Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering
Abstract
1. Introduction
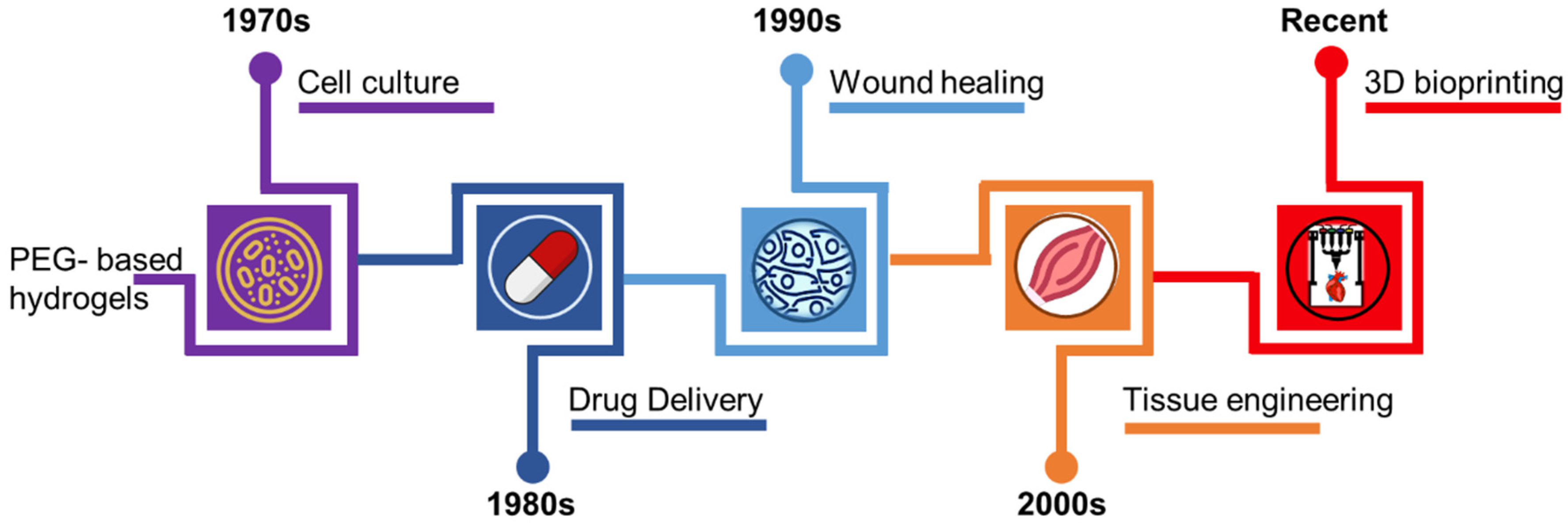
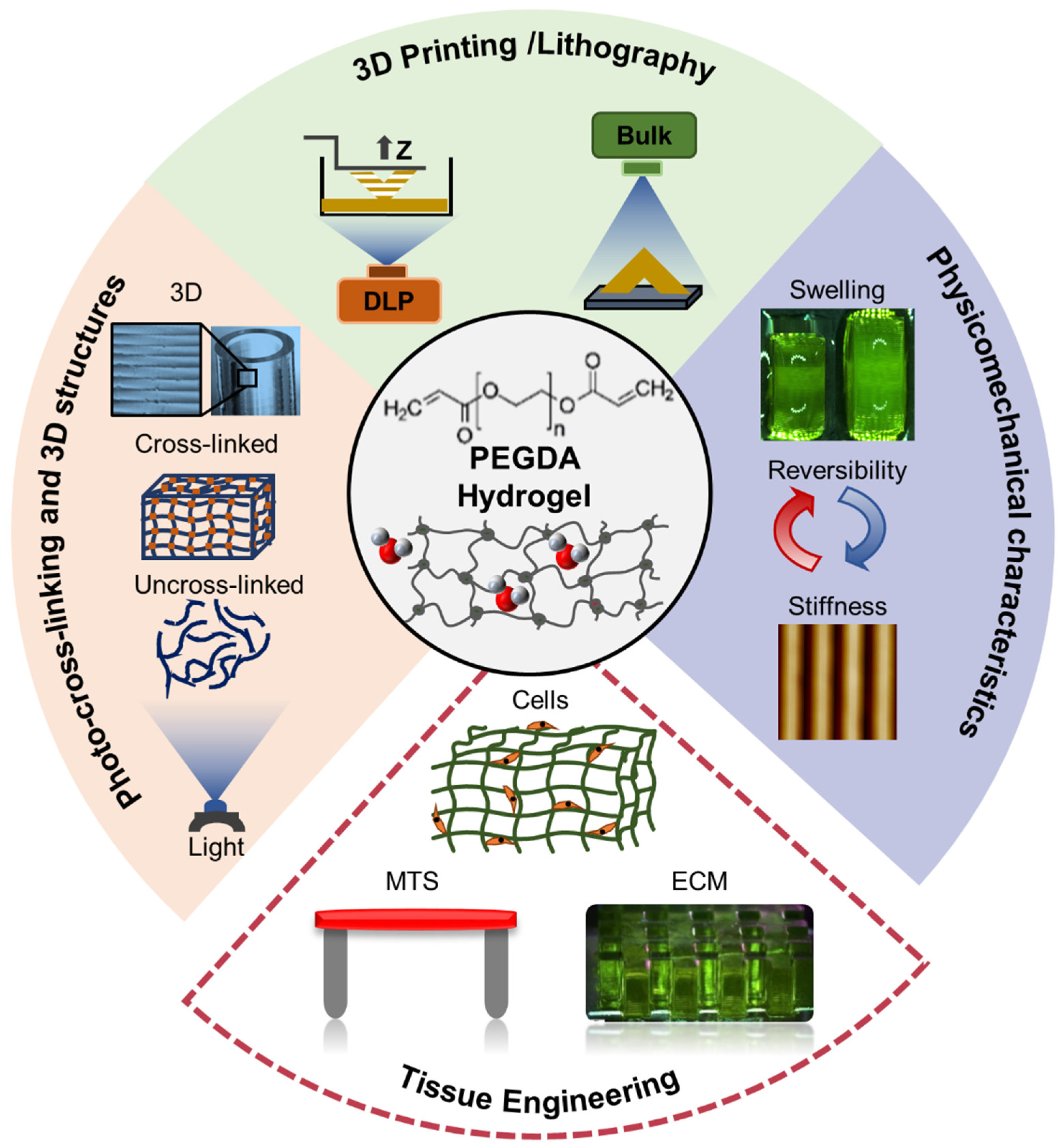
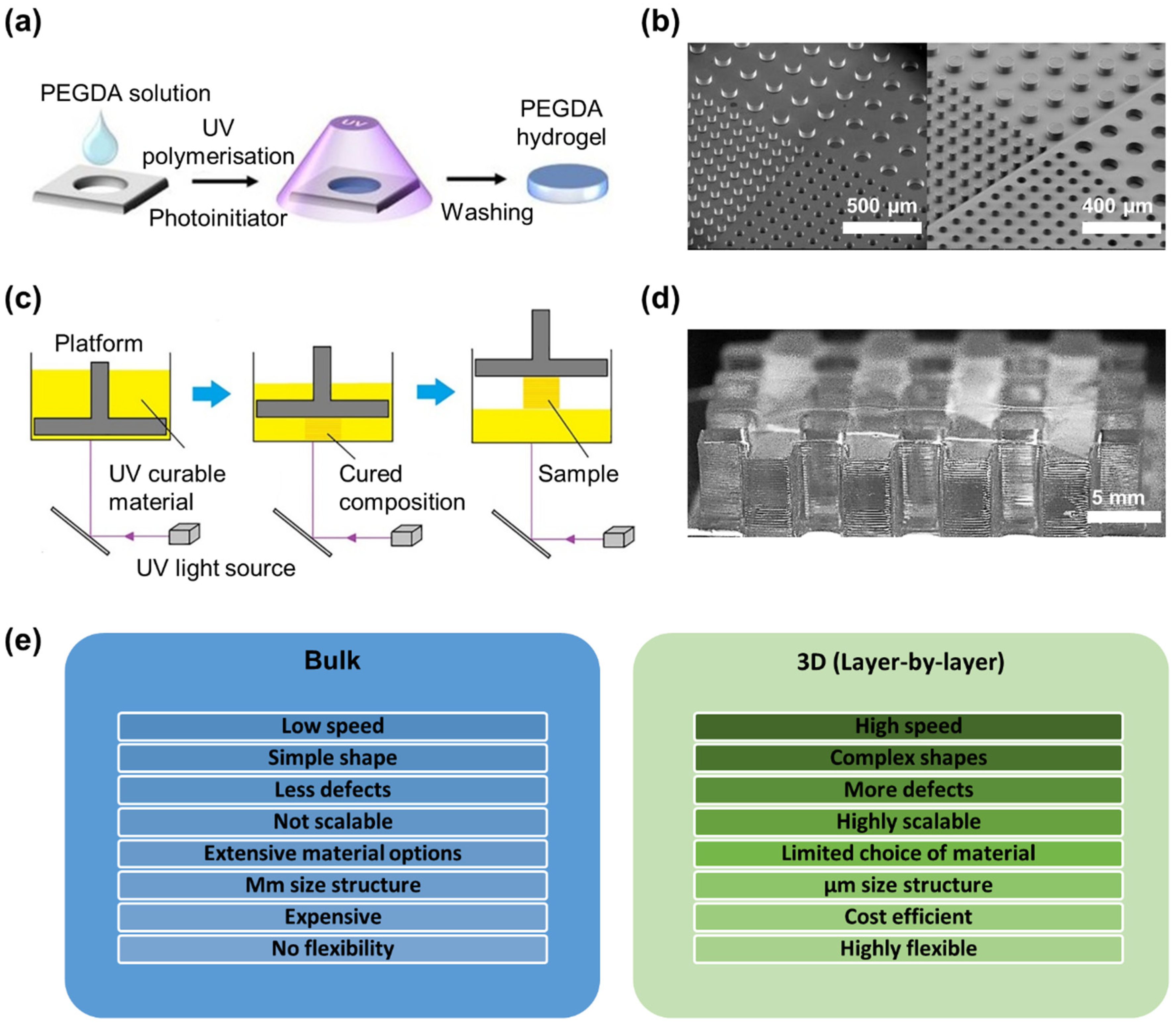
2. Manufacture of 3D PEGDA Biostructures
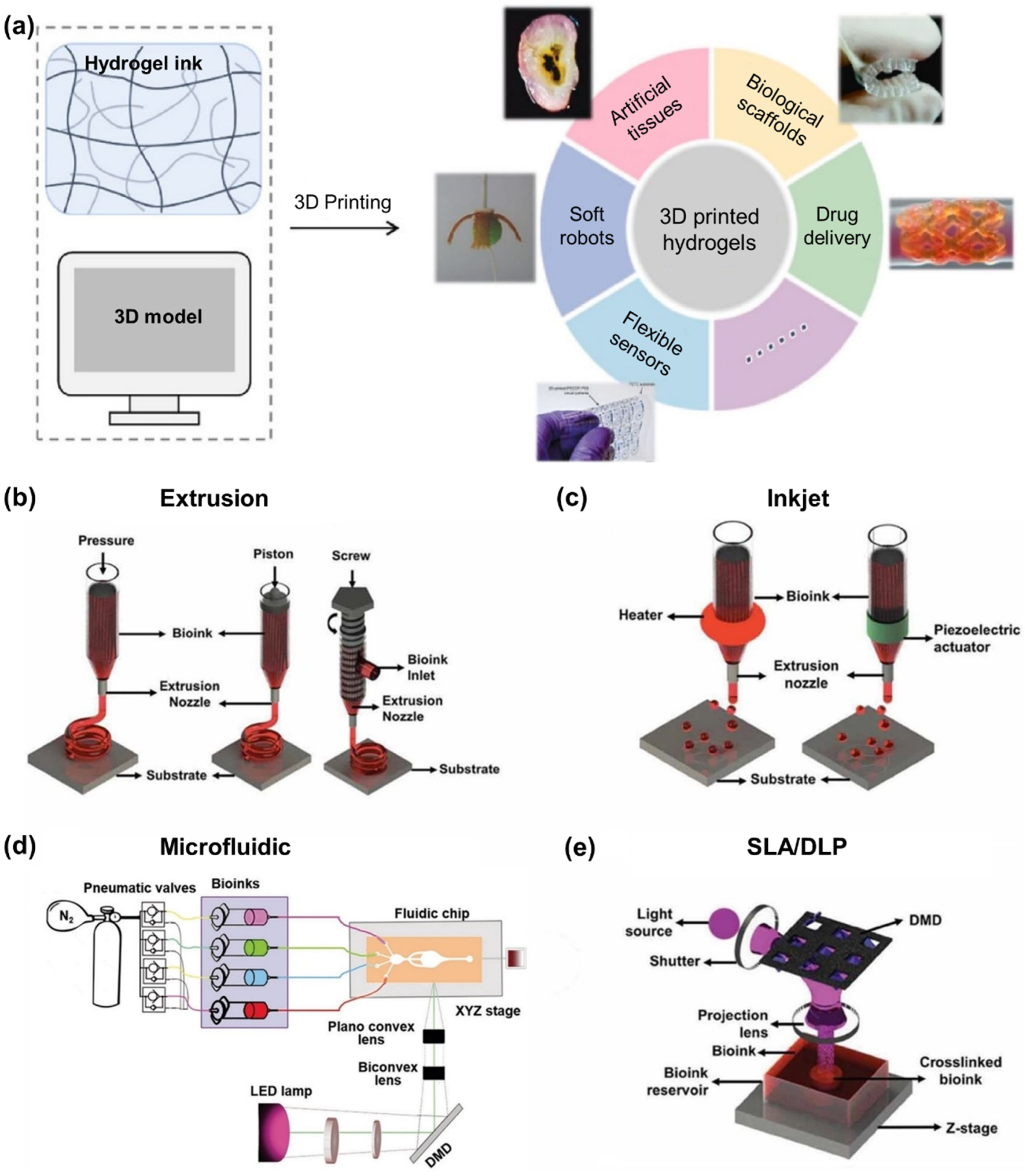
2.1. 3D Bioprinting Techniques
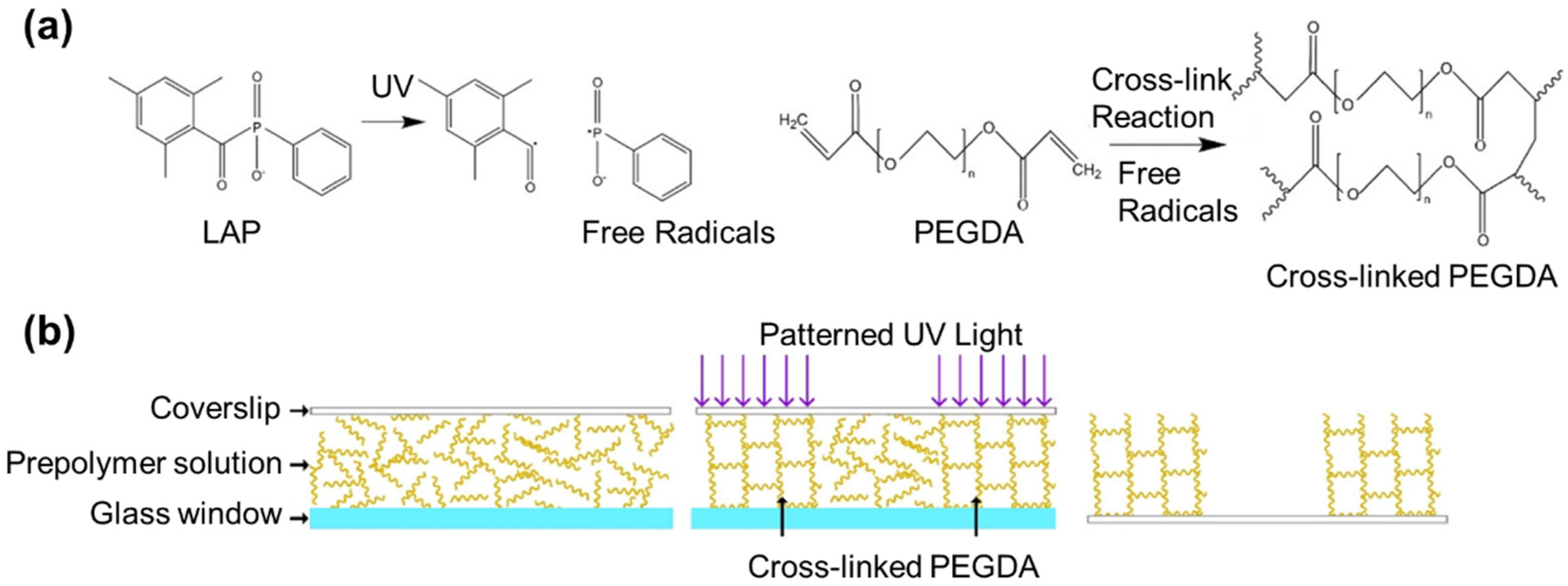
2.2. Photo-Cross-Linking Mechanism
2.3. Bulk vs. Layer-by-Layer Photo-Cross-Linking
3. Properties of Photo-Cross-Linked PEGDA Hydrogels
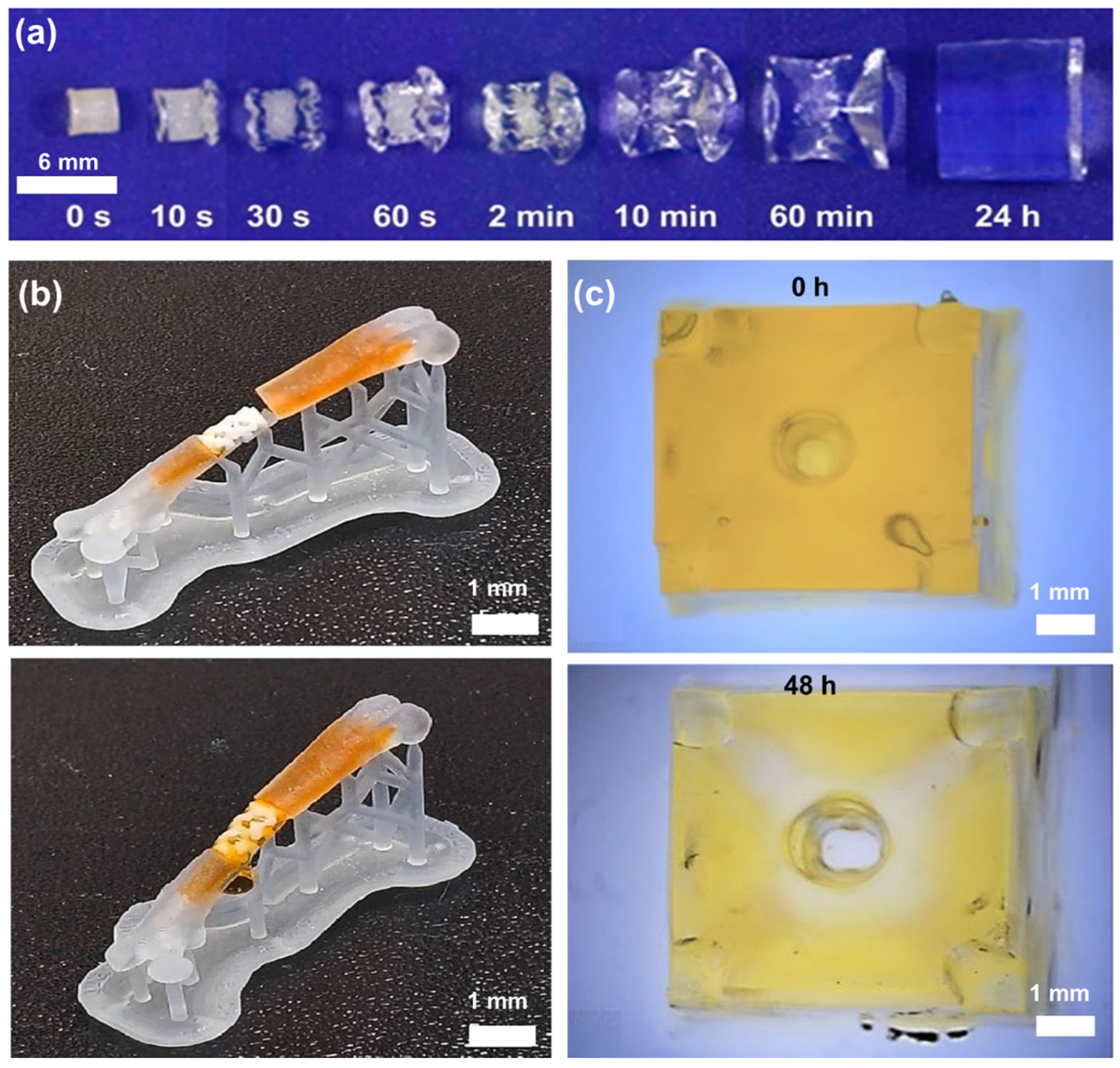
3.1. Swelling Behavior and the Effect of the Environment
| PEGDA/Comonomer | Molecular Weight (kDa) | Conc (%) | Bulk/3D | Type of Study | Cells Used | Sample Type | Storage Solution | pH | Temp (°C) | Volumetric Change Yes/No | Duration (Day) | Degradation | Swelling Ratio | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEGDA | 0.7 | 20 | 3D-projection lithography | Materials only | * N/a | Cuboid, 5 × 5 × 10 mm, 20 and 150 µm layer thicknesses | Deionized water | 7.4 | 8–45 | Yes | 7 | No | (−)10–(+)24 | [56] |
| PEGDA | 0.7 | 20 | 3D-projection lithography | Materials only | N/a | Cuboid, 5 × 5 × 5.1 mm, 20 and 150 µm layer thicknesses | Deionized water/cell culture media | 7.4 | 8–37 | Yes | 30 | No | (−)6–(+)20 | [57] |
| PEGDA/poly-(ε-caprolactone) (PCL) | 1 | 15 | 3D-SLA-DLP | In vitro viability of tissue constructs | Human umbilical vein endothelial cells (HUVECs) | n/a | Water | n/a | RT | No | 3 | No | 5 | [72] |
| PEGDA | 0.7, 3.4, 5, 10 | 40 | 3D-SLA | Long-term viability of SMT | NIH/3T3 cells | Disks 100 µm, dia 5 mm, 1 mm thickness | PBS | 7.4 | 37 | No | 1 | No | 5–35 | [100] |
| Poly-N-isopropylacrylamide (PNIPAM)/PEGDA | N/a | 10 | 3D-extrusion | Materials only | N/a | Multilayer, 260 µm | Water | N/a | RT | Yes, reversible between 20–50 °C | 1 | No | 0.1–3 | [129] |
| GelMA/PEGDA | 0.7 | 0–15 | Bulk and 3D-extrusion | Drug delivery | N/a | Multilayer, 200 µm, | PBS | 7.4 | 36.1 | No | 1 | Yes, 7 days | 4–7 | [141] |
| PEGDA/poly(ethylene glycol) methyl ethyl methacrylate (PEGMEMA) | 0.25, 0.575 | 45–100 | 3D-SLA | In vitro Viability of cell encapsulation in micro-stereolithography | HUVECs | Multilayer 50 µm, Φ9 mm, 1 mm | PBS | N/a | RT | No | 1 | No | 0.03–0.74 | [142] |
| PEGDA/poly(3,4-ethylenedioxythiophene) (PEDOT) | 0.575 | 50, 70, 90 | 3D-SLA | Materials only | N/a | Multilayer | PBS | N/a | 37 | No | 1 | No | 0.13–0.35 | [143] |
| Poly(ε-caprolactone) maleic acid (PGCL-Ma) and PEGDA | 2, 3.6, and 8 | 0–100 | Bulk | In vitro release of bovine serum albumin (BSA) for drug delivery | N/a | No specific shape, a section 25 mg | PBS | 7.4 | 37 | N/a | 40 | No degradation for 20 days | 4.1–73 | [144] |
| PEGDA/gelatine methacrylate (GelMA) | 0.7 | 5-20 | Bulk | Cell-laden cartilage tissue construct | Human bone marrow MSCs | Nanospheres | PBS | N/a | 37 | No | N/a | N/a | 6–10 | [145] |
| PEGDA/SiO2 | 0.575 | N/a | 3D-SLA | Materials only | N/a | Multilayer 25, 50, 100 µm (145 × 145 × 175 µm) | Water | N/a | 25 | Yes, 22% in thicknees and 15% on width | 4 | No | 0.35 | [146] |
| PEGDA | N/a | 10 | Bulk | Materials only | N/a | Cylinder, diam 3 mm, 15 mm thickness | Deionized water | N/a | 37 | No | 3 | N/a | 5–13.5 | [147] |
| PEGDA/GelMA | 1, 4, 8 | 15, 20, 30 | Bulk | 3D cell culture platform for studying cell invasion | MDA-MB-231 cells | N/a | PBS | 4, 7, 7.4 | 37 | No | 3 | No degradation for 21 days | 10–12 | [148] |
| PEGDA/tendon tECM | N/a | 10 | 3D-SLA | Tendon extracellular matrix for bone regeneration | Mesenchymal stem cell | Multilayer | Water | N/a | 25 | No | 1/2 | No | 0.06–0.12 | [150] |
| Water-miscible acrylate (HBA)/poly(ethylene glycol) methyl ethyl methacrylate (PEGMEMA)/PEGDA | 0.575, 0.7, | 2.5,7.5 | 3D-SLA | Materials only | N/a | Multilayer, 50 µm, dia 9 mm, 0.8 mm thickness | Mili-Q waer and PBS | N/a | RT | Yes | 3–24 h | No | 0.1–0.8 | [152] |
| PEGDA | 0.575 | 2–80 | 3D-DLP | Materials only | N/a | Multilayer, 50, 100, 500 µm, | Deionized water | N/a | RT | Yes | 1 | No | 0.01–0.2 | [153] |
| PEGDA | 0.7, 3.4 | 10,20,40 | Bulk | In vitro 3D synthetic matrices | NIH 3T3 cells | Cylinder, dia 10 mm, thickness 1 mm | Deionized water | N/a | RT | No | N/a | No | 2–9 | [155] |
| GelMA-PEGDA | 20 | 10 | Bulk | In vitro 3D hydrogels to mimic the neural tissue | Mouse neuroblastoma cell line Neuro2a | Cylinder, dia 10 mm, 3 mm thickness | Collagenase type II | N/a | RT | No | 2 | Yes, | 0.01–0.8 | [156] |
| PEGDA | 0.575 | N/a | 3D-SLA | Materials only | N/a | Multilayer, 100 µm, 200 × 200 µm | Water | N/a | 4–15 | Yes, reversible depending on cross-linking | N/a | N/a | 0.2–2.3 | [161] |
| PEGDA | 10 | 7.5 | Bulk | Materials only | N/a | Tubes with diameters 3.85–7.2 | Mili-Q | 7.4 | 37 | Yes | 2 | No | 30 | [162] |
| Calcium phosphates (Cap)/PEGDA | 0.250, 0.575, 0.7 | N/a | 3D-SLA | In Vivo 3D bone grafts | N/a | Multilayer. 6 mm dia, 200 µm thickness | Water | N/a | RT | No | 7 | No | 0.20–0.60 | [163] |
| PEGDA | 0.575 | N/a | 3D-SLA | Materials only | N/a | Multilayer, cantilevers | Ethanol, Water, acetone | N/a | RT | N/a | 60 min | no | 1.2–1.7 | [170] |
| PEGDA/Acrylic acid (AA) | 1, 4, 10 | 9–36 | Bulk | In vitro contractile SMT | C2C12 mouse myoblast cell | Single layer, 0.4 mm thickness | PBS | 7.4 | RT | Yes | 3 | Yes, after 4 weeks | 1–3.5 | [171] |
| PEGDA | 0.7 | 20 | 3D-SLA | In vitro contractile SMT | C2C12 mouse myoblast | Multilayer, 20 um, dia 6 mm, 5 mm thickness | Deionized water | N/a | RT | No | 1 | No | 5.1–5.6 | [149,154] |
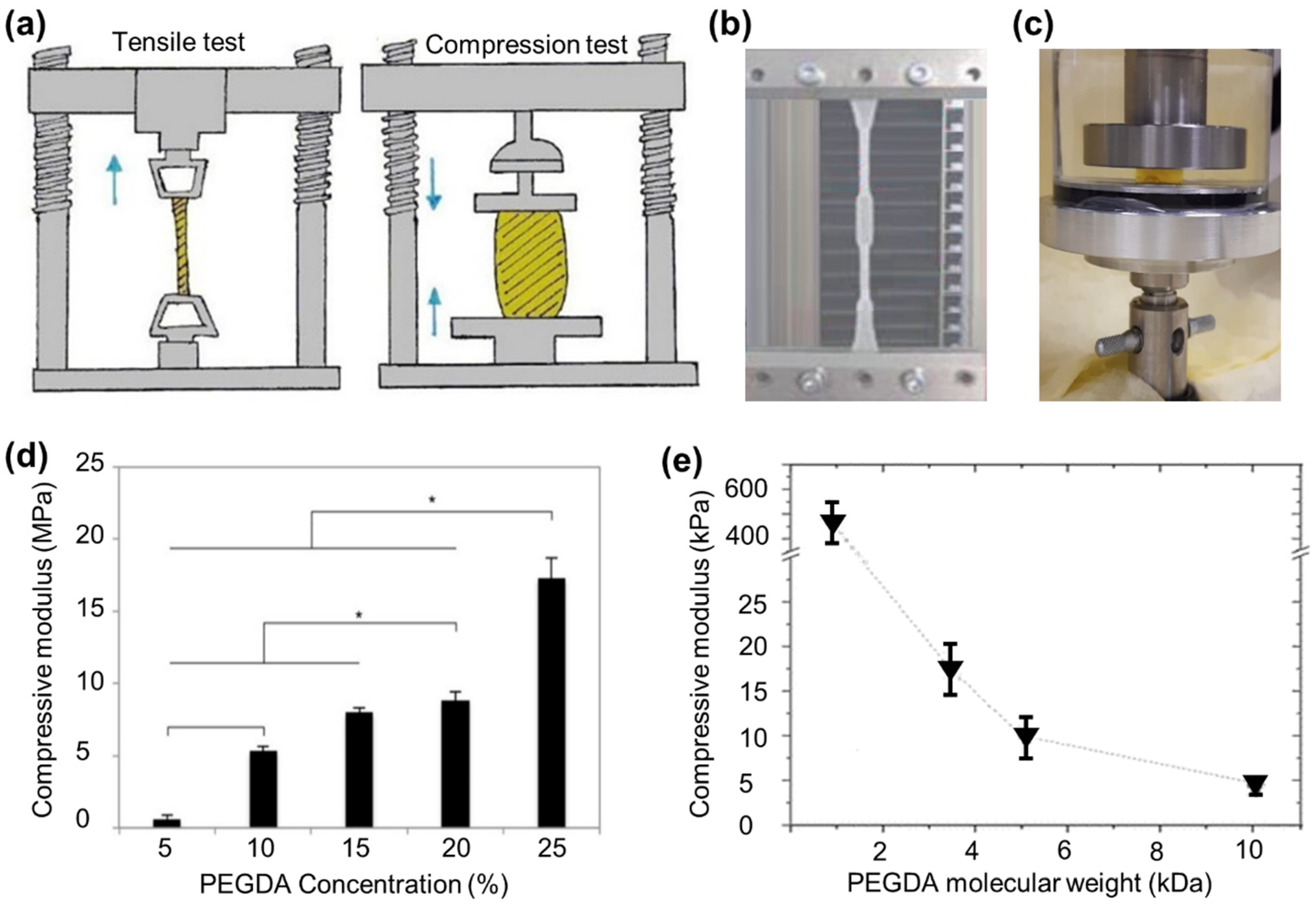
3.2. Mechanical Properties of PEGDA Hydrogels
3.2.1. Bulk Characteristic of PEGDA Hydrogels
| PEGDA/Comonomer | Molecular Weight (kDa) | Conc (%) | Bulk/3D | Type of Study | Cells Used | Sample Shape | UV Variations | Storage Solution | Temperature (°C) | Mechanical Test | Modulus | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEGDA/poly-(ε-caprolactone) (PCL) | 1 | 15 | 3D-SLA-DLP | In vitro viability of tissue constructs | HUVECs | Disks, 100 µm, dia 8 mm, 3 mm thickness | Yes | * N/a | N/a | Compressive modulus, 1 N load cell, 0.1–30% strain | 50–250 kPa | [72] |
| PEGDA | 0.7, 3.4, 5, 10 | 40 | 3D-SLA | Long-term viability of SMT | NIH/3T3 cells | Disks 100 um, dia 5 mm, 1 mm thickness | No | PBS | 25 | Compressive modulus, compression test, 1 mm/s, strain at 10% | 5–503 kPa | [100] |
| Poly-N-isopropylacrylamide (PNIPAM)/PEGDA | N/a | 10 | 3D-extrusion | Materials only | N/a | Multilayer, 260 µm, | No | Solution | 25 | Tensile strength, uniaxial tension, loading rate 2 mm/s | 85 kPa | [129] |
| PEGDA/gelatine methacrylate (GelMA) | 0.7 | 5–20 | Bulk | Cell-laden cartilage tissue construct | Human bone marrow MSCs | N/a | No | Dry | RT | Compression/100 N load, 2 mm/min | 5–20 MPa | [145] |
| PEGDA/SiO2 | 0.575 | N/a | 3D-SLA | Materials only | N/a | Multilayer 25, 50, 100 µm (145 × 145 × 175 µm) | No | Swollen (water) | 25 | Compression testing, 2 mm/min | 50–85 MPa | [146] |
| PEGDA | N/a | 10 | Bulk | Materials only | N/a | Cylinder, diameter 15 × 3 mm thickness | Yes | Dry | RT | Compression/ 2 kN, 0.5 mm/min | 0.49–0.7 MPa | [147] |
| PEGDA/GelMA | 1, 4, 8 | 15, 20, 30 | Bulk | 3D cell culture platform for studying cell invasion | MDA-MB-231 cells | N/a | No | Hydrated (water) | 37 | Storage modulus, rheometry, 1 to 400 rad/s, amplitude of 0.3%. | 1–8 kPa | [148] |
| PEGDA/tendon tECM | N/a | 10 | 3D-SLA | Tendon extracellular matrix for bone regeneration | Mesenchymal stem cell | Multilayer | No | Dry | RT | Compression, compressive modulus | 0.2–0.3 MPa | [150] |
| PEGDA | 0.7, 3.4 | 10, 20, 40 | Bulk | In vitro 3D synthetic matrices | NIH 3T3 cells | Cylinder, dia 10 mm, thickness 3 mm | No | Swollen (water) | RT | Confined compression, 19 µm/min, 10% deformation | 22–118 kPa | [155] |
| GelMA-PEGDA | 20 | 10 | Bulk | In vitro 3D hydrogels to mimic the neural tissue | Mouse neuroblastoma cell line Neuro2a | Tensile 30 × 10 × 3 mm, compression dia 10 mm, 5 mm thickness | No | PBS | 37 | Tensile modulus, max strain at 50%, 1mm/min compressive modulus, 0.5 mm/min | 10–60 kPa 0.8–6 kPa | [156] |
| Calcium phosphates (Cap)/PEGDA | 0.250, 0.575, 0.7 | N/a | 3D-SLA | In vivo 3D bone grafts | N/a | Multilayer. 200 um. 6 mm dia, 12 mm thickness | No | Dry and swollen (water) | 25 and 37 | Compression testing, 1 mm/min | 0.21–19.87 MPa | [163] |
