Chitosan Grafted with Thermoresponsive Poly(di(ethylene glycol) Methyl Ether Methacrylate) for Cell Culture Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Chitosan Films
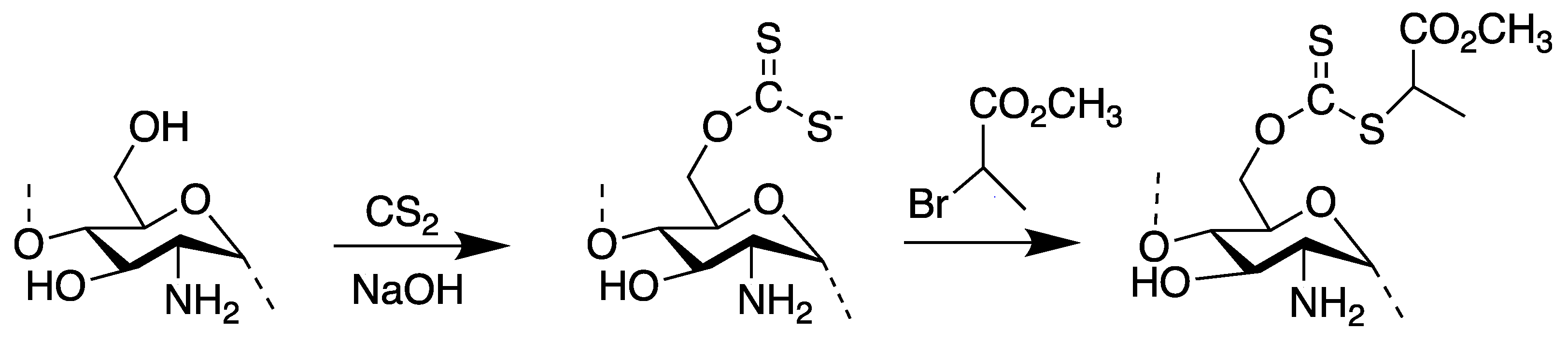
2.3. Synthesis of Xanthated Chitosan Films
2.4. Synthesis of RAFT Agent
2.5. Synthesis of Chito-g-PMEO2MA
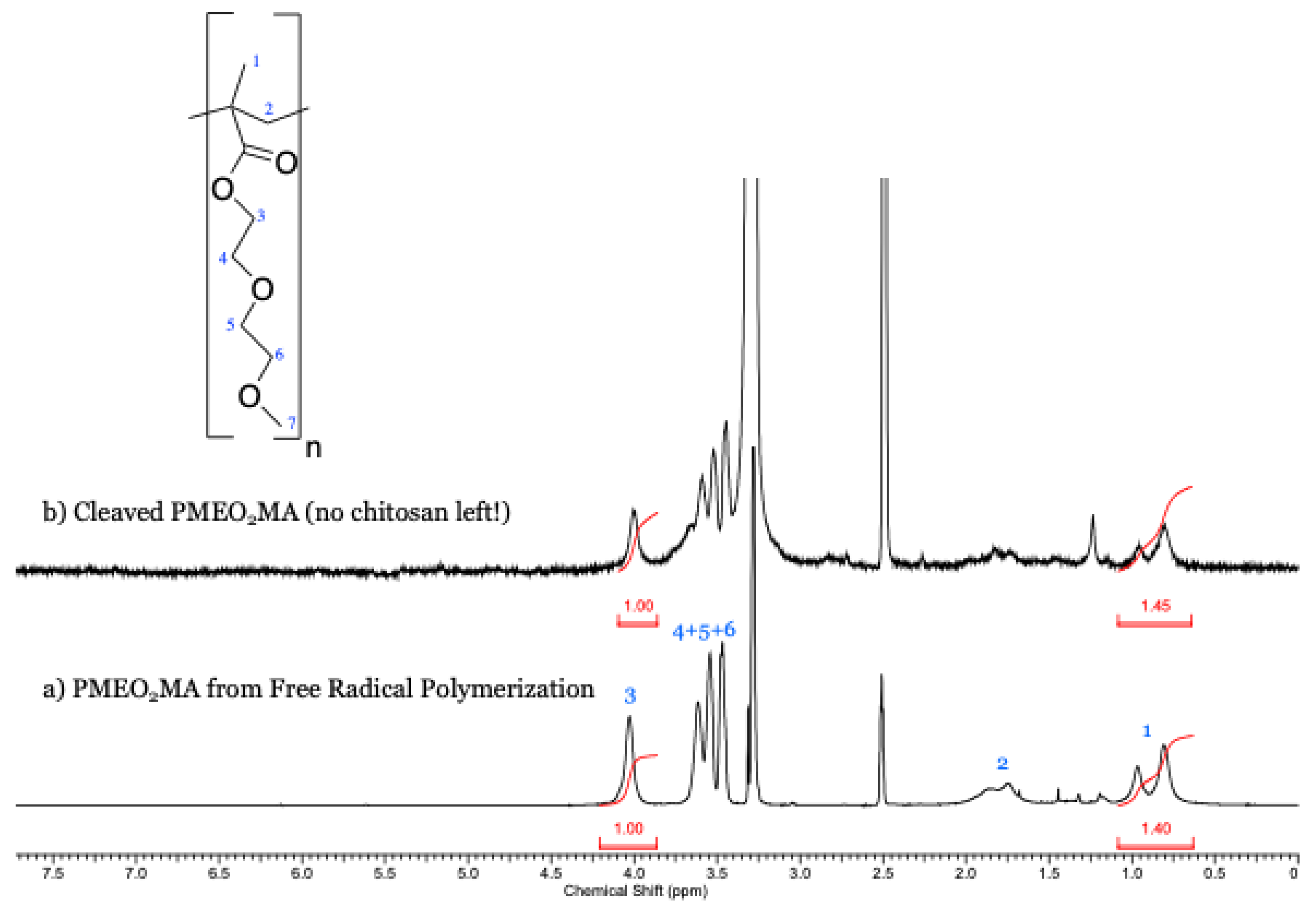
2.6. Cleavage of PMEO2MA Chains from Chito-g-PMEO2MA
2.7. Material Characterization
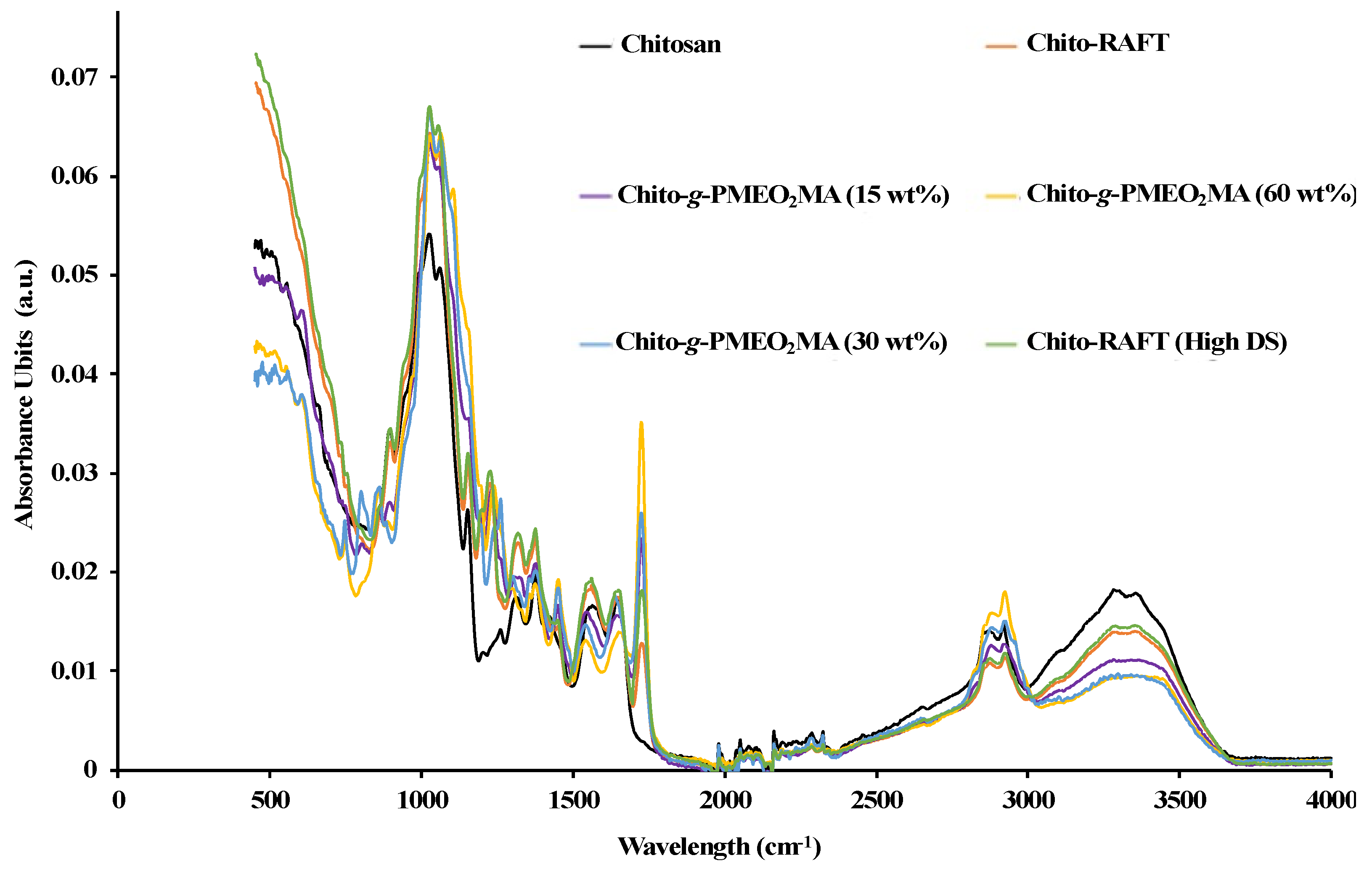
2.7.1. ATR–FTIR
2.7.2. 1H NMR
2.7.3. Gel Permeation Chromatography (GPC)
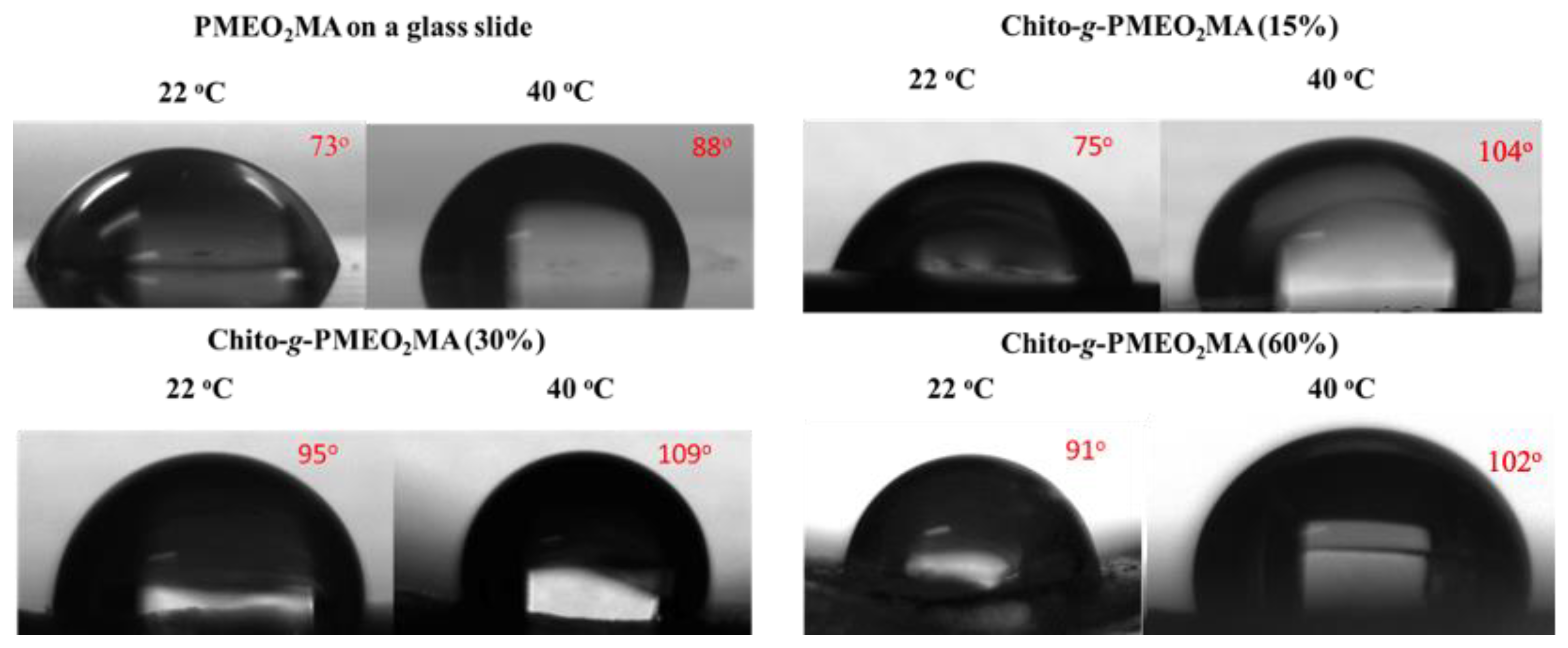
2.7.4. Contact Angle Measurements
2.7.5. Water Uptake Tests
2.8. Cell Study with Chitosan Films
2.8.1. Film Sterilization
2.8.2. Cell Culture and Seeding on Films
2.8.3. Proliferation as Measured Using the XTT Assay
2.8.4. Live and Dead Assay
2.8.5. Temperature-Induced Cell Detachment and Viability
2.8.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Chitosan-Based RAFT Agent and Chito-g-PMEO2MA
3.2. Cleavage of PMEO2MA Chains from Chito-g-PMEO2MA
3.3. Contact Angle Measurements
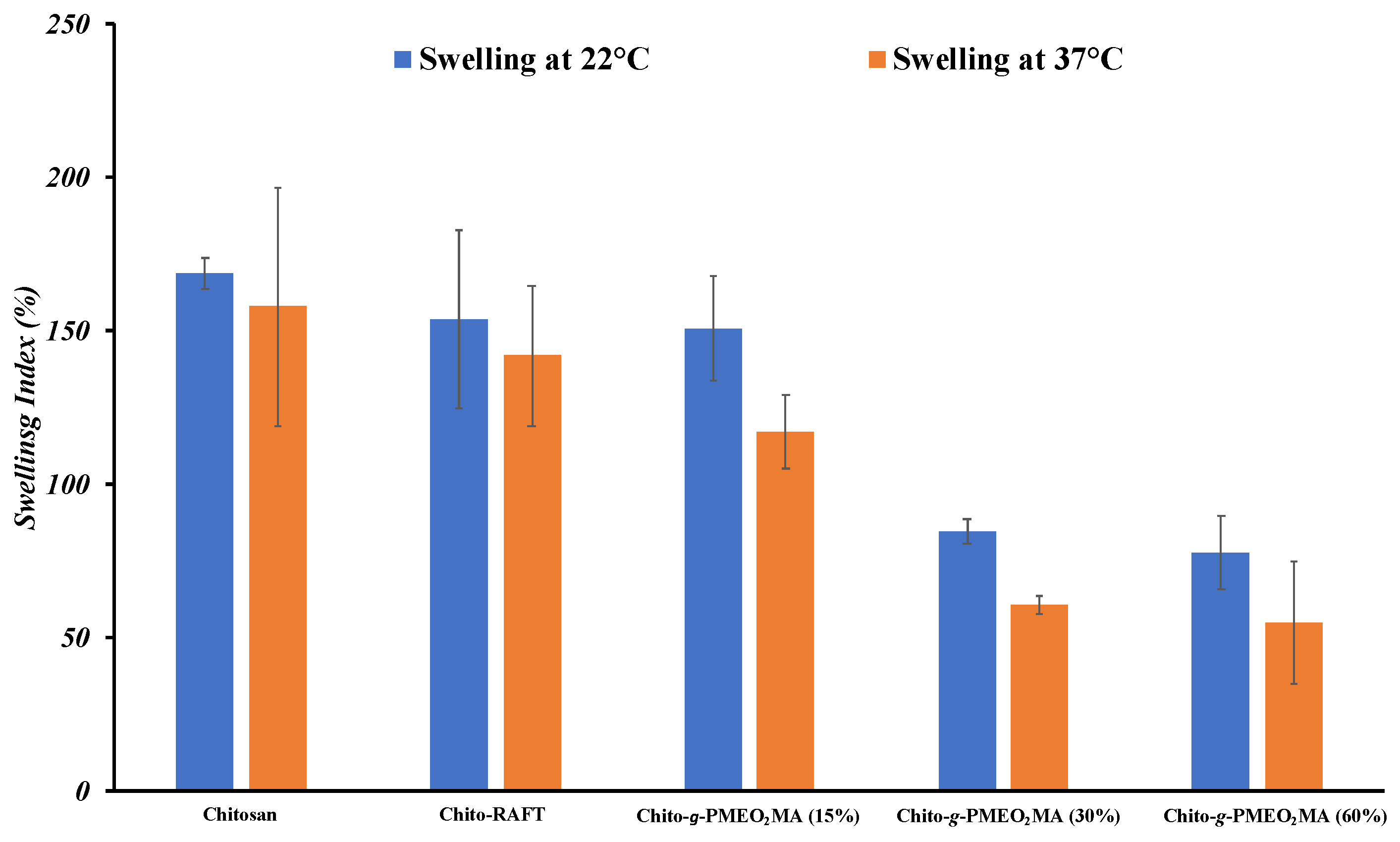
3.4. Water Uptake Tests
3.5. Cell Adhesion, Viability, and Proliferation on Chitosan Films
