Synthesis of Furan-Based Diamine and Its Application in the Preparation of Bio-Based Polyimide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
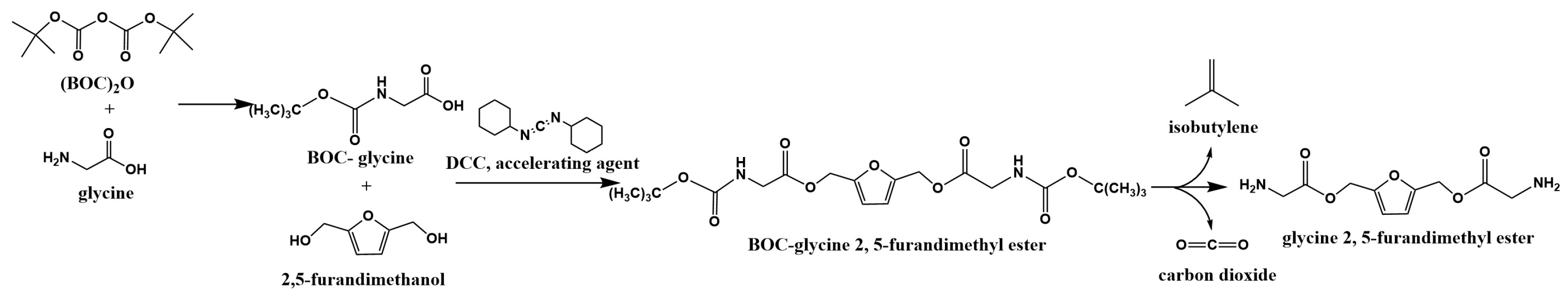
2.2. Preparation of BOC-Glycine
2.3. Preparation of BOC-Glycine 2,5-Furandimethyl Ester
2.4. The Removal of BOC Protection
2.5. Preparation of Polyimide Membrane
2.6. Characterization
3. Results and Discussion
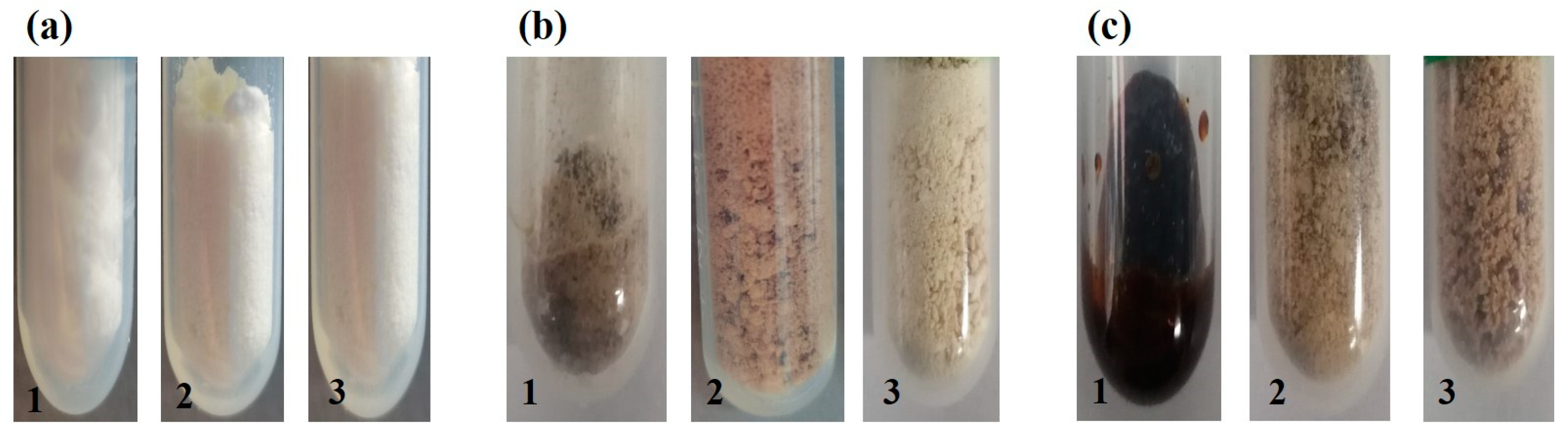
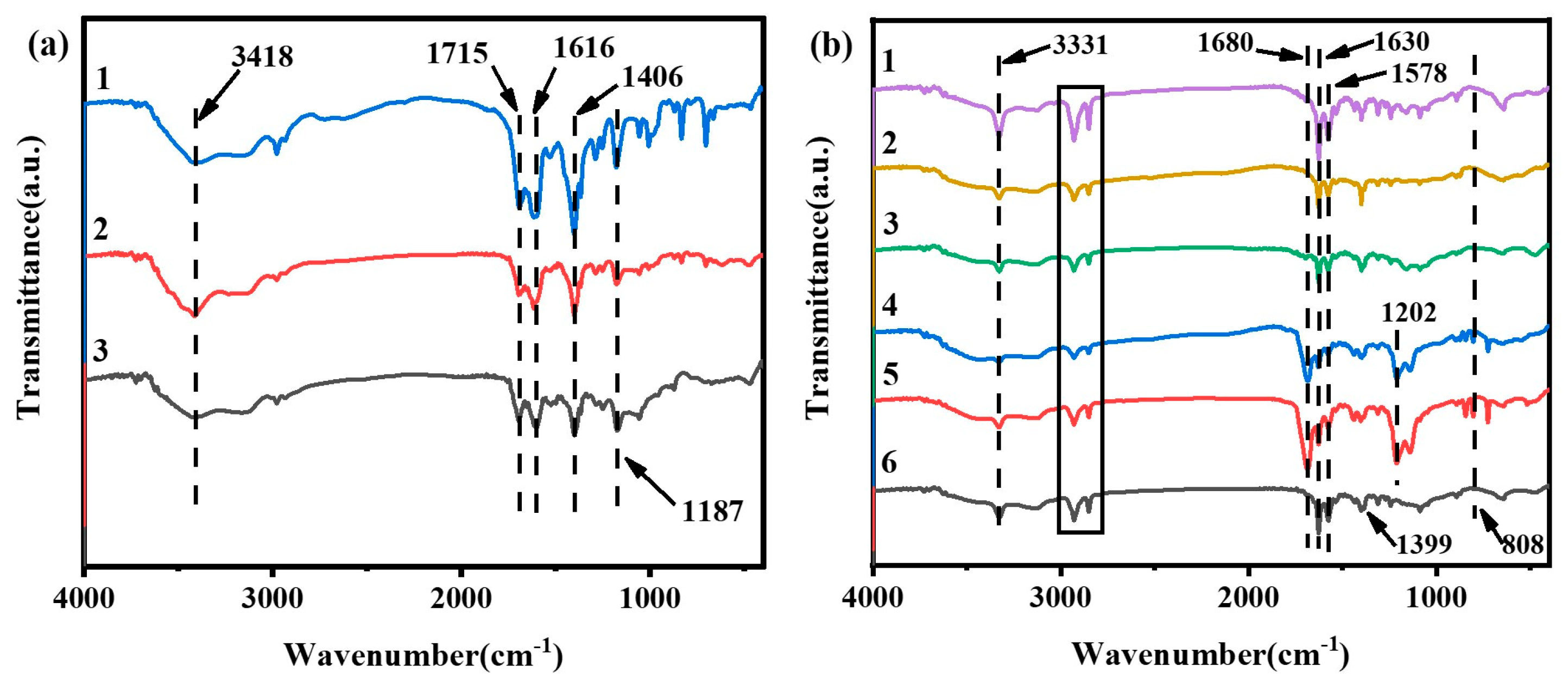
3.1. Characterization of BOC-Glycine and Ester
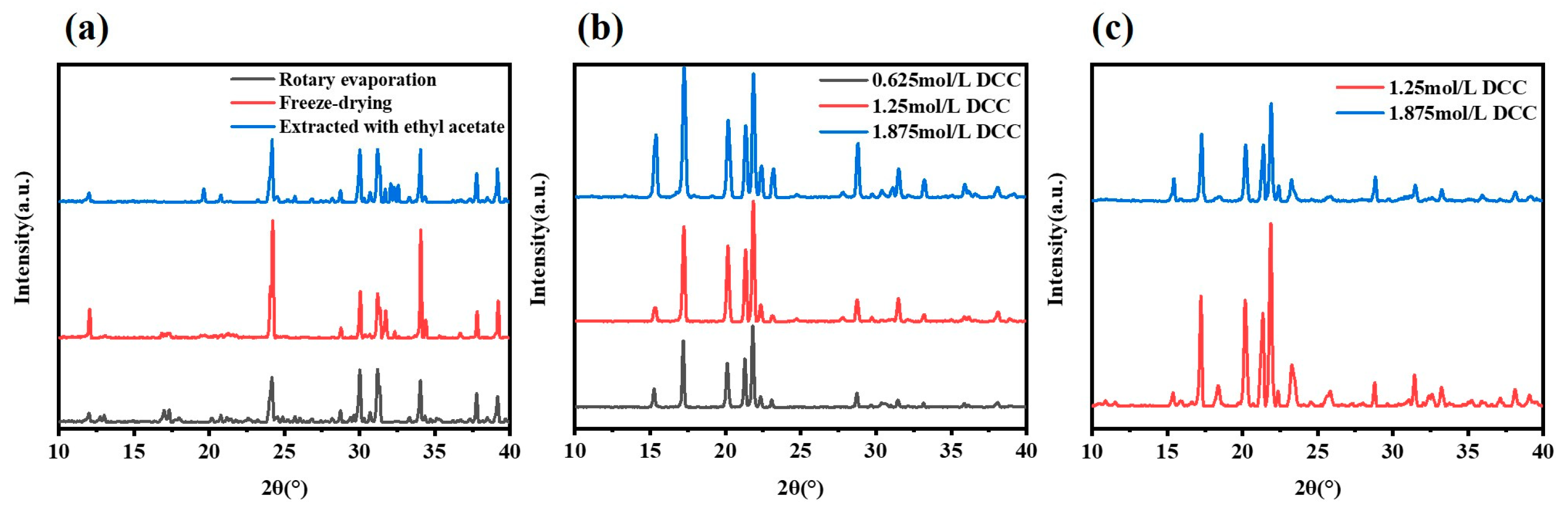
3.2. Attempt of Application of Glycine 2,5-Furandimethyl Ester
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, H.; Wang, Y.; Hua, R. Correction: Acid-catalyzed carboxylic acid esterification and ester hydrolysis mechanism: Acylium ion as a sharing active intermediate via a spontaneous trimolecular reaction based on density functional theory calculation and supported by electrospray ionization-mass spectrometry. Phys. Chem. Chem. Phys. 2015, 17, 32571–32573. [Google Scholar] [CrossRef]
- Ahmad, N.; Javed, F.; Awan, J.A.; Ali, S.; Fazal, T.; Hafeez, A.; Aslam, R.; Rashid, N.; Rehman, M.S.U.; Zimmerman, W.B.; et al. Biodiesel production intensification through microbubble mediated esterification. Fuel 2019, 253, 25–31. [Google Scholar] [CrossRef]
- Ganesh, B.; Rani, K.Y.; Satyavathi, B.; Patnaik, K.S.K.R. Experimental analysis in different batch operating units for process intensification: Methyl acetate production case study. Int. J. Ind. Chem. 2014, 5, 85–93. [Google Scholar] [CrossRef]