| PEGDA/Acrylic acid (AA) | 1, 4, 10 | 9–36 | Bulk | In vitro contractile SMT | C2C12 mouse myoblast cell | Single layer, 2 × 5 cm 0.4 mm | No | PBS | RT | Tensile test, elastic modulus, 1.5 mm/min | 68–214 kPa | [171] |
| PEGDA | 0.6 | N/a | 3D-DLP | Materials only | N/a | 10 × 80 × 0.7 mm | No | N/a | N/a | Tensile test, Young’s modulus, load cell 500 N | 1.4 MPa | [175] |
| PEGDA | 0.258 | 7–40 | Bulk | Human articular cartilage | N/a | Cylinder, dia 11 mm, height 8 mm | No | Dry wet | RT | Tensile test, elastic modulus, 20 N, compression test, elastic modulus | 4–20 MPa 0.05–3.19 MPa | [182] |
| PEGDA | 0.7 | 20 | 3D-SLA | In vitro contractile SMT | C2C12 mouse myoblast | Multilayer, 20 um, dia 6 mm, 5 mm thickness | No | Swollen (water) | RT | Compression testing shear modulus, 0.5 mm/min | 0.43 MPa | [149,154] |
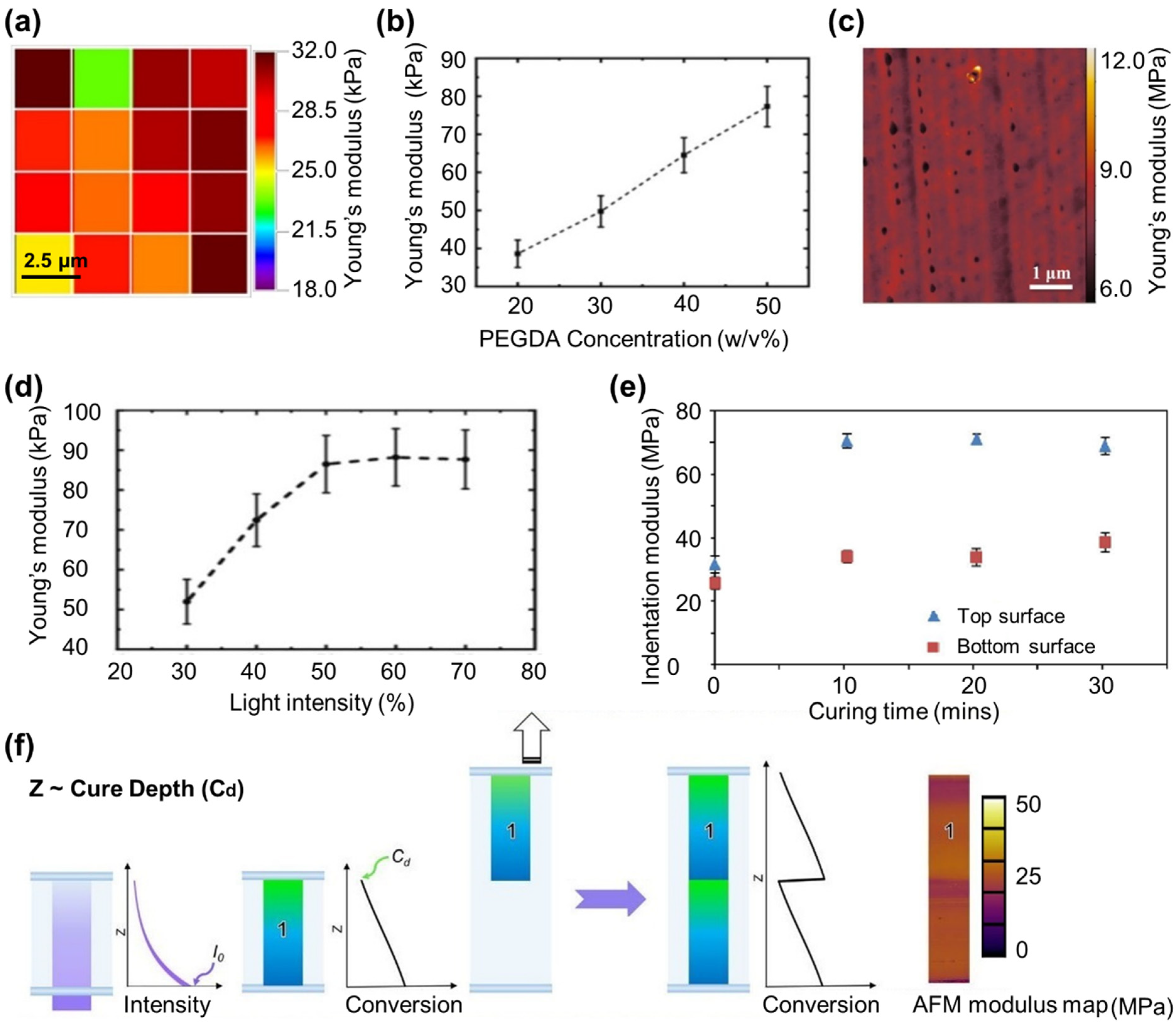
3.2.2. Localized Characteristics of PEGDA Hydrogels
| PEGDA/Comonomer | Molecular Weight (kDa) | Conc (%) | Bulk/3D | Type of Study | Cells Used | Sample Shape | UV Variations | Storage Solution | Temperature (°C) | Mechanical Test | Modulus | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEGDA | 0.7 | 20 | 3D-projection lithography | Materials only | * N/a | Cuboid, 5 × 5 × 1.2 mm, 20 and 150 µm layer thicknesses | Yes | Deionized water/cell culture media | RT | Nanoindentation | 0.67–1.69 MPa | [57] |
| PEGDA | 0.7 | 20 | 3D-projection lithography | Materials only | N/a | Cuboid, 5 × 5 × 3 mm, 20 µm layer thickness | Yes | Deionized water | RT | AFM | 2.8–13.1 kPa | [61] |
| Polycaprolactone di-methacrylated (PCLDMA)/PEGDA | 0.250 | 0–50 | 3D-Inkjet printing | Biocompatibility test | NIH3T3 fibroblasts | Multilayer, 5 µm, 100 layers, cuboid, 5 × 5 × 0.5 mm | Yes | N/a | RT | Nanoindentation, spherical indenter 50 µm radius | 25–75 MPa | [86] |
| PEGDA | 0.575 | N/a | 3D-SLA | Materials only | N/a | Multilayer, 100 µm, 200 × 200 µm | Yes | N/a | N/a | AFM, elastic modulus | 1.25–4 MPa | [161] |
| PEGDA | 0.6 | N/a | 3D-DLP | Materials only | N/a | Multilayer, 10–100 µm thickness | No | Dry | N/a | Nanoindentation, radius 1 µm diameter, | 7 MPa | [175] |
| PEGDA | 0.258 | 7–40 | Bulk | Human articular cartilage | N/a | Cylinder, dia 11 mm, height 8 mm | No | Wet | RT | Nanoindentatio, flat punch. 54 µm diameter | 1.84–3.29 MPa | [182] |
| PEGDA/Hyaluronic acid (HA) | 0.575 | N/a | Bulk | Influence of the network architecture | Murine L929 fibroblasts | N/a | No | No | 25 | AFM, | 2.5–25 kPa | [193] |
| PEGDA | 0.258, 0.575, 0.7 | N/a | Bulk | Materials only | N/a | Single layer, 1.5 mm thickness | No | Water | N/a | AFM | 2.48–4.33 MPa | [194] |
| PEGDA | 0.7 | 20–50 | Bulk | Materials only | N/a | Cuboid, 6 × 6 × 0.4 mm | Yes | N/a | N/a | AFM | 35–95 kPa | [195] |
| PEGDA | 0.7 | N/a | 3D-Direct Laser writing (DLW) | Materials only | N/a | Single layer, | Yes | No | N/a | AFM, elastic modulus | 6.9–9.5 MPa | [196] |
| PEGDA | 0.7 | N/a | 3D-DLP | Materials only | N/a | Multilayer, 10, 30, 100 µm | Yes | Dry | RT | AFM | 9.5–34.5 MPa | [197] |
| PEGDA | 0.7 | 50 | 3D-DLW | In vitro woodpile structures, model for leukemic disease | Bone marrow mesenchymal stem cells (BM-MSCs) | Multilayer, 100 × 100 × 50 µm | Yes | Water | RT | Nanoindentation, spherical 28 µm radius | 100–1500 kPa | [200] |
| PEGDA (no photoinitiator) | 0.7 | N/a | 3D-laser SLA | In vitro cell viability and biocompatibility | Chinese hamster ovarian cells (CHO) | Multilayer, 100 µm thickness | Yes | Dry | N/a | Nanoindentation, Berkovich tip | 10–100 MPa | [202] |
| PEGDA | 6, 20 | 15 | 3D-SLA | In vitro 3D microenvironment and viability test | Cardiac progenitor cells (hCPCs) | Multilayer, 250 µm layer thickness, 10 layers | No | Dry and wet | Water | Nanoindentation | 5 MPa 1 MPa | [204] |
| PEGDA | 0.575 | 5–20 | Bulk | Materials only | N/a | Single layer, 70 µm | No | Water | 20 | AFM, 2.5 µm radius spherical tip | 2.8–228.9 kPa | [205] |
| PEGDA | 0.7 | N/a | 3D-SLA | Materials only | N/a | Multilayer, 600 um height, diameter 300 µm | No | N/a | N/a | AFM | 200 MPa | [206] |
| PEGDA (PEG-fibrinogen) | 0.4, 4 | 5–25 | Microfluidic | Material stiffness and cell viability | human foreskin fibroblasts (HFFs) | Single layer, | No | N/a | N/a | AFM | 0.7–50 kPa | [207] |
| Polyacrylamide (PAM)/PEGDA | 0.7 | 5–16 | Microfluidic | Materials only | N/a | Hydrogel particles | No | Water, dry | RT | AFM | 2–7 kPa (hydrated) 25–180 kPa (dry) | [208] |
4. 3D PEGDA Hydrogel for Cardiac Tissue Engineering
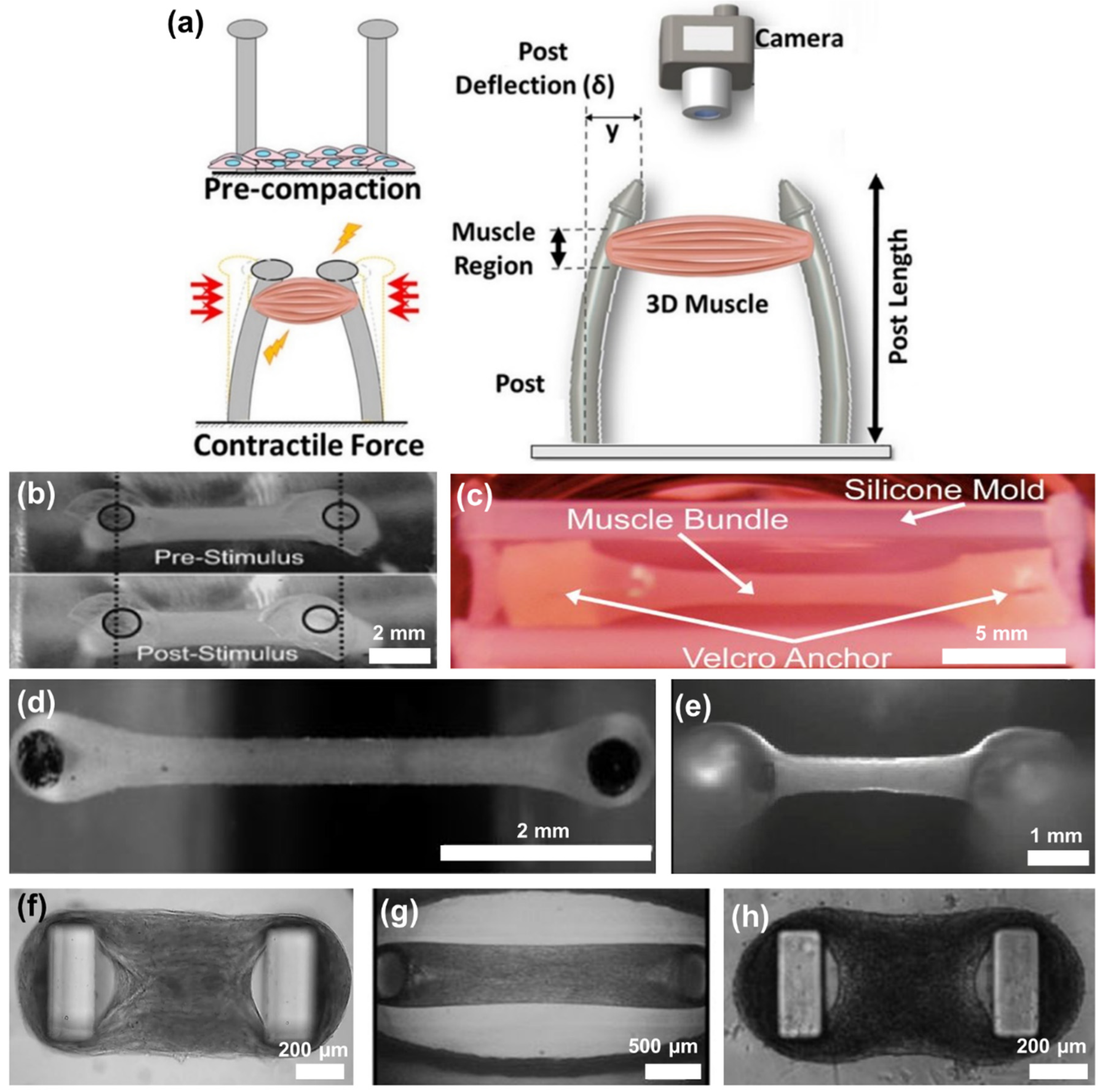
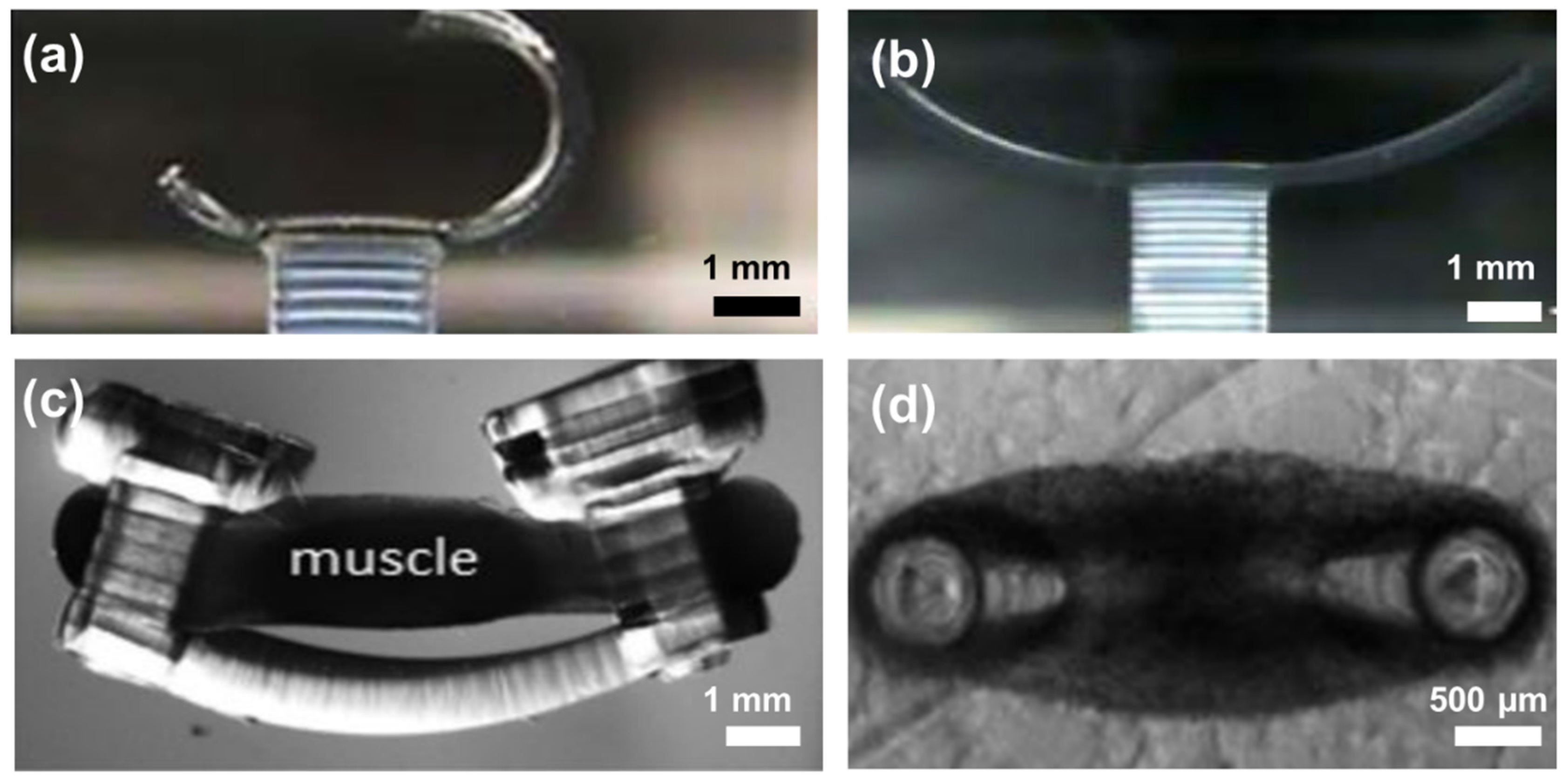
5. Knowledge Gap and Future Perspective
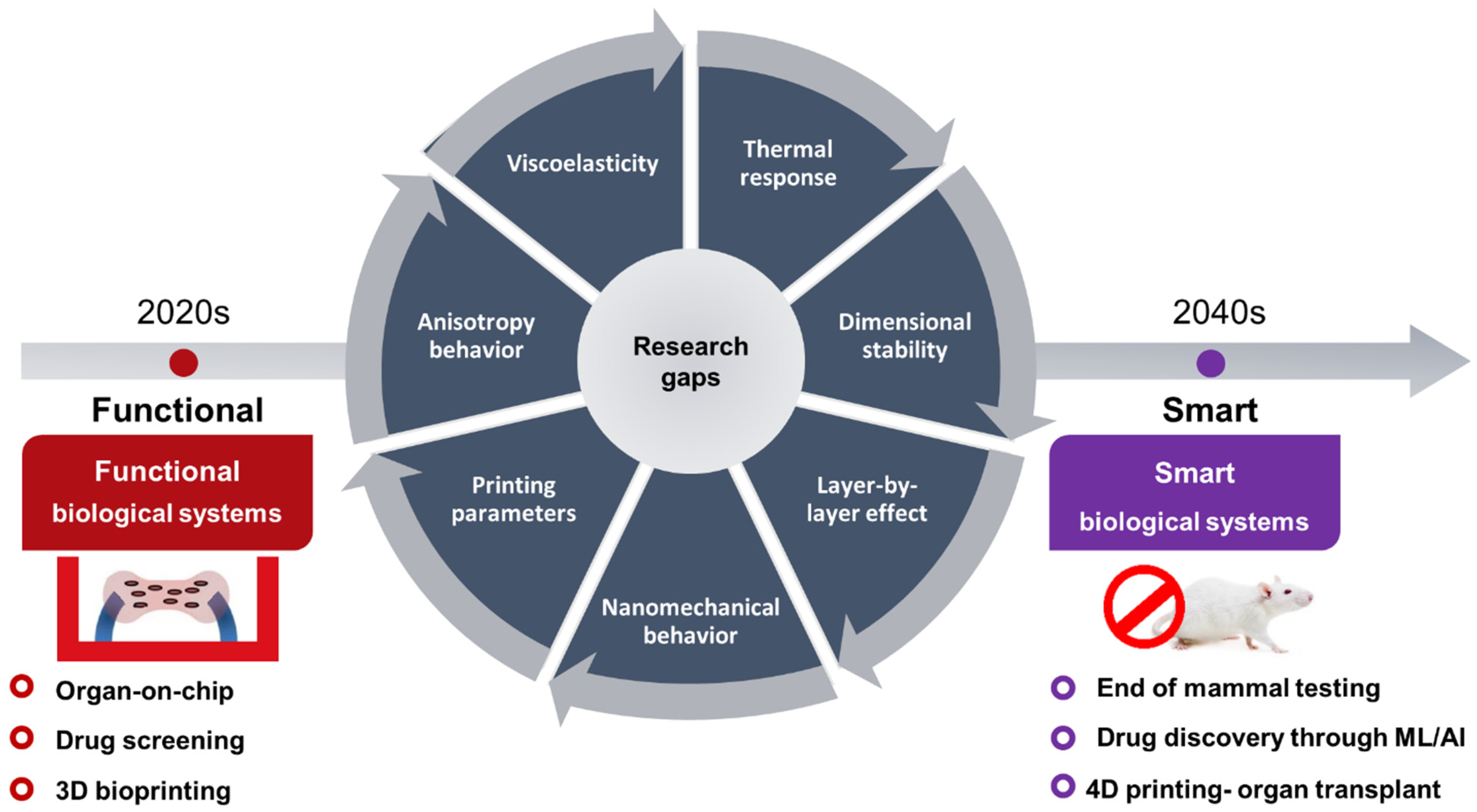
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, J.R.; Yong, K.W.; Tang, R.; Gong, Y.; Wen, T.; Yang, H.; Li, A.; Chia, Y.C.; Pingguan-Murphy, B.; Xu, F. Lateral Flow Assay Based on Paper–Hydrogel Hybrid Material for Sensitive Point-of-Care Detection of Dengue Virus. Adv. Healthc. Mater. 2017, 6, 1600920. [Google Scholar] [CrossRef]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent Advances in Hydrogel Based Drug Delivery Systems for the Human Body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef]
- Li, J.; Mo, L.; Lu, C.H.; Fu, T.; Yang, H.H.; Tan, W. Functional Nucleic Acid-Based Hydrogels for Bioanalytical and Biomedical Applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef]
- Lee, T.T.; García, J.R.; Paez, J.; Singh, A.; Phelps, E.A.; Weis, S.; Shafiq, Z.; Shekaran, A.; Campo, A.; Andrés, J. Light-Triggered in Vivo Activation of Adhesive Peptides Regulates Cell Adhesion, Inflammation and Vascularization of Biomaterials. Nat. Mater. 2015, 14, 352–360. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Alexis, K.; Jayant, R.D.; Sagar, V.; Vashist, A.; Nair, M. Bioresponsive Injectable Hydrogels for On-Demand Drug Release and Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3595–3602. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 38, 317–342. [Google Scholar] [CrossRef]
- Barthes, J.; Özçelik, H.; Hindié, M.; Ndreu-halili, A.; Vrana, N.E. Cell Microenvironment Engineering and Monitoring for Tissue Engineering and Regenerative Medicine: The Recent Advances. BioMed Res. Int. 2014, 2014, 921905. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. Native-Mimicking in vitro Microenvironment: An Elusive and Seductive Future for Tumor Modeling and Tissue Engineering. J. Biol. Eng. 2018, 12, 20. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Argentiere, S.; Siciliano, P.A.; Blasi, L. How Microgels Can Improve the Impact of Organ-on-Chip and Microfluidic Devices for 3d Culture: Compartmentalization, Single Cell Encapsulation and Control on Cell Fate. Polymers 2021, 13, 3216. [Google Scholar] [CrossRef]
- Liu, X.; Su, Q.; Zhang, X.; Yang, W.; Ning, J.; Jia, K.; Xin, J.; Li, H.; Yu, L.; Liao, Y.; et al. Recent Advances of Organ-on-a-Chip in Cancer Modeling Research. Biosensors 2022, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Chakraborty, E. Hydrogel Based Tissue Engineering and Its Future Applications in Personalized Disease Modeling and Regenerative Therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, S.; Ahn, S.I. Design and Engineering of Organ-on-a-Chip. Biomed. Eng. Lett. 2023, 13, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current Understanding of the Applications of Photocrosslinked Hydrogels in Biomedical Engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef]
- De Masi, A.; Scognamiglio, P.L.; Battista, E.; Netti, P.A.; Causa, F. PEG-Based Cleavable Hydrogel Microparticles with Controlled Porosity for Permiselective Trafficking of Biomolecular Complexes in Biosensing Applications. J. Mater. Chem. B 2022, 10, 1980–1990. [Google Scholar] [CrossRef]
- Fedi, A.; Vitale, C.; Giannoni, P.; Caluori, G.; Marrella, A. Biosensors to Monitor Cell Activity in 3D Hydrogel-Based Tissue Models. Sensors 2022, 22, 1517. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, S.; Zhang, X.; Wei, G.; Su, Z. Recent Advances in Peptide Engineering of PEG Hydrogels: Strategies, Functional Regulation, and Biomedical Applications. Macromol. Mater. Eng. 2022, 11, 2200385. [Google Scholar] [CrossRef]