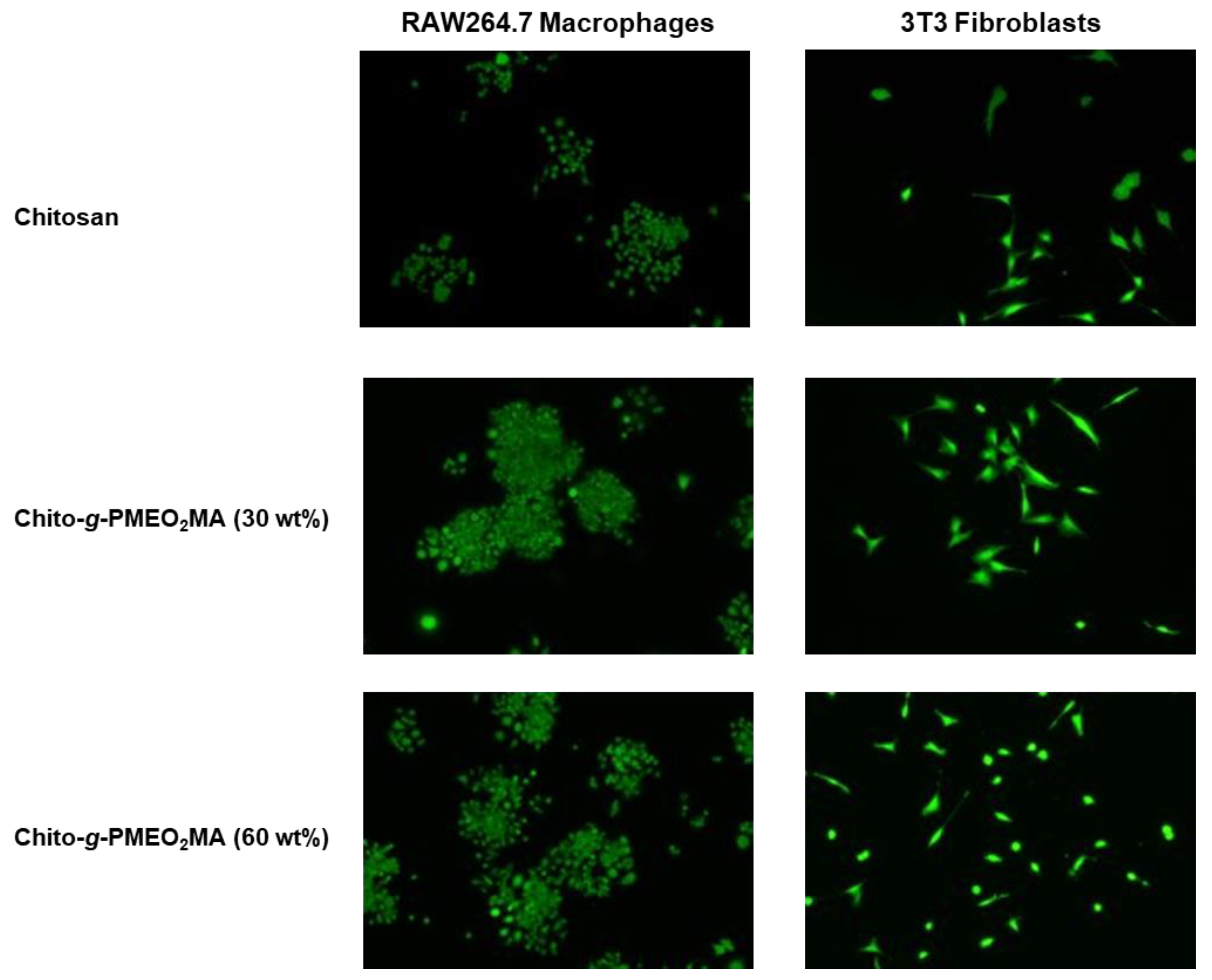
3.5.1. Macrophage Interactions with Chitosan Films
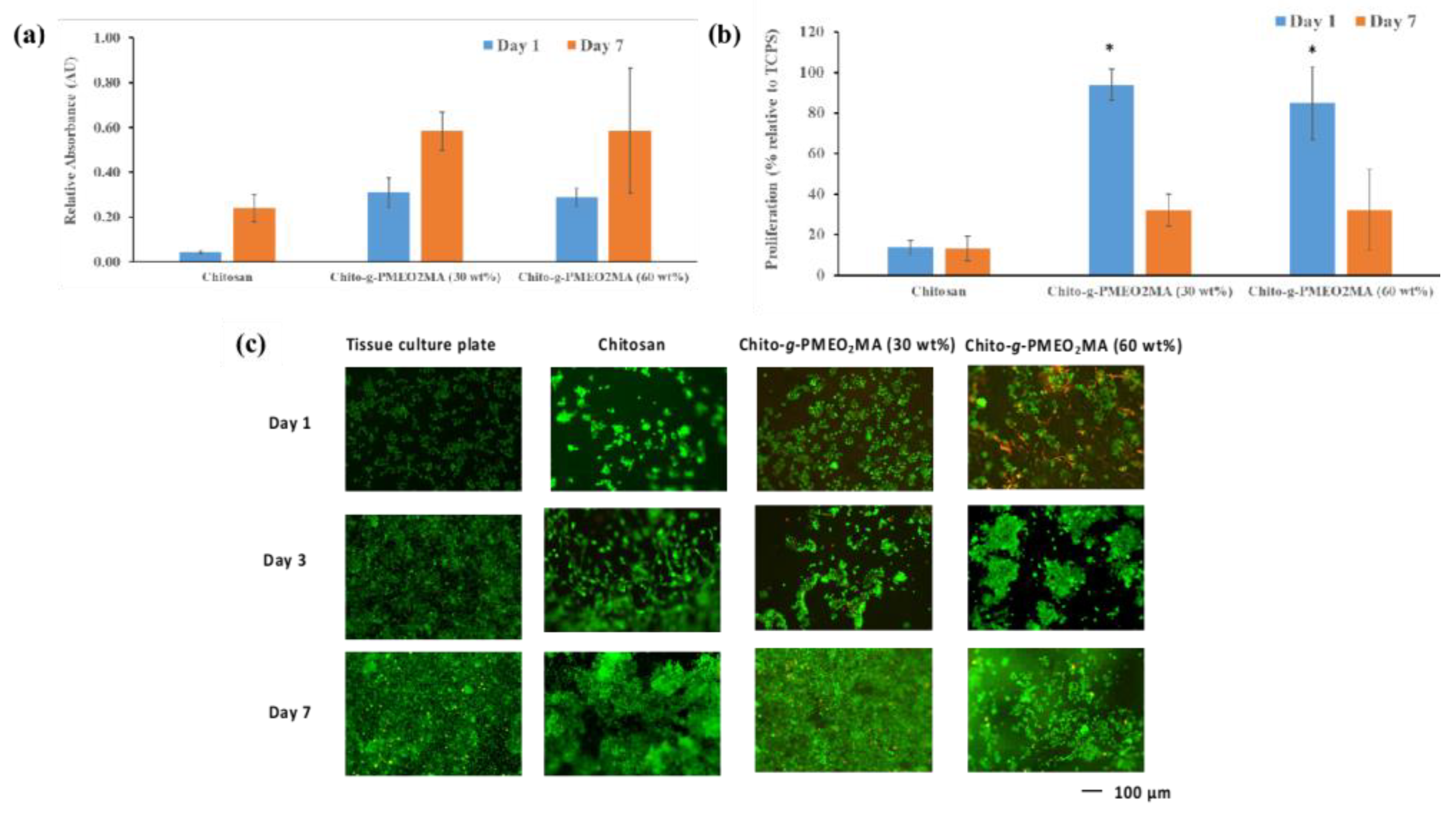
3.5.2. Fibroblast Interactions with Chitosan Films
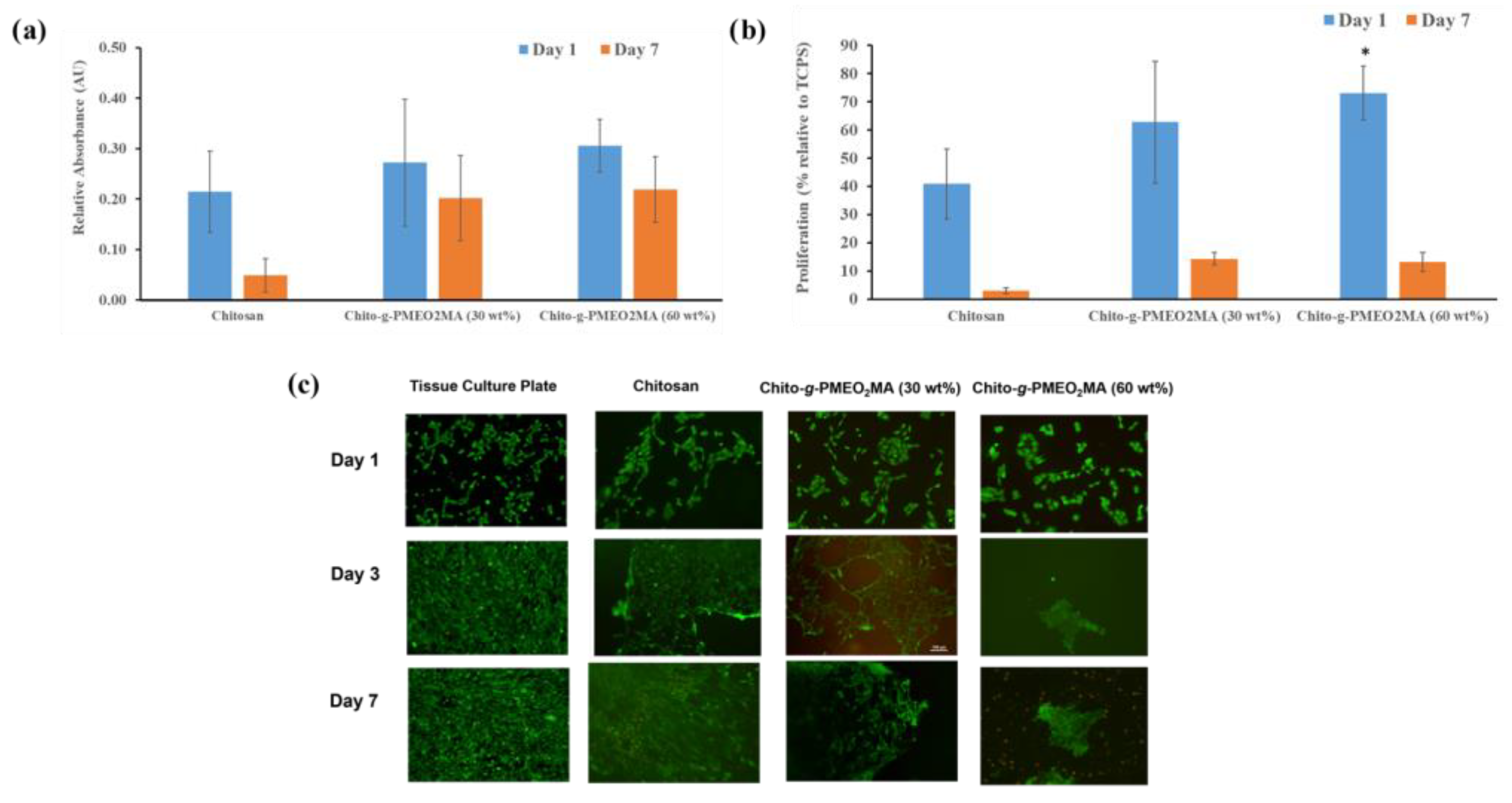
3.5.3. Human Corneal Epithelial Cell Interactions with Chitosan Films
3.6. Temperature-Induced Cell Detachment and Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knaul, J.Z.; Kasaai, M.R.; Bui, V.T.; Creber, K.A. Characterization of deacetylated chitosan and chitosan molecular weight review. Can. J. Chem. 1998, 76, 1699–1706. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Hamilton, V.; Yuan, Y.; Rigney, D.A.; Puckett, A.D.; Ong, J.L.; Yang, Y.; Elder, S.H.; Bumgardner, J.D. Characterization of chitosan films and effects on fibroblast cell attachment and proliferation. J. Mater. Sci. Mater. Med. 2006, 17, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Okumura, M.; Matsuura, M.; Ueno, K.; Tokura, S.; Okamoto, Y.; Minami, S.; Fujinaga, T. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials 1997, 18, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marini, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Thermo-Responsive Chitosan-graft-poly(N-isopropylacrylamide) Injectable Hydrogel for Cultivation of Chondrocytes and Meniscus Cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef]