- Zhen, B.; Jiao, Q.; Zhang, Y.; Wu, Q.; Li, H. Acidic ionic liquid immobilized on magnetic mesoporous silica: Preparation and catalytic performance in esterification. Appl. Catal. A Gen. 2012, 445–446, 239–245. [Google Scholar] [CrossRef]
- Tan, S.X.; Ong, H.C.; Lim, S.; Pang, Y.L.; Milano, J. Process intensification of biodiesel synthesis via ultrasound-assistedin situesterification ofJatrophaoil seeds. J. Chem. Technol. Biotechnol. 2018, 94, 1362–1373. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwinjr, J. A comparison of the esterification of acetic acid with methanol using heterogeneous versus homogeneous acid catalysis. J. Catal. 2006, 242, 278–286. [Google Scholar] [CrossRef]
- Peng, X.; Wang, L. Design and Control of Ionic Liquid-Catalyzed Reactive Distillation forn-Butyl Acetate Production. Chem. Eng. Technol. 2015, 38, 223–234. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, F.; Deng, Y. Esterification of aliphatic acids with olefin promoted by Brønsted acidic ionic liquids. J. Mol. Catal. A Chem. 2004, 212, 71–75. [Google Scholar] [CrossRef]
- Duan, Z.; Gu, Y.; Zhang, J.; Zhu, L.; Deng, Y. Protic pyridinium ionic liquids: Synthesis, acidity determination and their performances for acid catalysis. J. Mol. Catal. A Chem. 2006, 250, 163–168. [Google Scholar] [CrossRef]
- Liu, S.; Xie, C.; Yu, S.; Liu, F.; Ji, K. Esterification of α-pinene and acetic acid using acidic ionic liquids as catalysts. Catal. Commun. 2008, 9, 1634–1638. [Google Scholar] [CrossRef]
- Wang, A.-Q.; Wu, X.-L.; Wang, J.-X.; Pan, H.; Tian, X.-Y.; Xing, Y.-L. A novel solid acid catalyst synthesized from ZnAl2O4 spinel and its application in the esterification of acetic acid and n-butyl alcohol. RSC Adv. 2015, 5, 19652–19658. [Google Scholar] [CrossRef]
