Ring Opening Polymerization Used for the Production of VOC Free High-Performance Ecofriendly Novel PBZ/PDA/CeO2 Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Monomer Synthesis
2.3. Polymer Synthesis
2.4. Fabrication of NCs
3. Results
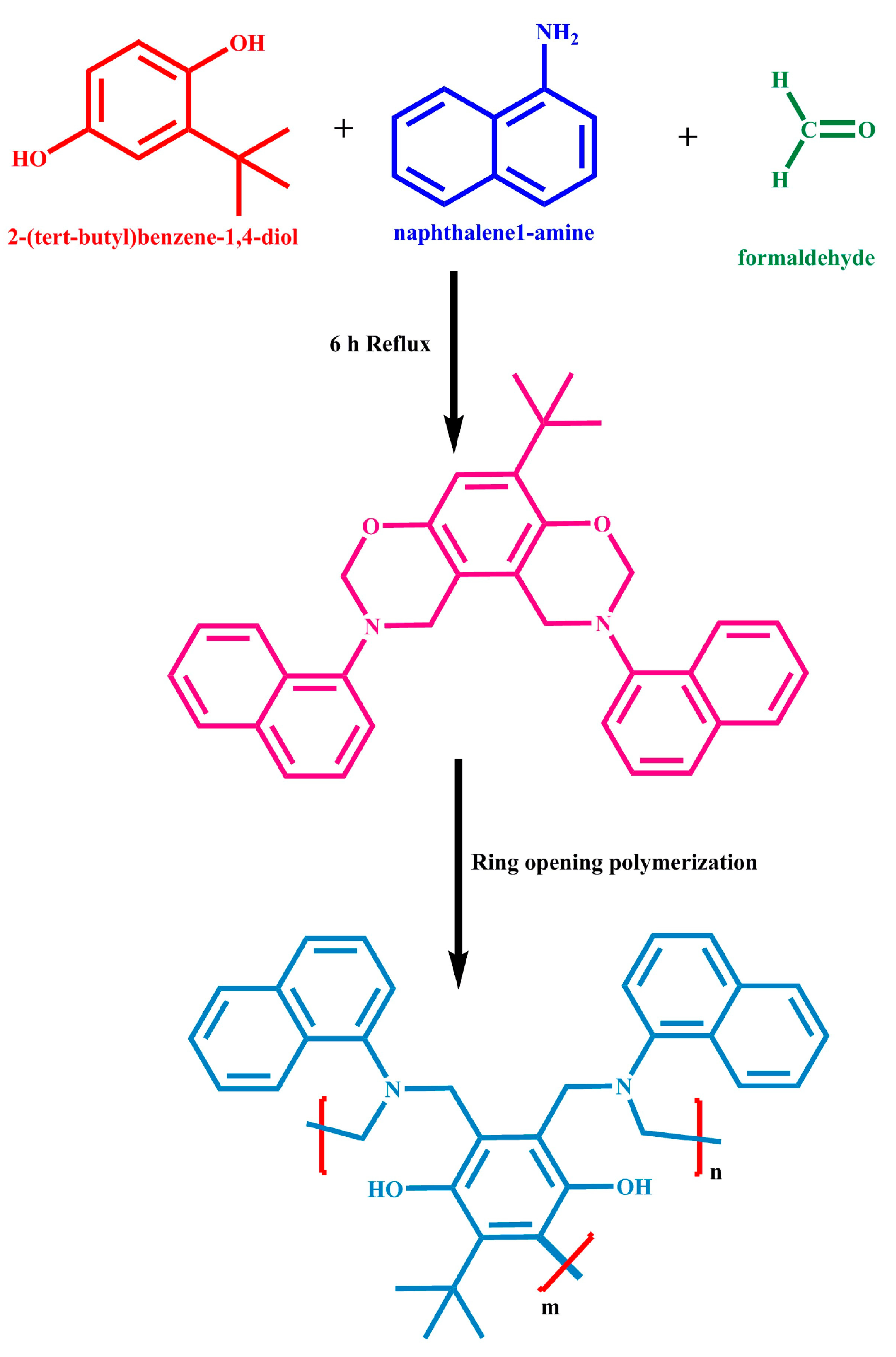
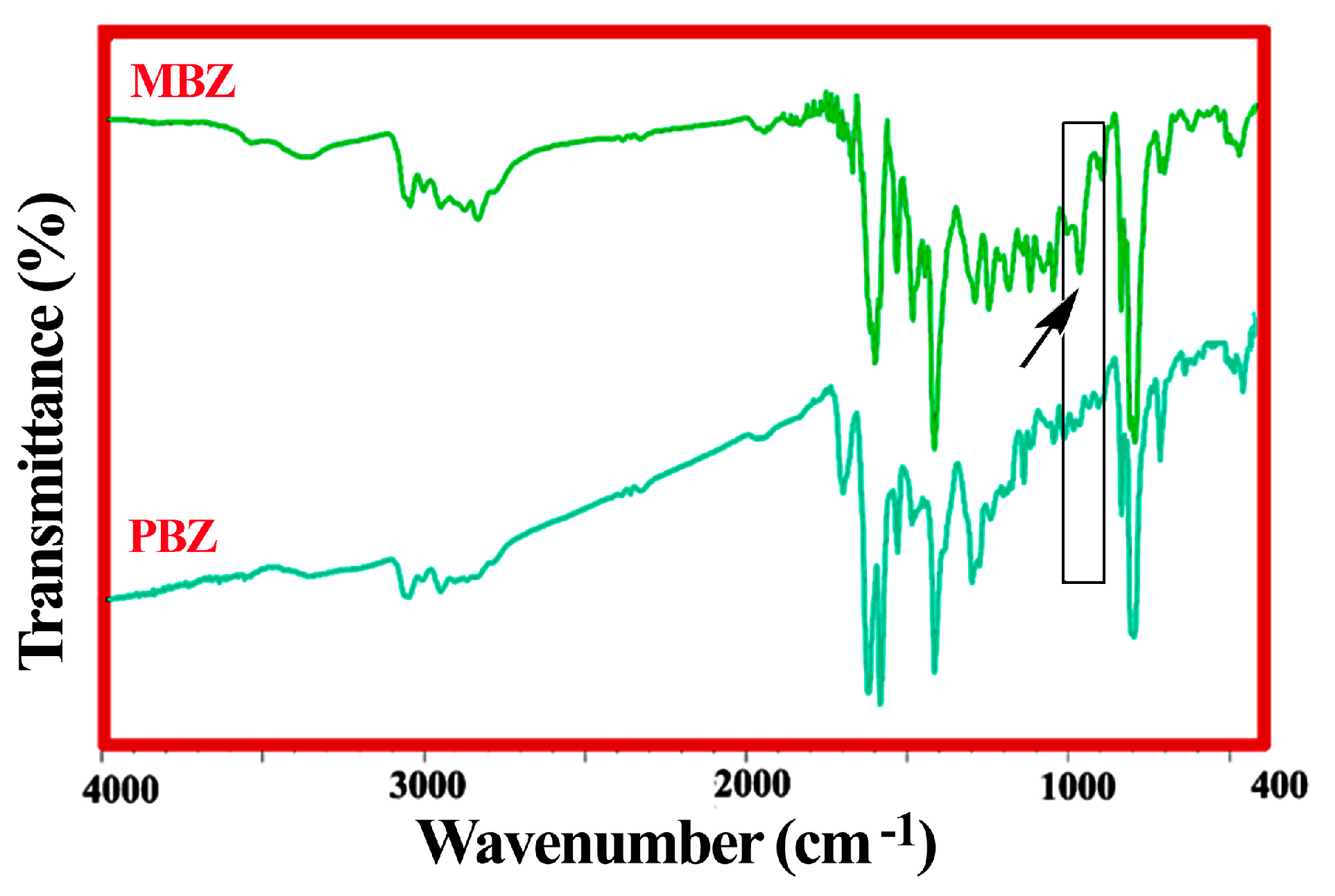
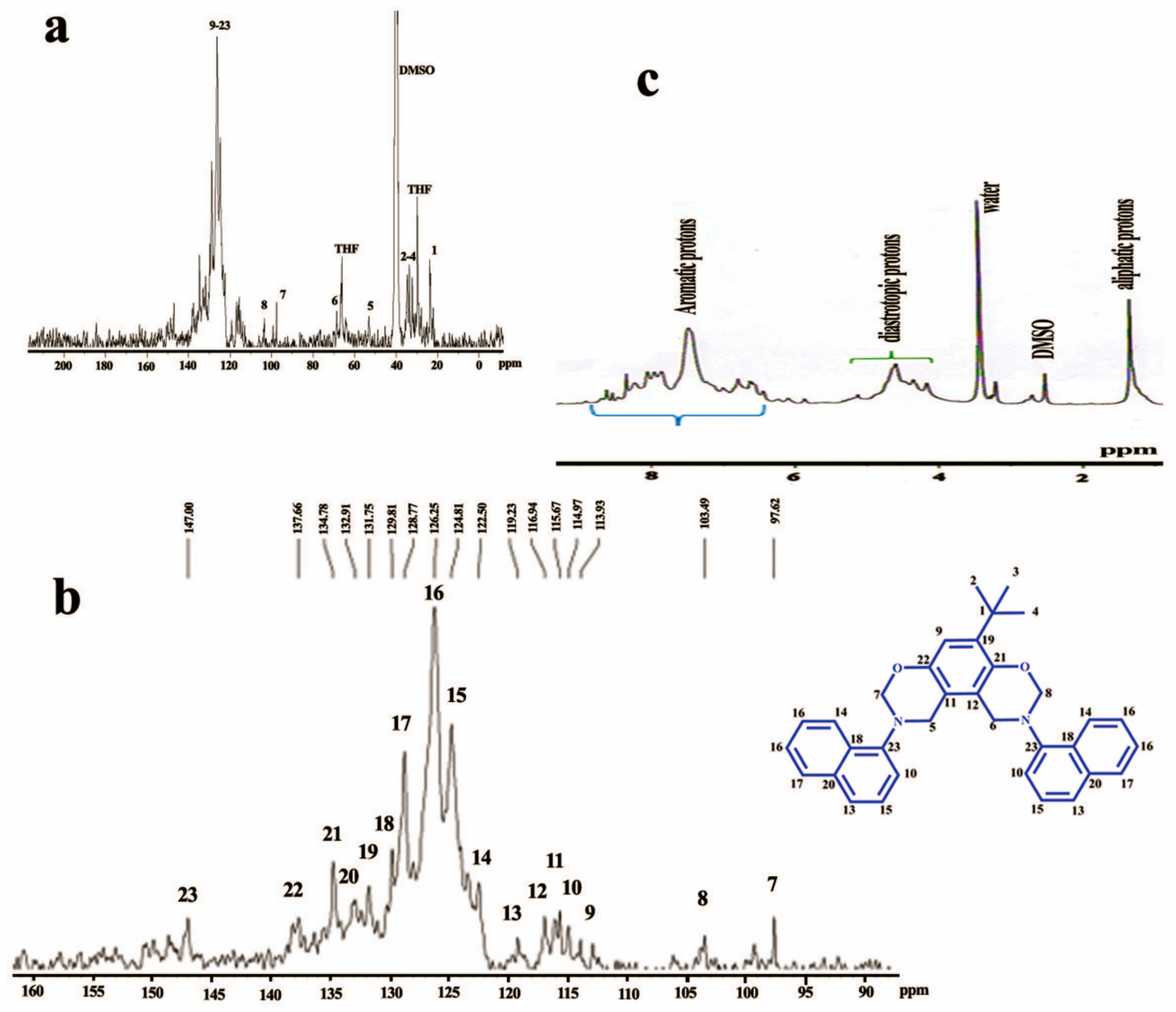
3.1. Design and Production of the MBZ Monomer, and the PBZ Polymer
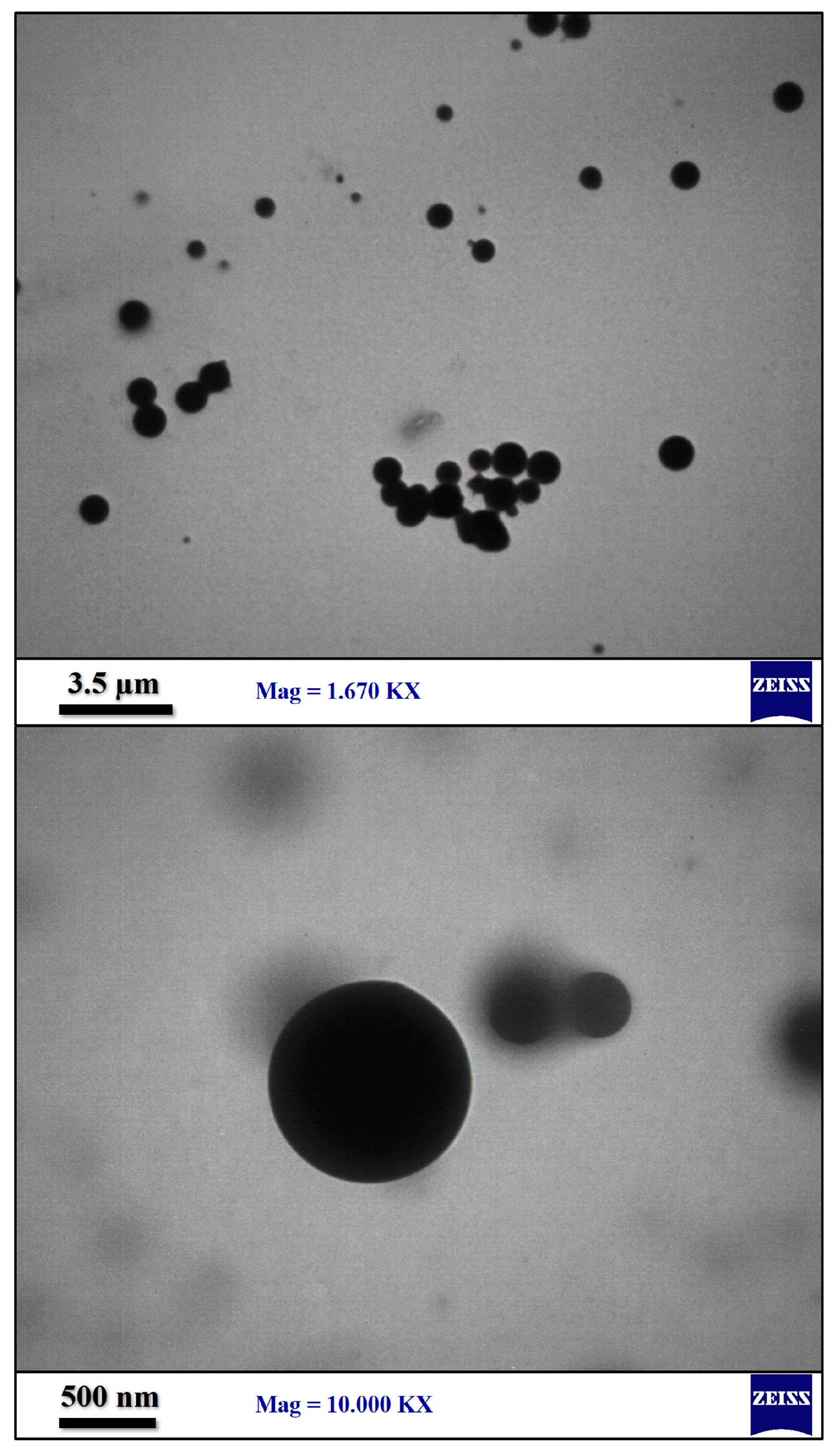
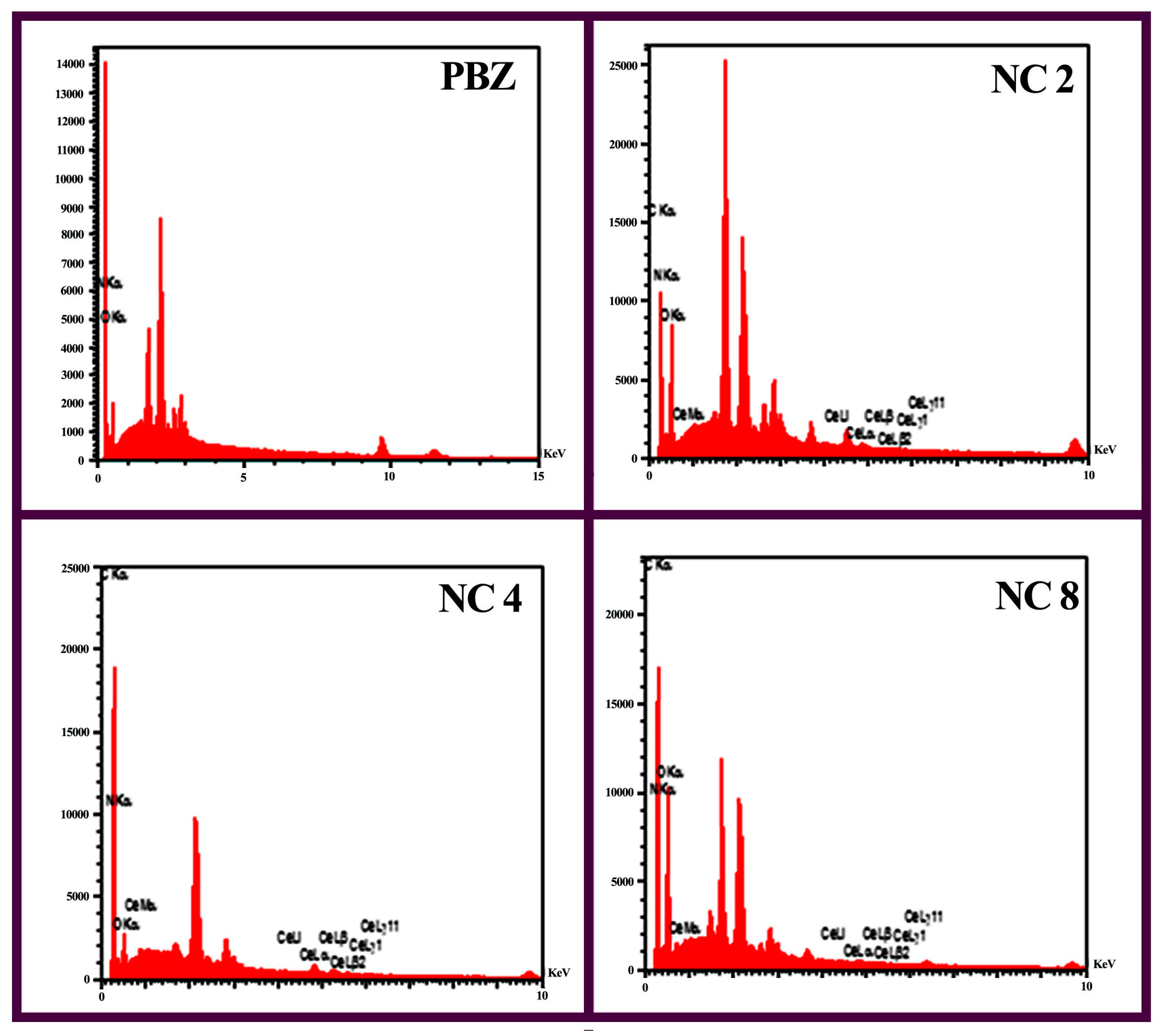
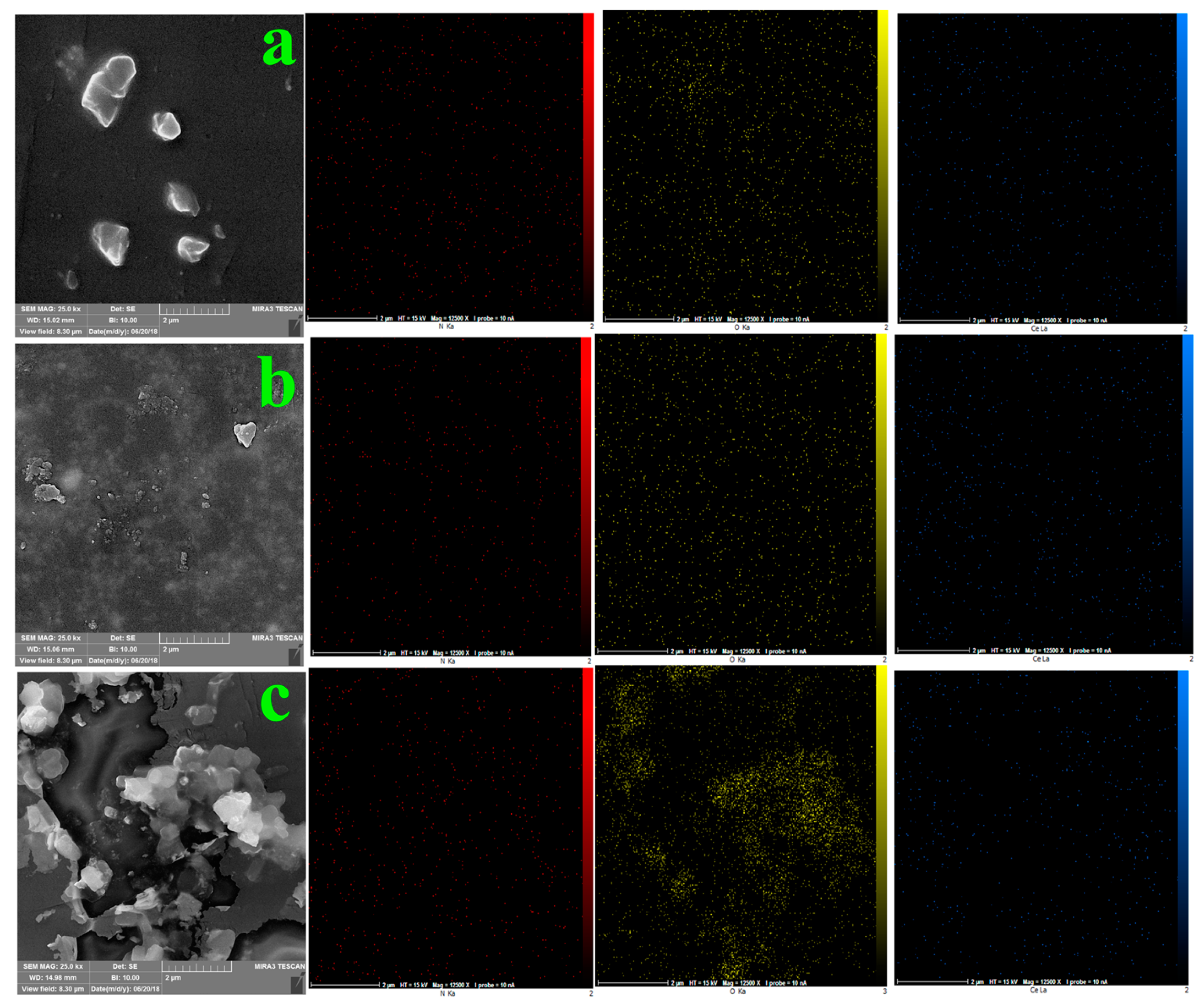
3.2. Surface Modification of Ceria NPs by Mussel Inspired Sonochemistry
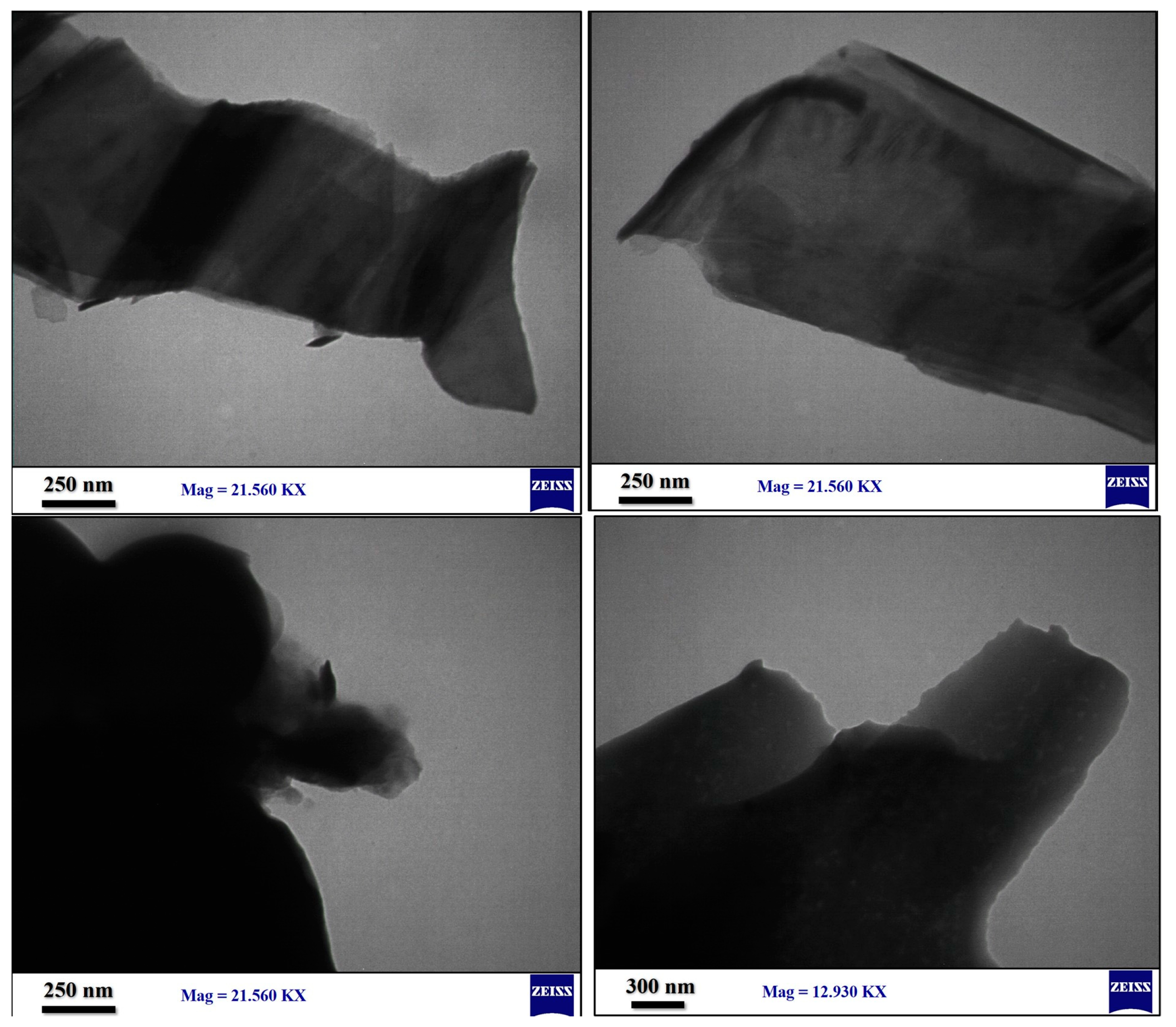
3.3. Nanocomposite Preparation and Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nair, C.P.R. Advances in Addition-Cure Phenolic Resins. Prog. Polym. Sci. 2004, 29, 401–498. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Li, T.; Zhu, P.; Zhuang, Q. Novel Fully Biobased Benzoxazines from Rosin: Synthesis and Properties. ACS Sustain. Chem. Eng. 2017, 5, 10682–10692. [Google Scholar] [CrossRef]
- Jin, L.; Agag, T.; Ishida, H. Bis(Benzoxazine-Maleimide)s as a Novel Class of High Performance Resin: Synthesis and Properties. Eur. Polym. J. 2010, 46, 354–363. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New High Performance Thermosetting Resins: Synthesis and Properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Kiskan, B.; Aydogan, B.; Yagci, Y. Synthesis, Characterization, and Thermally Activated Curing of Oligosiloxanes Containing Benzoxazine Moieties in the Main Chain. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 804–811. