Photo- and Water-Degradation Phenomena of ZnO Bio-Blend Based on Poly(lactic acid) and Polyamide 11
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blending Procedure
2.3. Size Exclusion Chromatography (SEC)
2.4. Matrix-Assisted Laser Desorption Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
2.5. Thermogravimetric Analysis (TGA)
2.6. Scanning Electron Microscopy (SEM)
2.7. Transmission Electron Microscopy (TEM)
2.8. Aging Tests
3. Results and Discussion
3.1. TGA Measurements
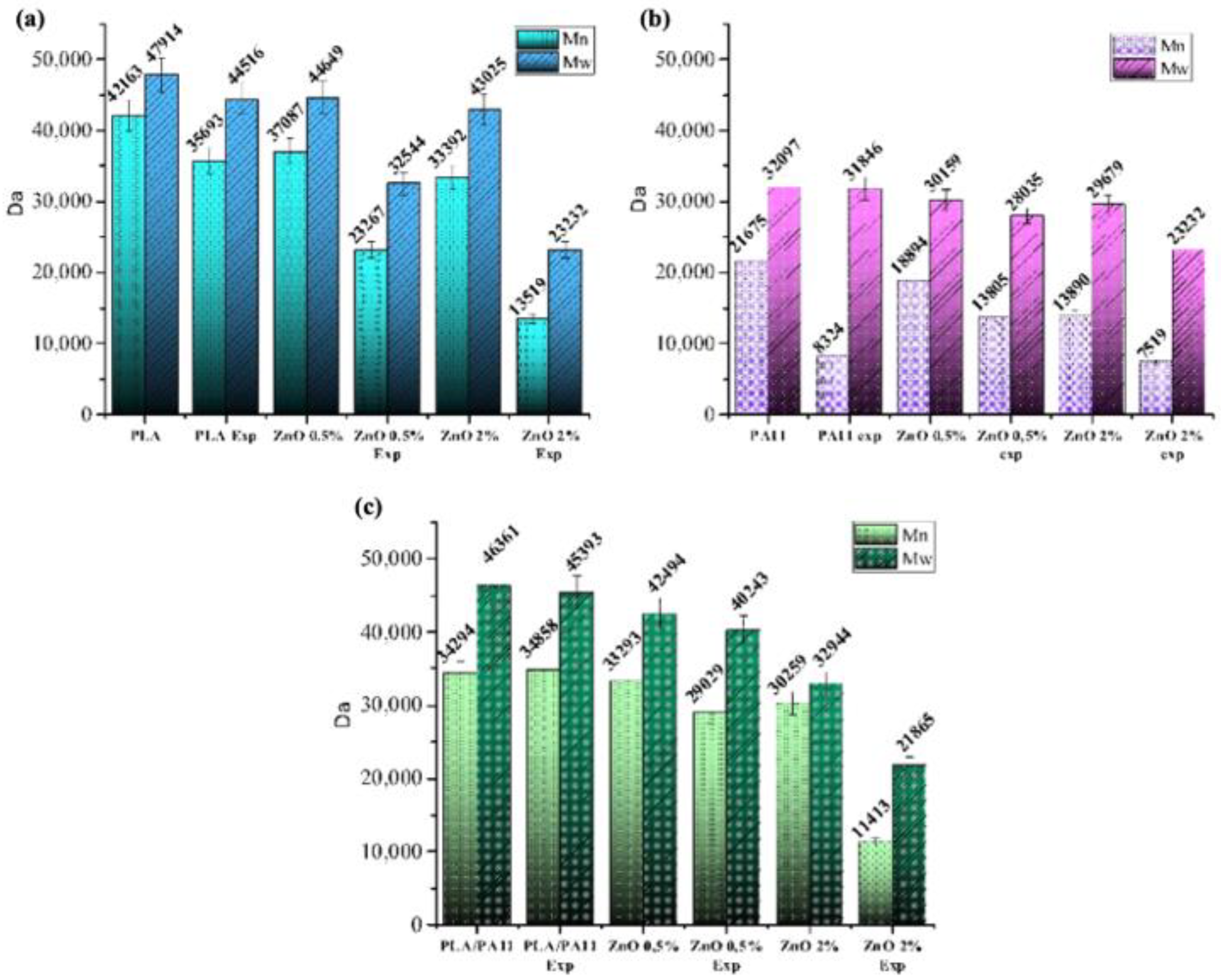
3.2. SEC Analysis
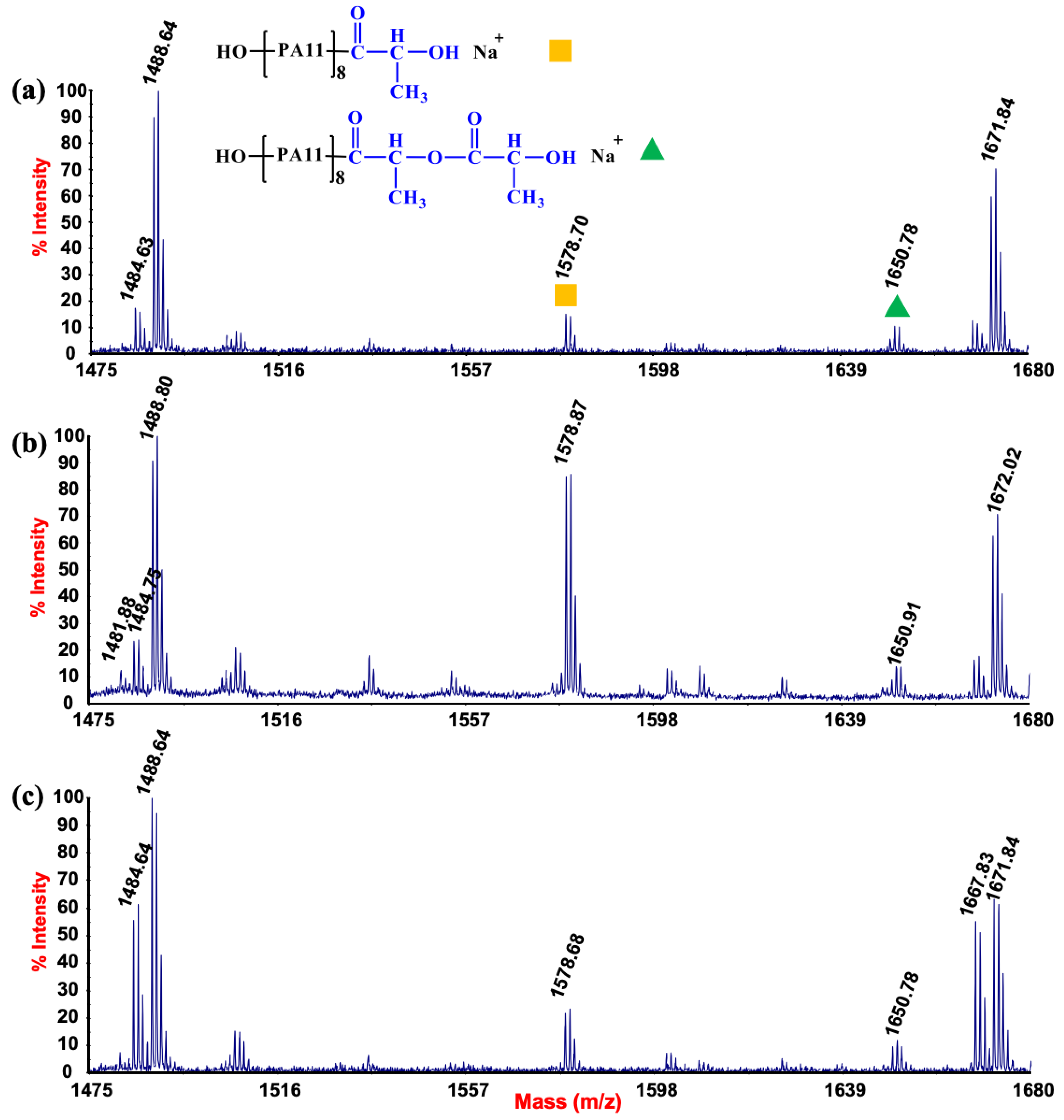
3.3. MALDI Mass Spectrometry Measurements
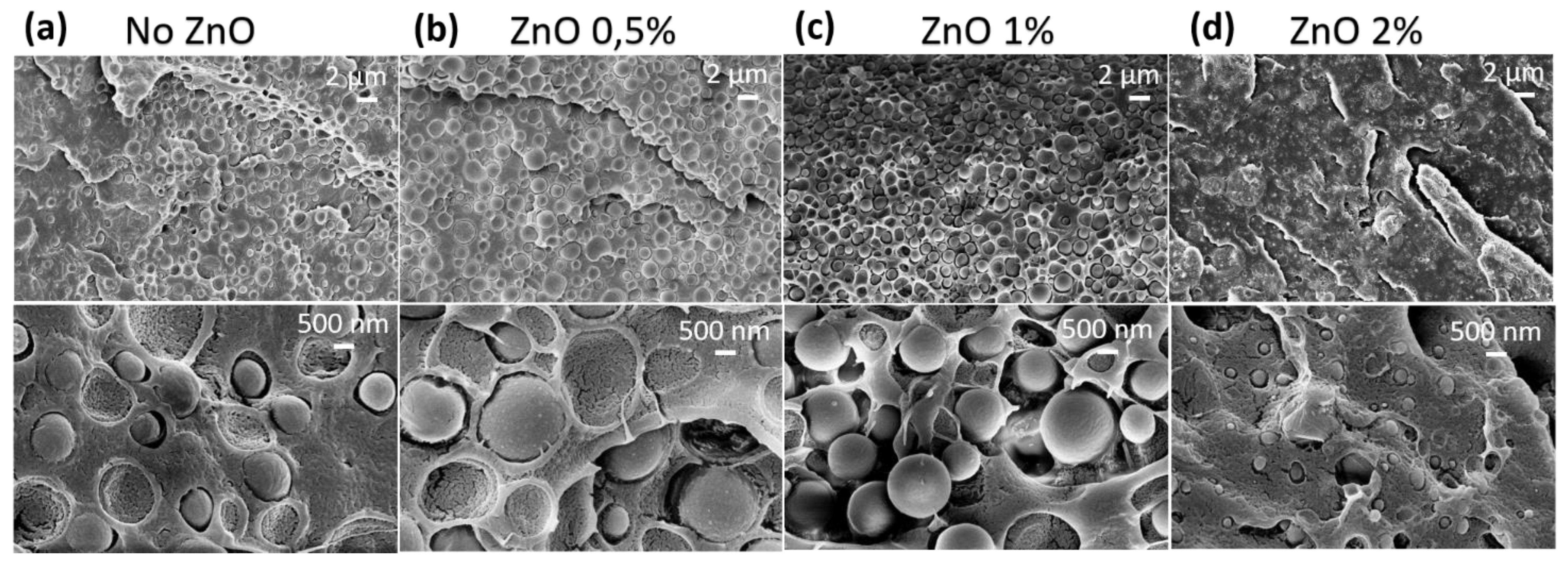
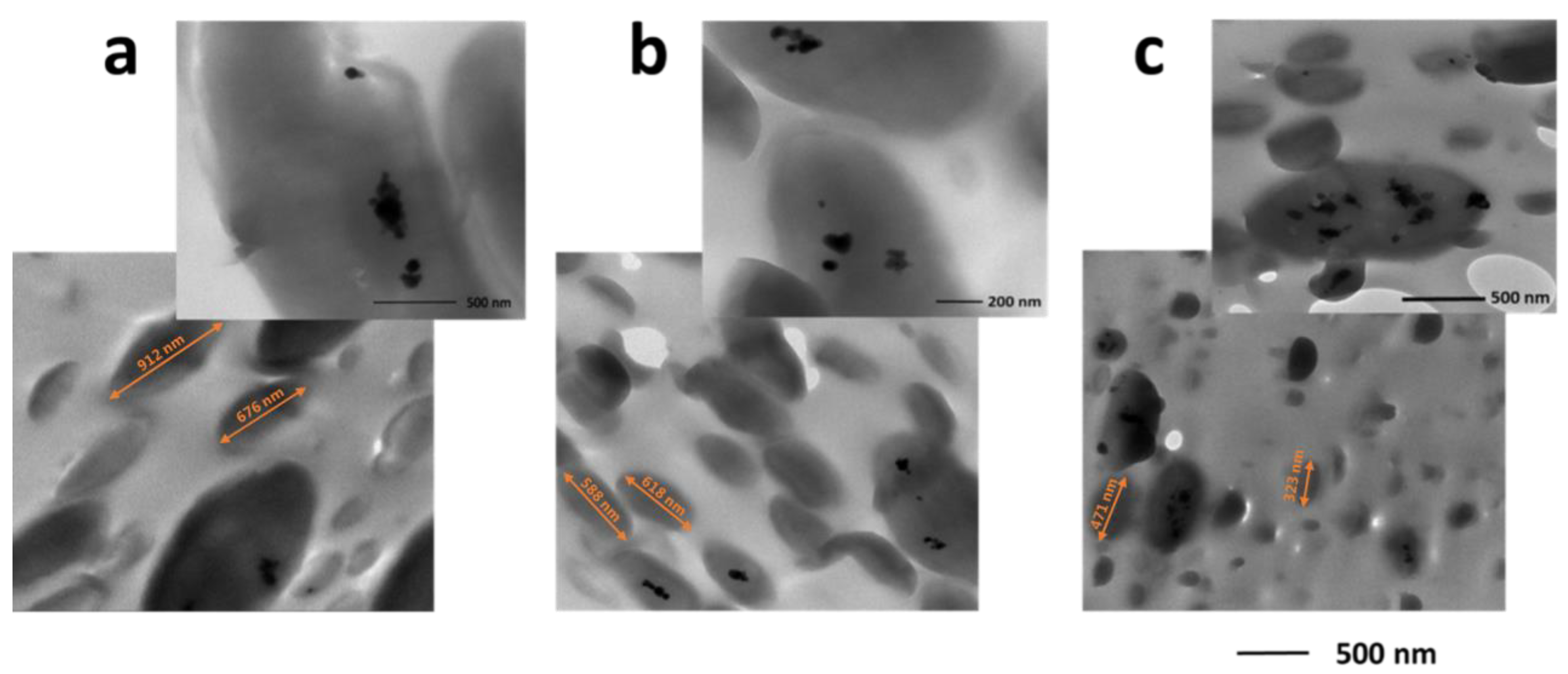
3.4. SEM and TEM Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoclet, G.; Seguela, R.; Lefebvre, J.M. Morphology, thermal behavior and mechanical properties of binary blends of compatible biosourced polymers: Polylactide/polyamide11. Polymer 2011, 52, 1417–1425. [Google Scholar] [CrossRef]
- Heshmati, V.; Favis, B.D. High performance poly (lactic acid)/bio-polyamide11 through controlled chain mobility. Polymer 2017, 123, 184–193. [Google Scholar] [CrossRef]
- Heshmati, V.; Kamal, M.R.; Favis, B.D. Tuning the localization of finely dispersed cellulose nanocrystal in poly (lactic acid)/bio-polyamide11 blends. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 576–587. [Google Scholar] [CrossRef]
- Heshmati, V.; Kamal, M.R.; Favis, B.D. Cellulose nanocrystal in poly(lactic acid)/polyamide11 blends: Preparation, morphology and co-continuity. Eur. Polym. J. 2018, 98, 11–20. [Google Scholar] [CrossRef]
- Heshmati, V.; Zolali, A.M.; Favis, B.D. Morphology development in poly (lactic acid)/polyamide11 biobased blends: Chain mobility and interfacial interactions. Polymer 2017, 120, 197–208. [Google Scholar] [CrossRef]