- Radhakumary, C.; Antonty, M.; Sreenivasan, K. Drug loaded thermoresponsive and cytocompatible chitosan based hydrogel as a potential wound dressing. Carbohydr. Polym. 2011, 83, 705–713. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Thermoresponsive surfaces for cell culture and enzyme-free cell detachment. Prog. Polym. Sci. 2010, 35, 1311–1324. [Google Scholar] [CrossRef]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and Poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8, 19. [Google Scholar] [CrossRef]
- Deng, H.; Dai, F.; Ma, G.; Zhang, X. Theranostic Gold Nanomicelles made from Biocompatible Comb-like Polymers for Thermochemotherapy and Multifunctional Imaging with Rapid Clearance. Adv. Mater. 2015, 27, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, A.; Dorigato, A.; Brugnara, M.; Penati, A. Contact angle measurements as a tool to investigate the filler–matrix interactions in polyurethane–clay nanocomposites from blocked prepolymer. Eur. Polym. J. 2008, 44, 1662–1672. [Google Scholar] [CrossRef]
- Mondal, D.; Haghpanah, Z.; Huxman, C.J.; Tanter, S.; Sun, D.; Gorbet, M.; Willett, T.L. MSLA-Based 3D Printing of Acrylated Epoxidized Soybean Oil-Nano-Hydroxyapatite Composites for Bone Repair. Mater. Sci. Eng. C 2021, 130, 112456. [Google Scholar]
- Norowski, P.A.; Mishra, S.; Adatrow, P.C.; Haggard, W.O.; Bumgardner, J.D. Suture pullout strength and in vitro fibroblast and RAW 264.7 monocyte biocompatibility of genipin crosslinked nanofibrous chitosan mats for guided tissue regeneration. J. Biomed. Mater. Res. Part A 2012, 100A, 2890–2896. [Google Scholar] [CrossRef]
- Postnikoff, C.K.; Pintwala, R.; Williams, S.; Wright, A.M.; Hileeto, D.; Gorbet, M.B. Development of a Curved, Stratified, In Vitro Model to Assess Ocular Biocompatibility. PLoS ONE 2014, 9, e96448. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, Q.; Shen, W.; An, Z. Aqueous Dispersion Polymerization of 2-Methoxyethyl Acrylate for the Synthesis of Biocompatible Nanoparticles Using a Hydrophilic RAFT Polymer and a Redox Initiator. Macromolecules 2011, 44, 5237–5245. [Google Scholar] [CrossRef]
- Tang, J.; Hua, D.; Cheng, J.; Jiang, J.; Zhu, X. Synthesis and properties of temperature-responsive chitosan by controlled free radical polymerization with chitosan-RAFT agent. Int. J. Biol. Macromol. 2008, 43, 383–389. [Google Scholar] [CrossRef]