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, Z.; Ma, H.; Qiu, J.; Liu, C. Dendritic Organic–Inorganic Hybrid Polyphenol and Branched Benzoxazine Monomers with Low Curing Temperature. RSC Adv. 2014, 4, 53505–53513. [Google Scholar] [CrossRef]
- Zhu, C.; Gao, X.; Fan, W.; Fu, X. Synthesis, Characterization, and Properties of a Novel Aromatic Ester-Based Polybenzoxazine. RSC Adv. 2020, 10, 6953–6959. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K. Studies on the Isomeric Effect of Nitrile Functionality on the Polymerization and Thermal Properties of Ortho-Norbornene-Based Benzoxazine Resins. J. Polym. Res. 2020, 27, 1–8. [Google Scholar] [CrossRef]
- Zeng, K.; Li, H.; Shi, H.; Wu, J.; Xu, J.; Li, Y.; Zhao, C. Synthesis and Thermal Properties of Silicon-Containing Benzoxazine. High Perform. Polym. 2020, 32, 59–64. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Ohashi, S.; Liu, X.; Han, Z.; Ishida, H. Synthesis of High Thermal Stability Polybenzoxazoles via Ortho-Imide-Functional Benzoxazine Monomers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1330–1338. [Google Scholar] [CrossRef]
- Takeichi, T.; Kawauchi, T.; Agag, T. High Performance Polybenzoxazines as a Novel Type of Phenolic Resin. Polym. J. 2008, 40, 1121–1131. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, P.; Bai, Y.; Zhou, T.; Zhu, R.; Gu, Y. Polybenzoxazines: Thermal Responsiveness of Hydrogen Bonds and Application as Latent Curing Agents for Thermosetting Resins. ACS Omega 2017, 2, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, X.; Xin, Z.; Zhou, C. lu Surface Properties and Hydrogen Bonds of Mono-Functional Polybenzoxazines with Different N-Substituents. Chin. J. Polym. Sci. 2016, 34, 919–932. