Assessment and Optimization of Thermal Stability and Water Absorption of Loading Snail Shell Nanoparticles and Sugarcane Bagasse Cellulose Fibers on Polylactic Acid Bioplastic Films
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.1.1. Techniques for Cellulose Fibers Extraction from Sugarcane Bagasse
2.1.2. Experimental Design and Optimization of Bioplastic Film Production
2.1.3. Bioplastic Preparation
2.1.4. Water Immersion
2.1.5. Thermal Properties
2.1.6. Fourier Transform Infrared Spectroscopic Analysis
2.1.7. Scanning Electron Microscopy (SEM) Analysis
2.1.8. Transmission Electron Microscope (TEM)
3. Results and Discussion
3.1. Bionaoparticle Optimization for Bioplastic Films Development
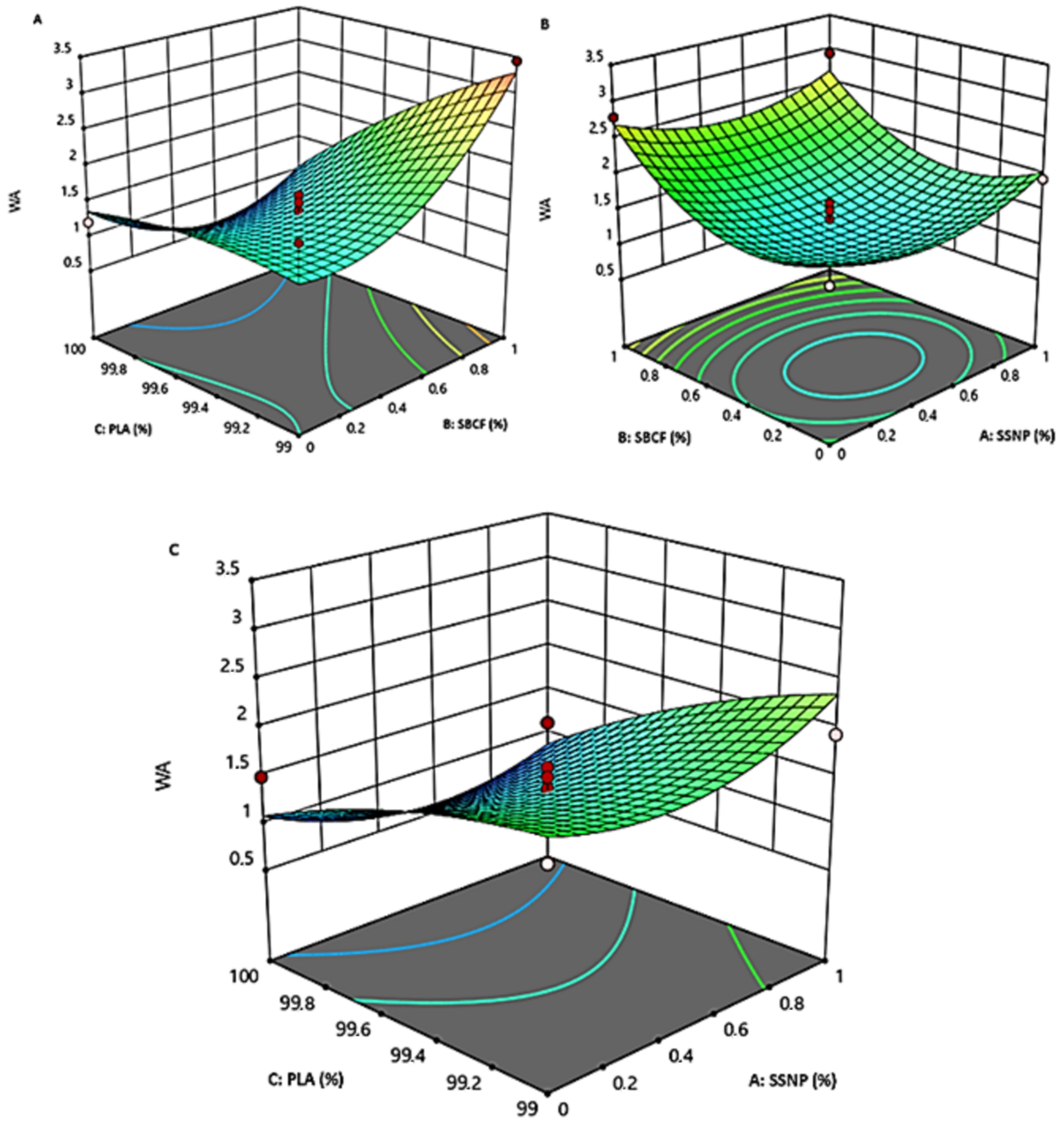
3.2. The Impact and Interaction Effects of Parameters on Water Absorption and Thermal Stability
3.2.1. The Interaction Effect of PLA and SBCF on Bioplastic Water Absorption and Thermal Stability
3.2.2. The Interaction Effect of SBCF and SSNP on Bioplastic Water Absorption and Thermal Stability
3.2.3. The Interaction Effect of PLA and SSNP on Bioplastic on Bioplastic Water Absorption and Thermal Stability
3.3. Justification of Water Absorption and Thermal Stability of Snail Shell Nanoparticles and Sugar Bagasse Cellulose Fibers Reinforced Polylactic Acid Bioplastic Films
3.4. Characterization
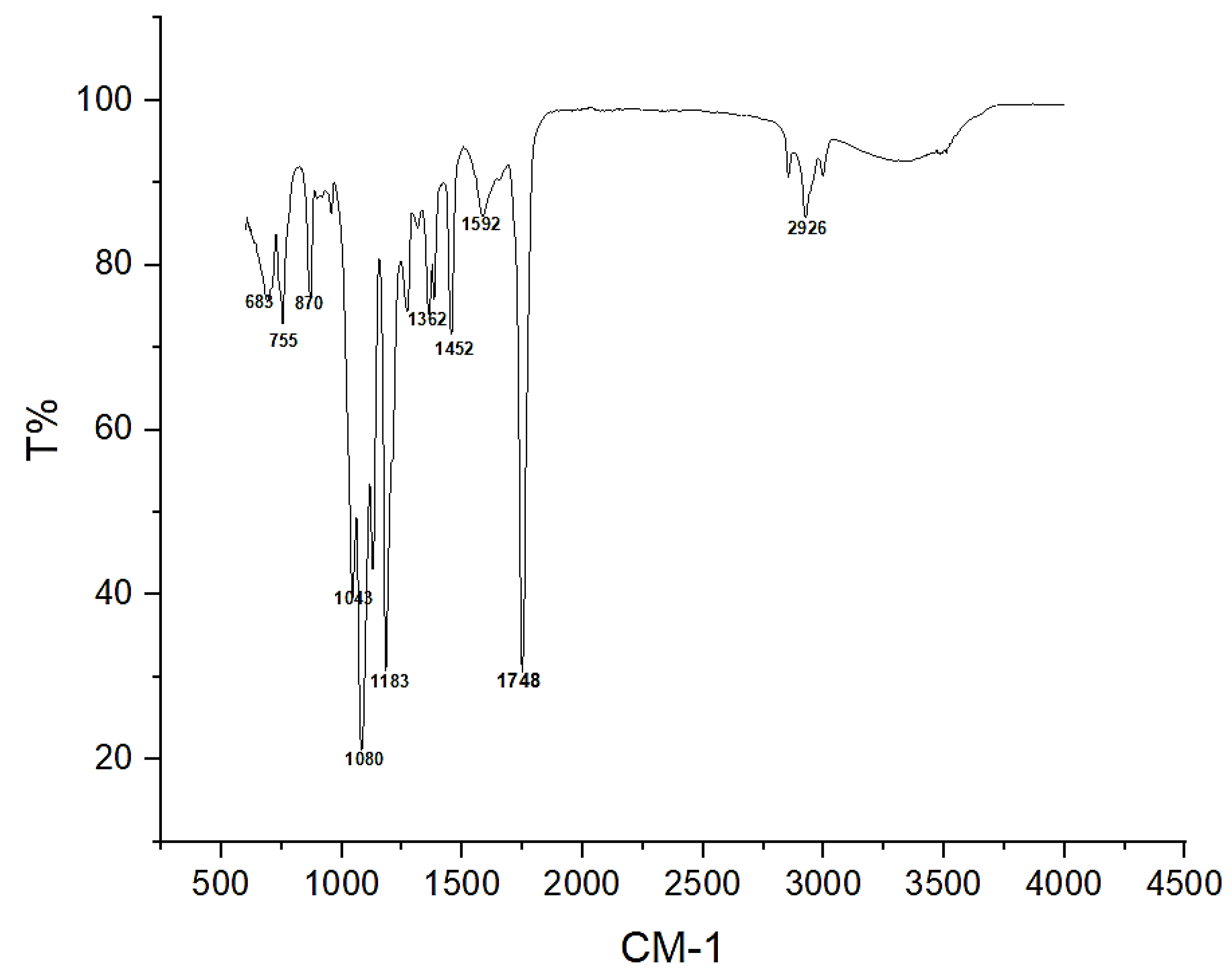
3.4.1. Fourier Transform Infrared Spectrometry
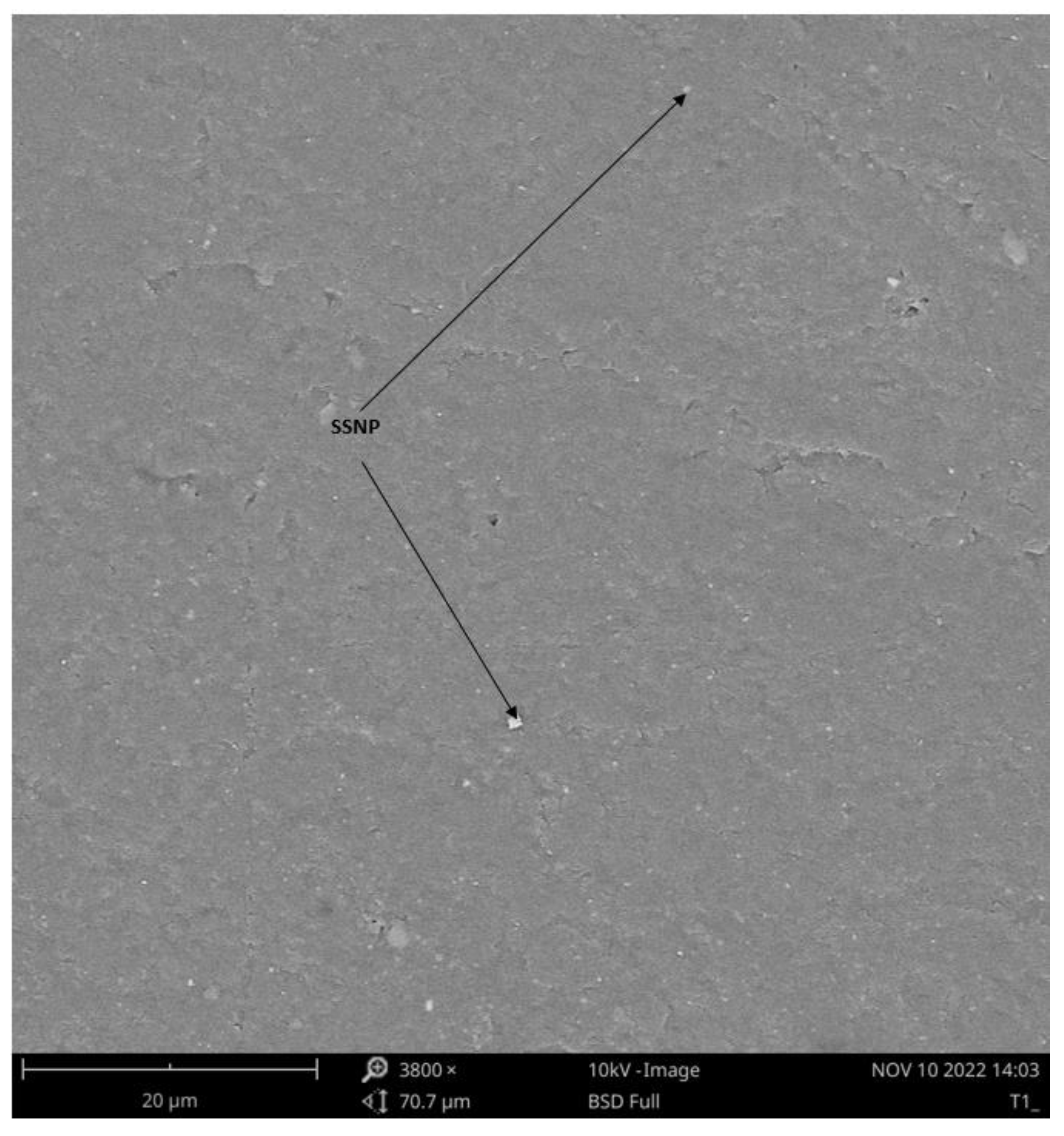
3.4.2. Microstructure of Bioplastic Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Akan, O.D.; Udofia, G.E.; Okeke, E.S.; Mgbechidinma, C.L.; Okoye, C.O.; Zoclanclounon, Y.A.B.; Atakpa, E.O.; Adebanjo, O.O. Plastic waste: Status, degradation and microbial management options for Africa. J. Environ. Manag. 2021, 292, 112758. [Google Scholar] [CrossRef] [PubMed]