- Dong, W.; Cao, X.; Li, Y. High-performance biosourced poly(lactic acid)/polyamide 11 blends with controlled salami structure. Polym. Int. 2014, 63, 1094–1100. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Rheological, Morphological and Mechanical Studies of Sustainably Sourced Polymer Blends Based on Poly(Lactic Acid) and Polyamide 11. Polymers 2016, 8, 61. [Google Scholar] [CrossRef]
- Nam, B.U.; Son, Y. Enhanced impact strength of compatibilized poly(lactic acid)/polyamide 11 blends by a crosslinking agent. J. Appl. Polym. Sci. 2020, 137, 49011. [Google Scholar] [CrossRef]
- Rasselet, D.; Pucci, M.F.; Caro-Bretelle, A.-S.; Lopez-Cuesta, J.-M.; Taguet, A. Peculiar Morphologies Obtained for 80/20 PLA/PA11 Blend with Small Amounts of Fumed Silica. Nanomaterials 2021, 11, 1721. [Google Scholar] [CrossRef]
- Wu, J.-H.; Chen, C.-W.; Kuo, M.C.; Yen, M.-S.; Lee, K.-Y. High Toughness and Fast Crystallization Poly(Lactic Acid)/Polyamide 11/SiO2 Composites. J. Polym. Environ. 2017, 26, 626–635. [Google Scholar] [CrossRef]
- Nuzzo, A.; Coiai, S.; Carroccio, S.C.; Dintcheva, N.T.; Gambarotti, C.; Filippone, G. Heat-Resistant Fully Bio-Based Nanocomposite Blends Based on Poly(lactic acid). Macromol. Mater. Eng. 2014, 299, 31–40. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial Membranes of Bio-Based PA 11 and HNTs Filled with Lysozyme Obtained by an Electrospinning Process. Nanomaterials 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Patel, R.; Ruehle, D.A.; Dorgan, J.R.; Halley, P.; Martin, D. Biorenewable blends of polyamide-11 and polylactide. Polym. Eng. Sci. 2014, 54, 1523–1532. [Google Scholar] [CrossRef]
- Nuzzo, A.; Bilotti, E.; Peijs, T.; Acierno, D.; Filippone, G. Nanoparticle-induced co-continuity in immiscible polymer blends—A comparative study on bio-based PLA-PA11 blends filled with organoclay, sepiolite, and carbon nanotubes. Polymer 2014, 55, 4908–4919. [Google Scholar] [CrossRef]