- Dechakhumwat, S.; Hongmanorom, P.; Thunyaratchatanon, C.; Smith, S.M.; Boonyuen, S.; Luengnaruemitchai, A. Catalytic activity of heterogeneous acid catalysts derived from corncob in the esterification of oleic acid with methanol. Renew. Energy 2020, 148, 897–906. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.; Shi, K.; Wu, T.; He, F.; Yang, H.; Zhang, S.; Peng, C. Nanocarbon-based catalysts for esterification: Effect of carbon dimensionality and synergistic effect of the surface functional groups. Carbon 2019, 147, 134–145. [Google Scholar] [CrossRef]
- Yadav, G.D.; Goel, P.K. Selective synthesis of perfumery grade cyclohexyl esters from cyclohexene and carboxylic acids over ion exchange resins: An example of 100% atom economy. Green Chem. 2000, 2, 71–78. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, Q.W.; Yang, X.L.; Chatterjee, S.; Pittman, C.U., Jr. Sulfonic acid resin-catalyzed addition of phenols, carboxylic acids, and water to olefins: Model reactions for catalytic upgrading of bio-oil. Bioresour. Technol. 2010, 101, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Dijs, I.J.; Ochten, H.L.F.V.; Heijden, A.J.M.V.D.; Geus, J.W.; Jenneskens, L.W. The catalytic performance of sulphonated cross-linked polystyrene beads in the formation of isobornyl acetate. Appl. Catal. A Gen. 2003, 241, 185–203. [Google Scholar] [CrossRef]
- Van Zadelhoff, A.; Vincken, J.P.; de Bruijn, W.J.C. Facile Amidation of Non-Protected Hydroxycinnamic Acids for the Synthesis of Natural Phenol Amides. Molecules 2022, 27, 2203. [Google Scholar] [CrossRef]
- Fahim, A.M.; Mohamed, A.; Ibrahim, M.A. Experimental and theoretical studies of some propiolate esters derivatives. J. Mol. Struct. 2021, 1236, 130281. [Google Scholar] [CrossRef]