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ebrahium, S.M.; Hammam, A.S.; Kuo, S.W.; Aly, K.I. Enhanced CO2 Capture in Nitrogen-Enriched Microporous Carbons Derived from Polybenzoxazines Containing Azobenzene and Carboxylic Acid Units. J. Polym. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Vinnik, R.M.; Roznyatovsky, V.A. Kinetic Method by Using Calorimetry to Mechanism of Epoxy-Amine Cure Reaction. J. Therm. Anal. Calorim. 2004, 75, 753–764. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.Q.; Fang, Z.P.; Peng, M. Synthesis of Aromatic Diamine-Based Benzoxazines and Effect of Their Backbone Structure on Thermal and Flammability Properties of Polymers. Chin. J. Polym. Sci. 2013, 31, 1359–1371. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, J.; Lin, R.; Li, P. Improving the Thermal Stability of Polybenzoxazines Through Incorporation of Eugenol-Based Benzoxazine. Macromol. Res. 2019, 28, 472–479. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Benzoxazine-Based Thermosets with Autonomous Self-Healing Ability. Macromolecules 2015, 48, 1329–1334. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Post-Modification of Polybutadienes by Photoinduced Hydrogen Abstraction from Benzoxazines and Their Thermally Activated Curing. Macromolecules 2016, 49, 5026–5032. [Google Scholar] [CrossRef]
- Du, J.; Chen, A.; Liu, L.; Li, B.; Zhang, Y. N-Doped Hollow Mesoporous Carbon Spheres Prepared by Polybenzoxazines Precursor for Energy Storage. Carbon N. Y. 2020, 160, 265–272. [Google Scholar] [CrossRef]