- Minh, N.C.; Nguyen, V.H.; Schwarz, S.; Stevens, W.F.; Trung, T.S. Preparation of water soluble hydrochloric chitosan from low molecular weight chitosan in the solid state. Int. J. Biol. Macromol. 2019, 121, 718–726. [Google Scholar] [CrossRef]
- Aljbour, N.D.; Beg, M.D.H.; Gimbun, J. Acid Hydrolysis of Chitosan to Oligomers Using Hydrochloric Acid. Chem. Eng. Technol. 2019, 42, 1741–1746. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Jiang, J.; Pan, X.; Cao, J.; Jiang, J.; Hua, D.; Zhu, X. Synthesis and property of chitosan graft copolymer by RAFT polymerization with tosylic acid–chitosan complex. Int. J. Biol. Macromol. 2012, 50, 586–590. [Google Scholar] [CrossRef]
- Pandey, V.K.; Upadhyay, S.N.; Niranjan, K.; Mishra, P.K. Antimicrobial biodegradable chitosan-based composite Nano-layers for food packaging. Int. J. Biol. Macromol. 2020, 157, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Duhamel, J. Extraction of Oil from Oil Sands Using Thermoresponsive Polymeric Surfactants. ACS Appl. Mater. Interfaces 2015, 7, 5879–5889. [Google Scholar] [CrossRef]
- Conzatti, G.; Ayadi, F.; Cavalie, S.; Carrère, N.; Tourrette, A. Thermosensitive PNIPAM grafted alginate/chitosan PEC. Appl. Surf. Sci. 2019, 467–468, 940–948. [Google Scholar] [CrossRef]
