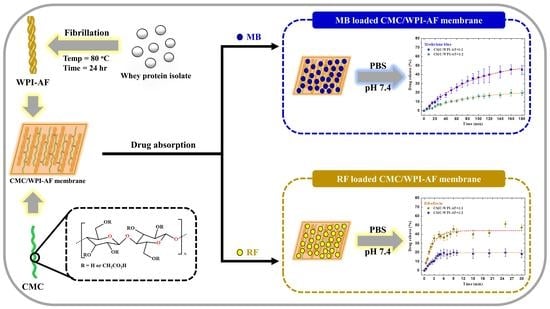
Application of Amyloid-Based Hybrid Membranes in Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Amyloid Fibril Formation of the Whey Protein Isolate (WPI)
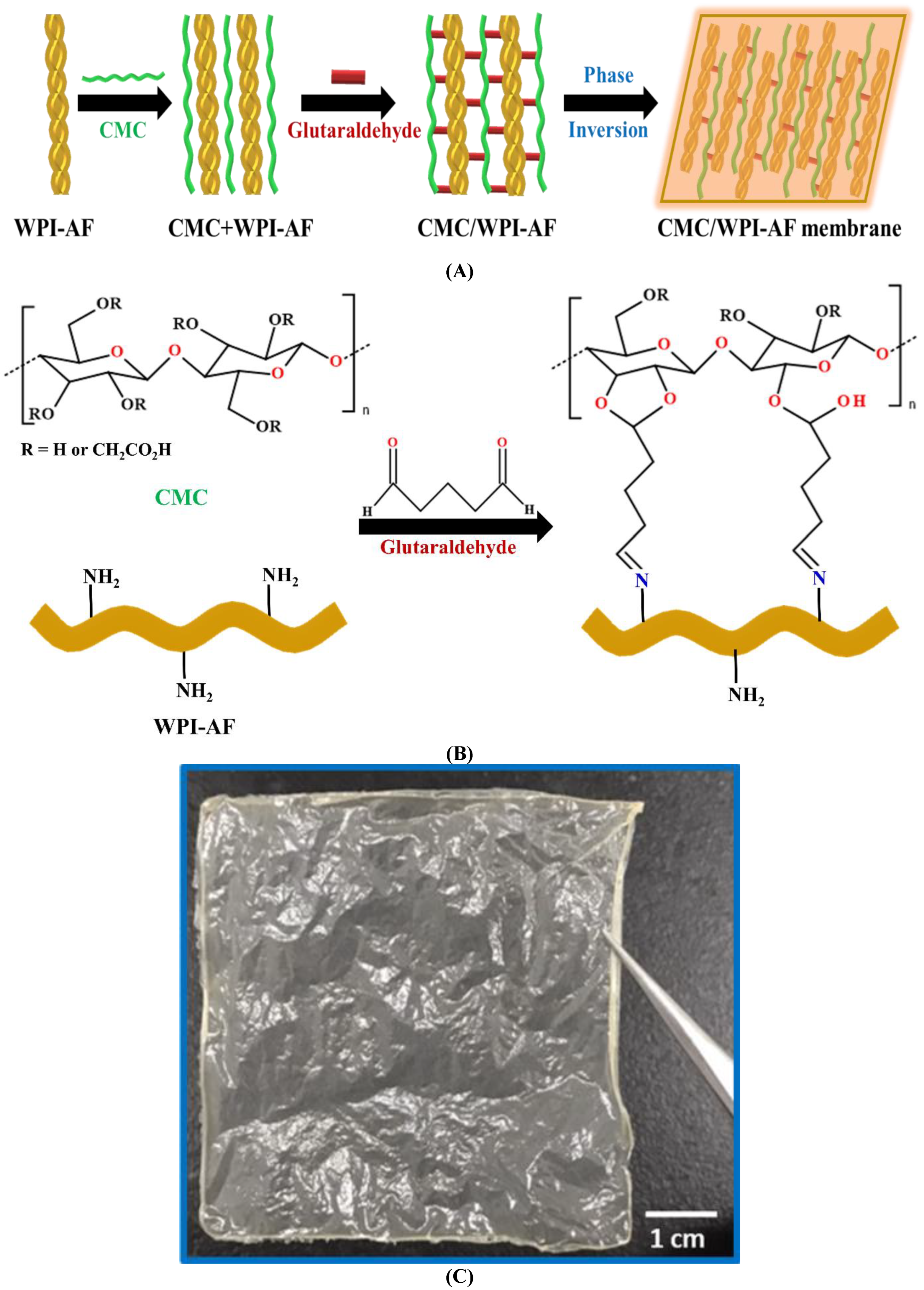
2.3. Fabrication of the Carboxymethyl Cellulose/Whey Protein Isolate Amyloid Fibril (CMC/WPI-AF) Membrane
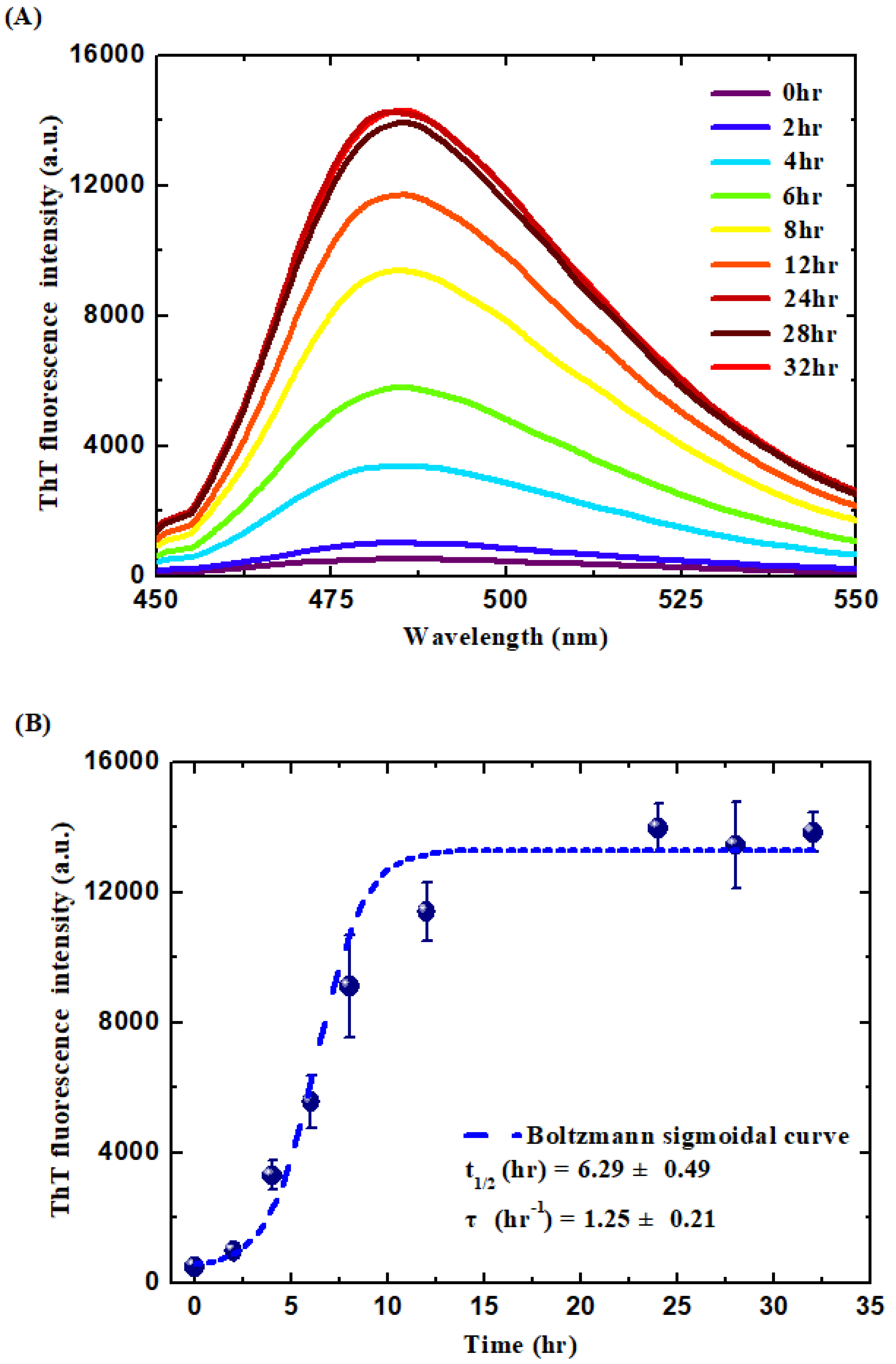
2.4. Thioflavin T (ThT) Binding Assay
2.5. Transmission Electron Microscopy (TEM)
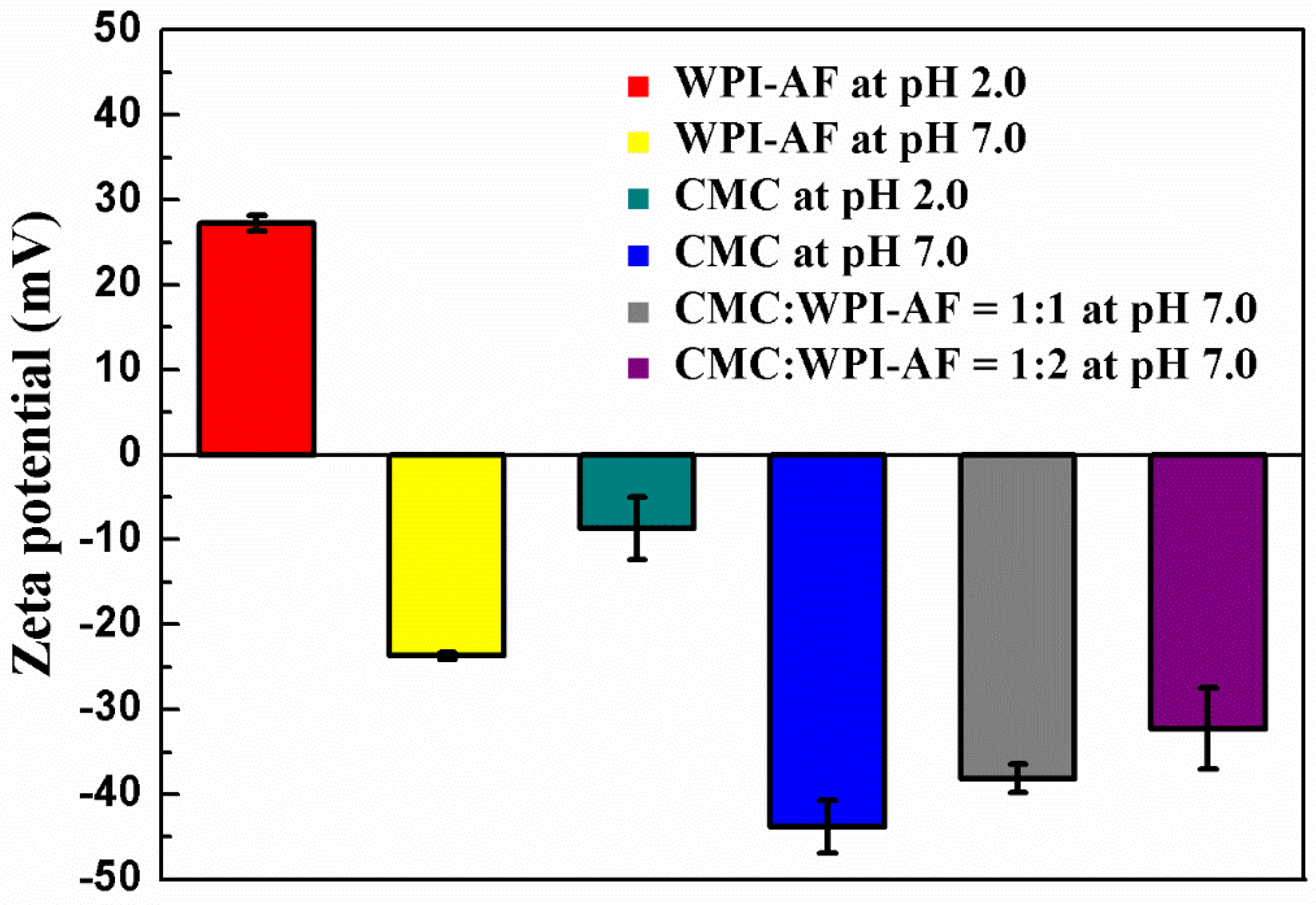
2.6. Zeta-Potential Measurements
2.7. Fourier Transform Infrared (FT-IR) Spectroscopy
2.8. Scanning Electron Microscopy (SEM)
2.9. Absorption of the Drug in the CMC/WPI-AF Membrane
2.10. In Vitro Passive Drug Delivery Studies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Formation and Characterization of the WPI-AF
3.2. Synthetic Mechanism of the CMC/WPI-AF Membranes
3.3. Zeta Potential Properties of the CMC/WPI-AF Membranes
3.4. Surface Microstructure Characterization of the CMC/WPI-AF Membranes
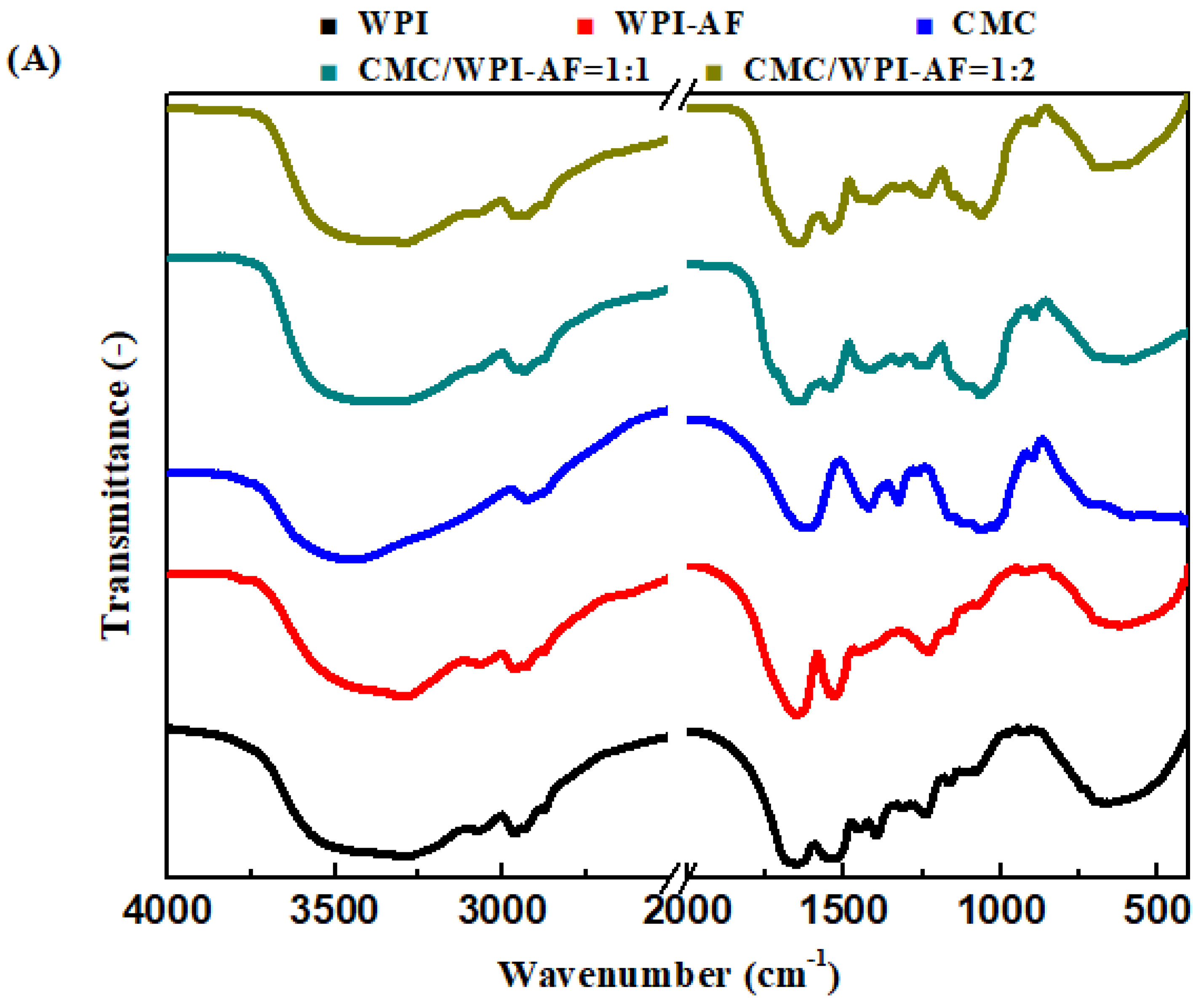
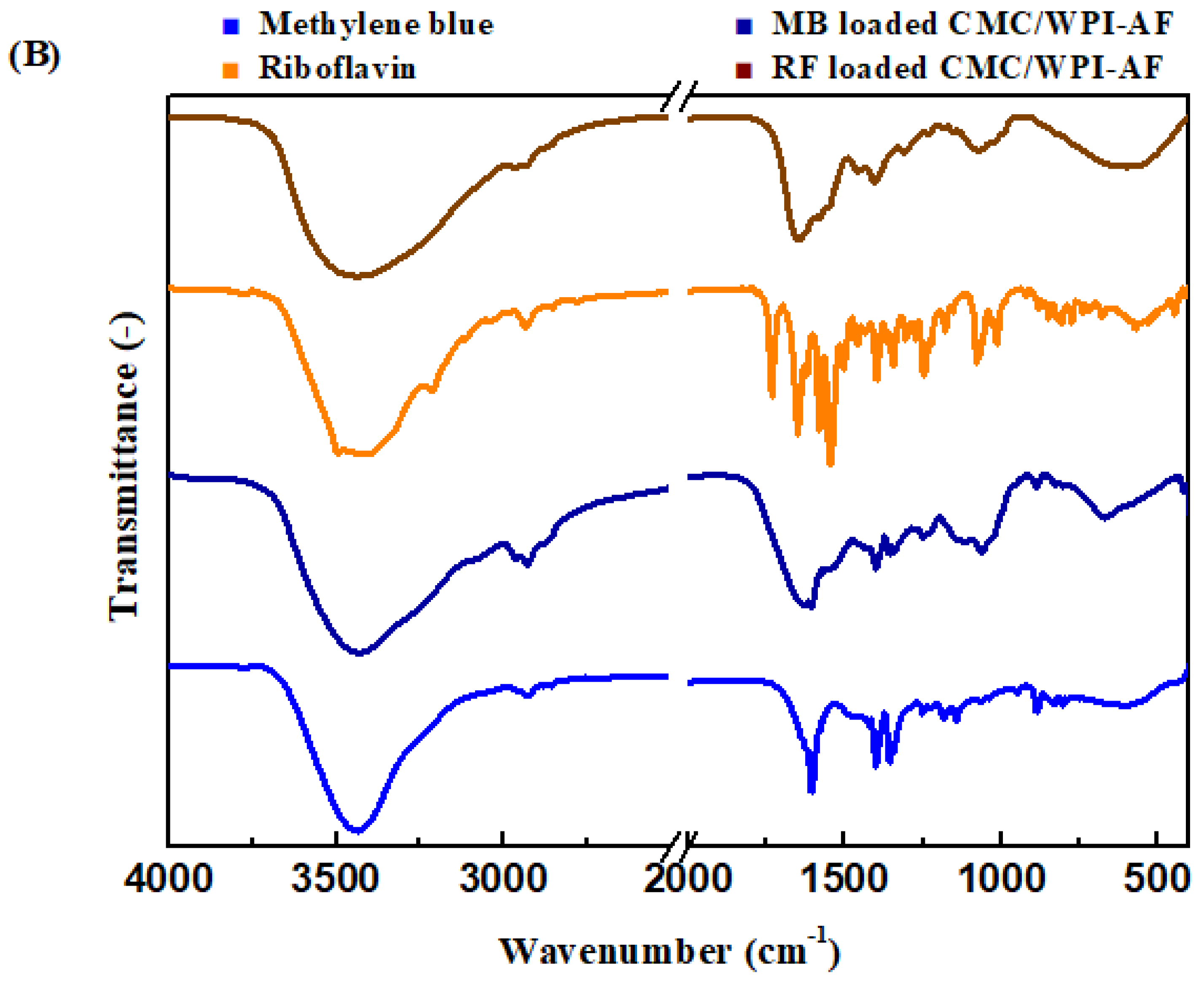
3.5. FT-IR Analysis of the CMC/WPI-AF Membranes
3.6. EE and LC of the Drugs on the CMC/WPI-AF Membranes
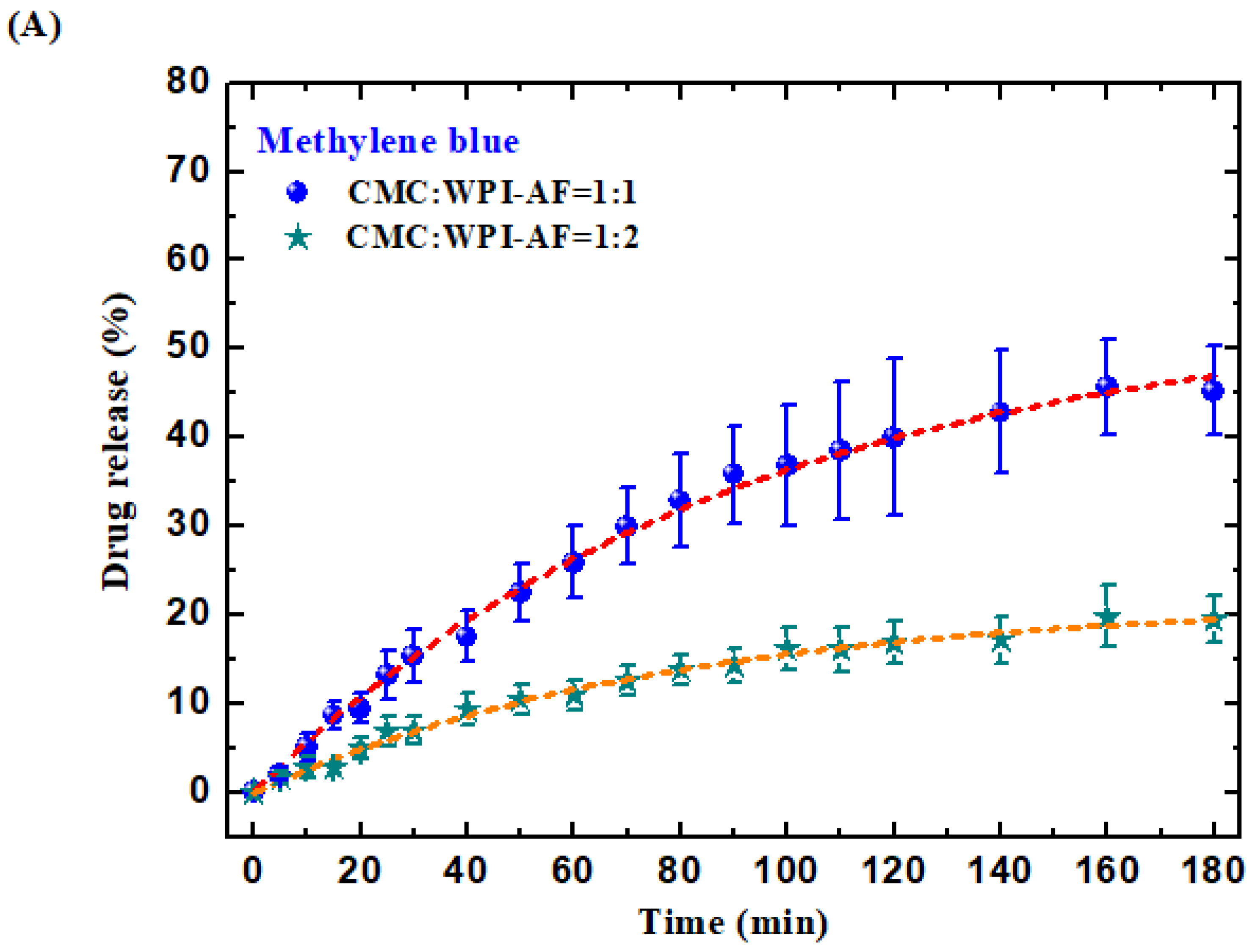
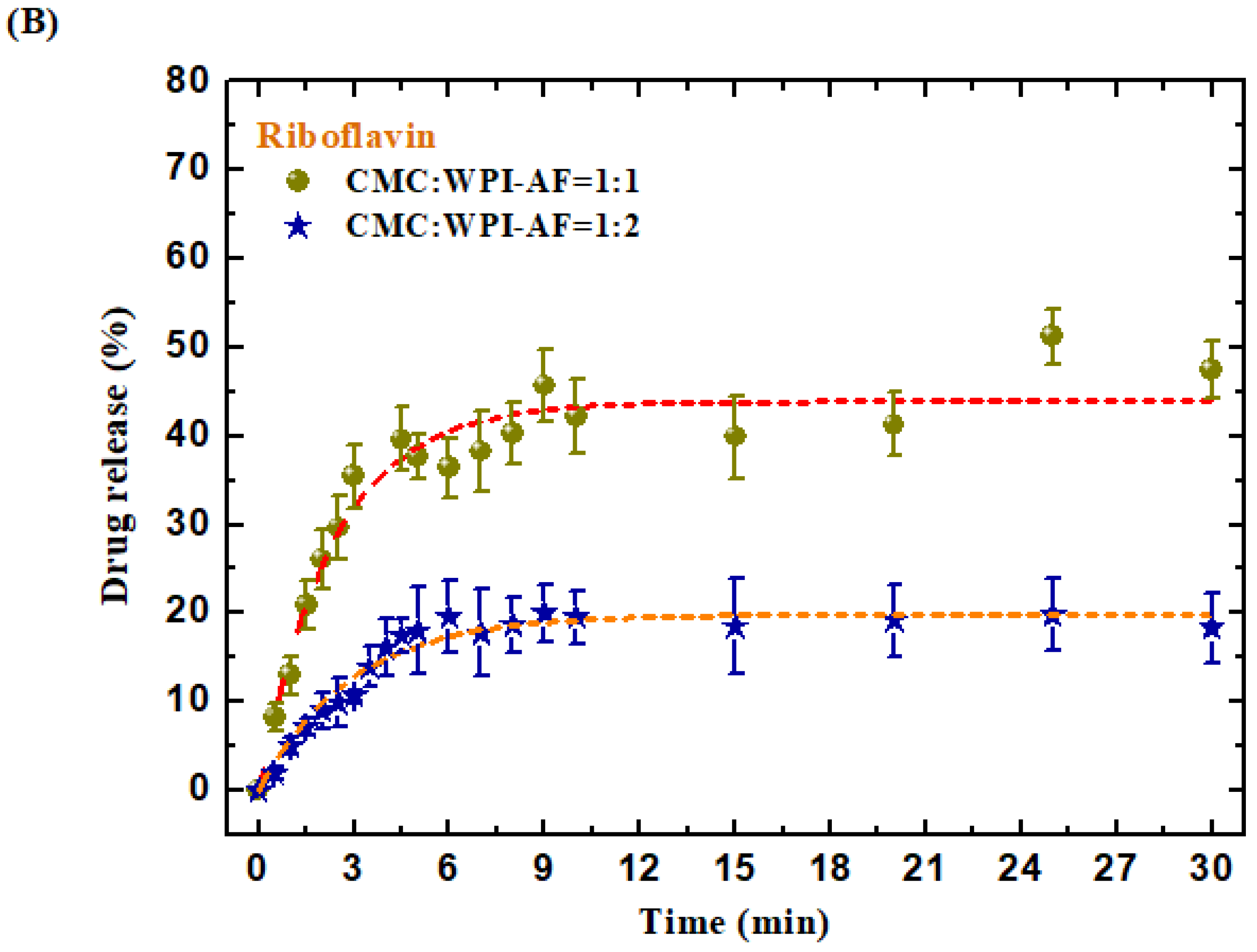
3.7. In Vitro Passive Drug Release of MB and RF from the CMC/WPI-AF Membranes
3.8. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, S.R.; Wu, Y.; Sui, S.F. Study of the correlation of secondary structure of beta-amyloid peptide (Abeta40) with the hydrophobic exposure under different conditions. Gen. Physiol. 2002, 21, 415–427. [Google Scholar]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Dharmadana, D.; Reynolds, N.P.; Conn, C.E.; Valery, C. Molecular interactions of amyloid nanofibrils with biological aggregation modifiers: Implications for cytotoxicity mechanisms and biomaterial design. Interface Focus 2017, 7, 20160160. [Google Scholar] [CrossRef]
- Bellesia, G.; Shea, J.E. Diversity of kinetic pathways in amyloid fibril formation. J. Chem. Phys. 2009, 131, 111102. [Google Scholar] [CrossRef]
- Knowles, T.P.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.S.; Wu, J.W.; Yamamoto, S.; Liu, H.S. Diseases of protein aggregation and the hunt for potential pharmacological agents. Biotechnol. J. Healthc. Nutr. Technol. 2008, 3, 165–192. [Google Scholar] [CrossRef] [PubMed]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef]