- Imran, M.; Kiskan, B.; Yagci, Y. Concise Synthesis and Characterization of Unsymmetric 1,3-Benzoxazines by Tandem Reactions. Tetrahedron Lett. 2013, 54, 4966–4969. [Google Scholar] [CrossRef]
- Martos, A.; Soto, M.; Schäfer, H.; Koschek, K.; Marquet, J.; Sebastián, R.M. Highly Crosslinked Polybenzoxazines from Monobenzoxazines: The Effect of Meta-Substitution in the Phenol Ring. Polymers 2020, 12, 254. [Google Scholar] [CrossRef]
- Baqar, M.; Agag, T.; Huang, R.; Maia, J.; Qutubuddin, S.; Ishida, H. Mechanistic Pathways for the Polymerization of Methylol-Functional Benzoxazine Monomers. Macromolecules 2012, 45, 8119–8125. [Google Scholar] [CrossRef]
- Wang, M.W.; Jeng, R.J.; Lin, C.H. Study on the Ring-Opening Polymerization of Benzoxazine through Multisubstituted Polybenzoxazine Precursors. Macromolecules 2015, 48, 530–535. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, Y.L. Polyelectrolyte Composite Membranes of Polybenzimidazole and Crosslinked Polybenzimidazole-Polybenzoxazine Electrospun Nanofibers for Proton Exchange Membrane Fuel Cells. J. Mater. Chem. A 2012, 1, 1171–1178. [Google Scholar] [CrossRef]
- Kim, S.K.; Ko, T.; Choi, S.W.; Park, J.O.; Kim, K.H.; Pak, C.; Chang, H.; Lee, J.C. Durable Cross-Linked Copolymer Membranes Based on Poly(Benzoxazine) and Poly(2,5-Benzimidazole) for Use in Fuel Cells at Elevated Temperatures. J. Mater. Chem. 2012, 22, 7194–7205. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, S.W.; Jeon, W.S.; Park, J.O.; Ko, T.; Chang, H.; Lee, J.C. Cross-Linked Benzoxazine-Benzimidazole Copolymer Electrolyte Membranes for Fuel Cells at Elevated Temperature. Macromolecules 2012, 45, 1438–1446. [Google Scholar] [CrossRef]