- Rashmi, B.J.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Toughening of poly(lactic acid) without sacrificing stiffness and strength by melt-blending with polyamide 11 and selective localization of halloysite nanotubes. Express Polym. Lett. 2015, 9, 721–735. [Google Scholar] [CrossRef]
- Ussia, M.; Curcuruto, G.; Zampino, D.; Dintcheva, N.T.; Filippone, G.; Mendichi, R.; Carroccio, S.C. Role of Organo-Modifier and Metal Impurities of Commercial Nanoclays in the Photo- and Thermo-Oxidation of Polyamide 11 Nanocomposites. Polymers 2020, 12, 1034. [Google Scholar] [CrossRef] [PubMed]
- Filippone, G.; Carroccio, S.C.; Curcuruto, G.; Passaglia, E.; Gambarotti, C.; Dintcheva, N.T. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt—Part II: Thermal and thermo-oxidative degradation of polyamide 11/organo-clay nanocomposites. Polymer 2015, 73, 102–110. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.C.; Mendichi, R.; Gioiella, L.; Dintcheva, N.T.; Gambarotti, C. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state—Part I: Thermal and thermo-oxidative degradation of polyamide 11. Polymer 2015, 72, 134–141. [Google Scholar] [CrossRef]
- Bocchini, S.; Fukushima, K.; Blasio, A.D.; Fina, A.; Frache, A.; Geobaldo, F. Polylactic Acid and Polylactic Acid-Based Nanocomposite Photooxidation. Biomacromolecules 2010, 11, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, L.; Kaci, M.; Bruzaud, S.; Bourmaud, A.; Grohens, Y. Effect of natural weather on the structure and properties of polylactide/Cloisite 30B nanocomposites. Polym. Degrad. Stab. 2010, 95, 1751–1758. [Google Scholar] [CrossRef]
- Kontou, E.; Georgiopoulos, P.; Niaounakis, M. The role of nanofillers on the degradation behavior of polylactic acid. Polym. Compos. 2012, 33, 282–294. [Google Scholar] [CrossRef]