- Plunkett, K.N.; Zhu, X.; Moore, J.S.; Leckband, D.E. PNIPAM Chain Collapse Depends on the Molecular Weight and Grafting Density. Langmuir 2006, 22, 4259–4266. [Google Scholar] [CrossRef]
- Barrere, F.; Mahmood, T.A.; De Groot, K.; Van Blitterswijk, C.A. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Kuriyama, T.; Fukuma, Y.; Imashiro, C.; Kabayama, K.; Kurashina, Y.; Takemura, K. Detachment of RAW264.7 macrophages from a culture dish using ultrasound excited by a Langevin transducer. J. Biosci. Bioeng. 2021, 131, 320–325. [Google Scholar] [CrossRef]
- Zan, Q.; Wang, C.; Dong, L.; Cheng, P.; Tian, J. Effect of surface roughness of chitosan-based microspheres on cell adhesion. Appl. Surf. Sci. 2008, 255, 401–403. [Google Scholar] [CrossRef]
- Lasocka, I.; Szulc-Dąbrowska, L.; Skibniewski, M.; Skibniewska, E.; Gregorczyk-Zboroch, K.; Pasternak, I.; Kalbacova, M.H. Cytocompatibility of Graphene Monolayer and Its Impact on Focal Cell Adhesion, Mitochondrial Morphology and Activity in Balb/3t3 Fibroblasts. Materials 2021, 14, 643. [Google Scholar] [CrossRef]