- Wang, S.S.S.; Chen, Y.T.; Chen, P.H.; Liu, K.N. A kinetic study on the aggregation behavior of beta-amyloid peptides in different initial solvent environments. Biochem. Eng. J. 2006, 29, 129–138. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; Rogers, S.S.; van der Goot, A.J.; Boom, R.M.; van der Linden, E. Shear pulses nucleate fibril aggregation. Food Biophys. 2006, 1, 144–150. [Google Scholar] [CrossRef]
- Giri, K.; Bhattacharyya, N.P.; Basak, S. pH-dependent self-assembly of polyalanine peptides. Biophys. J. 2007, 92, 293–302. [Google Scholar] [CrossRef]
- Kumar, E.K.; Haque, N.; Prabhu, N.P. Kinetics of protein fibril formation: Methods and mechanisms. Int. J. Biol. Macromol. 2017, 100, 3–10. [Google Scholar] [CrossRef]
- Arosio, P.; Knowles, T.P.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Aso, Y.; Shiraki, K.; Takagi, M. Systematic analysis of aggregates from 38 kinds of non disease-related proteins: Identifying the intrinsic propensity of polypeptides to form amyloid fibrils. Biosci. Biotechnol. Biochem. 2007, 71, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Badtke, M.P.; Hammer, N.D.; Chapman, M.R. Functional amyloids signal their arrival. Sci. Signal. 2009, 2, pe43. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, R.P.; Shewmaker, F.; Hu, K.-N.; McPhie, P.; Tycko, R.; Wickner, R.B. Repeat domains of melanosome matrix protein Pmel17 orthologs form amyloid fibrils at the acidic melanosomal pH. J. Biol. Chem. 2011, 286, 8385–8393. [Google Scholar] [CrossRef]
- Al-Halifa, S.; Babych, M.; Zottig, X.; Archambault, D.; Bourgault, S. Amyloid self-assembling peptides: Potential applications in nanovaccine engineering and biosensing. Pept. Sci. 2019, 111, e24095. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.; Debelouchina, G.T.; Bayro, M.J.; Clare, D.K.; Caporini, M.A.; Bajaj, V.S.; Jaroniec, C.P.; Wang, L.; Ladizhansky, V.; Müller, S.A. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc. Natl. Acad. Sci. USA 2013, 110, 5468–5473. [Google Scholar] [CrossRef]
- Navarro, S.; Ventura, S. Computational methods to predict protein aggregation. Curr. Opin. Struct. Biol. 2022, 73, 102343. [Google Scholar] [CrossRef]
- Taylor, A.I.; Staniforth, R.A. General Principles Underpinning Amyloid Structure. Front. Neurosci. 2022, 16, 878869. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Madadlou, A. Technological functionality and biological properties of food protein nanofibrils formed by heating at acidic condition. Trends Food Sci. Technol. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Shen, Y.; Levin, A.; Kamada, A.; Toprakcioglu, Z.; Rodriguez-Garcia, M.; Xu, Y.; Knowles, T.P.J. From Protein Building Blocks to Functional Materials. ACS Nano 2021, 15, 5819–5837. [Google Scholar] [CrossRef]