- Adel, F.; Shaaban, A.-F.F.; El-Dougdoug, W.; Tantawy, A.H.; Metwally, A.M. Novel synthesized amide-incorporating copolymeric surfactants based on natural wastes as petro-dispersing agents: Design, synthesis, and characterizations. J. Mol. Liq. 2022, 367, 120579. [Google Scholar] [CrossRef]
- Mohamed, M.; Kuo, S. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2018, 11, 26. [Google Scholar] [CrossRef]
- Govindaraj, B.; Sundararajan, P.; Sarojadevi, M. Synthesis and characterization of polyimide/polyhedral oligomeric silsesquioxane nanocomposites containing quinolyl moiety. Polym. Int. 2012, 61, 1344–1352. [Google Scholar] [CrossRef]
- Ando, S.; Harada, M.; Okada, T.; Ishige, R. Effective Reduction of Volumetric Thermal Expansion of Aromatic Polyimide Films by Incorporating Interchain Crosslinking. Polymers 2018, 10, 761. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, P.I.; Popoola, O.M.; Adeosun, S.O. A review on recent advances on improving polyimide matrix nanocomposites for mechanical, thermal, and tribological applications: Challenges and recommendations for future improvement. J. Thermoplast. Compos. Mater. 2021, 36, 1. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yu, H.C.; Lee, J.; Jeon, J.; Im, J.; Jang, J.; Jin, S.W.; Kim, K.K.; Cho, S.; Chung, C.M. Preparation of Polyimide/Graphene Oxide Nanocomposite and Its Application to Nonvolatile Resistive Memory Device. Polymers 2018, 10, 901. [Google Scholar] [CrossRef]
- Chi, Q.; Sun, J.; Zhang, C.; Liu, G.; Lin, J.; Wang, Y.; Wang, X.; Lei, Q. Enhanced dielectric performance of amorphous calcium copper titanate/polyimide hybrid film. J. Mater. Chem. C 2014, 2, 172–177. [Google Scholar] [CrossRef]