- Ishida, H.; Sanders, D.P. Regioselectivity and Network Structure of Difunctional Alkyl-Substituted Aromatic Amine-Based Polybenzoxazines. Macromolecules 2000, 33, 8149–8157. [Google Scholar] [CrossRef]
- Ishida, H.; Sanders, D.P. Regioselectivity of the Ring-Opening Polymerization of Monofunctional Alkyl-Substituted Aromatic Amine-Based Benzoxazines. Polymer 2001, 42, 3115–3125. [Google Scholar] [CrossRef]
- Xu, G.; Mahmood, Q.; Lv, C.; Yang, R.; Zhou, L.; Wang, Q. Asymmetric kinetic resolution polymerization. Coord. Chem. Rev. 2020, 414, 213296. [Google Scholar] [CrossRef]
- Doma, A.S.; Kamoun, E.A.; Abboudy, S.; Belal, M.A.; Khattab, S.N.; El-Bardan, A.A. Influence of grafting of a polymeric antistatic membrane doped with orthophosphoric acid on its electrical properties. Chem. Eng. Technol. 2022, 45, 1158–1171. [Google Scholar] [CrossRef]
- Hatami, M.; Sharifi, A.; Karimi-Maleh, H.; Agheli, H.; Karaman, C. Simultaneous Improvements in Antibacterial and Flame Retardant Properties of PET by Use of Bio-Nanotechnology for Fabrication of High Performance PET Bionanocomposites. Environ. Res. 2022, 206, 112281. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Rahnama, N.; Karimi-Maleh, H.; Djafarzadeh, N.; Qandalee, M.; Setva, R.; Karimi, F.; Durán-Valle, C.J.; López-Coca, I.M.; Sharifi, A. Use of Phosphorylated Chitosan/Alumina Nanoadditives for Polymer Performance Improvement. Cellulose 2022, 29, 6677–6696. [Google Scholar] [CrossRef]