- Knowles, T.P.; Buehler, M.J. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 2011, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lendel, C.; Langton, M.; Olsson, R.T.; Hedenqvist, M.S. Protein nanofibrils: Preparation, properties, and possible applications in industrial nanomaterials. In Industrial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–63. [Google Scholar]
- Smith, J.F.; Knowles, T.P.; Dobson, C.M.; MacPhee, C.E.; Welland, M.E. Characterization of the nanoscale properties of individual amyloid fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.; Vanacore, G.M.; Zewail, A.H. Nanomechanics and intermolecular forces of amyloid revealed by four-dimensional electron microscopy. Proc. Natl. Acad. Sci. USA 2015, 112, 3380–3385. [Google Scholar] [CrossRef]
- Povilonienė, S.; Časaitė, V.; Bukauskas, V.; Šetkus, A.; Staniulis, J.; Meškys, R. Functionalization of α-synuclein fibrils. Beilstein J. Nanotechnol. 2015, 6, 124–133. [Google Scholar] [CrossRef]
- Knowles, T.P.; Mezzenga, R. Amyloid fibrils as building blocks for natural and artificial functional materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef]
- Li, C.; Alam, M.M.; Bolisetty, S.; Adamcik, J.; Mezzenga, R. New biocompatible thermo-reversible hydrogels from PNiPAM-decorated amyloid fibrils. Chem. Commun. 2011, 47, 2913–2915. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Li, D.; Xi, W.; Luo, F.; Zhang, X.; Zou, M.; Cao, M.; Hu, J.; Wang, W.; Wei, G.; et al. Tunable assembly of amyloid-forming peptides into nanosheets as a retrovirus carrier. Proc. Natl. Acad. Sci. USA 2015, 112, 2996–3001. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Shorter, J.; Lindquist, S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005, 6, 435–450. [Google Scholar] [CrossRef]
- Jacob, R.S.; Das, S.; Ghosh, S.; Anoop, A.; Jha, N.N.; Khan, T.; Singru, P.; Kumar, A.; Maji, S.K. Amyloid formation of growth hormone in presence of zinc: Relevance to its storage in secretory granules. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Su, Z.; Reynolds, N.P.; Arosio, P.; Hamley, I.W.; Gazit, E.; Mezzenga, R. Self-assembling peptide and protein amyloids: From structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017, 46, 4661–4708. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Roberts, S.J.; Meade, S.J.; Gerrard, J.A. Amyloid fibrils as a nanoscaffold for enzyme immobilization. Biotechnol. Prog. 2010, 26, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.S.; Anoop, A.; Maji, S.K. Protein nanofibrils as storage forms of peptide drugs and hormones. Biol. Bio-Inspired Nanomater. 2019, 1174, 265–290. [Google Scholar]