- Gbadeyan, O.J.; Fagbemi, O.D.; Andrew, J.; Adali, S.; Glen, B.; Sithole, B. Cellulose nanocrystals and snail shell-reinforced polyvinyl alcohol bioplastic films: Additive concentration optimization and mechanical properties assessment. J. Appl. Polym. Sci. 2022, 139, e52839. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Linganiso, L.Z.; Deenadayalu, N. Thermomechanical characterization of bioplastic films produced using a combination of polylactic acid and bionano calcium carbonate. Sci. Rep. 2022, 12, 15538. [Google Scholar] [CrossRef]
- Adeniran, A.A.; Shakantu, W. The Health and Environmental Impact of Plastic Waste Disposal in South African Townships: A Review. Int. J. Environ. Res. Public Health 2022, 19, 779. [Google Scholar] [CrossRef] [PubMed]
- Williams-Wynn, M.D.; Naidoo, P. A review of the treatment options for marine plastic waste in South Africa. Mar. Pollut. Bull. 2020, 161, 111785. [Google Scholar] [CrossRef]
- Galafassi, S.; Nizzetto, L.; Volta, P. Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 2019, 693, 133499. [Google Scholar] [CrossRef]
- Lin, C.; Nakamura, S. Approaches to solving China’s marine plastic pollution and CO2 emission problems. Econ. Syst. Res. 2019, 31, 143–157. [Google Scholar] [CrossRef]
- Mondal, M.; Bose, B.; Bansal, P. Recycling waste thermoplastic for energy efficient construction materials: An experimental investigation. J. Environ. Manag. 2019, 240, 119–125. [Google Scholar] [CrossRef]
- Dauvergne, P. Why is the global governance of plastic failing the oceans? Glob. Environ. Chang. 2018, 51, 22–31. [Google Scholar] [CrossRef]