- Zhu, A.P.; Fang, N. Adhesion Dynamics, Morphology, and Organization of 3T3 Fibroblast on Chitosan and Its Derivative: The Effect of O-Carboxymethylation. Biomacromolecules 2005, 6, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the Degree of Acetylation on Some Biological Properties of Chitosan Films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Wu, M.-F.; Stachon, T.; Seitz, B.; Langenbucher, A.; Szentmáry, N. Effect of human autologous serum and fetal bovine serum on human corneal epithelial cell viability, migration and proliferation in vitro. Int. J. Ophthalmol. 2017, 10, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Benhabbour, S.R.; Sheardown, H.; Adronov, A. Cell adhesion and proliferation on hydrophilic dendritically modified surfaces. Biomaterials 2008, 29, 4177–4186. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.G.; Xiao, F.; Zhang, A.-H.; Yao, K.-D. Preparation of Thermoresponsive Chitosan Copolymer and Its Cytocompatibility and Detachability. Chem. J. Chin. Univ. 2008, 29, 1061–1064. [Google Scholar]
- Lu, Y.-T.; Zeng, K.; Fuhrmann, B.; Woelk, C.; Zhang, K.; Groth, T. Engineering of Stable Cross-Linked Multilayers Based on Thermo-Responsive PNIPAM-Grafted-Chitosan/Heparin to Tailor Their Physiochemical Properties and Biocompatibility. ACS Appl. Mater. Interfaces 2022, 14, 29550–29562. [Google Scholar] [CrossRef]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef]