- Fedunova, D.; Antosova, A.; Marek, J.; Vanik, V.; Demjen, E.; Bednarikova, Z.; Gazova, Z. Effect of 1-Ethyl-3-methylimidazolium Tetrafluoroborate and Acetate Ionic Liquids on Stability and Amyloid Aggregation of Lysozyme. Int. J. Mol. Sci. 2022, 23, 783. [Google Scholar] [CrossRef]
- Ziaunys, M.; Sakalauskas, A.; Mikalauskaite, K.; Snieckute, R.; Smirnovas, V. Temperature-dependent structural variability of prion protein amyloid fibrils. Int. J. Mol. Sci. 2021, 22, 5075. [Google Scholar] [CrossRef]
- How, S.-C.; Lin, T.-H.; Chang, C.-C.; Wang, S.S.-S. Examining the effect of bovine serum albumin on the properties and drug release behavior of β-lactoglobulin-derived amyloid fibril-based hydrogels. Int. J. Biol. Macromol. 2021, 184, 79–91. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Jansens, K.J.; Rombouts, I.; Brijs, K.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Conditions governing food protein amyloid fibril formation. part II: Milk and legume proteins. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1277–1291. [Google Scholar] [CrossRef]
- Gade Malmos, K.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: A primer on the use of thioflavin T to investigate amyloid formation. Amyloid 2017, 24, 1–16. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumbhakar, M.; Pal, H.; Nath, S. Ultrafast bond twisting dynamics in amyloid fibril sensor. J. Phys. Chem. B 2010, 114, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-R.; Lai, J.-T.; Wang, S.S.-S.; Kuo, Y.-C.; Lin, T.-H. Silver nanoparticle-deposited whey protein isolate amyloid fibrils as catalysts for the reduction of methylene blue. Int. J. Biol. Macromol. 2022, 213, 1098–1114. [Google Scholar] [CrossRef]
- Buhus, G.; Popa, M.; Desbrieres, J. Hydrogels based on carboxymethylcellulose and gelatin for inclusion and release of chloramphenicol. J. Bioact. Compat. Polym. 2009, 24, 525–545. [Google Scholar] [CrossRef]
- Chinyerenwa, A.C.; Wang, H.; Zhang, Q.; Zhuang, Y.; Munna, K.H.; Ying, C.; Yang, H.; Xu, W. Structure and thermal properties of porous polylactic acid membranes prepared via phase inversion induced by hot water droplets. Polymer 2018, 141, 62–69. [Google Scholar] [CrossRef]