- Rekowska, N.; Eickner, T.; Grabow, N.; Teske, M.; Konasch, J.; Riess, A.; Mau, R.; Seitz, H. PEGDA Drug Delivery Scaffolds Prepared with UV Curing Process. Curr. Dir. Biomed. Eng. 2020, 6, 193–195. [Google Scholar] [CrossRef]
- Peyton, S.R.; Raub, C.B.; Keschrumrus, V.P.; Putnam, A.J. The Use of Poly(Ethylene Glycol) Hydrogels to Investigate the Impact of ECM Chemistry and Mechanics on Smooth Muscle Cells. Biomaterials 2006, 27, 4881–4893. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mapili, G.; Suhali, G.; Chen, S.; Roy, K. A Digital Micro-Mirror Device-Based System for the Microfabrication of Complex, Spatially Patterned Tissue Engineering Scaffolds. J. Biomed. Mater. Res. Part A 2006, 77, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.N.; Zhu, J.; Marchant, R.; West, J.L.; Yoo, J.U.; Johnstone, B. Design and Characterization of Poly(Ethylene Glycol) Photopolymerizable Semi-Interpenetrating Networks for Chondrogenesis of Human Mesenchymal Stem Cells. Tissue Eng. 2007, 13, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, S.; Farber, J.L.; Baserga, R. A Simple Method for Decreasing the Toxicity of Polyethylene Glycol in Mammalian Cell Hybridization. Somatic Cell Genet. 1979, 5, 263–269. [Google Scholar] [CrossRef]
- Kedar, E.; Unger, E.; Schwartzbach, M. In vitro Induction of Cell-Mediated Immunity to Murine Leukemia Cells. I. Optimization of Tissue Cultre Conditions for the Generation of Cytotoxic Lymphocytes. J. Immunol. Methods 1976, 13, 1–19. [Google Scholar] [CrossRef]
- Yamini, C.; Shantha, K.L.; Panduranga Rao, K. Synthesis and Characterization of Poly[N-Vinyl-2-Pyrrolidone-Polyethylene Glycol Diacrylate] Copolymeric Hydrogels for Drug Delivery. J. Macromol. Sci. Pure Appl. Chem. 1997, 34, 2461–2470. [Google Scholar] [CrossRef]
- Graham, N.B.; McNeill, M.E. Hydrogels for Controlled Drug Delivery. Biomaterials 1984, 5, 27–36. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Shahani, S.; Sun, D.D.N.; Sharma, B.; Elisseeff, J.H.; Leong, K.W. Biodegradable and Photocrosslinkable Polyphosphoester Hydrogel. Biomaterials 2006, 27, 1027–1034. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.W.; Hefferan, T.E.; Currier, B.L.; Yaszemski, M.J.; Lu, L. Synthesis and Evaluation of Novel Biodegradable Hydrogels Based on Poly(Ethylene Glycol) and Sebacic Acid as Tissue Engineering Scaffolds. Biomacromolecules 2008, 9, 149–157. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Prestwich, G.D. In Situ Crosslinkable Hyaluronan Hydrogels for Tissue Engineering. Biomaterials 2004, 25, 1339–1348. [Google Scholar] [CrossRef]
- Schmedlen, R.H.; Nyugen, K.T.; West, J.L. Tissue Engineered Vascular Grafts: Elastic Polyethylene Glycol Hydrogel Scaffolds for Culture under Pulsatile Flow Conditions. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Houston, TX, USA, 23–26 October 2002; Volume 1, pp. 813–814. [Google Scholar] [CrossRef]
- Troken, A.; Marion, N.; Hollister, S.; Mao, J. Tissue Engineering of the Synovial Joint: The Role of Cell Density. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2007, 221, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Grover, G.N.; Rao, N.; Christman, K.L. Myocardial Matrix-Polyethylene Glycol Hybrid Hydrogels for Tissue Engineering. Nanotechnology 2014, 25, 014011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, P.; Chen, Y.; Li, M.; Chen, C.; Lu, H. 3D-Printed Extracellular Matrix/Polyethylene Glycol Diacrylate Hydrogel Incorporating the Anti-Inflammatory Phytomolecule Honokiol for Regeneration of Osteochondral Defects. Am. J. Sports Med. 2020, 48, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Guan, J.; Mao, Y.J.; Zhou, P. Hydrogel: A Potential Therapeutic Material for Bone Tissue Engineering. AIP Adv. 2021, 11, 010701. [Google Scholar] [CrossRef]
- Han, Y.; Lian, M.; Zhang, C.; Jia, B.; Wu, Q.; Sun, B.; Qiao, Z.; Sun, B.; Dai, K. Study on Bioactive PEGDA/ECM Hybrid Bi-Layered Hydrogel Scaffolds Fabricated by Electro-Writing for Cartilage Regeneration. Appl. Mater. Today 2022, 28, 101547. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Z.; Guan, G.; Lu, Z.; Yan, S.; Du, A.; Wang, L.; Li, Q. Polyethylene Glycol Diacrylate Scaffold Filled with Cell-Laden Methacrylamide Gelatin/Alginate Hydrogels Used for Cartilage Repair. J. Biomater. Appl. 2022, 36, 1019–1032. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D Bioprinting of Photo-Crosslinkable Silk Methacrylate (SilMA)-Polyethylene Glycol Diacrylate (PEGDA) Bioink for Cartilage Tissue Engineering. J. Biomed. Mater. Res. Part A 2022, 110, 884–898. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, N.; Prestwich, G.D.; Wen, X. Recruitment of Endogenous Stem Cells for Tissue Repair. Macromol. Biosci. 2008, 8, 836–842. [Google Scholar] [CrossRef]
- Kim, J.; Hefferan, T.E.; Yaszemski, M.J.; Lu, L. Potential of Hydrogels Based on Poly(Ethylene Glycol) and Sebacic Acid as Orthopedic Tissue Engineering Scaffolds. Tissue Eng. Part A 2009, 15, 2299–2307. [Google Scholar] [CrossRef]
- Paxton, J.Z.; Donnelly, K.; Keatch, R.P.; Baar, K. Engineering the Bone-Ligament Interface Using Polyethylene Glycol Diacrylate Incorporated with Hydroxyapatite. Tissue Eng. Part A 2009, 15, 1201–1209. [Google Scholar] [CrossRef]
- Tan, G.; Wang, Y.; Li, J.; Zhang, S. Synthesis and Characterization of Injectable Photocrosslinking Poly (Ethylene Glycol) Diacrylate Based Hydrogels. Polym. Bull. 2008, 61, 91–98. [Google Scholar] [CrossRef]
- Curley, J.L.; Jennings, S.R.; Moore, M.J. Fabrication of Micropatterned Hydrogels for Neural Culture Systems Using Dynamic Mask Projection Photolithography. J. Vis. Exp. 2011, 3–9. [Google Scholar] [CrossRef]
- Kang, K.H.; Hockaday, L.A.; Butcher, J.T. Quantitative Optimization of Solid Freeform Deposition of Aqueous Hydrogels. Biofabrication 2013, 5, 035001. [Google Scholar] [CrossRef] [PubMed]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.C.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D Printing of Anatomically Accurate and Mechanically Heterogeneous Aortic Valve Hydrogel Scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [PubMed]
- Joas, S.; Tovar, G.E.M.; Celik, O.; Bonten, C.; Southan, A. Extrusion-Based 3d Printing of Poly(Ethylene Glycol) Diacrylate Hydrogels Containing Positively and Negatively Charged Groups. Gels 2018, 4, 69. [Google Scholar] [CrossRef]
- Kim, Y.T.; Choi, J.S.; Choi, E.; Shin, H. Additive Manufacturing of a 3D Vascular Chip Based on Cytocompatible Hydrogel. Eur. Polym. J. 2021, 151, 110451. [Google Scholar] [CrossRef]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.M.; Rigat-Brugarolas, L.G.; Nallapatti, U.; Samitier, J.; Deforest, C.A.; Posas, F.; Garcia-Cordero, J.L.; et al. 3D-Printing of Transparent Bio-Microfluidic Devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Preobrazhenskiy, I.I.; Tikhonov, A.A.; Evdokimov, P.V.; Shibaev, A.V.; Putlyaev, V.I. DLP Printing of Hydrogel/Calcium Phosphate Composites for the Treatment of Bone Defects. Open Ceram. 2021, 6, 100115. [Google Scholar] [CrossRef]
- Nguyen, M.T.H.; Kim, S.Y.; Jeong, T.H.; Kim, J.H.; Cho, H.S.; Ha, T.H.; Ahn, S.J.; Kim, Y.H. Preparation and Stability of PEGDA/GO Conductive Materials by DLP 3D Printing. Electron. Mater. Lett. 2022, 18, 275–281. [Google Scholar] [CrossRef]
- Sheng, L.; Li, M.; Zheng, S.; Qi, J. Adjusting the Accuracy of PEGDA-GelMA Vascular Network by Dark Pigments via Digital Light Processing Printing. J. Biomater. Appl. 2022, 36, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zou, B.; Lai, Q.; Zhu, K. SLA-3d Printing and Compressive Strength of PEGDA/NHAP Biomaterials. Ceram. Int. 2022, 48, 30917–30926. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive Modification of Poly(Ethylene Glycol) Hydrogels for Tissue Engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; West, J.L. Bioactive Poly(Ethylene Glycol) Acrylate Hydrogels for Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 167–179. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Afsar, A.; Zhang, R.; Wilson, S.; Dossi, E.; Goel, S.; Impey, S.A.; Aria, A.I. Thermal Response of Multi-Layer UV Crosslinked PEGDA Hydrogels. Polym. Degrad. Stab. 2022, 195, 109805. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Williams, C.J.; Micallef, C.; Duarte-Martinez, F.; Afsar, A.; Zhang, R.; Wilson, S.; Dossi, E.; Impey, S.A.; Goel, S.; et al. Nanoindentation Response of 3D Printed PEGDA Hydrogels in Hydrated Environment. ACS Appl. Polym. Mater. 2023, 5, 1180–1190. [Google Scholar] [CrossRef]