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018, 4, eaat0131. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Alpizar, F.; Carlsson, F.; Lanza, G.; Carney, B.; Daniels, R.; Jaime, M.; Ho, T.; Nie, Z.; Salazar, C.; Tibesigwa, B.; et al. A framework for selecting and designing policies to reduce marine plastic pollution in developing countries. Environ. Sci. Policy 2020, 109, 25–35. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Marelli, B.; Patel, N.; Duggan, T.; Perotto, G.; Shirman, E.; Li, C.; Kaplan, D.L.; Omenetto, F.G. Programming function into mechanical forms by directed assembly of silk bulk materials. Proc. Natl. Acad. Sci. USA 2016, 114, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Perotto, G.; Simonutti, R.; Ceseracciu, L.; Mauri, M.; Besghini, D.; Athanassiou, A. Water-induced plasticization in vegetable-based bioplastic films: A structural and thermo-mechanical study. Polymer 2020, 200, 122598. [Google Scholar] [CrossRef]
- Nan, N.; DeVallance, D.B.; Xie, X.; Wang, J. The effect of bio-carbon addition on the electrical, mechanical, and thermal properties of polyvinyl alcohol/biochar composites. J. Compos. Mater. 2016, 50, 1161–1168. [Google Scholar] [CrossRef]
- Judawisastra, H.; Sitohang, R.D.R.; Marta, L. Mardiyati Water absorption and its effect on the tensile properties of tapioca starch/polyvinyl alcohol bioplastics. IOP Conf. Series: Mater. Sci. Eng. 2017, 223, 012066. [Google Scholar] [CrossRef]
- Sa’Adah, S.M.; Saepudin, E. Effect of citric acid on physical and mechanical properties of Starch/PLA/PVA bioplastic films. AIP Conf. Proc. 2021, 2370, 060011. [Google Scholar] [CrossRef]
- Lim, R.; Kiew, P.L.; Lam, M.K.; Yeoh, W.M.; Ho, M.Y. Corn starch/PVA bioplastics—The properties and biodegradability study using Chlorella vulgaris cultivation. Asia-Pac. J. Chem. Eng. 2021, 16, e2622. [Google Scholar] [CrossRef]