- Duhoranimana, E.; Karangwa, E.; Lai, L.; Xu, X.; Yu, J.; Xia, S.; Zhang, X.; Muhoza, B.; Habinshuti, I. Effect of sodium carboxymethyl cellulose on complex coacervates formation with gelatin: Coacervates characterization, stabilization and formation mechanism. Food Hydrocoll. 2017, 69, 111–120. [Google Scholar] [CrossRef]
- Cai, T.; Yang, Z.; Li, H.; Yang, H.; Li, A.; Cheng, R. Effect of hydrolysis degree of hydrolyzed polyacrylamide grafted carboxymethyl cellulose on dye removal efficiency. Cellulose 2013, 20, 2605–2614. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Saharan, V.; Kukkar, V.; Kataria, M.; Gera, M.; Choudhury, P.K. Dissolution enhancement of drugs. Part I: Technologies and effect of carriers. Int. J. Health Res. 2009, 2, 2. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Hedenqvist, M.S.; Langton, M.; Lendel, C. On the role of peptide hydrolysis for fibrillation kinetics and amyloid fibril morphology. RSC Adv. 2018, 8, 6915–6924. [Google Scholar] [CrossRef]
- Habibi, N. Preparation of biocompatible magnetite-carboxymethyl cellulose nanocomposite: Characterization of nanocomposite by FTIR, XRD, FESEM and TEM. Spectrochim. Acta A Mol. 2014, 131, 55–58. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Musale, D.A.; Kumar, A. Effects of surface crosslinking on sieving characteristics of chitosan/poly (acrylonitrile) composite nanofiltration membranes. Sep. Purif. Technol. 2000, 21, 27–37. [Google Scholar] [CrossRef]
- Giraldo, S.; Robles, I.; Godínez, L.A.; Acelas, N.; Flórez, E. Experimental and theoretical insights on methylene blue removal from wastewater using an adsorbent obtained from the residues of the orange industry. Molecules 2021, 26, 4555. [Google Scholar] [CrossRef]
- Liu, F.; Zou, H.; Hu, J.; Liu, H.; Peng, J.; Chen, Y.; Lu, F.; Huo, Y. Fast removal of methylene blue from aqueous solution using porous soy protein isolate based composite beads. Chem. Eng. J. 2016, 287, 410–418. [Google Scholar] [CrossRef]
- Chakraborty, P.; Bairi, P.; Roy, B.; Nandi, A.K. Rheological and fluorescent properties of riboflavin-poly (N-isopropylacrylamide) hybrid hydrogel with a potentiality of forming Ag nanoparticle. RSC Adv. 2014, 4, 54684–54693. [Google Scholar] [CrossRef]
- Ge, X.; Sun, Y.; Kong, J.; Mao, M.; Yu, H.; Arora, A.; Suppavorasatit, I.; Wang, Y. The thermal resistance and targeting release of zein-sodium alginate binary complexes as a vehicle for the oral delivery of riboflavin. J. Food Sci. Technol. 2022, 60, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Prasanna, G.; Jing, P. Polyphenol binding disassembles glycation-modified bovine serum albumin amyloid fibrils. Spectrochim. Acta A Mol. 2021, 246, 119001. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bolisetty, S.; Mohammadi, T.; Mezzenga, R. Assessing the binding performance of amyloid-carbon membranes toward heavy metal ions. Langmuir 2019, 35, 4161–4170. [Google Scholar] [CrossRef]