- Bai, J.; Yuan, S.; Shen, F.; Zhang, B.; Chua, C.K.; Zhou, K.; Wei, J. Toughening of polyamide 11 with carbon nanotubes for additive manufacturing. Virtual Phys. Prototyp. 2017, 12, 235–240. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.-J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef] [PubMed]
- Esthappan, S.K.; Nair, A.B.; Joseph, R. Effect of crystallite size of zinc oxide on the mechanical, thermal and flow properties of polypropylene/zinc oxide nanocomposites. Compos. Part B Eng. 2015, 69, 145–153. [Google Scholar] [CrossRef]
- Abbas, M.; Buntinx, M.; Deferme, W.; Peeters, R. (Bio)polymer/ZnO Nanocomposites for Packaging Applications: A Review of Gas Barrier and Mechanical Properties. Nanomaterials 2019, 9, 1494. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, F.; Chu, Z.; Fan, C.; Qin, Y. Barrier Properties and Characterizations of Poly(lactic Acid)/ZnO Nanocomposites. Molecules 2020, 25, 1310. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Hezma, A.M.; Rajeh, A.; Mannaa, M.A. An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123821. [Google Scholar] [CrossRef]
- Therias, S.; Larché, J.-F.; Bussière, P.-O.; Gardette, J.-L.; Murariu, M.; Dubois, P. Photochemical Behavior of Polylactide/ZnO Nanocomposite Films. Biomacromolecules 2012, 13, 3283–3291. [Google Scholar] [CrossRef]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak, D.; Kyratzis, I.; Sola, A.; Wen, C. Additive manufacturing of antibacterial PLA-ZnO nanocomposites: Benefits, limitations and open challenges. J. Mater. Sci. Technol. 2022, 111, 120–151. [Google Scholar] [CrossRef]
- Nonato, R.C.; Mei, L.H.I.; Bonse, B.C.; Leal, C.V.; Levy, C.E.; Oliveira, F.A.; Delarmelina, C.; Duarte, M.C.T.; Morales, A.R. Nanocomposites of PLA/ZnO nanofibers for medical applications: Antimicrobial effect, thermal, and mechanical behavior under cyclic stress. Polym. Eng. Sci. 2022, 62, 1147–1155. [Google Scholar] [CrossRef]