- Chong, T.Y.; Law, M.C.; Chan, Y.S. The Potentials of Corn Waste Lignocellulosic Fibre as an Improved Reinforced Bioplastic Composites. J. Polym. Environ. 2021, 29, 363–381. [Google Scholar] [CrossRef]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. Mechanical Performance of Soy-Hull-Reinforced Bioplastic Green Composites: A Comparison with Polypropylene Composites. Macromol. Mater. Eng. 2012, 297, 184–194. [Google Scholar] [CrossRef]
- Wahyuningtiyas, N.E.; Suryanto, H. Properties of Cassava Starch based Bioplastic Reinforced by Nanoclay. J. Mech. Eng. Sci. Technol. 2018, 2, 20–26. [Google Scholar] [CrossRef]
- Agustin, M.B.; Ahmmad, B.; Alonzo, S.M.M.; Patriana, F.M. Bioplastic based on starch and cellulose nanocrystals from rice straw. J. Reinf. Plast. Compos. 2014, 33, 2205–2213. [Google Scholar] [CrossRef]
- Gbadeyan, O.; Adali, S.; Bright, G.; Sithole, B.; Awogbemi, O. Studies on the mechanical and absorption properties of achatina fulica snail and eggshells reinforced composite materials. Compos. Struct. 2020, 239, 112043. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B.; Onwubu, S. Optimization of Milling Procedures for Synthesizing Nano-CaCO3 from Achatina fulica Shell through Mechanochemical Techniques. J. Nanomater. 2020, 2020, 4370172. [Google Scholar] [CrossRef]
- Gbadeyan, O.; Adali, S.; Bright, G.; Sithole, B.; Lekha, P. Mechanical, microstructure, and dynamic mechanical analysis of nano-shell and plant fiber hybrid biocomposite. J. Compos. Mater. 2021, 55, 3345–3358. [Google Scholar] [CrossRef]
- Bessa, W.; Trache, D.; Derradji, M.; Tarchoun, A.F. Kevlar fabric reinforced polybenzoxazine composites filled with silane treated microcrystalline cellulose in the interlayers: The next generation of multi-layered armor panels. Def. Technol. 2021, 18, 2000–2007. [Google Scholar] [CrossRef]