- Hate, S.S.; Reutzel-Edens, S.M.; Taylor, L.S. Influence of Drug-Silica Electrostatic Interactions on Drug Release from Mesoporous Silica-Based Oral Delivery Systems. Mol. Pharm. 2020, 17, 3435–3446. [Google Scholar] [CrossRef]
- Feng, Z.; Zheng, Y.; Zhao, L.; Zhang, Z.; Sun, Y.; Qiao, K.; Xie, Y.; Wang, Y.; He, W. An ultrasound-controllable release system based on waterborne polyurethane/chitosan membrane for implantable enhanced anticancer therapy. Mater. Sci. Eng. C 2019, 104, 109944. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.; Molina, E.; Chiavacci, L.; Santilli, C.V.; Briois, V.; Pulcinelli, S.H. Drug-matrix interaction of sodium diclofenac incorporated into ureasil-poly (ethylene oxide) hybrid materials. RSC Adv. 2012, 2, 5629–5636. [Google Scholar] [CrossRef]
- Zandi, M.; Mohebbi, M.; Varidi, M.; Ramezanian, N. Evaluation of diacetyl encapsulated alginate-whey protein microspheres release kinetics and mechanism at simulated mouth conditions. Food Res. Int. 2014, 56, 211–217. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Hu, C.-C.; Li, C.-Y.; Li, S.-C.; Cai, Z.-W.; Wei, Y.; Don, T.-M. Theophylline-Loaded Pectin/Chitosan Hydrochloride Submicron Particles Prepared by Spray Drying with a Continuous Feeding Ultrasonic Atomizer. Polymers 2022, 14, 4538. [Google Scholar] [CrossRef]
- Rac, V.; Lević, S.; Balanč, B.; Graells, B.O.; Bijelić, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B 2019, 180, 441–448. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Radovanović, F. Polyethersulfone/poly (acrylic acid) composite hydrogel membrane reservoirs for controlled delivery of cationic drug formulations. Polymer 2018, 147, 56–66. [Google Scholar] [CrossRef]
- Im, J.S.; Bai, B.C.; Lee, Y.-S. The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system. Biomaterials 2010, 31, 1414–1419. [Google Scholar] [CrossRef]
- Sheng, S.; Yin, X.; Chen, F.; Lv, Y.; Zhang, L.; Cao, M.; Sun, Y. Preparation and characterization of PVA-co-PE drug-loaded nanofiber membrane by electrospinning technology. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Qiao, Z.; Tran, L.; Parks, J.; Zhao, Y.; Hai, N.; Zhong, Y.; Ji, H.F. Highly stretchable gelatin-polyacrylamide hydrogel for potential transdermal drug release. Nano Select 2021, 2, 107–115. [Google Scholar] [CrossRef]