- Kloxin, A.; Kloxin, C.; Bowman, C.; Anseth, K. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef]
- Zafeiris, K.; Brasinika, D.; Karatza, A.; Koumoulos, E.; Karoussis, I.K.; Kyriakidou, K.; Charitidis, C.A. Additive Manufacturing of Hydroxyapatite–Chitosan–Genipin Composite Scaffolds for Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 119, 111639. [Google Scholar] [CrossRef]
- Schwach, V.; Passier, R. Native Cardiac Environment and Its Impact on Engineering Cardiac Tissue. Biomater. Sci. 2019, 7, 3566–3580. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Panchal, V.; Dulebo, A.; Hawi, S.; Zhang, R.; Wilson, S.; Dossi, E.; Goel, S.; Impey, S.A.; Aria, A.I. Mechanical Behavior of 3D Printed Poly (Ethylene Glycol) Diacrylate Hydrogels in Hydrated Conditions Investigated Using Atomic Force Microscopy. ACS Appl. Polym. Mater. 2023, 5, 3034. [Google Scholar] [CrossRef]
- Chen, J.Y.; Hwang, J.V.; Ao-Ieong, W.S.; Lin, Y.C.; Hsieh, Y.K.; Cheng, Y.L.; Wang, J. Study of Physical and Degradation Properties of 3D-Printed Biodegradable, Photocurable Copolymers, PGSA-Co-PEGDA and PGSA-Co-PCLDA. Polymers 2018, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, T.H.; Kang, D.L.; Baek, S.Y.; Lee, Y.; Koh, Y.G.; Kim, Y. Il Chondrogenic Differentiation of Human ASCs by Stiffness Control in 3D Fibrin Hydrogel. Biochem. Biophys. Res. Commun. 2020, 522, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, C.; Wang, Y.; Zhang, L.; Zhang, J.; Shi, J.; Si, J.; Yuan, Y.; Liu, C. Direct Three-Dimensional Printing of a Highly Customized Freestanding Hyperelastic Bioscaffold for Complex Craniomaxillofacial Reconstruction. Chem. Eng. J. 2021, 411, 128541. [Google Scholar] [CrossRef]
- Ye, K.; Wang, X.; Cao, L.; Li, S.; Li, Z.; Yu, L.; Ding, J. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Lett. 2015, 15, 4720–4729. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Lü, D.; Luo, C.; Zhang, C.; Li, Z.; Long, M. Differential Regulation of Morphology and Stemness of Mouse Embryonic Stem Cells by Substrate Stiffness and Topography. Biomaterials 2014, 35, 3945–3955. [Google Scholar] [CrossRef]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes Differentiate Optimally on Substrates with Tissue-like Stiffness: Pathological Implications for Soft or Stiff Microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef]
- Petzold, J.; Gentleman, E. Intrinsic Mechanical Cues and Their Impact on Stem Cells and Embryogenesis. Front. Cell Dev. Biol. 2021, 9, 3112. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An Overview of Substrate Stiffness Guided Cellular Response and Its Applications in Tissue Regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Zhou, C.; Duan, M.; Guo, D.; Du, X.; Zhang, D.; Xie, J. Microenvironmental Stiffness Mediates Cytoskeleton Re-Organization in Chondrocytes through Laminin-FAK Mechanotransduction. Int. J. Oral Sci. 2022, 15, 14. [Google Scholar] [CrossRef]
- Shanjani, Y.; Pan, C.C.; Elomaa, L.; Yang, Y. A Novel Bioprinting Method and System for Forming Hybrid Tissue Engineering Constructs. Biofabrication 2015, 7, 045008. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Harrysson, O.L.A.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion Bioprinting: Recent Progress, Challenges, and Future Opportunities. Bioprinting 2021, 21, e00116. [Google Scholar] [CrossRef]
- Murphy, C.A.; Lim, K.S.; Woodfield, T.B.F. Next Evolution in Organ-Scale Biofabrication: Bioresin Design for Rapid High-Resolution Vat Polymerization. Adv. Mater. 2022, 34, 2107759. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Farris, G.; Cinelli, P.; Anguillesi, I.; Salvadori, S.; Coltelli, M.B.; Lazzeri, A. Effect of Ageing Time on Mechanical Properties of Plasticized Poly(Hydroxybutyrate) (PHB). In Proceedings of the AIP Conference, Ischia, Italy, 22–26 June 2014; Volume 1599, pp. 294–297. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A New Chapter in Pharmaceutical Manufacturing: 3D-Printed Drug Products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, Y.; Tan, J.; Huang, L.; Luo, B.; Lu, L.; Zhou, C. 3D Bioprinting of Gellan Gum and Poly (Ethylene Glycol) Diacrylate Based Hydrogels to Produce Human-Scale Constructs with High-Fidelity. Mater. Des. 2018, 160, 486–495. [Google Scholar] [CrossRef]
- Cristovão, A.F.; Sousa, D.; Silvestre, F.; Ropio, I.; Gaspar, A.; Henriques, C.; Velhinho, A.; Baptista, A.C.; Faustino, M.; Ferreira, I. Customized Tracheal Design Using 3D Printing of a Polymer Hydrogel: Influence of UV Laser Cross-Linking on Mechanical Properties. 3D Print. Med. 2019, 5, 12. [Google Scholar] [CrossRef]
- Lei, I.M.; Sheng, Y.; Lei, C.L.; Leow, C.; Huang, Y.Y.S. A Hackable, Multi-Functional, and Modular Extrusion 3D Printer for Soft Materials. Sci. Rep. 2022, 12, 12294. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.; Li, L. Review of 3D Printable Hydrogels and Constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Xia, Z.; Jin, S.; Ye, K. Tissue and Organ 3D Bioprinting. SLAS Technol. 2018, 23, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, M.; Saunders, R.E.; Tirelli, N.; Derby, B. Inkjet Printing and Cell Seeding Thermoreversible Photocurable Gel Structures. Soft Matter 2011, 7, 2639–2646. [Google Scholar] [CrossRef]
- He, Y.; Tuck, C.J.; Prina, E.; Kilsby, S.; Christie, S.D.R.; Edmondson, S.; Hague, R.J.M.; Rose, F.R.A.J.; Wildman, R.D. A New Photocrosslinkable Polycaprolactone-Based Ink for Three-Dimensional Inkjet Printing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1645–1657. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Rodriguez-Umanzor, F.E.; Sarabia-Vallejos, M.A.; Terraza, C.A.; Martínez-Campos, E.; Rodriguez-Hernandez, J. Innovative Procedure for Precise Deposition of Wrinkled Hydrogel Films Using Direct Inkjet Printing. Mater. Des. 2020, 194, 108959. [Google Scholar] [CrossRef]
- Xia, B.; Jiang, Z.; Debroy, D.; Li, D.; Oakey, J. Cytocompatible Cell Encapsulation via Hydrogel Photopolymerization in Microfluidic Emulsion Droplets. Biomicrofluidics 2017, 11, 044102. [Google Scholar] [CrossRef]
- Chen, M.; Aluunmani, R.; Bolognesi, G.; Vladisavljević, G.T. Facile Microfluidic Fabrication of Biocompatible Hydrogel Microspheres in a Novel Microfluidic Device. Molecules 2022, 27, 4013. [Google Scholar] [CrossRef]
- Guo, S.; Kang, G.; Phan, D.T.; Hsu, M.N.; Por, Y.C.; Chen, C.H. Polymerization-Induced Phase Separation Formation of Structured Hydrogel Particles via Microfluidics for Scar Therapeutics. Sci. Rep. 2018, 8, 2245. [Google Scholar] [CrossRef]
- Dang, T.D.; Kim, Y.H.; Kim, H.G.; Kim, G.M. Preparation of Monodisperse PEG Hydrogel Microparticles Using a Microfluidic Flow-Focusing Device. J. Ind. Eng. Chem. 2012, 18, 1308–1313. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel Microparticles for Biomedical Applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Xia, B.; Krutkramelis, K.; Oakey, J. Oxygen-Purged Microfluidic Device to Enhance Cell Viability in Photopolymerized PEG Hydrogel Microparticles. Biomacromolecules 2016, 17, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Morimura, C.; Ozeki, T. Effective and Simple Prediction Model of Drug Release from “Ghost Tablets” Fabricated Using a Digital Light Projection-Type 3D Printer. Int. J. Pharm. 2021, 604, 120721. [Google Scholar] [CrossRef] [PubMed]
- Baruffaldi, D.; Palmara, G.; Pirri, C.; Frascella, F. 3D Cell Culture: Recent Development in Materials with Tunable Stiffness. ACS Appl. Bio Mater. 2021, 4, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Stillman, Z.; Jarai, B.M.; Raman, N.; Patel, P.; Fromen, C.A. Degradation Profiles of Poly(Ethylene Glycol)Diacrylate (PEGDA)-Based Hydrogel Nanoparticles. Polym. Chem. 2020, 11, 568–580. [Google Scholar] [CrossRef]
- García-Lizarribar, A.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Castaño, A.G.; Samitier, J.; Ramon-Azcon, J. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol. Biosci. 2018, 18, e1800167. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Nieto, D.; Iglesias, L.; Goodarzi Hosseinabadi, H.; Maharjan, S.; Ruiz-Esparza, G.U.; Khoshakhlagh, P.; Manbachi, A.; Dokmeci, M.R.; Chen, S.; et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv. Mater. 2018, 30, e1800242. [Google Scholar] [CrossRef] [PubMed]
