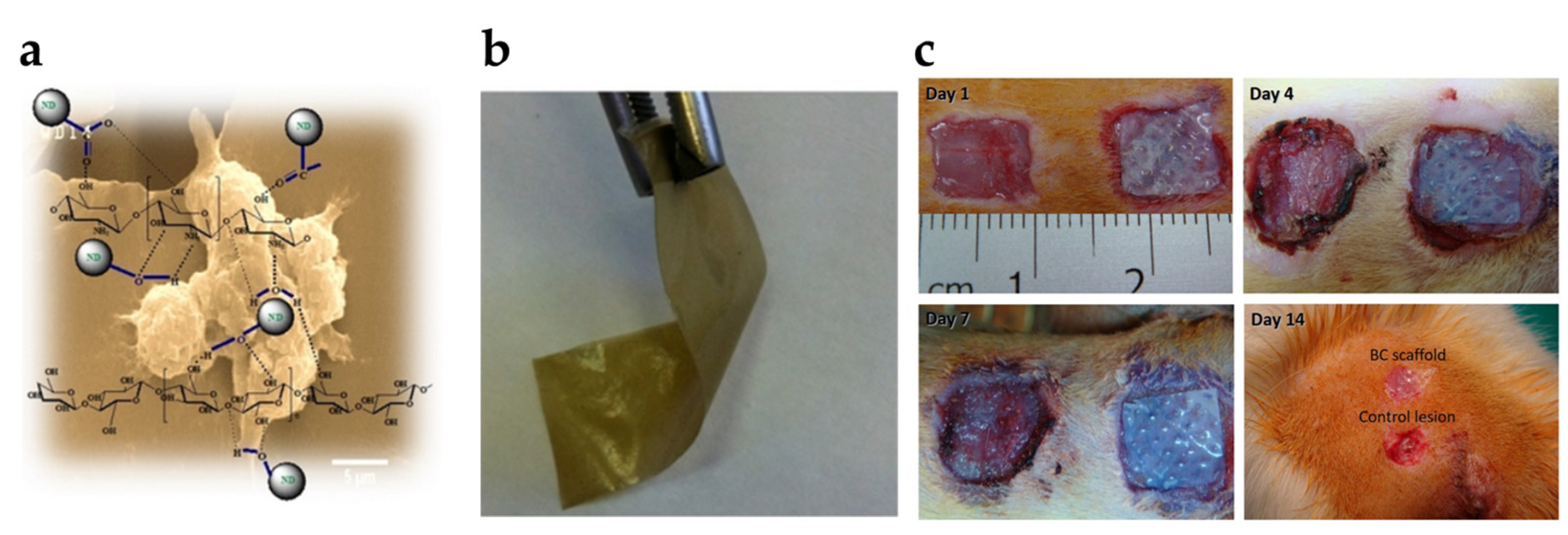


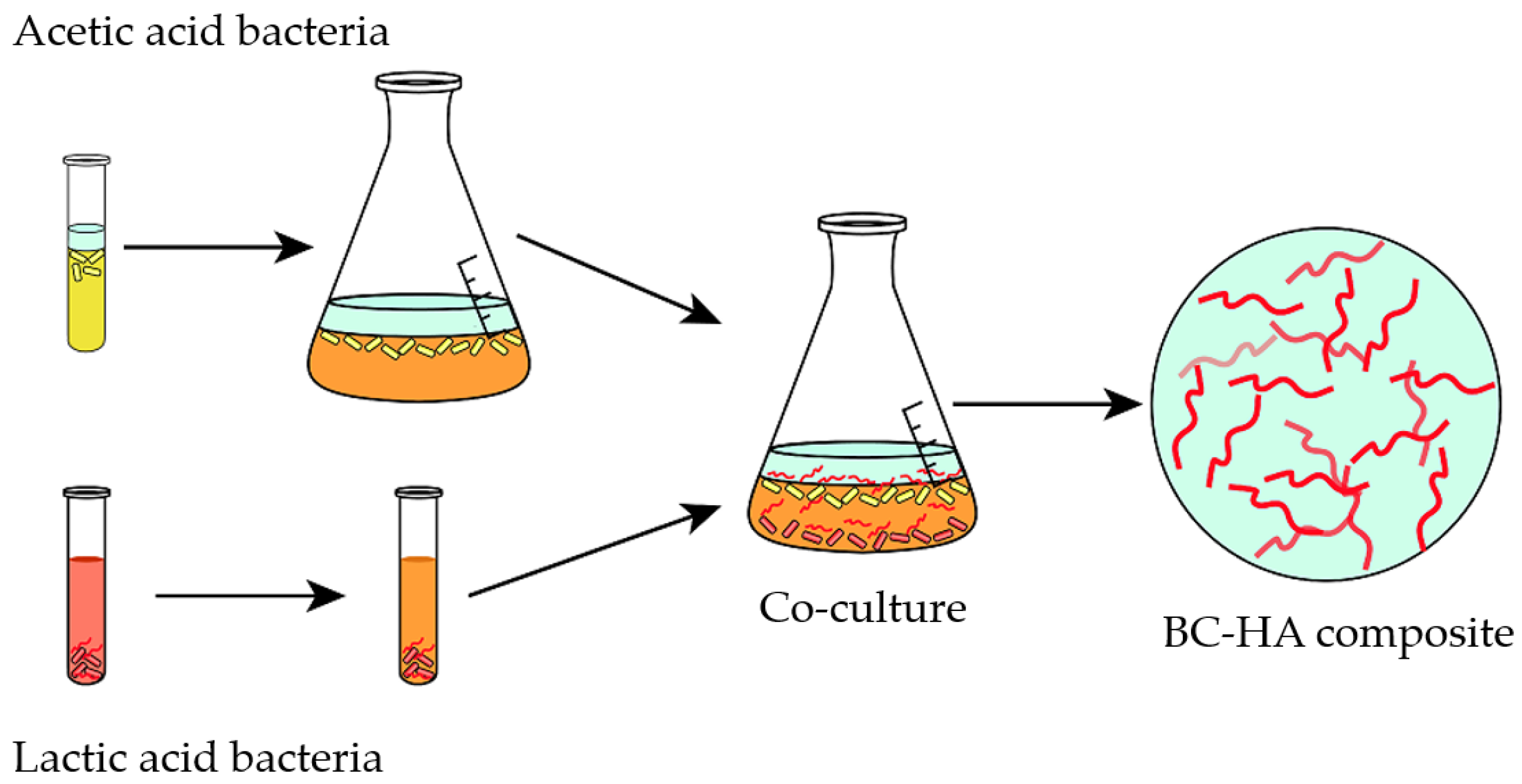

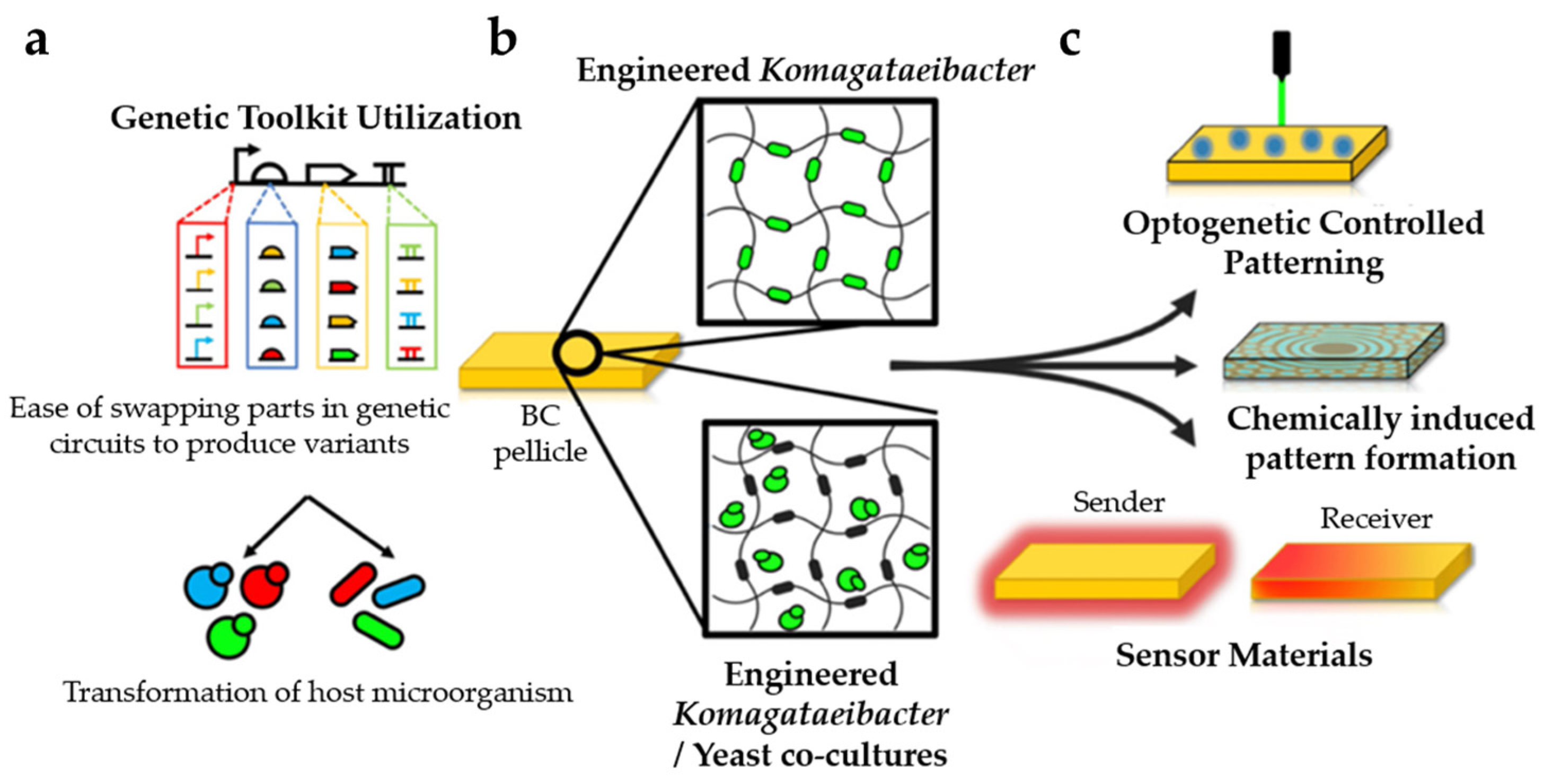

| Year | Test Organism | Short Description | Reference |
|---|---|---|---|
| 1999 | Acetobacter xylinum subsp. sucrofermentans BPR 2001 (K. sucrofermentans) | Introducing the sucrose synthase gene enables sucrose metabolism and improves BC production | [129] |
| 2004 | Acetobacter ITDI 2.1 (K. xylinus) | Introducing the β-galactosidase-encoding gene (lacZ) enables lactose metabolism and improves BC production | [130] |
| 2005 | G. xylinus BPR 2001 (K. sucrofermentans) | Knocking out the glucose dehydrogenase-encoding gene (gdh) improves BC production | [131] |
| 2006 | A. xylinum BCRC 12334 (K. xylinus) | Expressing the Vitreoscilla hemoglobin gene (vhb) improves oxygen regulation, cell growth, and BC production | [132] |
| 2006 | G. xylinus st-60-12 (K. xylinus), L. mali JCM 1116 | Co-culturing K. xylinus and L. mali improves BC production | [123] |
| 2010 | G. xylinus ATCC 10245 (K. xylinus) | Introducing the UDP-N-acetylglucosamine-synthesizing operon enables the production of BC/chitin copolymer with improved in vivo degradability | [133] |