- Langer, R. Invited review polymeric delivery systems for controlled drug release. Chem. Eng. Commun. 1980, 6, 1–48. [Google Scholar] [CrossRef]
- Lassé, M.; Ulluwishewa, D.; Healy, J.; Thompson, D.; Miller, A.; Roy, N.; Chitcholtan, K.; Gerrard, J.A. Evaluation of protease resistance and toxicity of amyloid-like food fibrils from whey, soy, kidney bean, and egg white. Food Chem. 2016, 192, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, F.D.; Rodero, C.F.; Lutz-Bueno, V.; Chorilli, M.; Mezzenga, R. Amyloid Fibrils Enhance the Topical Bio-Adhesivity of Liquid Crystalline Mesophase-Based Drug Formulations. Adv. Healthc. Mater. 2023, 2202720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, P. Amyloid-Mediated Fabrication of Organic—Inorganic Hybrid Materials and Their Biomedical Applications. Adv. Mater. Interfaces 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Bolisetty, S.; Boddupalli, C.S.; Handschin, S.; Chaitanya, K.; Adamcik, J.; Saito, Y.; Manz, M.G.; Mezzenga, R. Amyloid fibrils enhance transport of metal nanoparticles in living cells and induced cytotoxicity. Biomacromolecules 2014, 15, 2793–2799. [Google Scholar] [CrossRef]
- Shen, Y.; Posavec, L.; Bolisetty, S.; Hilty, F.M.; Nyström, G.; Kohlbrecher, J.; Hilbe, M.; Rossi, A.; Baumgartner, J.; Zimmermann, M.B. Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron. Nat. Nanotechnol. 2017, 12, 642–647. [Google Scholar] [CrossRef]
- Yue, J.; Shu, M.; Yao, X.; Chen, X.; Li, D.; Yang, D.; Liu, N.; Nishinari, K.; Jiang, F. Fibrillar assembly of whey protein isolate and gum Arabic as iron carrier for food fortification. Food Hydrocoll. 2022, 128, 107608. [Google Scholar] [CrossRef]
- Shimanovich, U.; Efimov, I.; Mason, T.O.; Flagmeier, P.; Buell, A.K.; Gedanken, A.; Linse, S.; Åkerfeldt, K.S.; Dobson, C.M.; Weitz, D.A. Protein microgels from amyloid fibril networks. ACS Nano 2015, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.; Lamprou, D.A.; McIntosh, L.; Oswald, I.D.; Urquhart, A.J. Beta-adrenoceptor antagonists affect amyloid nanostructure; amyloid hydrogels as drug delivery vehicles. Chem. Commun. 2013, 49, 5082–5084. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Kwon, Y.; Nam, E.-J.; Park, H.; Paik, S.R. Disulfide-mediated elongation of amyloid fibrils of α-synuclein for use in producing self-healing hydrogel and dye-absorbing aerogel. Acta Biomater. 2022, 145, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, H.; Yao, L.; Yu, Y.; Ma, G. Amyloid-based injectable hydrogel derived from hydrolyzed hen egg white lysozyme. Acs Omega 2019, 4, 8071–8080. [Google Scholar] [CrossRef]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef]