- Hatami, M. Production of Polyimide Ceria Nanocomposites by Development of Molecular Hook Technology in Nano-Sonochemistry. Ultrason. Sonochem. 2018, 44, 261–271. [Google Scholar] [CrossRef]
- Hatami, M.; Azarkar, B.F.; Qandalee, M.; Hasanabadi, H. Morphological Investigation of Synthetic Poly(Amic Acid)/Cerium Oxide Nanostructures. Polym. Eng. Sci. 2015, 55, 2339–2348. [Google Scholar] [CrossRef]
- Abdel-Gawad, A.M.; Ramadan, A.R.; Flores, A.; Esawi, A.M.K. Fabrication of Nylon 6-Montmorillonite Clay Nanocomposites with Enhanced Structural and Mechanical Properties by Solution Compounding. Polymers 2022, 14, 4471. [Google Scholar] [CrossRef]
- Alshaikhi, H.A.; Asiri, A.M.; Alamry, K.A.; Marwani, H.M.; Alfifi, S.Y.; Khan, S.B. Copper Nanoparticles Decorated Alginate/Cobalt-Doped Cerium Oxide Composite Beads for Catalytic Reduction and Photodegradation of Organic Dyes. Polymers 2022, 14, 4458. [Google Scholar] [CrossRef] [PubMed]
- Elmesallamy, S.M.; Fekry, M.; Hussein, L.I.; Abdelwahab, M.A.; Bakry, A. Polybenzoxazine/Carbon Nanotube Nanocomposites as a Polymeric Sensing Material for Volatile Organic Compounds. J. Polym. Res. 2022, 29, 1–10. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Ran, Q. Facile Preparation and Properties of Polybenzoxazine/Graphene Porous Nanocomposites for Electromagnetic Wave Absorption. Polym. Eng. Sci. 2022, 62, 2580–2591. [Google Scholar] [CrossRef]
- Farhanian, S.; Hatami, M. Thermal and Morphological Aspects of Silver Decorated Halloysite Reinforced Polypropylene Nanocomposites. J. Therm. Anal. Calorim. 2017, 130, 2069–2078. [Google Scholar] [CrossRef]