- Orts, W.J.; Imam, S.; Shey, J.; Glenn, G.M.; Inglesby, M.; Guttman, M.; Nguyen, A.; Revol, J.F. Effect of fiber source on cellulose reinforced polymer nanocomposites. In Proceedings of the Annual Technical Conference—ANTEC, Conference Proceedings, Chicago, IL, USA, 16–20 May 2004; Volume 2, pp. 2427–2431. [Google Scholar]
- Xie, F.; Bao, J.; Zhuo, L.; Zhao, Y.; Dang, W.; Si, L.; Yao, C.; Zhang, M.; Lu, Z. Toward high-performance nanofibrillated cellulose/aramid fibrid paper-based composites via polyethyleneimine-assisted decoration of silica nanoparticle onto aramid fibrid. Carbohydr. Polym. 2020, 245, 116610. [Google Scholar] [CrossRef]
- Lu, Z.; Ning, D.; Dang, W.; Wang, D.; Jia, F.; Li, J.; E, S. Comparative study on the mechanical and dielectric properties of aramid fibrid, mica and nanofibrillated cellulose based binary composites. Cellulose 2020, 27, 8027–8037. [Google Scholar]
- Mohit, H.; Selvan, V.A.M. Effect of a Novel Chemical Treatment on the Physico-Thermal Properties of Sugarcane Nanocellulose Fiber Reinforced Epoxy Nanocomposites. Int. Polym. Process. 2020, 35, 211–220. [Google Scholar] [CrossRef]
- Greeves, N. Calcium Carbonate—CaCO3—Polymorphs. ChemTube3D. Available online: https://www.chemtube3d.com/ss-caco3/ (accessed on 8 February 2023).
- Alamy. Polylactic acid Stock Photos and Images. Available online: https://www.alamy.com/stock-photo/polylactic-acid.html?sortBy=relevant (accessed on 9 February 2023).
- Beyene, D.; Chae, M.; Dai, J.; Danumah, C.; Tosto, F.; Demesa, A.G.; Bressler, D.C. Characterization of Cellulase-Treated Fibers and Resulting Cellulose Nanocrystals Generated through Acid Hydrolysis. Materials 2018, 11, 1272. [Google Scholar] [CrossRef] [PubMed]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B. Comparative