| 2014 | E. coli XL1-Blue | Co-expressing cellulose synthase genes for proteins BcsA, BcsB, and diguanylate cyclase (DGC) enables the production of amorphous BC in E. coli | [134] |
| 2015 | G. xylinus AY201 (K. xylinus) | Introducing the curdlan-synthesizing gene (crdS) enables the production of BC/curdlan composite | [135] |
| 2016 | K. rhaeticus iGEM |
| [136] |
| 2018 | E. coli GM HMS174 (DE3), E. coli GM C41 (DE3) | Introducing cellulose synthase-encoding operon (bcsABCD) enables recombinant biosynthesis of BC in E. coli | [137] |
| 2019 | K. xylinus DSM 2325 | Overexpressing two carbon metabolism genes (pgi and gnd) improves BC production | [138] |
| 2019 | K. xylinus DSM 2325 | Introducing the phosphofructokinase-encoding gene (pfkA) establishes a glycolytic pathway, increases the level of intracellular ATP, enhances cell growth, and improves BC production | [139] |
| 2019 | K. rhaeticus iGEM | Co-culturing AHL-inducible and AHL-synthesizing cells enables the developing BC to initiate its red fluorescence | [140] |
| 2019 | G. hansenii ATCC 53582 (N. hansenii), G. xylinus ATCC 700178 (K. xylinus), K. rhaeticus iGEM |
| [141] |
| 2019 | K. sucrofermentans | Adding 6-carboxyfluorescein glucose (6-CF-Glc) to the growth medium enables the production of BC with unnatural green fluorescing capabilities | [122] |
| 2019 | K. hansenii ATCC 23769 (N. hansenii) | Varying the expression of two motility genes (motA and motB) changes BC morphology | [142] |
| 2019 | Enterobacter sp. FY-07 | Inducing the expression of the colanic acid-encoding operon (wca) improves BC water-holding capacity | [143] |
| 2019 | G. hansenii ATCC 23769 (N. hansenii), E. coli ATCC 700728 | Co-culturing N. hansenii and E. coli enables the production of BC with improved mechanical properties | [124] |
| 2019 | G. hansenii ATCC 23769 (N. hansenii), L. lactis APJ3 | Co-culturing N. hansenii and L. lactis enables the production of BC/hyaluronic acid copolymer | [125] |
| 2019 | A. xylinum ATCC 23769 (N. hansenii), L. casei, L. lactis, S. cerevisiae, R. palustris | Co-culturing N. hansenii and effective microorganism (EM) improves BC water-holding capacity | [127] |
| 2020 | K. xylinus CGMCC 2955 | Varying the expression of the UGPase-encoding gene (galU) changes the crystallinity and porosity of BC | [144] |
| 2021 | K. rhaeticus iGEM, S. cerevisiae |
| [63] |
| 2021 | G. xylinus ATCC 700178 (K. xylinus), R. eutropha H16 (DSM-428) | Co-culturing K. xylinus and R. eutropha enables the production of mechanically superior BC/polyhydroxybutyrate nanocomposite | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. https://doi.org/10.3390/polym15163466
Potočnik V, Gorgieva S, Trček J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers. 2023; 15(16):3466. https://doi.org/10.3390/polym15163466
Chicago/Turabian StylePotočnik, Vid, Selestina Gorgieva, and Janja Trček. 2023. "From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology" Polymers 15, no. 16: 3466. https://doi.org/10.3390/polym15163466
APA StylePotočnik, V., Gorgieva, S., & Trček, J. (2023). From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers, 15(16), 3466. https://doi.org/10.3390/polym15163466