- Roghabadi, F.A.; Ahmadi, V.; Nejand, B.A.; Aghmiuni, K.O. Enhancing Lifetime and Efficiency of Organic Solar Cell by Applying an In Situ Synthesized Low-Crystalline ZnO Layer. ChemSusChem 2017, 10, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Guedri, L.; Ben Amor, S.; Gardette, J.L.; Jacquet, M.; Rivaton, A. Lifetime improvement of poly(ethylene naphthalate) by ZnO adhesive coatings. Polym. Degrad. Stab. 2005, 88, 199–205. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhang, Z.; Peng, S.; Chen, H.; Zhao, X. High-performance fully bio-based poly(lactic acid)/polyamide11 (PLA/PA11) blends by reactive blending with multi-functionalized epoxy. Polym. Test. 2019, 78, 105980. [Google Scholar] [CrossRef]
- Wang, A.; Quan, W.; Zhang, H.; Li, H.; Yang, S. Heterogeneous ZnO-containing catalysts for efficient biodiesel production. RSC Adv. 2021, 11, 20465–20478. [Google Scholar] [CrossRef] [PubMed]
- Therias, S.; Murariu, M.; Dubois, P. Bionanocomposites based on PLA and halloysite nanotubes: From key properties to photooxidative degradation. Polym. Degrad. Stab. 2017, 145, 60–69. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.-C.; Södergård, A. From Lactic Acid to Poly(lactic acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Luo, Y.; Cao, Y.; Guo, G. Effects of TiO2 nanoparticles on the photodegradation of poly(lactic acid). J. Appl. Polym. Sci. 2018, 135, 46509. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the Gap for Bioplastic Use in Food Packaging: An Update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef]
- Vitalini, D.; Spina, E.; Dattilo, S.; Mineo, P.; Scamporrino, E. Synthesis, characterization, and nucleotidic chain cleavage ability of uncharged water soluble poly(ethylene glycol)-fullerene derivatives with an amphiphilic character. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2145–2153. [Google Scholar] [CrossRef]