Reinforcement Effect of Achatina fulica Snail Shell Nanoparticles, Montmorillonite, and Kaolinite Nanoclay on the Mechanical and Physical Properties of Greenpoxy Biocomposite. Polymers 2022, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B. The investigation of reinforcement properties of nano-CaCO3 synthesized from Achatina fulica snail shell through mechanochemical methods on epoxy nanocomposites. Nanocomposites 2021, 7, 79–86. [Google Scholar] [CrossRef]
- Mohan, T.; Kanny, K. Thermal, mechanical and physical properties of nanoegg shell particle-filled epoxy nanocomposites. J. Compos. Mater. 2018, 52, 3989–4000. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, L.; Sun, J.; Liu, J. Cellulose Nanofibrils Filled Poly(Lactic Acid) Biocomposite Filament for FDM 3D Printing. Molecules 2020, 25, 2319. [Google Scholar] [CrossRef]
- Lafia-Araga, R.A.; Sabo, R.; Nabinejad, O.; Matuana, L.; Stark, N. Influence of Lactic Acid Surface Modification of Cellulose Nanofibrils on the Properties of Cellulose Nanofibril Films and Cellulose Nanofibril–Poly(lactic acid) Composites. Biomolecules 2021, 11, 1346. [Google Scholar] [CrossRef]
- Fu, K.; Su, Y.; Yang, L.; Ji, N.; Song, C.; Ma, D.; Lv, X.; Han, R.; Liu, Q. Pt loaded manganese oxide nanoarray-based monolithic catalysts for catalytic oxidation of acetone. Chem. Eng. J. 2022, 432, 134397. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A Renewable Lignin–Lactide Copolymer and Application in Biobased Composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Li, Y.; Mi, J.; Fu, H.; Zhou, H.; Wang, X. Nanocellular Foaming Behaviors of Chain-Extended Poly(lactic acid) Induced by Isothermal Crystallization. ACS Omega 2019, 4, 12512–12523. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.; Huang, C.; Zheng, K.; Luo, Y.; Dong, Y.; Shen, Z.; Li, W.; Qin, C. Preparation of Modified Chitosan Microsphere-Supported Copper Catalysts for the Borylation of α,β-Unsaturated Compounds. Polymers 2019, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