- Hatami, M. Production and Morphological Characterization of Low Resistance Polyimide/Silver Nanowire Nanocomposites: Potential Application in Nanoconductive Adhesives. J. Mater. Sci. Mater. Electron. 2017, 28, 3897–3908. [Google Scholar] [CrossRef]
- Vengatesan, M.R.; Devaraju, S.; Kannaiyan, D.; Song, J.K.; Alagar, M. Ultrasound-Assisted Synthesis of Benzoxazine Monomers: Thermal and Mechanical Properties of Polybenzoxazines. Polym. Int. 2013, 62, 127–133. [Google Scholar] [CrossRef]
- Rajamanikam, R.; Pichaimani, P.; Kumar, M.; Muthukaruppan, A. Optical and Thermomechanical Behavior of Benzoxazine Functionalized ZnO Reinforced Polybenzoxazine Nanocomposites. Polym. Compos. 2017, 38, 1881–1889. [Google Scholar] [CrossRef]
- Hatami, M.; Yazdan Panah, M. Ultrasonic Assisted Synthesis of Nanocomposite Materials Based on Resole Resin and Surface Modified Nano CeO2: Chemical and Morphological Aspects. Ultrason. Sonochem. 2017, 39, 160–173. [Google Scholar] [CrossRef]
- Ahmadi, M.; Rad-Moghadam, K.; Hatami, M. From Parkinson’s Chemotropic Agent l-Dopa to Thermally Resistive Carbonaceous Nanocomposite of a New Catechol-Grafted Poly(Amide-Imide). Polymer 2018, 149, 1–12. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khani, Z. An Eco-Friendly Method for the Preparation of Poly(N-Vinyl-2-Pyrrolidone)–Poly(Vinyl Alcohol) Blend Nanocomposite Films Containing Vitamin B1-Modified Silica Nanoparticles to Enhance Thermal and Wettability Properties. Polym. Bull. 2020, 77, 1489–1502. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 1997; p. 875. [Google Scholar]









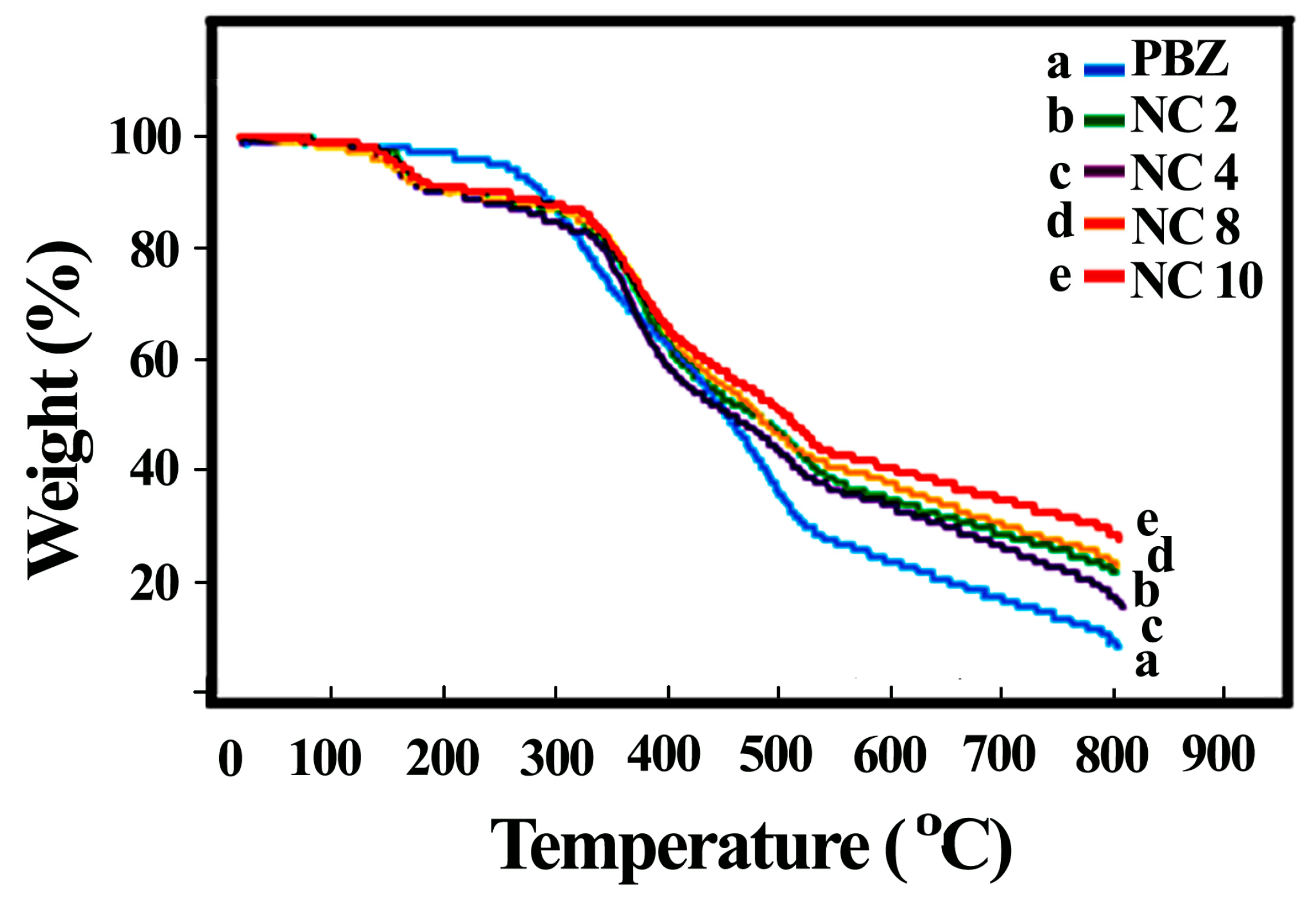
| Sample | T5 (°C) a | T10 (°C) b | Char Yield (%) c | LOI d | ∆Hcomb |