- Montaudo, G.; Samperi, F.; Montaudo, M.S.; Carroccio, S.; Puglisi, C. Current Trends in Matrix-Assisted Laser Desorption/Ionization of Polymeric Materials. Eur. J. Mass Spectrom. 2017, 11, 1–14. [Google Scholar] [CrossRef]
- Montaudo, G.; Carroccio, S.; Montaudo, M.S.; Puglisi, C.; Samperi, F. Recent Advances in MALDI Mass Spectrometry of Polymers. Macromol. Symp. 2001, 169, 101–112. [Google Scholar] [CrossRef]
- Carroccio, S.; Puglisi, C.; Montaudo, G. MALDI Investigation of the Photooxidation of Nylon-66. Macromolecules 2004, 37, 6037–6049. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Ussia, M.; Mendichi, R.; Zampino, D.; Puglisi, C.; Carroccio, S.C. Halloysite nanotubes and thymol as photo protectors of biobased polyamide 11. Polym. Degrad. Stab. 2018, 152, 43–51. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Qi, X.-D.; Yang, J.-H.; Zhang, N.; Huang, T.; Zhou, Z.-W.; Kühnert, I.; Pötschke, P.; Wang, Y. Selective localization of carbon nanotubes and its effect on the structure and properties of polymer blends. Prog. Polym. Sci. 2021, 123, 101471. [Google Scholar] [CrossRef]
- Wu, D.; Lin, D.; Zhang, J.; Zhou, W.; Zhang, M.; Zhang, Y.; Wang, D.; Lin, B. Selective Localization of Nanofillers: Effect on Morphology and Crystallization of PLA/PCL Blends. Macromol. Chem. Phys. 2011, 212, 613–626. [Google Scholar] [CrossRef]
- Helal, E.; Pottier, C.; David, E.; Fréchette, M.; Demarquette, N.R. Polyethylene/thermoplastic elastomer/Zinc Oxide nanocomposites for high voltage insulation applications: Dielectric, mechanical and rheological behavior. Eur. Polym. J. 2018, 100, 258–269. [Google Scholar] [CrossRef]
| Tmax (°C) | Tmax2 (°C) | T5% a | Residue at 700 °C (%/°C) | |
|---|---|---|---|---|
| PA11 | 425 | 381.3 | 0.00 | |
| PA11_0.5 ZnO | 455 | 397.2 | 0.52 | |
| PA11_1 ZnO | 453 | 414.2 | 1.04 | |
| PA11_2 ZnO | 445 | 397.6 | 2.9 | |
| PLA | 377 | 256.9 | 0.19 | |
| PLA_0.5 ZnO | 281 | 242.5 | 0.39 | |
| PLA_1 ZnO | 291 | 239.4 | 1.42 | |