- Vannozzi, L.; Yasa, I.C.; Ceylan, H.; Menciassi, A.; Ricotti, L.; Sitti, M. Self-Folded Hydrogel Tubes for Implantable Muscular Tissue Scaffolds. Macromol. Biosci. 2018, 18, e1700377. [Google Scholar] [CrossRef]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-Dimensional Photopatterning of Hydrogels Using Stereolithography for Long-Term Cell Encapsulation. Lab Chip 2010, 10, 2062–2070. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Trindade, G.F.; Taresco, V.; Zhou, Z.; He, Y.; Burroughs, L.; Clark, E.A.; Rose, F.R.A.J.; Tuck, C.; Hague, R.; et al. Bespoke 3D-Printed Polydrug Implants Created via Microstructural Control of Oligomers. ACS Appl. Mater. Interfaces 2021, 13, 38969–38978. [Google Scholar] [CrossRef]
- Burke, G.; Devine, D.M.; Major, I. Effect of Stereolithography 3d Printing on the Properties of Pegdma Hydrogels. Polymers 2020, 12, 2015. [Google Scholar] [CrossRef]
- He, B.; Wang, J.; Xie, M.; Xu, M.; Zhang, Y.; Hao, H.; Xing, X.; Lu, W.; Han, Q.; Liu, W. 3D Printed Biomimetic Epithelium/Stroma Bilayer Hydrogel Implant for Corneal Regeneration. Bioact. Mater. 2022, 17, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Ouyang, L.; Xiong, Z.; Zhang, T. Advances in Digital Light Processing of Hydrogels. Biomed. Mater. 2022, 17, 042002. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wei, W.; Gong, L.; Li, C.; Hong, Y.; Wang, X.; Liang, R.; Shao, Q.; Liang, Q.; Huang, W.; et al. Biomacromolecule-Based Agent for High-Precision Light-Based 3D Hydrogel Bioprinting. Cell Rep. Phys. Sci. 2022, 3, 100985. [Google Scholar] [CrossRef]
- Taormina, G.; Sciancalepore, C.; Messori, M.; Bondioli, F. 3D Printing Processes for Photocurable Polymeric Materials: Technologies, Materials, and Future Trends. J. Appl. Biomater. Funct. Mater. 2018, 16, 151–160. [Google Scholar] [CrossRef]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. Part B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, e1805510. [Google Scholar] [CrossRef]
- Schesny, M.K.; Monaghan, M.; Bindermann, A.H.; Freund, D.; Seifert, M.; Eble, J.A.; Vogel, S.; Gawaz, M.P.; Hinderer, S.; Schenke-Layland, K. Preserved Bioactivity and Tunable Release of a SDF1-GPVI Bi-Specific Protein Using Photo-Crosslinked PEGda Hydrogels. Biomaterials 2014, 35, 7180–7187. [Google Scholar] [CrossRef]
- Tian, F.; Lyu, J.; Shi, J.; Tan, F.; Yang, M. A Polymeric Microfluidic Device Integrated with Nanoporous Alumina Membranes for Simultaneous Detection of Multiple Foodborne Pathogens. Sens. Actuators B Chem. 2016, 225, 312–318. [Google Scholar] [CrossRef]
- Schweller, R.M.; West, J.L. Encoding Hydrogel Mechanics via Network Cross-Linking Structure. ACS Biomater. Sci. Eng. 2015, 1, 335–344. [Google Scholar] [CrossRef]
- Patel, P.N.; Smith, C.K.; Patrick, C.W. Rheological and Recovery Properties of Poly(Ethylene Glycol) Diacrylate Hydrogels and Human Adipose Tissue. J. Biomed. Mater. Res. Part A 2005, 73, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Song, L.; Niu, G.; Cao, H.; Wang, G.; Yang, H.; Zhu, S. Controllable Properties and Microstructure of Hydrogels Based on Crosslinked Poly(Ethylene Glycol) Diacrylates with Different Molecular Weights. J. Appl. Polym. Sci. 2011, 121, 531–540. [Google Scholar] [CrossRef]
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The Effects of Monoacrylated Poly(Ethylene Glycol) on the Properties of Poly(Ethylene Glycol) Diacrylate Hydrogels Used for Tissue Engineering. J. Biomed. Mater. Res. Part A 2010, 92, 441–450. [Google Scholar] [CrossRef]
- Rekowska, N.; Arbeiter, D.; Seitz, H.; Mau, R.; Riess, A.; Eickner, T.; Grabow, N.; Teske, M. The Influence of PEGDA’s Molecular Weight on Its Mechanical Properties in the Context of Biomedical Applications. Curr. Dir. Biomed. Eng. 2022, 8, 181–184. [Google Scholar] [CrossRef]
- Jabbari, E.; Sarvestani, S.K.; Daneshian, L.; Moeinzadeh, S. Optimum 3D Matrix Stiffness for Maintenance of Cancer Stem Cells Is Dependent on Tissue Origin of Cancer Cells. PLoS ONE 2015, 10, e0132377. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Wong, K.W.; Choi, J.Y.; Cowie, A.C. Recent Advances in Photo-Crosslinkable Hydrogels for Biomedical Applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef]
- Xue, D.; Wang, Y.; Zhang, J.; Mei, D.; Wang, Y.; Chen, S. Projection-Based 3D Printing of Cell Patterning Scaffolds with Multiscale Channels. ACS Appl. Mater. Interfaces 2018, 10, 19428–19435. [Google Scholar] [CrossRef]
- Yasar, O.; Orock, A.; Tarantini, S.; White, J.; Khandaker, M. Mechanical Characterization of Polyethylene Glycol Diacrylate (PEGDA) for Tissue Engineering Applications. In Mechanics of Biological Systems and Materials; Springer: Cham, Switzerland, 2013; pp. 189–196. ISBN 9781461444268. [Google Scholar]
- Castro, D.; Conchouso, D.; Fan, Y.; Foulds, I.G. Surface Treatments of Soft Molds for High Aspect Ratio Molding of Poly-PEGDA. In Proceedings of the 16th International Conference Miniaturized Systems for Chemistry and Life Sciences, Okinawa, Japan, 28 October–1 November 2012; pp. 1231–1233. [Google Scholar]
- Mazzoccoli, J.P.; Feke, D.L.; Baskaran, H.; Pintauro, P.N. Mechanical and Cell Viability Properties of Crosslinked Low and High MW PEGDA Blends. J. Biomed. Mater. Res. A 2010, 93, 558–566. [Google Scholar]
- Shibayama, M. Small-Angle Neutron Scattering on Polymer Gels: Phase Behavior, Inhomogeneities and Deformation Mechanisms. Polym. J. 2011, 43, 18–34. [Google Scholar] [CrossRef]
- Choi, M.; Choi, J.W.; Kim, S.; Nizamoglu, S.; Hahn, S.K.; Yun, S.H. Light-Guiding Hydrogels for Cell-Based Sensing and Optogenetic Synthesis in Vivo. Nat. Photonics 2013, 7, 987–994. [Google Scholar] [CrossRef]
- De Molina, P.M.; Lad, S.; Helgeson, M.E. Heterogeneity and Its Influence on the Properties of Difunctional Poly(Ethylene Glycol) Hydrogels: Structure and Mechanics. Macromolecules 2015, 48, 5402–5411. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Caparras, A.M.; Canales-Galarza, Z.; Barrow, M.; Dietrich, B.; Laüger, J.; Nemeth, M.; Draper, E.R.; Adams, D.J. Mechanical Characterization of Multilayered Hydrogels: A Rheological Study for 3D-Printed Systems. Biomacromolecules 2021, 22, 1625–1638. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Qian, J.; Wu, Z.; Xiao, R. Multi-Responsive PNIPAM-PEGDA Hydrogel Composite. Soft Matter 2021, 17, 10421–10427. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Chen, Z.; Cheng, J.; Zhang, B.; Zhang, Y.F.; Li, H.; He, X.; Yuan, C.; Liu, J.; Magdassi, S.; et al. 3D Printing of Highly Stretchable Hydrogel with Diverse UV Curable Polymers. Sci. Adv. 2021, 7, eaba4261. [Google Scholar] [CrossRef]
- Kaufman, C.D.; Liu, S.C.; Cvetkovic, C.; Lee, C.A.; Naseri Kouzehgarani, G.; Gillette, R.; Bashir, R.; Gillette, M.U. Emergence of Functional Neuromuscular Junctions in an Engineered, Multicellular Spinal Cord-Muscle Bioactuator. APL Bioeng. 2020, 4, 026104. [Google Scholar] [CrossRef]
- Mau, R.; Nazir, J.; John, S.; Seitz, H. Preliminary Study on 3D Printing of PEGDA Hydrogels for Frontal Sinus Implants Using Digital Light Processing (DLP). Curr. Dir. Biomed. Eng. 2019, 5, 249–252. [Google Scholar] [CrossRef]
- Mau, R.; Reske, T.; Eickner, T.; Grabow, N.; Seitz, H. DLP 3D Printing of Dexamethasone-Incorporated PEGDA-Based Photopolymers: Compressive Properties and Drug Release. Curr. Dir. Biomed. Eng. 2020, 6, 406–409. [Google Scholar] [CrossRef]
- Kim, Y.S.; Cho, K.; Lee, H.J.; Chang, S.; Lee, H.; Kim, J.H.; Koh, W.G. Highly Conductive and Hydrated PEG-Based Hydrogels for the Potential Application of a Tissue Engineering Scaffold. React. Funct. Polym. 2016, 109, 15–22. [Google Scholar] [CrossRef]
- Yang, W.; Hu, R.; Zheng, L.; Yan, G.; Yan, W. Fabrication and Investigation of 3D-Printed Gun Propellants. Mater. Des. 2020, 192, 108761. [Google Scholar] [CrossRef]