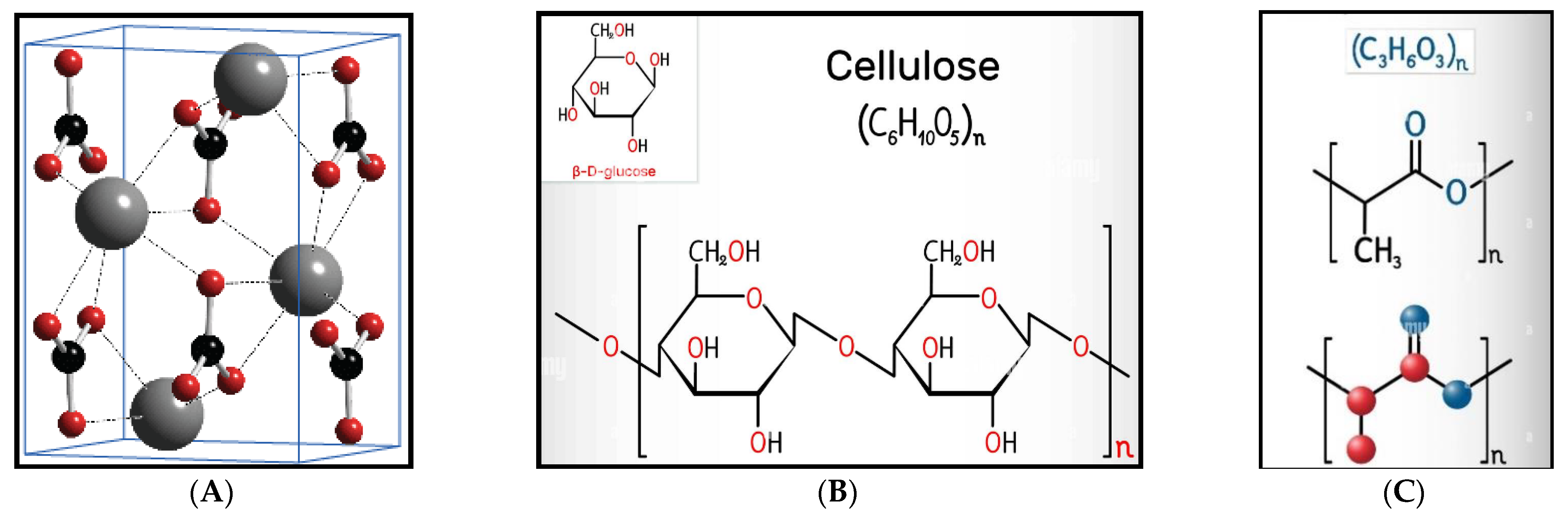



| Run | Factor 1 A (SSNP) % | Factor 2 B (SBCF) % | Factor 3 C (PLA) % |
|---|---|---|---|
| 1 | 0.5 | 0 | 100 |
| 2 | 0.5 | 0.5 | 99.5 |
| 3 | 0 | 0.5 | 100 |
| 4 | 1 | 1 | 99.5 |
| 5 | 0.5 | 0 | 99 |
| 6 | 0.5 | 1 | 99 |
| 7 | 0 | 0.5 | 99 |
| 8 | 0.5 | 1 | 100 |
| 9 | 0 | 0 | 99.5 |
| 10 | 1 | 0.5 | 100 |
| 11 | 0.5 | 0.5 | 99.5 |
| 12 | 1 | 0 | 99.5 |
| 13 | 0 | 1 | 99.5 |
| 14 | 0.5 | 0.5 | 99.5 |
| 15 | 0.5 | 0.5 | 99.5 |
| 16 | 0.5 | 0.5 | 99.5 |
| 17 | 1 | 0.5 | 99 |
| Response 1 (WA) % | Response 2 (TS) °C |
|---|---|
| 1.2027 | 251 |
| 1.0693 | 245 |
| 1.494 | 335 |
| 2.9277 | 290.833 |
| 2.0166 | 241.333 |
| 3.444 | 262.833 |
| 1.7278 | 280.5 |
| 0.532 | 261 |
| 1.5867 | 270.833 |
| 1.1093 | 285 |
| 1.5981 | 251.5 |
| 1.934 | 242.333 |
| 2.7894 | 241 |
| 1.2452 | 238.667 |
| 1.4921 | 241.333 |
| 1.3711 | 263 |
| 1.9356 | 271.167 |
| Source | Sum of Squares | dF | Mean Squares | F-Value | p-Values | R2 |
|---|---|---|---|---|---|---|
| Water absorption (model) | 7.34 | 9 | 0.8150 | 4.15 | 0.0369 | 0.8422 |
| Thermal stability (model) | 8254.06 | 9 | 917.12 | 3.85 | 0.0447 | 0.8318 |
| Factor | Water Absorption CE | Water Absorption SE | Thermal Stability CE | Thermal Stability SE |
|---|---|---|---|---|
| Intercept | 1.36 | 0.1981 | 247.90 | 6.91 |
| A-SSNP | 0.0386 | 0.1566 | −4.75 | 5.46 |
| B-SBCF | 0.3691 | 0.1566 | 6.27 | 5.46 |
| C-PLA | −0.5983 | 0.1566 | 9.52 | 5.46 |
| AB | −0.0523 | 0.2215 | 19.58 | 7.72 |
| AC | −0.1481 | 0.2215 | −10.17 | 7.72 |