- Kabay, G.; Meydan, A.E.; Can, G.K.; Demirci, C.; Mutlu, M. Controlled release of a hydrophilic drug from electrospun amyloid-like protein blend nanofibers. Mater. Sci. Eng. C 2017, 81, 271–279. [Google Scholar] [CrossRef]


| CMC/WPI-AF Membranes | Methylene Blue (MB) | |
|---|---|---|
| Encapsulation Efficiency (%) | Loading Capacity (%) | |
| CMC:WPI-AF = 1:1 | 18.31 ± 3.14 | 106.71 ± 7.97 |
| CMC:WPI-AF = 1:2 | 46.80 ± 2.88 | 197.38 ± 9.43 |
| CMC/WPI-AF membranes | Riboflavin (RF) | |
| Encapsulation efficiency (%) | Loading capacity (%) | |
| CMC:WPI-AF = 1:1 | 10.74 ± 1.63 | 21.89 ± 1.21 |
| CMC:WPI-AF = 1:2 | 16.49 ± 3.48 | 24.80 ± 1.49 |
| CMC/WPI-AF Membranes | Methylene Blue (MB) | ||
|---|---|---|---|
| M∞ | kf (min−1) | R2 | |
| CMC:WPI-AF = 1:1 | 54.35 ± 1.41 | 0.0111 ± 0.0005 | 0.996 |
| CMC:WPI-AF = 1:2 | 21.66 ± 0.67 | 0.0127 ± 0.0008 | 0.993 |
| CMC/WPI-AF membranes | Riboflavin (RF) | ||
| M∞ | kf (min−1) | R2 | |
| CMC:WPI-AF = 1:1 | 43.92 ± 1.19 | 0.4294 ± 0.0439 | 0.951 |
| CMC:WPI-AF = 1:2 | 19.86 ± 0.53 | 0.3442 ± 0.0293 | 0.961 |
| CMC/WPI-AF Membranes | Methylene Blue (MB) | ||
|---|---|---|---|
| kp (min−n) | N | R2 | |
| CMC:WPI-AF = 1:1 | 0.015 ± 0.002 | 0.852 ± 0.025 | 0.996 |
| CMC:WPI-AF = 1:2 | 0.024 ± 0.005 | 0.755 ± 0.055 | 0.976 |
| CMC/WPI-AF membranes | Riboflavin (RF) | ||
| kp (min−n) | N | R2 | |
| CMC:WPI-AF = 1:1 | 0.326 ± 0.014 | 0.821 ± 0.057 | 0.992 |
| CMC:WPI-AF = 1:2 | 0.249 ± 0.020 | 0.750 ± 0.090 | 0.973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-R.; Wang, S.S.-S.; Hsu, T.-L.; Chou, S.-H.; How, S.-C.; Lin, T.-H. Application of Amyloid-Based Hybrid Membranes in Drug Delivery. Polymers 2023, 15, 1444. https://doi.org/10.3390/polym15061444
Lai Y-R, Wang SS-S, Hsu T-L, Chou S-H, How S-C, Lin T-H. Application of Amyloid-Based Hybrid Membranes in Drug Delivery. Polymers. 2023; 15(6):1444. https://doi.org/10.3390/polym15061444
Chicago/Turabian StyleLai, You-Ren, Steven S.-S. Wang, Ti-Lun Hsu, Szu-Hui Chou, Su-Chun How, and Ta-Hsien Lin. 2023. "Application of Amyloid-Based Hybrid Membranes in Drug Delivery" Polymers 15, no. 6: 1444. https://doi.org/10.3390/polym15061444
APA StyleLai, Y.-R., Wang, S. S.-S., Hsu, T.-L., Chou, S.-H., How, S.-C., & Lin, T.-H. (2023). Application of Amyloid-Based Hybrid Membranes in Drug Delivery. Polymers, 15(6), 1444. https://doi.org/10.3390/polym15061444