- Hayman, E.G.; Pierschbacher, M.D.; Suzuki, S.; Ruoslahti, E. Vitronectin—A major cell attachment-promoting protein in fetal bovine serum. Exp. Cell Res. 1985, 160, 245–258. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, N.; Sun, D.; Gorbet, M.; Gauthier, M. Chitosan Grafted with Thermoresponsive Poly(di(ethylene glycol) Methyl Ether Methacrylate) for Cell Culture Applications. Polymers 2023, 15, 1515. https://doi.org/10.3390/polym15061515
Dasgupta N, Sun D, Gorbet M, Gauthier M. Chitosan Grafted with Thermoresponsive Poly(di(ethylene glycol) Methyl Ether Methacrylate) for Cell Culture Applications. Polymers. 2023; 15(6):1515. https://doi.org/10.3390/polym15061515
Chicago/Turabian StyleDasgupta, Natun, Duo Sun, Maud Gorbet, and Mario Gauthier. 2023. "Chitosan Grafted with Thermoresponsive Poly(di(ethylene glycol) Methyl Ether Methacrylate) for Cell Culture Applications" Polymers 15, no. 6: 1515. https://doi.org/10.3390/polym15061515
APA StyleDasgupta, N., Sun, D., Gorbet, M., & Gauthier, M. (2023). Chitosan Grafted with Thermoresponsive Poly(di(ethylene glycol) Methyl Ether Methacrylate) for Cell Culture Applications. Polymers, 15(6), 1515. https://doi.org/10.3390/polym15061515