| BC | −0.5245 | 0.2215 | −2.88 | 7.72 |
| A² | 0.3611 | 0.2159 | 26.11 | 7.53 |
| B² | 0.5932 | 0.2159 | −12.76 | 7.53 |
| C² | −0.1496 | 0.2159 | 18.90 | 7.53 |
| Independent Variables | Predicted Optimum Levels (%) |
|---|---|
| Polylactic acid | 99.815 |
| Sugar bagasse cellulose fibers | 0.036 |
| Snail shell nanoparticle | 0.634 |
| Responses | Predicted Values | Observed Values |
|---|---|---|
| Water absorption % (model) | 1.42 | 0.45 |
| Thermal stability °C (model) | 240.248 | 259.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gbadeyan, O.J.; Linganiso, L.Z.; Deenadayalu, N. Assessment and Optimization of Thermal Stability and Water Absorption of Loading Snail Shell Nanoparticles and Sugarcane Bagasse Cellulose Fibers on Polylactic Acid Bioplastic Films. Polymers 2023, 15, 1557. https://doi.org/10.3390/polym15061557
Gbadeyan OJ, Linganiso LZ, Deenadayalu N. Assessment and Optimization of Thermal Stability and Water Absorption of Loading Snail Shell Nanoparticles and Sugarcane Bagasse Cellulose Fibers on Polylactic Acid Bioplastic Films. Polymers. 2023; 15(6):1557. https://doi.org/10.3390/polym15061557
Chicago/Turabian StyleGbadeyan, Oluwatoyin J., Linda Z. Linganiso, and Nirmala Deenadayalu. 2023. "Assessment and Optimization of Thermal Stability and Water Absorption of Loading Snail Shell Nanoparticles and Sugarcane Bagasse Cellulose Fibers on Polylactic Acid Bioplastic Films" Polymers 15, no. 6: 1557. https://doi.org/10.3390/polym15061557
APA StyleGbadeyan, O. J., Linganiso, L. Z., & Deenadayalu, N. (2023). Assessment and Optimization of Thermal Stability and Water Absorption of Loading Snail Shell Nanoparticles and Sugarcane Bagasse Cellulose Fibers on Polylactic Acid Bioplastic Films. Polymers, 15(6), 1557. https://doi.org/10.3390/polym15061557