- Fan, H.; Yang, R. Flame-Retardant Polyimide Cross-Linked with Polyhedral Oligomeric Octa(aminophenyl)silsesquioxane. Ind. Eng. Chem. Res. 2013, 52, 2493–2500. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Ruan, B.; Zhang, X.; Jiang, T.; Ma, N.; Tsai, F.C. Design and Synthesis of Polyimide Covalent Organic Frameworks. Macromol. Rapid Commun. 2020, 41, 2000402. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. Furan Polymers: State of the Art and Perspectives. Macromol. Mater. Eng. 2022, 307, 2100902. [Google Scholar] [CrossRef]
- Kumar, A.; Tateyama, S.; Yasaki, K.; Ali, M.A.; Takaya, N.; Singh, R.; Kaneko, T. Ultrahigh performance bio-based polyimides from 4,4’-diaminostilbene. Polymer 2016, 83, 182–189. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Ullah, A.; Iftikhar, F.; Arfan, M.; Batool Kazmi, S.T.; Anjum, M.N.; Haq, I.U.; Ayaz, M.; Farooq, S.; Rashid, U. Amino acid conjugated antimicrobial drugs: Synthesis, lipophilicity- activity relationship, antibacterial and urease inhibition activity. Eur. J. Med. Chem. 2018, 145, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Man, D.; Zhengliu, X.; Hui, Z.; Xing, L.; Lu, K.; Ziru, H.; Liyuan, G.; Shi, W. Effect of Different Solvent Systems on Amino Protection of Amino Acids by Boc. Chem. Bioeng. 2020, 37, 33–36. [Google Scholar]
- Tarikere, L.G.; Ramasubbu, N.; Levine, M.J. Solid-phase synthesis of human salivary mucin-derived O-linked glycopeptides. Lett. Pept. Sci. 1996, 3, 79–88. [Google Scholar]
- Narayan, B.; Dharmaprakash, S. Growth of nonlinear optical g-glycine crystals. J. Cryst. Growth 2002, 236, 376–380. [Google Scholar]