| PLA_2 ZnO | 292 | 240.4 | 2.23 | |
| PLA/PA11 | 335.0 | 441.5 | 312.5 | 0.26 |
| PLA/PA11_0.5 ZnO | 350.9 | 453.3 | 301.2 | 0.83 |
| PLA/PA11_1 ZnO | 356.8 | 457.4 | 330.3 | 1.17 |
| PLA/PA11_2 ZnO | 278.5 | 456.8 | 241.7 | 3.72 |
| Sample | Mw a | PD b | ΔMw (%) c |
|---|---|---|---|
| PLA | 91,100 | 1.61 | 441 |
| PLA@ZnO 0.5 | 51,200 | 1.87 | 43 |
| PLA@ZnO 1 | 50,200 | 1.87 | 44 |
| PLA@ZnO 2 | 42,000 | 1.87 | 53 |
| Sample | Mw a | PD b | ΔMw (%) c |
|---|---|---|---|
| PLA/PA11 | 87,800 | 1.23 | 7 |
| PLA/PA11@ZnO_0.5 | 58,200 | 1.42 | 33.7 |
| PLA/PA11@ZnO_1 | 47,000 | 1.70 | 46.4 |
| PLA/PA11@ZnO_2 | 26,400 | 1.99 | 69.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puglisi, R.; Scamporrino, A.A.; Dintcheva, N.T.; Filippone, G.; Bruno, E.; Scarfato, P.; Cerruti, P.; Carroccio, S.C. Photo- and Water-Degradation Phenomena of ZnO Bio-Blend Based on Poly(lactic acid) and Polyamide 11. Polymers 2023, 15, 1434. https://doi.org/10.3390/polym15061434
Puglisi R, Scamporrino AA, Dintcheva NT, Filippone G, Bruno E, Scarfato P, Cerruti P, Carroccio SC. Photo- and Water-Degradation Phenomena of ZnO Bio-Blend Based on Poly(lactic acid) and Polyamide 11. Polymers. 2023; 15(6):1434. https://doi.org/10.3390/polym15061434
Chicago/Turabian StylePuglisi, Roberta, Andrea Antonino Scamporrino, Nadka Tzankova Dintcheva, Giovanni Filippone, Elena Bruno, Paola Scarfato, Pierfrancesco Cerruti, and Sabrina Carola Carroccio. 2023. "Photo- and Water-Degradation Phenomena of ZnO Bio-Blend Based on Poly(lactic acid) and Polyamide 11" Polymers 15, no. 6: 1434. https://doi.org/10.3390/polym15061434
APA StylePuglisi, R., Scamporrino, A. A., Dintcheva, N. T., Filippone, G., Bruno, E., Scarfato, P., Cerruti, P., & Carroccio, S. C. (2023). Photo- and Water-Degradation Phenomena of ZnO Bio-Blend Based on Poly(lactic acid) and Polyamide 11. Polymers, 15(6), 1434. https://doi.org/10.3390/polym15061434