|---|---|---|---|---|---|
| PBZ | 243 | 294 | 9 | 17.53 | 456.36 |
| NC 2 | 164 | 209 | 22 | 17.58 | 455.06 |
| NC 4 | 155 | 195 | 16 | 17.56 | 455.58 |
| NC 8 | 154 | 210 | 23 | 17.59 | 454.80 |
| NC 10 | 163 | 221 | 28 | 17.61 | 454.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatami, M.; Abadi, Z.S.N.; Yousefi, A.; Qandalee, M.; Djafarzadeh, N.; Ghasemi, Y.; López-Coca, I.M.; Durán-Valle, C.J. Ring Opening Polymerization Used for the Production of VOC Free High-Performance Ecofriendly Novel PBZ/PDA/CeO2 Nanocomposites. Polymers 2023, 15, 1416. https://doi.org/10.3390/polym15061416
Hatami M, Abadi ZSN, Yousefi A, Qandalee M, Djafarzadeh N, Ghasemi Y, López-Coca IM, Durán-Valle CJ. Ring Opening Polymerization Used for the Production of VOC Free High-Performance Ecofriendly Novel PBZ/PDA/CeO2 Nanocomposites. Polymers. 2023; 15(6):1416. https://doi.org/10.3390/polym15061416
Chicago/Turabian StyleHatami, Mehdi, Zohreh Seifi Najaf Abadi, Alireza Yousefi, Mohammad Qandalee, Nader Djafarzadeh, Yaser Ghasemi, Ignacio M. López-Coca, and Carlos J. Durán-Valle. 2023. "Ring Opening Polymerization Used for the Production of VOC Free High-Performance Ecofriendly Novel PBZ/PDA/CeO2 Nanocomposites" Polymers 15, no. 6: 1416. https://doi.org/10.3390/polym15061416
APA StyleHatami, M., Abadi, Z. S. N., Yousefi, A., Qandalee, M., Djafarzadeh, N., Ghasemi, Y., López-Coca, I. M., & Durán-Valle, C. J. (2023). Ring Opening Polymerization Used for the Production of VOC Free High-Performance Ecofriendly Novel PBZ/PDA/CeO2 Nanocomposites. Polymers, 15(6), 1416. https://doi.org/10.3390/polym15061416