- Mercado-Montijo, J.; Anstine, D.M.; Rukmani, S.J.; Colina, C.M.; Andrew, J.S. PEGDA Hydrogel Structure from Semi-Dilute Concentrations: Insights from Experiments and Molecular Simulations. Soft Matter 2022, 18, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Sangaj, N.; Razafiarison, T.; Zhang, C.; Varghese, S. Influence of Physical Properties of Biomaterials on Cellular Behavior. Pharm. Res. 2011, 28, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Sokic, S.; Papavasiliou, G. Controlled Proteolytic Cleavage Site Presentation in Biomimetic PEGDA Hydrogels Enhances Neovascularization in vitro. Tissue Eng. Part A 2012, 18, 2477–2486. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-Crosslinked Poly(Ethylene Glycol) Diacrylate (PEGDA) Hydrogels from Low Molecular Weight Prepolymer: Swelling and Permeation Studies. Appl. Polym. 2017, 134, 44380. [Google Scholar] [CrossRef]
- Browning, M.B.; Cosgriff-hernandez, E. Development of a Biostable Replacement for PEGDA Hydrogels. Bio Macromol. 2012, 13, 779–786. [Google Scholar] [CrossRef]
- Zidan, G.; Greene, C.A.; Etxabide, A.; Rupenthal, I.D.; Seyfoddin, A. Gelatine-Based Drug-Eluting Bandage Contact Lenses: Effect of PEGDA Concentration and Manufacturing Technique. Int. J. Pharm. 2021, 599, 120452. [Google Scholar] [CrossRef]
- Männel, M.J.; Fischer, C.; Thiele, J. A Non-Cytotoxic Resin for Micro-Stereolithography for Cell Cultures of HUVECs. Micromachines 2020, 11, 246. [Google Scholar] [CrossRef]
- Abdullah, H.; Phairatana, T. A Study on the Performances of PEGDA and PEDOT: PSS Composite Resin for 3D-Printed Biosensor Applications. ECS Trans. 2022, 107, 15001–15010. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, X.; Chu, C.C. Synthesis, Characterization and Drug Release from Three-Arm Poly(ε-Caprolactone) Maleic Acid/Poly(Ethylene Glycol) Diacrylate Hydrogels. J. Biomater. Sci. Polym. Ed. 2003, 14, 777–802. [Google Scholar] [CrossRef]
- Zhu, W.; Cui, H.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.Y.; Zhang, L.G. 3D Bioprinting Mesenchymal Stem Cell-Laden Construct with Core-Shell Nanospheres for Cartilage Tissue Engineering. Nanotechnology 2018, 29, 185101. [Google Scholar] [CrossRef] [PubMed]
- Dizon, J.R.C.; Chen, Q.; Valino, A.D.; Advincula, R.C. Thermo-Mechanical and Swelling Properties of Three-Dimensional-Printed Poly (Ethylene Glycol) Diacrylate/Silica Nanocomposites. MRS Commun. 2019, 9, 209–217. [Google Scholar] [CrossRef]
- Tang, A.; Wang, Q.; Zhao, S.; Liu, W. Fabrication of Nanocellulose/PEGDA Hydrogel by 3D Printing. Rapid Prototyp. J. 2018, 24, 1265–1271. [Google Scholar] [CrossRef]
- Peter, M.; Singh, A.; Mohankumar, K.; Jeenger, R.; Joge, P.A.; Gatne, M.M.; Tayalia, P. Gelatin-Based Matrices as a Tunable Platform to Study in vitro and in Vivo 3D Cell Invasion. ACS Appl. Bio Mater. 2019, 2, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.K.; Von Halling Laier, C.; Kiziltay, A.; Wilson, S.; Larsen, N.B. 3D Printed Hydrogel Multiassay Platforms for Robust Generation of Engineered Contractile Tissues. Biomacromolecules 2019, 21, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, H.; Jiang, J.; Zhao, C.; Zhang, J.; Chen, P.; Lin, X.; Fan, S. Desktop-Stereolithography 3D Printing of a Polyporous Extracellular Matrix Bioink for Bone Defect Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 589094. [Google Scholar] [CrossRef]
- Yang, X.; Dargaville, B.L.; Hutmacher, D.W. Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure. Polymers 2021, 13, 845. [Google Scholar] [CrossRef]
- Weigel, N.; Thiele, J. Photopolymer Formulations for ΜSL Printing of Hydrogel Microstructures as Swellable Functional Elements. Proc. SPIE 2021, 11637, 116370A. [Google Scholar] [CrossRef]
- Hisham, M.; Saravana Kumar, G.; Deshpande, A.P. Process Optimization and Optimal Tolerancing to Improve Dimensional Accuracy of Vat-Photopolymerized Functionally Graded Hydrogels. Results Eng. 2022, 14, 100442. [Google Scholar] [CrossRef]
- Zhang, R.; Larsen, N.B. Stereolithographic Hydrogel Printing of 3D Culture Chips with Biofunctionalized Complex 3D Perfusion Networks. Lab Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical Behavior of Bioactive Poly(Ethylene Glycol) Diacrylate Matrices for Biomedical Application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Yuen, H.Y.; Zhao, X. Mechanical Stretching of 3D Hydrogels for Neural Stem Cell Differentiation. Bio-Des. Manuf. 2022, 5, 714–728. [Google Scholar] [CrossRef]
- Roy, T.; James, B.D.; Allen, J.B. Anti-VEGF-R2 Aptamer and RGD Peptide Synergize in a Bifunctional Hydrogel for Enhanced Angiogenic Potential. Macromol. Biosci. 2021, 21, e2000337. [Google Scholar] [CrossRef]
- Obregon-Miano, F.; Fathi, A.; Rathsam, C.; Sandoval, I.; Deheghani, F.; Spahr, A. Injectable Porcine Bone Demineralized and Digested Extracellular Matrix—PEGDA Hydrogel Blend for Bone Regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 21. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Shih, Y.-R.V.; Velez, D.O.V.; Cabrales, P.; Varghese, S. Poly(Ethylene Glycol) Hydrogels with Cell Cleavable Groups for Autonomous Cell Delivery. Biomaterials 2016, 77, 186–197. [Google Scholar] [CrossRef]
- Xin, A.X.; Gaydos, C.; Mao, J.J. In Vitro Degradation Behavior of Photopolymerized PEG Hydrogels as Tissue Engineering Scaffold. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2091–2093. [Google Scholar] [CrossRef]
- Alketbi, A.S.; Shi, Y.; Li, H.; Raza, A.; Zhang, T.J. Impact of PEGDA Photopolymerization in Micro-Stereolithography on 3D Printed Hydrogel Structure and Swelling. Soft Matter 2021, 17, 7188–7195. [Google Scholar] [CrossRef] [PubMed]
- Salvekar, A.V.; Huang, W.M.; Xiao, R.; Wong, Y.S.; Venkatraman, S.S.; Tay, K.H.; Shen, Z.X. Water-Responsive Shape Recovery Induced Buckling in Biodegradable Photo-Cross-Linked Poly(Ethylene Glycol) (PEG) Hydrogel. Acc. Chem. Res. 2017, 50, 141–150. [Google Scholar] [CrossRef]
- Tikhonov, A.; Evdokimov, P.; Klimashina, E.; Tikhonova, S.; Karpushkin, E.; Scherbackov, I.; Dubrov, V.; Putlayev, V. Stereolithographic Fabrication of Three-Dimensional Permeable Scaffolds from CaP/PEGDA Hydrogel Biocomposites for Use as Bone Grafts. J. Mech. Behav. Biomed. Mater. 2020, 110, 103922. [Google Scholar] [CrossRef]
- Kalossaka, L.M.; Mohammed, A.A.; Sena, G.; Barter, L.; Myant, C. 3D Printing Nanocomposite Hydrogels with Lattice Vascular Networks Using Stereolithography. J. Mater. Res. 2021, 36, 4249–4261. [Google Scholar] [CrossRef]
- Oyen, M.L. Mechanical Characterisation of Hydrogel Materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Yang, X. Fabrication, Properties and Applications of PEGDA Hydrogels in Biomedical Science. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2021. [Google Scholar]
- Kang, G.; Cao, Y.; Zhao, H.; Yuan, Q. Preparation and Characterization of Crosslinked Poly(Ethylene Glycol) Diacrylate Membranes with Excellent Antifouling and Solvent-Resistant Properties. J. Memb. Sci. 2008, 318, 227–232. [Google Scholar] [CrossRef]
- Wu, Y.H.; Park, H.B.; Kai, T.; Freeman, B.D.; Kalika, D.S. Water Uptake, Transport and Structure Characterization in Poly(Ethylene Glycol) Diacrylate Hydrogels. J. Memb. Sci. 2010, 347, 197–208. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, L.; Xu, C.; Zhai, Z.; Zhou, J.A.; Yao, Y.; Xia, H.; Wang, Y. Synthesis and Characterization of Glucosamine Modified Poly(Ethylene Glycol) Hydrogels via Photopolymerization. J. Appl. Polym. Sci. 2013, 128, 89–96. [Google Scholar] [CrossRef]
- Lee, H.; Xia, C.; Fang, N.X. Biomimetic Microactuator Powered by Polymer Swelling. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Boston, MA, USA, 31 October–6 November 2008; pp. 765–769. [Google Scholar]
- Browe, D.P.; Wood, C.; Sze, M.T.; White, K.A.; Scott, T.; Olabisi, R.M.; Freeman, J.W. Characterization and Optimization of Actuating Poly(Ethylene Glycol) Diacrylate/Acrylic Acid Hydrogels as Artificial Muscles. Polymer 2017, 117, 331–341. [Google Scholar] [CrossRef]
- Lee, S.H.; Shim, K.Y.; Kim, B.; Sung, J.H. Hydrogel-Based Three-Dimensional Cell Culture for Organ-on-a-Chip Applications. Biotechnol. Prog. 2017, 33, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Vizetto-Duarte, C.; Moay, Z.K.; Setyawati, M.I.; Rakshit, M.; Kathawala, M.H.; Ng, K.W. Composite Hydrogels in Three-Dimensional in Vitro Models. Front. Bioeng. Biotechnol. 2020, 8, 611. [Google Scholar] [CrossRef]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation versus Tensile Measurements of Young’s Modulus for Soft Biological Tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef]