- Zhang, S.; Lu, X.; Cai, M.; Wang, Z.; Han, Z.; Chen, Z.; Liu, R.; Li, K.; Min, Y. Attapulgite Nanorod-Incorporated Polyimide Membrane for Enhanced Gas Separation Performance. Polymers 2022, 14, 5391. [Google Scholar] [CrossRef]



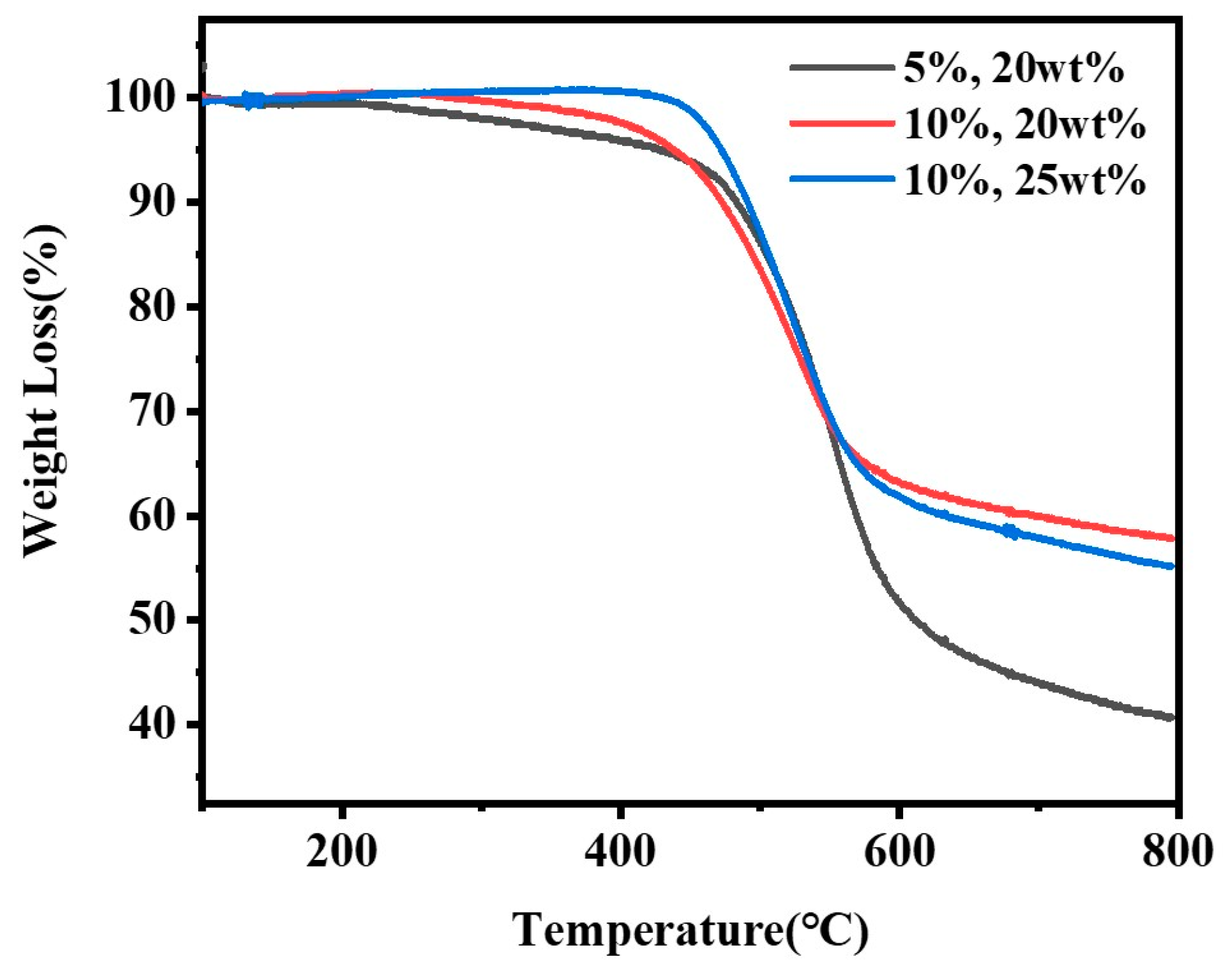
| Number | The Dosage of Diamine/g | The Dosage of Dianhydride/g | The Dosage of Solvent/g | Maximum Molecular Weight Average/Mw | Name | |
|---|---|---|---|---|---|---|
| Glycine 2,5-Furandimethyl Ester | ODA | PMDA | DMAc | |||
| 1 | 0.1924(5 wt%) | 3.6552(95 wt%) | 4.1524 | 32(80 wt%) | 392,046 | 5%, 20 wt% |
| 2 | 0.3867(10 wt%) | 3.4806(90 wt%) | 4.1327 | 32(80 wt%) | 459,361 | 10%, 20 wt% |
| 3 | 0.4809(10 wt%) | 4.3286(90 wt%) | 5.1905 | 30(75 wt%) | 563,731 | 10%, 25 wt% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, L.; He, Y.; Luo, W.; Li, K.; Min, Y. Synthesis of Furan-Based Diamine and Its Application in the Preparation of Bio-Based Polyimide. Polymers 2023, 15, 1088. https://doi.org/10.3390/polym15051088
Zhang Y, Chen L, He Y, Luo W, Li K, Min Y. Synthesis of Furan-Based Diamine and Its Application in the Preparation of Bio-Based Polyimide. Polymers. 2023; 15(5):1088. https://doi.org/10.3390/polym15051088
Chicago/Turabian StyleZhang, Yao, Lei Chen, Yima He, Weiyu Luo, Kaixin Li, and Yonggang Min. 2023. "Synthesis of Furan-Based Diamine and Its Application in the Preparation of Bio-Based Polyimide" Polymers 15, no. 5: 1088. https://doi.org/10.3390/polym15051088
APA StyleZhang, Y., Chen, L., He, Y., Luo, W., Li, K., & Min, Y. (2023). Synthesis of Furan-Based Diamine and Its Application in the Preparation of Bio-Based Polyimide. Polymers, 15(5), 1088. https://doi.org/10.3390/polym15051088