- Chiappone, A.; Fantino, E.; Roppolo, I.; Lorusso, M.; Manfredi, D.; Fino, P.; Pirri, C.F.; Calignano, F. 3D Printed PEG-Based Hybrid Nanocomposites Obtained by Sol-Gel Technique. ACS Appl. Mater. Interfaces 2016, 8, 5627–5633. [Google Scholar] [CrossRef]
- Swetha, S.; Lavanya, K.; Sruthi, R.; Selvamurugan, N. An Insight into Cell-Laden 3D-Printed Constructs for Bone Tissue Engineering. J. Mater. Chem. B 2020, 8, 9836–9862. [Google Scholar] [CrossRef]
- Fan, L.; Liu, C.; Chen, X.; Zou, Y.; Zhou, Z.; Lin, C.; Tan, G.; Zhou, L.; Ning, C.; Wang, Q. Directing Induced Pluripotent Stem Cell Derived Neural Stem Cell Fate with a Three-Dimensional Biomimetic Hydrogel for Spinal Cord Injury Repair. ACS Appl. Mater. Interfaces 2018, 10, 17742–17755. [Google Scholar] [CrossRef]
- Tirella, A.; Mattei, G.; La Marca, M.; Ahluwalia, A.; Tirelli, N. Functionalized Enzyme-Responsive Biomaterials to Model Tissue Stiffening in Vitro. Front. Bioeng. Biotechnol. 2020, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Valero, D. Compression Testing and Measurement of Material Properties of a Double Network Hydrogel. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2015. [Google Scholar]
- Gojzewski, H.; Sadej, M.; Andrzejewska, E.; Kokowska, M. Nanoscale Young’s Modulus and Surface Morphology in Photocurable Polyacrylate/Nanosilica Composites. Eur. Polym. J. 2017, 88, 205–220. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Gäbler, S.; Stampfl, J.; Koch, T.; Seidler, S.; Schüller, G.; Redl, H.; Juras, V.; Trattnig, S.; Weidisch, R. Determination of the Viscoelastic Properties of Hydrogels Based on Polyethylene Glycol Diacrylate (PEG-DA) and Human Articular Cartilage. Int. J. Mater. Eng. Innov. 2009, 1, 3. [Google Scholar] [CrossRef]
- Mighani, S.; Bernabé, Y.; Boulenouar, A.; Mok, U.; Evans, B. Creep Deformation in Vaca Muerta Shale from Nanoindentation to Triaxial Experiments. J. Geophys. Res. Solid Earth 2019, 124, 7842–7868. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Liu, Y.; Li, Y.; Xiong, Y. Using Nanoindentation to Characterize the Mechanical and Creep Properties of Shale: Load and Loading Strain Rate Effects. ACS Omega 2022, 7, 14317–14331. [Google Scholar] [CrossRef]
- Korhonen, R.K.; Laasanen, M.S.; Töyräs, J.; Helminen, H.J. Jurvelin Comparison of the Equilibrium Response of Articular Cartilage in Unconfined Compression, Confined Compression and Indentation. Biomechanics 2002, 35, 903–909. [Google Scholar] [CrossRef]
- Xia, T.; Liu, W.; Yang, L. A Review of Gradient Stiffness Hydrogels Used in Tissue Engineering and Regenerative Medicine. J. Biomed. Mater. Res. Part A 2017, 105, 1799–1812. [Google Scholar] [CrossRef]
- Laird, N.Z.; Acri, T.M.; Chakka, J.L.; Quarterman, J.C.; Malkawi, W.I.; Elangovan, S.; Salem, A.K. Applications of Nanotechnology in 3D Printed Tissue Engineering Scaffolds. Eur. J. Pharm. Biopharm. 2021, 161, 15–28. [Google Scholar] [CrossRef]
- Kilpatrick, J.I.; Revenko, I.; Rodriguez, B.J. Nanomechanics of Cells and Biomaterials Studied by Atomic Force Microscopy. Adv. Healthc. Mater. 2015, 4, 2456–2474. [Google Scholar] [CrossRef]
- Vinckier, A.; Semenza, G. Measuring Elasticity of Biological Materials by Atomic Force Microscopy. FEBS Lett. 1998, 430, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Almasi, D.; Sharifi, R.; Kadir, M.R.A.; Krishnamurithy, G.; Kamarul, T. Study on the AFM Force Curve Common Errors and Their Effects on the Calculated Nanomechanical Properties of Materials. J. Eng. 2016, 2016, 2456378. [Google Scholar] [CrossRef]
- Bush, B.G.; Shapiro, J.M.; DelRio, F.W.; Cook, R.F.; Oyen, M.L. Mechanical Measurements of Heterogeneity and Length Scale Effects in PEG-Based Hydrogels. Soft Matter 2015, 11, 7191–7200. [Google Scholar] [CrossRef]
- Simič, R.; Mandal, J.; Zhang, K.; Spencer, N.D. Oxygen Inhibition of Free-Radical Polymerization Is the Dominant Mechanism behind the “Mold Effect” on Hydrogels. Soft Matter 2021, 17, 6394–6403. [Google Scholar] [CrossRef] [PubMed]
- Ouasti, S.; Donno, R.; Cellesi, F.; Sherratt, M.J.; Terenghi, G.; Tirelli, N. Network Connectivity, Mechanical Properties and Cell Adhesion for Hyaluronic Acid/PEG Hydrogels. Biomaterials 2011, 32, 6456–6470. [Google Scholar] [CrossRef] [PubMed]
- Drira, Z.; Yadavalli, V.K. Nanomechanical Measurements of Polyethylene Glycol Hydrogels Using Atomic Force Microscopy. J. Mech. Behav. Biomed. Mater. 2013, 18, 20–28. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Wang, X.; Yu, T.; Xie, S.; Ge, Z. 3D Printing of Bioinspired Hydrogel Microstructures with Programmable and Complex Shape Deformations Based on a Digital Micro-Mirror Device. Opt. Laser Technol. 2023, 157, 108759. [Google Scholar] [CrossRef]
- Belqat, M.; Wu, X.; Gomez, L.P.C.; Malval, J.P.; Dominici, S.; Leuschel, B.; Spangenberg, A.; Mougin, K. Tuning Nanomechanical Properties of Microstructures Made by 3D Direct Laser Writing. Addit. Manuf. 2021, 47, 102232. [Google Scholar] [CrossRef]
- Uzcategui, A.C.; Higgins, C.I.; Hergert, J.E.; Tomaschke, A.E.; Crespo-Cuevas, V.; Ferguson, V.L.; Bryant, S.J.; McLeod, R.R.; Killgore, J.P. Microscale Photopatterning of Through-Thickness Modulus in a Monolithic and Functionally Graded 3D-Printed Part. Small Sci. 2021, 1, 2000017. [Google Scholar] [CrossRef]
- Pathak, S.; Kalidindi, S.R. Spherical Nanoindentation Stress-Strain Curves. Mater. Sci. Eng. R Rep. 2015, 91, 1–36. [Google Scholar] [CrossRef]
- Akhtar, R.; Schwarzer, N.; Sherratt, M.J.; Watson, R.E.; Graham, H.; Trafford, A.; Mummery, P.; Derby, B. Nanoindentation of Histological Specimens: Mapping the Elastic Properties of Soft Tissues. Mater. Res. Soc. 2009, 24, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.N.L.; Adão, R.M.R.; Maibohm, C.; Accardo, A.; Cardoso, V.F.; Nieder, J.B. Cellular Interaction of Bone Marrow Mesenchymal Stem Cells with Polymer and Hydrogel 3D Microscaffold Templates. ACS Appl. Mater. Interfaces 2022, 14, 13013–13024. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Kumamoto, Y.; Minoshima, M.; Kikuchi, K.; Taguchi, A.; Fujita, K. Photoinitiator-Free Two-Photon Polymerization of Biocompatible Materials for 3D Micro/Nanofabrication. Adv. Opt. Mater. 2022, 6, 1800257. [Google Scholar] [CrossRef]
- Farkas, B.; Dante, S.; Brandi, F. Photoinitiator-Free 3D Scaffolds Fabricated by Excimer Laser Photocuring. Nanotechnology 2017, 28, 034001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, S.; Xu, X.; Yu, T.; Guo, Z.; Ma, M.; Zhang, Y.; Gu, Z.; Feng, Y.; Du, C.; et al. Three-Dimensional Cell-Culture Platform Based on Hydrogel with Tunable Microenvironmental Properties to Improve Insulin-Secreting Function of MIN6 Cells. Biomaterials 2021, 270, 120687. [Google Scholar] [CrossRef] [PubMed]
- Ciocci, M.; Mochi, F.; Carotenuto, F.; Di Giovanni, E.; Prosposito, P.; Francini, R.; De Matteis, F.; Reshetov, I.; Casalboni, M.; Melino, S.; et al. Scaffold-in-Scaffold Potential to Induce Growth and Differentiation of Cardiac Progenitor Cells. Stem Cells Dev. 2017, 26, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Corbin, E.A.; Millet, L.J.; Pikul, J.H.; Johnson, C.L.; Georgiadis, J.G.; King, W.P.; Bashir, R. Micromechanical Properties of Hydrogels Measured with MEMS Resonant Sensors. Biomed. Microdevices 2013, 15, 311–319. [Google Scholar] [CrossRef]
- Chandbadshah, S.B.V.J.; Mannayee, G. PEGDA Microneedle Arrays Fabrication and Investigation of Mechanical Properties for Transdermal Vaccine Delivery. Int. J. Eng. Trends Technol. 2022, 70, 15–23. [Google Scholar] [CrossRef]
- Cheung, Y.K.; Azeloglu, E.U.; Shiovitz, D.; Costa, K.D.; Seliktar, D.; Sia, S.K. Patterning Micro-Stiffness in Cell-Adhesive Substrate Using Microfluidics-Based Lithography. In Proceedings of the 2010 IEEE 36th Annual Northeast Bioengineering Conference, NEBEC, Haifa, Israel, 26–28 March 2010; pp. 1–2. [Google Scholar]
- Nozdriukhin, D.V.; Filatov, N.A.; Evstrapov, A.A.; Bukatin, A.S. Formation of Polyacrylamide and PEGDA Hydrogel Particles in a Microfluidic Flow Focusing Droplet Generator. Tech. Phys. 2018, 63, 1328–1333. [Google Scholar] [CrossRef]
- Zhan, C.; Hu, Y.; Zhou, A.; Zhang, S. Three-Dimensional Printing of Cell-Laden Bioink for Blood Vessel Tissue Engineering: Influence of Process Parameters and Components on Cell Viability; Authorea: New York, NY, USA, 2021; pp. 1–26. [Google Scholar] [CrossRef]
- Kim, H.N.; Choi, N. Consideration of the Mechanical Properties of Hydrogels for Brain Tissue Engineering and Brain-on-a-Chip. Biochip J. 2019, 13, 8–19. [Google Scholar] [CrossRef]
- Munoz-Pinto, D.J.; Jimenez-Vergara, A.C.; Gharat, T.P.; Hahn, M.S. Characterization of Sequential Collagen-Poly(Ethylene Glycol) Diacrylate Interpenetrating Networks and Initial Assessment of Their Potential for Vascular Tissue Engineering. Biomaterials 2015, 40, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Beldjilali-Labro, M.; Jellali, R.; Brown, A.D.; Garcia Garcia, A.; Lerebours, A.; Guenin, E.; Bedoui, F.; Dufresne, M.; Stewart, C.; Grosset, J.F.; et al. Multiscale-Engineered Muscle Constructs: PEG Hydrogel Micro-Patterning on an Electrospun PCL Mat Functionalized with Gold Nanoparticles. Int. J. Mol. Sci. 2021, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Vesga-Castro, C.; Aldazabal, J.; Vallejo-Illarramendi, A.; Paredes, J. Contractile Force Assessment Methods for in Vitro Skeletal Muscle Tissues. eLife 2022, 11, e77204. [Google Scholar] [CrossRef] [PubMed]
- Lev, R.; Seliktar, D. Hydrogel Biomaterials and Their Therapeutic Potential for Muscle Injuries and Muscular Dystrophies. J. R. Soc. Interface 2018, 15, 20170380. [Google Scholar] [CrossRef] [PubMed]
- Dessauge, F.; Schleder, C.; Perruchot, M.H.; Rouger, K. 3D in Vitro Models of Skeletal Muscle: Myopshere, Myobundle and Bioprinted Muscle Construct. Vet. Res. 2021, 52, 72. [Google Scholar] [CrossRef]
- Schätzlein, E.; Blaeser, A. Recent Trends in Bioartificial Muscle Engineering and Their Applications in Cultured Meat, Biorobotic Systems and Biohybrid Implants. Commun. Biol. 2022, 5, 737. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J. Recent Trends in Biofabrication Technologies for Studying Skeletal Muscle Tissue-Related Diseases. Front. Bioeng. Biotechnol. 2021, 9, 782333. [Google Scholar] [CrossRef]
- Vandenburgh, H.; Shansky, J.; Benesch-Lee, F.; Barbata, V.; Reid, J.; Thorrez, L.; Valentini, R.; Crawford, G. Drug-Screening Platform Based on the Contractility of Tissue-Engineered Muscle. Muscle Nerve 2008, 37, 438–447. [Google Scholar] [CrossRef]
- Hinds, S.; Bian, W.; Dennis, R.G.; Bursac, N. The Role of Extracellular Matrix Composition in Structure and Function of Bioengineered Skeletal Muscle. Biomaterials 2011, 23, 3575–3583. [Google Scholar] [CrossRef]
- Cashman, T.J.; Josowitz, R.; Gelb, B.D.; Li, R.A.; Dubois, N.C.; Costa, K.D. Construction of Defined Human Engineered Cardiac Tissues to Study Mechanisms of Cardiac Cell Therapy. J. Vis. Exp. 2016, 2016, e53447. [Google Scholar] [CrossRef]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Parker, B.L.; Monnot, P.; Needham, E.J.; Vivien, C.J.; Ferguson, C.; Parton, R.G.; James, D.E.; Porrello, E.R.; Hudson, J.E. Development of a Human Skeletal Micro Muscle Platform with Pacing Capabilities. Biomaterials 2019, 198, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.E.; Abraha, H.Y.; Bakooshli, M.A.; Davoudi, S.; Thavandiran, N.; Tung, K.; Ahn, H.; Ginsberg, H.J.; Zandstra, P.W.; Gilbert, P.M. A 96-Well Culture Platform Enables Longitudinal Analyses of Engineered Human Skeletal Muscle Microtissue Strength. Sci. Rep. 2020, 10, 6918. [Google Scholar] [CrossRef] [PubMed]
- Dostanić, M.; Windt, L.M.; Stein, J.M.; Van Meer, B.J.; Bellin, M.; Orlova, V.; Mastrangeli, M.; Mummery, C.L.; Sarro, P.M. A Miniaturized EHT Platform for Accurate Measurements of Tissue Contractile Properties. J. Microelectromech. Syst. 2020, 29, 881–887. [Google Scholar] [CrossRef]
- Iuliano, A.; van der Wal, E.; Ruijmbeek, C.W.B.; in‘t Groen, S.L.M.; Pijnappel, W.W.M.P.; de Greef, J.C.; Saggiomo, V. Coupling 3D Printing and Novel Replica Molding for In House Fabrication of Skeletal Muscle Tissue Engineering Devices. Adv. Mater. Technol. 2020, 5, 2000344. [Google Scholar] [CrossRef]
- Nagashima, T.; Hadiwidjaja, S.; Ohsumi, S.; Murata, A.; Hisada, T.; Kato, R.; Okada, Y.; Honda, H.; Shimizu, K. In Vitro Model of Human Skeletal Muscle Tissues with Contractility Fabricated by Immortalized Human Myogenic Cells. Adv. Biosyst. 2020, 4, e2000121. [Google Scholar] [CrossRef]
- Fernández-Garibay, X.; Gómez-Florit, M.; Domingues, R.M.A.; Gomes, M.E.; Fernández-Costa, J.M.; Ramón-Azcón, J. Xeno-Free Bioengineered Human Skeletal Muscle Tissue Using Human Platelet Lysate-Based Hydrogels. Biofabrication 2022, 14, 045015. [Google Scholar] [CrossRef]
- Winston, T.S.; Chen, C.; Suddhapas, K.; Tarris, B.A.; Elattar, S.; Sun, S.; Zhang, T.; Ma, Z. Controlling Mesenchyme Tissue Remodeling via Spatial Arrangement of Mechanical Constraints. Front. Bioeng. Biotechnol. 2022, 10, 3. [Google Scholar] [CrossRef]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Brenière, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef]
- Mills, R.J.; Titmarsh, D.M.; Koenig, X.; Parker, B.L.; Ryall, J.G.; Quaife-Ryan, G.A.; Voges, H.K.; Hodson, M.P.; Ferguson, C.; Drowley, L.; et al. Functional Screening in Human Cardiac Organoids Reveals a Metabolic Mechanism for Cardiomyocyte Cell Cycle Arrest. Proc. Natl. Acad. Sci. USA 2017, 114, 8372–8381. [Google Scholar] [CrossRef]
- Chan, V.; Park, K.; Collens, M.B.; Kong, H.; Saif, T.A.; Bashir, R. Development of Miniaturized Walking Biological Machines. Sci. Rep. 2012, 2, srep00857. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Jeong, J.H.; Bajaj, P.; Collens, M.; Saif, T.; Kong, H.; Bashir, R. Multi-Material Bio-Fabrication of Hydrogel Cantilevers and Actuators with Stereolithography. Lab Chip 2012, 12, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Carag-krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, E. Elasticity: Scar-Like Rigidity Inhibits Beating. J. Cell Sci. 2009, 121, 3794–3802. [Google Scholar] [CrossRef]
- Sakar, M.S.; Neal, D.; Boudou, T.; Borochin, M.A.; Li, Y.; Weiss, R.; Kamm, R.D.; Chen, C.S.; Asada, H.H. Formation and Optogenetic Control of Engineered 3D Skeletal Muscle Bioactuators. Lab Chip 2012, 12, 4976–4985. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Cvetkovic, C.; Bashir, R. A Modular Approach to the Design, Fabrication, and Characterization of Muscle-Powered Biological Machines. Nat. Protoc. 2017, 12, 519–533. [Google Scholar] [CrossRef]
- Grant, L.; Raman, R.; Cvetkovic, C.; Ferrall-Fairbanks, M.C.; Pagan-Diaz, G.J.; Hadley, P.; Ko, E.; Platt, M.O.; Bashir, R. Long-Term Cryopreservation and Revival of Tissue-Engineered Skeletal Muscle. Tissue Eng. Part A 2019, 25, 1023–1036. [Google Scholar] [CrossRef]
- Kumar, P.; Schmidleithner, C.; Larsen, N.B.; Sigmund, O. Topology Optimization and 3D Printing of Large Deformation Compliant Mechanisms for Straining Biological Tissues. Struct. Multidiscip. Optim. 2021, 63, 1351–1366. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Kaplan, D.L.; Black, L.D., III. Electrical and Mechanical Stimulation of Cardiac Cells and Tissue Constructs. Adv. Drug Deliv. Rev. 2016, 96, 135–155. [Google Scholar] [CrossRef]
- Santoni, S.M.; Winston, T.; Hoang, P.; Ma, Z. Microsystems for Electromechanical Stimulations to Engineered Cardiac Tissues. Microphysiol. Syst. 2018, 2, 11. [Google Scholar] [CrossRef]
- Ferrigno, B.; Bordett, R.; Duraisamy, N.; Moskow, J.; Arul, M.R.; Rudraiah, S.; Nukavarapu, S.P.; Vella, A.T.; Kumbar, S.G. Bioactive Polymeric Materials and Electrical Stimulation Strategies for Musculoskeletal Tissue Repair and Regeneration. Bioact. Mater. 2020, 5, 468–485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. https://doi.org/10.3390/polym15102341
Hakim Khalili M, Zhang R, Wilson S, Goel S, Impey SA, Aria AI. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers. 2023; 15(10):2341. https://doi.org/10.3390/polym15102341
Chicago/Turabian StyleHakim Khalili, Mohammad, Rujing Zhang, Sandra Wilson, Saurav Goel, Susan A. Impey, and Adrianus Indrat Aria. 2023. "Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering" Polymers 15, no. 10: 2341. https://doi.org/10.3390/polym15102341
APA StyleHakim Khalili, M., Zhang, R., Wilson, S., Goel, S., Impey, S. A., & Aria, A. I. (2023). Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers, 15(10), 2341. https://doi.org/10.3390/polym15102341









